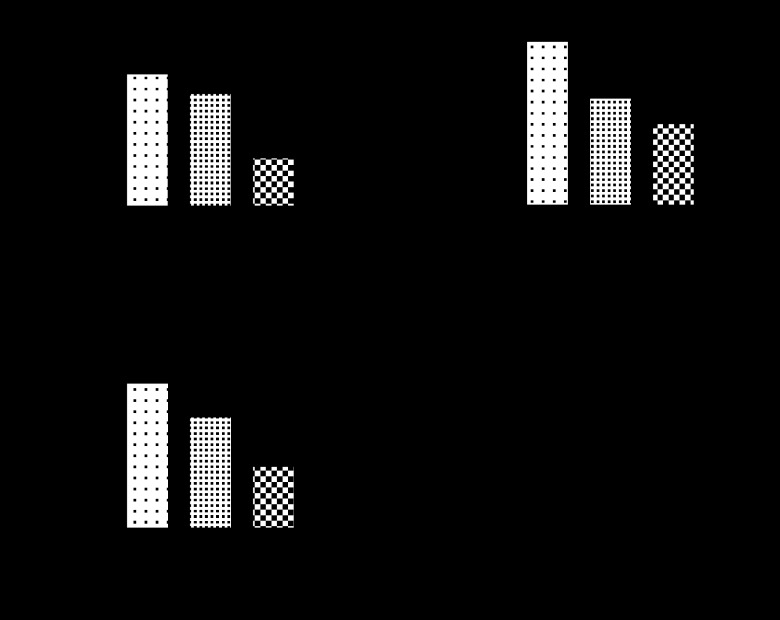Abstract
Stroke is a major cause of both death and disability. However, there are no pharmacological treatments used in most countries other than recombinant tissue plasminogen activator, a thrombolytic, and this is only used in about 4% of patients presenting after an acute ischaemic stroke. One novel thrombolytic (desmoteplase) has just been reported to have failed in a Phase IIb/III trial, but other thrombolytics and reperfusion agents remain in development. The picture with neuroprotectant agents, that is compounds that act to preserve neurones following an acute cerebral ischaemic insult, is even more bleak. Despite the development of over 1000 compounds, many proving effective in animal models of stroke, none has demonstrated efficacy in patients in the over 100 clinical trials conducted. This includes NXY-059, which was developed in accordance with the guidelines proposed by an academic-industry roundtable group (STAIR). This review examines the available data on compounds currently in development. It also proposes that the failure of translation between efficacy in preclinical models and patients is likely to terminate most current neuroprotective drug development. It is suggested that animal models must be made more representative of the patient condition (with other co-morbid conditions) and suggests that since stroke is primarily a cardiovascular disease with a neurological outcome, more research on the neurovascular unit would be valuable. New approaches on neuroinflammation, neurorestoration and neurorepair are also likely to gain prominence in the search for new drugs to treat this major clinical problem.
Keywords: tissue plasminogen activator, NXY-059, STAIR criteria, neuroprotection, thrombolysis, cerebral ischaemia, stroke
The burden of stroke
There are many excellent reviews on the economic and social consequences of stroke (Payne et al., 2002) and this will only be covered very briefly here to emphasize how vital it is that effective therapy be developed quickly.
Stroke is the third major cause of death in the major industrialized countries after cardiovascular disease and cancer. The overall incidence of stroke is predicted to increase over the next decade by 12% but by around 20% in low-income families (Editorial, 2005). More than 30% of the stroke survivors will have severe disability, and calculations suggest that over 50 million healthy life-years will be lost by 2015 (Editorial, 2005). The burden of stroke to the individual, the family of stroke victims and society as a whole is clearly enormous.
These figures, however, do not give any insight to the consequences to the individual stroke patient. Saver (2006) undertook the task of calculating the impact a stroke has on nervous tissue. His calculations are sobering. Patients experiencing a typical large-vessel acute ischaemic stroke will lose 120 million neurones, 830 billion synapses and 714 km of myelinated fibres each hour. Compared with the normal rate of neurone loss during aging, the ischaemic brain will age 3.6 years for every hour the stroke goes untreated. Little wonder that the phrase ‘time is brain' was coined to emphasize the need to try and treat a stroke rapidly.
The large majority (85%) of strokes in the western world are ischaemic, that is, a stroke resulting from an occlusion of a major cerebral artery, commonly the middle cerebral artery (MCA) by a thrombus or embolism. The other strokes are haemorrhagic, where a blood vessel bursts either in the brain or on its surface. These figures differ in China where haemorrhagic strokes are more common (Zhang et al., 2003b).
Some risk factors can be mitigated. For example, the use of antihypertensives to lower blood pressure and statins to treat hyperlipidaemia has proven value. Furthermore, changing lifestyle (stopping smoking, decreasing body weight) undoubtedly decreases the risk of suffering a stroke.
However, providing effective pharmacological treatment immediately following an acute stroke to lessen the cerebral damage remains an elusive goal. Only one drug is approved for clinical use in most countries and that is the thrombolytic agent recombinant tissue plasminogen activator (rt-PA; alteplase) and this is only given to 4–5% of patients for reasons discussed below. The need to develop an effective treatment for stroke, therefore, remains paramount. This review deals only with acute pharmacological treatments, therefore mechanical approaches such as the MERCI retriever are not reviewed, but details of this therapy can be found elsewhere (Molina and Saver, 2005).
The classification of thrombolytics and neuroprotectants
Experimental drugs developed to treat acute ischaemic stroke have tended to be grouped by investigators into two distinct groups: thrombolytics and neuroprotectants. The former group encompasses compounds that restore blood flow, while the latter term covers drugs that act to preserve neurones (see Green and Ashwood, 2005).
Although this classification has proved useful, it has weaknesses and limitations. For example, drugs are now being developed that restore blood flow by mechanisms other than thrombolysis. Furthermore, if a drug restoring blood flow also preserves cerebral tissue compromised by the ischaemia, why should this compound not also be called a neuroprotective agent?
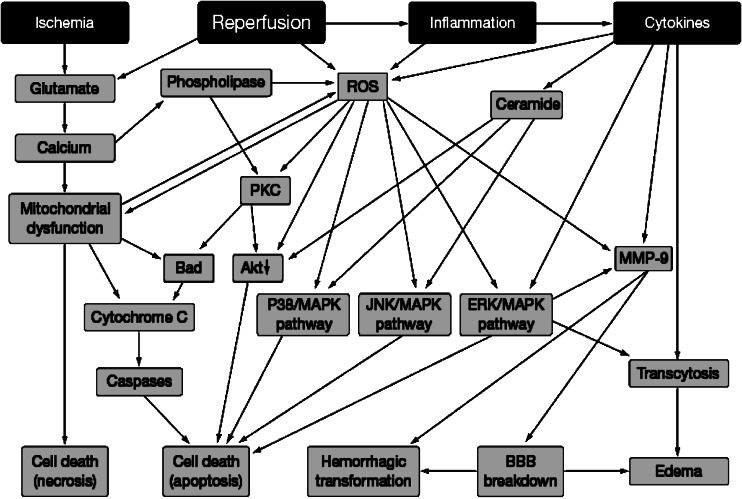
The idea of neuroprotection evolved in response to the idea that drugs could be developed that would interfere with the biochemical mechanisms that follow an ischaemic insult and thereby prevent cell death. Over the last 25 years, neurochemists have provided a great deal of information on the chain of events that follows an ischaemic episode (the so-called ‘ischaemic cascade'; see Figure 1). Many compounds that act on one or more of these mechanisms have been developed and some are shown in Figure 1 and these compounds (and many others) do indeed prevent ischaemia-induced cell death in the brain of experimental animals. Such compounds have generally been referred to as neuroprotectants.
Figure 1.

A simplified diagram of the mechanisms involved in the ‘ischaemic cascade' together with some of the compounds developed to interfere with the mechanisms involved in the cascade and thereby provide neuroprotection. Reproduced from (Green et al., 2003b) with permission of Elsevier Ltd.
However, recent research suggesting that drugs working on the neurovascular unit (Lo et al., 2003; Abbott et al., 2006) may also prove to be useful in protecting neurones following an experimentally induced stroke (del Zoppo, 2006), further weakens the division of neuroprotection and thrombolysis, since these drugs by acting primarily on the vasculature are nevertheless providing neuroprotection.
Therefore, while I will continue (for ease of discussion) to use these terms for the purposes of grouping experimental compounds in this review, I do question whether we can continue to use this simplistic classification in the future.
Thrombolytics and other antiaggregation compounds
Thrombolytics
As stated above, the only compound licensed in most countries for clinical use in acute ischaemic stroke is rt-PA (alteplase), a drug initially in use for the treatment of myocardial infarction. It was approved on the basis of two Phase III trials, which studied its efficacy when given within 3 h of onset of the symptom (NINDS t-PA Stroke Study Group, 1995). It is also effective in a rat thromboembolic stroke model when given within 3 h, but not at later times (Brinker et al. (1999a) and at present clinical trials have also failed to demonstrate unequivocal benefit using later times in stroke patients (Hacke et al., 1998). This immediately limits the usefulness of the drug, as drug administration within 3 h of diagnosis is a challenge in many countries and this challenge is further increased by the need for a computed tomography scan to confirm that the stroke is ischaemic and not haemorrhagic, a condition that would be exacerbated by a thrombolytic drug.
A further reason for the low use of rt-PA in stroke is that the compound increases the incidence of cerebral haemorrhage (Wardlaw et al., 1997), a problem that can be mimicked in experimental animals (Brinker et al., 1999b; Asahi et al., 2000) and which appears to be associated with the drug increasing the levels of matrix metalloproteinase-9 in the endothelium (Lapchak et al., 2000; Pfefferkorn and Rosenberg, 2003).
Looking to the future, desmoteplase is another thrombolytic in development. This is a genetically engineered version of a protein in the saliva of Vampire Bats (Schleuning, 2001). Because desmoteplase has a requirement for fibrin as a cofactor, it is more potent than rt-PA in the presence of fibrin (Bringmann et al., 1995). It has been suggested that this characteristic will give desmoteplase the ability to dissolve clots without increasing systemic clotting and thereby increases the haemorrhagic transformation, a problem that occurs with rt-PA. Although this hypothesis was not supported by a preclinical study (Montoney et al., 1995), it appears that appropriate dosing in patients might assist in achieving this goal (Hacke et al., 2005).
Some preclinical evidence suggests that if rt-PA reaches the extracellular space in the CNS it can induce neurotoxicity (Kaur et al., 2004), and recent studies suggest that this problem is not seen with desmoteplase (Liberatore et al., 2003; Reddrop et al., 2005; Lopez-Atalaya et al., 2007). However, there is no evidence has been reported that rt-PA does induce neurotoxicity clinically.
The first Phase IIb/III trial (DIAS-2) has recently completed the investigation of two dose levels (90 and 125 μg kg−1) given 3–9 h post-onset of stroke symptoms. The trial was not large (186 patients) and a press announcement from the companies developing the compound on 31st May 2007 (www.frx.com/news/PressRelease.aspx?ID=1009782) reported no difference between the placebo- and drug-treated group (data were also presented at the European Stroke Conference in Glasgow). It is difficult to further evaluate these data until more information is available, but it is clearly a major setback in the development of this new thrombolytic.
Antiaggregation compounds and other perfusion-enhancing compounds
The monoclonal antibody abciximab is an antiaggregation compound that binds to the glycoprotein IIb/IIIa receptor on the surface of platelets, and which promotes fibrinolysis by preventing the platelets from sticking together, thereby inhibiting clot formation (Tam et al., 1998). It is in current clinical use for the restoration of coronary blood flow, often being given in combination with thrombolytics.
Preclinical studies on abciximab to investigate its possible use in stroke cannot be performed, because it is not active in rodents (Sassoli et al., 2001). Therefore, an analogue 7E3 F(ab′)2 has been used in rat thromboembolic stroke models and has provided encouraging data. Shuaib et al. (2002) reported that both rt-PA (10 or 20 mg kg−1) and 7E3 F(ab′)2 (6 mg kg−1) reduced infarct size as the two drugs did in combination. However, the cerebral haemorrhage rate increased following administration of either drug with the highest incidence of haemorrhage occurring in the group given the high-dose combination. Zhang et al. (2003a) also observed a decrease in infarct size in rats treated with a combination of 7E3 F(ab′)2 and rt-PA, but found no reduction in infarct size when the drugs were administered individually. A further study by this group found that administration of tenectaplase to rats together with 7E3 F(ab′)2 also resulted in a decrease in infarct size when both compounds were given at 4 h (Zhang et al., 2004).
Although the recent company press release announced the termination of the Phase III trial of abciximab in stroke, following a recommendation by the independent safety and efficacy monitoring committee, a new trial is now reported to be starting.
The problem of increased haemorrhage rate seen following rt-PA administration does not seem to be obviated by using other types of approach to increase blood flow in the brain of stroke patients. For example, intravenous administration of the defibrinogenating compound ancrod also increased cerebral haemorrhage rate in a Phase III trial, when it was given within 3 h of stroke onset, although it also produced a favourable neurological response compared with placebo (Sherman et al., 2000). A Phase III trial of the compound given up to 6 h after stroke onset was negative but a new trial, also examining the effect of administration within 6 h, began in September 2005.
Heparin may be beneficial if given within 3 h, but again increases the incidence of cerebral haemorrhage (Camerlingo et al., 2005). Plasmin and microplasmin, a truncated form of plasmin, are finbrinolytic agents that may be free of the problem of increasing cerebral haemorrhage rate, which again makes them candidates for development as therapeutic agents for stroke (Molina and Saver, 2005).
Finally, there are indications that early aspirin can be of some value to reduce the early recurrence, with no excess of subsequent haemorrhagic stroke, although the absolute benefit in terms of patient numbers is small (Chen et al., 2000) and aspirin may thus confer modest benefit if given within 48 h of stroke (Sherman, 2004).
Neuroprotective agents
Introduction
Despite the fact that the thrombolytic rt-PA has demonstrated efficacy in treating acute ischaemic stroke patients, the short time window, risk of haemorrhage and requirement for a computed tomography scan mean that few patients actually receive the drug and other approaches to treating stoke have been pursued vigorously. The increasing knowledge of the mechanisms involved in producing cell death following a stroke obtained in the 1980s and beyond produced a buoyant hope that interfering with the mechanisms involved in this so-called ‘ischaemic cascade' or pathway of ‘excitotoxic cell death' (Figure 1) would lead to lessening the neuronal damage that would normally occur. However, not a single one of the many compounds developed to produce neuroprotection, usually by interfering with one of the mechanisms shown, has achieved clinical success, despite many of them providing clear evidence of efficacy in animal models (Green et al., 2003a), a recent calculation has suggested that the number of experimental compounds that have failed at some stage of development now numbers over 1000 (O'Collins et al., 2006). During the late 1990s, this situation was considered so severe that a group of scientists from both academia and industry convened to produce guidelines to be used for preclinical drug development with the suggestion that no compound should progress to clinical development unless it fulfilled the guidelines. The group was called the Stroke Therapy Academic Industry Roundtable (STAIR) and the published guidelines (Stroke Therapy Academic Industry Roundtable, 1999) are now often referred to as the STAIR criteria. These have been modified by both the STAIR group itself (Stroke Therapy Academic Industry Roundtable, 2001) and others (see Table 1; Green et al., 2003a). However, it is now reasonable to question them in the light of recent results (see later).
Table 1. Outline of the recommendations of the Stroke Therapy Academic Industry Roundtable (1999) as modified by Green et al. (2003a) that should be met before a compound is progressed to clinical trial.
| STAIR recommendations |
|---|
| • Adequate dose–response plus serum concentrations measured, thereby defining minimally and maximally effective doses |
| • Time window studies confirming efficacy. Time and duration of drug administration appropriate to the mechanism of action and appropriate to the proposed clinical protocol |
| • Physiological monitoring of animals undertaken |
| • Randomized, blinded studies; reproducible effects (one independent) |
| • Infarct volume measured, compound provides subcortical as well as cortical protection. |
| • Histological protection of >70% in both transient and permanent focal ischaemia when drug is given 15–30 min post occlusion |
| • Attenuates white matter damage in brain |
| • Functional tests used, short and long term assessment |
| • Small rodent studied with permanent MCAO. Must show efficacy in permanent MCAO models; compound is efficacious as monotherapy |
| • Larger species used for novel, first-in-class compound |
| • Studies published in peer-reviewed journal |
Many of the compounds developed in the early days of neuroprotective research belonged to only one class, the glutamate antagonists; however, there have also been a substantial number of compounds synthesized and evaluated that act at other sites on the ischaemic cascade (see O'Collins et al., 2006).
In the following sections, I will only consider compounds that have failed if the information gained gives understanding of future research directions. Otherwise only compounds in continued development will be discussed.
Drugs altering glutamate function
Because a pathological increase in glutamate was one of the early changes to be shown to occur after an episode of cerebral ischaemia, many compounds interfering with glutamate function were developed during the late 1980s and early 1990s. These were predominantly N-methyl-D-aspartate (NMDA) receptor subtype antagonists, but some (RS)-α-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid (AMPA) receptor antagonists were investigated and also a glycine modulator site antagonist. It seems reasonable in retrospect to suggest that a major problem with glutamate antagonism as a mechanism suitable for therapeutic intervention target is that pathological glutamate release is an event occurring very early in the ischaemic cascade (Figure 1). This presumably makes it necessary to give such drugs very quickly after the ischaemic insult (Dirnagl et al., 1999; Green et al., 2003b; Hoyte et al., 2004), and rapid admission is often a major clinical problem. However, other problems that also arose with some of these drugs included poor pharmacokinetics, poor brain penetration and a failure to protect subcortical structures.
There may now be only one NMDA antagonist in development. This is traxoprodil (CP-101 606), which has NR2B receptor subtype selectivity (Chenard et al., 1995; Wang and Shuaib, 2005). It is an effective glutamate antagonist in a primary cortical neurone preparation (Menniti, 1997), but there is little information on its in vivo activity. It reduces damage after cortical compression induced brain ischaemia (Kundrotiene et al., 2004) and is neuroprotective when given 15 min prior to MCA occlusion (MCAO) in the cat (Di et al., 1997). It has also been reported to be an effective neuroprotectant when given 2 h after clot insertion in a rat thromboembolic stroke model, decreasing infarct volume dose dependently (Yang et al., 2003). There has been little recently published information about traxoprodil apart from a clinical pharmacokinetic study and a clinical investigation in severe traumatic brain injury, which did not reveal unequivocal evidence of efficacy (Yurkewicz et al., 2005). Its development for stroke may therefore have been terminated.
It is well established that magnesium ‘gates' the NMDA receptor channel and that reducing the Mg2+ concentration in vitro enhances NMDA-induced electrophysiological responses (Nowak et al., 1984). Magnesium also reduces presynaptic glutamate release (Lin et al., 2002). A dose of magnesium sulphate is neuroprotective in both transient (Marinov et al., 1996; Schmid-Elsaesser et al., 1999) and permanent (Izumi et al., 1991) MCAO models of stroke and also in an embolic stroke model (Yang et al., 2000).
Following the pilot studies on the effect of magnesium in stroke patients, a full clinical multicentre trial was undertaken in almost 2400 patients. Although no reduction in death or disability was detected, the study did observe a possible benefit in lacunar strokes (Muir et al., 2004). According to the investigators, this finding proposed was worthy of further investigation, even though the result was the opposite to what had been hypothesized before the trial took place.
Gavestinel is a compound that acts at the glycine-regulatory site of the NMDA receptor, thereby antagonizing the effect of pathological glutamate function (Di Fabio et al., 1998). The failure of the compound in a Phase III trial (Sacco et al., 2001) has probably prevented any further development of compounds with this mode of action.
AMPA antagonists have also received significant interest as neuroprotectants. However, compounds that have been developed have suffered from various safety problems, including sedation, visual disturbance, and memory impairment (Weiser, 2005), and the development of YM 872, the only AMPA antagonist to have been in recent clinical investigation, has been terminated.
5-HT1A agonists
Repinotan is a potent 5-HT1A receptor agonist that was suggested to act as a neuroprotectant by inhibiting neuronal firing in he dorsal raphé nuclei, thereby leading to the inhibition of excessive ischaemia-induced glutamate release. It may also produce hyperpolarization and thus reduce anoxia-induced depolarization.
In animal models of stroke, repinotan was reported to be effective in both transient and permanent MCAO models even when given up to 5 h after the occlusion (Mauler and Horváth, 2005).
A Phase III clinical trial was initiated and then modified and reclassified as a Phase IIb study with an inclusion time window of 4.5 h. However, the company terminated development in December 2004 due to the drugs failure to meet efficacy endpoints.
Piclozotan (SUN N4057) is another potent 5-HT1A agonist that has been reported to be an effective neuroprotective agent when given immediately following the start of a transient MCAO (Kamei et al., 2001). These authors also stated the compound to be in clinical development (Phase IIb) for stroke, but the lack of subsequent communication suggests that its development has been terminated.
Citicoline
Citicoline or cytidine-5′ diphosphocholine (CDP-choline), has been in clinical use in Europe and Japan for many years for a variety of degenerative neurological disorders. It is an intermediate in the biosynthesis of phosphotidylcholine, which is of major importance in regulating cell membrane integrity.
The interest in citicoline as a neuroprotectant is based on the fact that phosphotidylcholine is broken down to free fatty acids during ischaemia, thereby generating free radicals that in turn induce cell damage (Phillis and Regan, 2004). Citicoline, by reducing lipid metabolism following ischaemia, reduces the levels of free fatty acids (Trovarelli et al., 1981; Rao et al., 1999) and, therefore, decreases free radical production (Adibhatla and Hatcher, 2002). There is good evidence for citicoline being effective as a neuroprotectant in models of acute ischaemic stroke. It has been shown to reduce neurological deficits a rat global ischaemia model (Kakihana et al., 1988) and a rat transient focal ischaemia model (Sobrado et al., 2003). Citicoline also significantly reduced infarct size in a rat thromboembolic focal ischaemia model (Shuaib et al., 2000).
A total of four Phase III studies on citicoline have been conducted. However, all examined small numbers of patients with variable stroke severity and dosing varied across the studies (500–2000 mg). While the trials showed trends for improvement, conclusions were difficult because of either small cohort size or possible inappropriate primary outcome measures. Dávalos et al. (2002) performed a meta-analysis on the data from the four trials (total patient numbers 1372: 583 placebo; 789 citicoline) and this suggested a beneficial effect of the drug (P=0.034) with the greatest effect at the highest dose. A further Phase III trial was initiated in 2006.
Metal chelation
Metal ions are vital factors in controlling for enzymes, co-factors and cellular transporters, including matrix metalloproteinases, calpain and Cu/Zn superoxide dismutase, whose disruption have been proposed to be intimately associated with cell death following acute cerebral ischaemia. Disruption in metal ion homoeostasis has also been suggested to be involved in various chronic neurodegenerative conditions such as Parkinson's and Alzheimer's Diseases (Angel et al., 2002). The probability that disturbance in metal ion regulation could be associated with cerebral ischaemia is indicated by the observation that disturbances in zinc homoeostasis was associated with cerebral cell death following transient focal ischaemia (Koh et al., 1996), and this has resulted in the development of DP-b99. This is a derivative of BAPTA, a compound that chelates divalent metal ions including zinc, calcium, iron and copper (Angel et al., 2002). This chelating action may explain its beneficial effect of DP-b99 in inhibiting the oxidative stress-induced increases in calpain in vitro (Friedman et al., 2004) and reduction in ischaemia-induced matrix metalloproteinase activation in the brain of rats subjected to MCAO (Angel et al., 2004). The compound has also been reported to decrease infarct size in a rat MCAO model in an abstract publication (Angel et al., 2004). The compound appears to be well tolerated in healthy volunteers (Rosenberg et al., 2005) and a recent press release from the company reported efficacy in a Phase IIb clinical trial. No initiation of a Phase III trial has been announced.
Another metal chelator that decreases infarct volume in a rat transient focal ischaemia model is PAN-811, which was originally developed as an anticancer drug, due to its ability to chelate iron, but which also modulates calcium homoeostasis and, therefore, presumably reduces free radical production (Lu et al., 2004). The compound decreased infarct size by a modest 35% in a transient MCAO model and has a narrow dose range (Lu et al., 2004), making it unlikely that it will progress into clinical development. However, these data indicate that metal chelation may be a viable mechanistic approach to neuroprotection.
Arundic acid
Arundic acid (ONO-2506) is an astrocyte-modulating compound that inhibits the synthesis of the protein S-100β in cultured astrocytes and inhibits the increase in the concentration of S-100β in the colony-stimulating factor and plasma of rats subjected to transient or permanent MCAO (Tateishi et al., 2002). S-100β has been implicated in producing cell death through its activation of several intracellular signalling pathways (Asano et al., 2005). The compound also has actions on GABAA receptors, glutamate transporters and lipopolysaccharide-inducible nitric oxide synthase expression in cultured astrocyte preparations (Asano et al., 2005).
Arundic acid has been reported to have a very long therapeutic time window of 24 h in transient and 48 h in permanent MCAO. This has been proposed to be due to its effects on both S100β and the other diverse mechanisms outlined above (Asano et al., 2005). Other studies, including a neuroprotective effect on primates subjected to permanent MCAO were reviewed by Asano et al. (2005).
In May 2005, Ono Pharmaceuticals reported that the Phase II clinical study of the drug for acute stroke initiated in North America would be terminated following a futility analysis by an independent board of advisors. However, a new trial has recently been initiated in this continent and the study in Japan is continuing (see http://www.strokecenter.org/trials).
Albumin
Administration of albumin administration decreases infarct size in rats subjected to both transient (Huh et al., 1998) and permanent MCAO (Liu et al., 2001). The time window for administration in transient ischaemia is 4 h after occlusion (Belayev et al., 2001). It is proposed that only part of the neuroprotective effect of albumin is via haemodilution (Huh et al., 1998), as several other mechanisms can have also been proposed to be involved (Huh et al., 1998; Belayev et al., 2001). While a small open study suggested increased cardiopulmonary adverse events in stroke patients when albumin was given within 24 h of stroke onset (Koch et al., 2004), a subsequent Phase I trial indicated that albumin was well tolerated (Ginsberg et al., 2005) and a Phase III study was started in late 2006 with completion projected to be in 2010.
Cytokines
Cytokines, particularly the tumour necrosis factor (TNF)α and IL-1β (Figure 2), are the major factors involved in the inflammatory response of the brain to injury (Barone and Parsons, 2000; Allan and Rothwell, 2001), and both TNFα antibodies (Barone et al., 1997) and IL-1β antibodies (Relton and Rothwell, 1992) have been shown to inhibit ischaemia-induced cerebral damage. Although a recombinant IL-1α antibody has recently been found to be well tolerated in a Phase II study in stroke patients (Emsley et al., 2005), there is a problem in developing compounds antagonizing the action of TNFα in stroke, because TNFα can also assist cell survival (Hallenbeck, 2002; Heldmann et al., 2005). Nevertheless, efforts are now being made to produce nonpeptide anti-TNFα compounds in addition to the protein-based compounds. These approaches include targeting enzymes involved in the biosynthesis of TNFα. Another approach is to produce a compound targeting the protease (TNFα converting enzyme or TACE), which acts on membrane-bound TNFα and thus generates soluble TNFα (Lovering and Zhang, 2005). Some compounds with TACE inhibitory activity are neuroprotective in animal stroke models. However, they also inhibit matrix metalloproteinases, key enzymes involved in blood brain barrier damage, so their mechanism of neuroprotective action remains unclear (Lovering and Zhang, 2005).
Figure 2.
Some of the pathways proposed to be involved in cell death and cell survival following an acute cerebral ischaemia. This figure is simplified and illustrates only some of the pathways. Some pathways can initiate cell death or cell survival depending on the time after the onset of ischaemia and the severity of the ischaemia. There is also a lack of agreement among investigators as to the specific role of some of these signalling systems and the figure is used here merely to illustrate possible targets for pharmacological intervention. Figure adapted from Green and Shuaib (2006) with permission of Elsevier Ltd.
A cytokine that has distinct possibilities for clinical development is erythropoietin or a close congener. Erythropoietin not only is a primary stimulator of red blood cell formation but also appears to have functions in the brain (Masuda et al., 1993). In vitro, it protects cortical neurones from glutamate-induced neurotxicity and in vivo is neuroprotetive in the gerbil global ischaemia model (Sakanaka et al., 1998).
A clinical trial of erythropoietin in stroke patients suggested efficacy when it was given in three doses over 3 days (Ehrenreich et al., 2002). However, concerns over safety of the compound in non-anaemic patients have led to the development of related compounds that retain the neuroprotective properties but lacking the blood forming properties of erythropoietin (see Torup, 2007). One such compound is carbamylated erythropoietin and Wang et al. (2007) have recently examined both erythropoietin and carbamylated erythropoietin in rats using an embolic stroke model and found that all neuroprotective doses of erythropoietin increased the haematocrit. However, a low dose of the latter could produce effective neuroprotection without any haematological effects.
It has been suggested that erythropoietin and carbamylated erythropoietin might provide efficacy through several mechanisms of action including antiapoptotic effects and stimulation of angiogenesis and neurogenenesis (Torup, 2007), which may enhance the chances of success for this new approach to stroke therapy.
Free radical scavengers and trapping agents
The fact that there is clear evidence that free radicals are involved in the production of cerebral tissue damage following an ischaemic insult and also in the damage produced by reperfusion (Love, 1999; Chan, 2001) has resulted in the development of several compounds that have been designed to remove free radicals and, therefore, hopefully to lessen ischaemia-induced damage.
Ebselen is a selenium compound with glutathione peroxidase-like activity, which may act as a mimic for this enzyme rather than being a free radical scavenger (Noguchi et al., 1992). It is neuroprotective in rat models of transient ischaemia but not permanent focal ischaemia (Salom et al., 2004). The small clinical studies performed failed to provide clear evidence for efficacy in stroke and development was terminated (see Green and Ashwood, 2005).
The lazaroid compound tirilazad was also ineffective in rat permanent ischaemia models (Xue et al., 1992) and also failed to demonstrate efficacy in transient ischaemia models when given several hours after the insult. Several clinical trials failed to demonstrate the efficacy (see Green and Ashwood, 2005).
Available preclinical data on the effects of edaravone, a hydroxyl radical scavenger, in stroke models are sparse, although it has been examined in a variety of other disease models. In cerebral ischaemia models, nothing has been published on dose–response data and most studies gave the compound almost immediately after the ischaemic insult. The compound also failed to protect subcortical structures (see Green and Ashwood, 2005). Published clinical data are also limited. Nevertheless, the compound has been approved by the regulatory authority in Japan to treat stroke patients apparently on the basis of a single placebo-controlled study in a small number of patients. It is not known whether the compound will be developed outside Japan.
Disodium 2,4-disulphophenyl-N-tert-butylnitrone (NXY-059)
The development of the neuroprotectant disodium 2,4-disulphophenyl-N-tert-butylnitrone (NXY-059) will be considered in some detail, because this compound was, until recently, thought to be the most likely neuroprotective agent to succeed in the clinic and demonstrate unequivocal evidence of efficacy in stroke patients. Its recent failure therefore provides both lessons and questions on the future of neuroprotectant drug development.
NXY-059 is a nitrone-derived compound with free radical trapping properties (Maples et al., 2001; Green et al., 2003b; Williams et al., 2007). Because NXY-059 was in development at the time of the publication of the STAIR criteria, its development programme was focussed on following the guidelines (Table 1; Stroke Therapy Academic Industry Roundtable, 1999). Consequently, it has become the first compound to have been developed in accordance with the criteria (Lees et al., 2001; Green and Ashwood, 2005; Shuaib, 2006).
The compound has been shown to produce clear dose-dependent neuroprotection in rats in both transient (Kuroda et al., 1999; Sydserff et al., 2002) and permanent (Zhao et al., 2001; Sydserff et al., 2002) MCAO models of stroke, including subcortical protection (Figure 3; Sydserff et al., 2002) and in rat (Wang and Shuaib, 2004) and rabbit (Lapchak et al., 2002) embolic stroke models. NXY-059 also has a wide window of opportunity producing statistically significant neuroprotection, when given at 4 h after permanent focal ischaemia (Sydserff et al., 2002) and 5 h after transient ischaemia (Kuroda et al., 1999). In a primate model of permanent focal ischaemia, it lessened both the motor deficits in the paretic arm of marmosets and also spatial hemineglect, even when given 4 h after permanent MCAO (Marshall et al., 2001, 2003). Thus, the compound provided clear evidence for producing functional improvement, in addition to the histological evidence for it decreasing infarct size.
Figure 3.
The effect of increasing doses of NXY-059 on the volume of ischaemic damage in the (a) cortex, (b) subcortex and (c) total brain volume, together with the (d) dose–response versus neuroprotection. Reproduced from Sydserff et al. (2002) with permission of Nature Publishing Group.
Overall, therefore NXY-059 could be claimed to have met all the STAIR criteria (Table 1). In particular, it is worth emphasizing that the window of therapeutic opportunity (greater than 4 h) and the fact that NXY-059 had a maximal effective neuroprotection in rats at a plasma unbound concentration of 24 μmol l−1 in rat transient focal ischaemia and 140 μmol l−1 in rat permanent focal ischaemia models allowed direct translation to the design of the clinical trial, as NXY-059 was well tolerated in stroke patients at a plasma unbound concentration of 260 μmol l−1 (Lees et al., 2003).
The subsequent Phase III trial design was such that the plasma concentration of drug in the patients exceeded the concentration known to be maximally effective in rodent models and the time window of inclusion matched that known to be effective in both rodent and primate models. This is the first time that this has been achieved (Green and Ashwood, 2005).
The first Phase III trial was in 1700 patients (SAINT I) and the drug was found to significantly reduce disability (using the modified Rankin score) within 6 h of stroke onset without apparent tolerability or safety issues (Lees et al., 2006). NXY-059 did not improve neurological function as measured by the NIHSS score (Lees et al., 2006). However, the companion SAINT II study, which had a patient size of 3200 patients, failed to confirm efficacy (Shuaib et al., 2007).
This failure has naturally resulted in others proposing reasons for the negative outcome and it is worth reviewing these suggestions briefly. Proctor and Tamborello (2007) have proposed that the positive SAINT I result was due to an impurity in the drug substance not present in the SAINT II investigation. This hypothesis is based on the fact that a hydroxylamine derivative has been shown to be present in samples of another nitrone (phenylbutyl nitrone) and is a potent radical scavenger (Atamna et al., 2000). However, this proposal has been rapidly refuted by the investigators (Lees et al., 2007), and even cursory examination of the original in vitro study on the hydroxylamine (Atamna et al., 2000) indicates that the amount of impurity that has to be present in the drug substance is likely to be quite enormous to produce a pharmacological effect, which renders this suggestion untenable. In addition, a paper has appeared questioning the validity of the claim that NXY-059 did meet the STAIR criteria and criticizing some of the preclinical data (Savitz, 2007). However, a survey by a recent independent review group (O'Collins et al., 2006) did conclude that the work on NXY-059 did satisfy the original STAIR criteria and Feuerstein et al. (2007) suggested the clinical trial had been well founded on extensive preclinical data. It should also be emphasized that the major studies on the time window and dose dependence were performed with appropriate blinding and allocation concealment.
The clinical failure of the nitrone NXY-059 must cast a shadow over the further development of another nitrone-derived compound, stilbazulenyl nitrone, in transient MCAO. Stilbazulenyl nitrone is an effective neuroprotectant in transient ischaemia in rats (Ginsberg et al., 2003). A major claim for the value of this compound over NXY-059 is its greater lipophilicity and potency. However, while it does have greater penetration of cerebral tissue (Ley et al., 2005) than NXY-059 (Dehouck et al., 2002; Green et al., 2006), this increase is modest, and its importance remains unclear as there is some evidence that NXY-059 may be acting at the level of the endothelium (Culot et al., 2006; Hainsworth et al., 2007). This supports earlier proposal that NXY-059 might be acting at the microvasculature and enhances recent proposals that neuroprotection may be achieved by the drugs acting on the neurovascular unit (Abbott et al., 2006; del Zoppo, 2006). An action at this site is also likely to explain the neuroprotective effect of the nitrone 2-sulphophenyl-N-tert-butylnitrone, as this also has poor cerebral penetration. Given the suggestion that even the brain penetrating nitrone N-tert-butylnitrone may be acting outside the brain to produce its neuroprotective action in rats (Gido et al., 2000); one wonders if all nitrones therefore act at the same site in animals to produce neuroprotection. In any event, the failure of NXY-059 must surely make any company wary of developing any other nitrone compound.
Neuroprotection research: the current situation
Problems of animal models
I have previously argued strongly in favour of the use of animal models in preclinical stroke medicine research (Green et al., 2003a; Green and Shuaib, 2006). In support of the arguments, it was pointed out that most compounds that failed in the clinic did not replicate in the patients either the drug exposure required for efficacy in the respective animal models or the time window, or both. Furthermore, the fact that many of the physiological factors that influence the severity of ischaemic damage in animal models have the same effect on stroke outcome in patients seemed to indicate cross-species validity (Green et al., 2003a). This view was supported by studies in marmosets, in which it is observed that focal ischaemia results not only in motor problems in the contralateral arm, but also spatial hemineglect and both of these problems have exact clinical correlates (Marshall and Ridley, 1996; Marshall et al., 2001).
However, the failure of NXY-059 in the recent large Phase III study (Shuaib et al., 2007) does lead to major questions as to the value of animal models, at least as utilized at present. NXY-059 was not only developed in accordance with the STAIR criteria (Lees et al., 2001; Green and Ashwood, 2005; Shuaib, 2006), its development circumvented the major weaknesses of many earlier trials as listed above. Specifically, the plasma levels in patients were considerably in excess of those required for a maximum neuroprotective effect in rats, the time window for treatment initiation was equivalent to that reported to be required in rats and, in the marmoset model, the drug had been shown to improve both the use of the paretic arm and lessen spatial hemineglect, two problems with exact clinical correlates.
Given the likelihood that current neuroprotective drugs in development have also used the same or similar stroke models as those used in the NXY-059 development programme, one has to question their chances of clinical success.
Although there are several animal models of acute ischaemic stroke available, they are mostly closely related with modest modifications sometimes introduced by the scientists using them (Green and Cross, 1997; Traystman, 2003). Basically, most models induce an occlusion of the MCA because most human strokes result from an occlusion of this artery (Mohr et al., 1986). If this occlusion is temporary, then the model mimics the problem of both ischaemia and reperfusion, whereas permanent occlusion presumably produces the problem of long-term vessel blockade, as often occurs in humans (Ringelstein et al., 1992).
However, even before the clinical failure of NXY-059 was known, some stroke researchers questioned the value of the models and the data generated by them. For example Carney (2005) said: ‘For stroke, the [animal] models and the clinical condition are extremely different, which is reflected in the failure of molecules in the human condition'. This view was supported in another article published around the same time (Kaste, 2005), which was discussed further in succeeding articles (Donnan and Davis, 2005; Fisher and Tatlisumak, 2005).
A major criticism of the current animal models is that they nearly all utilize young healthy animals. In contrast, stoke patients are usually elderly, with a variety of other clinical problems such as hypertension, myocardial infarction and diabetes. With the exception of hypertension, which has been modelled by examining the effects of vessel occlusion in spontaneously hypertensive rats (Zhao et al., 2001), few of the other problems are routinely included in the animal models. Both Davis et al. (1995) and Schaller (2007) have examined older animals and data suggest that neuroprotective efficacy is reduced in these animals.
The question, therefore, arises as to whether the predictive value of animal models of stroke would improve if other comorbid conditions were included, and if so why? Until such modelling is performed, it can be shown that compounds like NXY-059 fail to provide neuroprotection we cannot know. However, it does seem reasonable in the light of current knowledge to suppose that other comorbid conditions may alter cerebrovascular functions such as the structure of the blood–brain barrier or the neuroimmune system. The future of neuroprotection research may, therefore, depend on the development of better animal models.
Another factor generally omitted in animal studies is white matter protection. It was noted as a weakness in preclinical studies some time ago (Muir and Grosset, 1999), but the requirement for stroke models to be performed in large animals to reliably measure this parameter means that little progress has been made since.
Animal models and future experimental design
One point that should be considered in all future studies is the design and size of primary pharmacodynamic trials. The CAMARADES collaboration (http://www.camarades.info/index.htm) is now proposing that all investigators conduct animal studies with full regard to random allocation, blinding and allocation concealment, and they further suggest, on the basis of their re-evaluation of existing preclinical publications, that failure to meet such methodology generally results in neuroprotective efficacy being overestimated. Although such techniques were not in regular use over 10 years ago when most of the preclinical work was performed on NXY-059 (for such is the time-line for drug development), the major studies on the time window and dose dependence were undertaken with appropriate blinding and allocation concealment. Furthermore, the constancy of the positive preclinical neuroprotection outcome data suggest that there must be a far more fundamental problem in translating the effect of drugs in animal models to that in human subjects, and one which all investigators must now confront if drugs are in future to be clinically successful.
The CAMARADES inspection of published data also indicates that the cohort size of the animal studies is generally not large (often 6–12 animals per group). Meta-analysis of preclinical studies can now being performed by systematic reviews of stroke compounds tested in preclinical stroke models (see http://www.camarades.info/index.htm). These reviews are performed using Cochrane Collaboration approaches (as used in clinical studies but applied to animal investigations) to evaluate more clearly the predictability and transferability of animal models to the clinical situation. One certainly suspects that experimental group size is, at present, generally not sufficient to provide good statistical power to many studies and that single high-quality studies are rare, a problem that has been discussed in detail elsewhere (Perel et al., 2007). This weakness in experimental design is apparent in both academic and pharmaceutical company studies. Given the enormous costs of clinical development of stroke drugs, it is obvious that patient studies should only commence following statistically compelling data from the relatively cheap preclinical studies. Equally pertinent is the argument that no patient should be exposed to the novel compounds without very persuasive preclinical data being available. All these considerations must be encompassed in the planning of research in the future.
The use of combination therapy
It has been suggested by several investigators (for example Wagner and Jauch, 2004) that combining a thrombolytic plus a neuroprotectant might offer advantages such as enhancing the degree of clinical improvement, or extending the treatment window for rt-PA, or both. However several preclinical studies have failed to demonstrate enhancement of the effect of rt-PA and a neuroprotectant compared with the neuroprotectant alone (Lapchak et al., 2002; Yang et al., 2003). Lu et al. (2005) have recently presented evidence as to why it is complicated to demonstrate the value of combination treatments in animals and thereby prove synergy rather than addition. They suggested that factorial designs must be used with group size of sufficient number to allow confidence in the result obtained. A final point is that assessment must be made to ensure that the presence of the neuroprotectant does not alter the thrombolytic properties of the thrombolytic (see Mutch et al., 2007).
Stroke therapy research: the future
The failure of NXY-059, a compound developed with close regard to the best currently established guidelines of preclinical and clinical methodology may result in the pharmaceutical industry switching strategies. Few companies are going to feel the urge to continue developing drugs that interfere with biochemical mechanisms known to be involved with the ischaemic cascade and burden themselves with the huge costs of clinical trials in the light of so many failures.
Several approaches to the treatment of stroke are now being evaluated, which involve either biopharmaceuticals or initial biopharmaceutical approaches with the aim of finally producing small molecules from the data obtained. Furthermore, I would suggest that we will see increasing research on related approaches which result in neurorestoration or neurorepair.
Growth factors
The studies that have examined growth factors as neuroprotectants in animal stroke models have recently been reviewed critically by Ren and Finkelstein (2005). Studies have included the effect of basic fibroblast growth factor, osteogenic protein-1 (OP-1), vascular endothelial growth factor and granulocyte colony-stimulating factor. While Ren and Finkelstein (2005) suggested that such compounds might have more applicability in assisting long term recovery rather than acute treatment, fibroblast growth factor is neuroprotective if given soon after an acute MCAO (Ellsworth et al., 2003).
Cell survival/cell death pathways
Considerable knowledge is now being gained about the mechanisms responsible for controlling cell death and cell survival. The pathways involved are complex (Figure 2), but one can be sure that research activity will increase in an effort to develop compounds that will modify the pathways. There are, however, major problems to be overcome, as some of the factors shown can lead to either cell survival or cell death depending on the duration or severity of the ischaemic insult or the isoform being targeted (see for example Resnick and Fennell, 2004). There is also controversy about the role of some of the pathways and the way that they interact (Martindale and Holbrook, 2002). While manipulation of these pathways may prove valuable following a stroke, we remain ignorant at present as to how we might ‘switch on' or ‘switch off' such mechanisms in the damaged brain without disrupting homoeostasis in many other regions of the body where such intervention might prove problematic.
Conclusions
The continued development of drug to induce reperfusion following an acute ischaemic will continue, as this is the only approach with proven therapeutic benefit at present. Success with compounds presently in Phase III and in preclinical development will have to encompass not only improved functional outcomes compared with placebo, but also a longer therapeutic window than rt-PA. The new compounds will also have to possess a satisfactory adverse event profile, particularly a low incidence of haemorrhagic transformation, a problem that has limited acceptance of rt-PA by clinicians.
The lack of success with the neuroprotectant approach to therapy is going to markedly inhibit future research and development. NXY-059 adhered to the STAIR criteria in its development programme and failed. New ideas are therefore desperately needed. Perhaps new animal models mimicking more closely the morbidity problems of the stroke patient might be the way forward. At present, any novel compounds in development have presumably gone through similar screening techniques in vivo to those employed in the development of NXY-059, so it is hard to imagine they will enjoy any greater clinical success than NXY-059.
Another major point raised by the NXY-059 clinical programme is that more than one large clinical trial will be required by any regulatory body to prove efficacy. And when one says large, this really means large in a way not contemplated by many earlier trials. The total patient population of the two NXY-059 trials (SAINT 1 and SAINT 2) was around 5000 patients. This is a huge investment for any company to make.
I have little doubt research in the pharmaceutical industry will continue, because the clinical need is great and the commercial rewards are strong. However, research may not continue with neuroprotection as such, but rather on neuro-restoration and neurorepair as we get to understand more about the molecular mechanisms involved in such approaches.
Glossary
- AMPA
(RS)-α-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid
- MCA
middle cerebral artery
- NMDA
N-methyl-D-aspartate
- NXY-059
disodium 2,4-disulphophenyl-N-tert-butylnitrone
- rt-PA
recombinant tissue plasminogen activator
- STAIR
Stroke Therapy Academic Industry Roundtable
Footnotes
Conflict of interest
The author is a former employee of AstraZeneca Pharmaceuticals.
References
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nature Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF. Citicoline mechanisms and clinical efficacy in cerebral ischemia. J Neurosci Res. 2002;70:133–139. doi: 10.1002/jnr.10403. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nature Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Angel I, Bar A, Horovitz T, Taler G, Krakovsky M, Resnitsky D, et al. Metal ion chelation in neurodegenerative disorders. Drug Develop Res. 2002;56:300–309. [Google Scholar]
- Angel I, Strein S, Schatz G, Krakovsky M, Finkelstein E, Dvir E, et al. The lipophilic transition modulator DP-b99 attenuates matrix metalloproteinase activity in a rat MCAO ischaemic model. Soc Neurosci Abstract. 2004;100:3. [Google Scholar]
- Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2000;20:452–457. doi: 10.1097/00004647-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Asano T, Mori T, Shimoda R, Shinagawa R, Satoh S, Yada S, et al. Arundic acid (ONO-2506) ameliorates delayed ischemic brain damage by preventing astrocyte overproduction of S100â. Curr Drug Targets-CNS & Neurol Dis. 2005;4:127–142. doi: 10.2174/1568007053544084. [DOI] [PubMed] [Google Scholar]
- Atamna H, Paler-Martinez A, Ames BN. N-t-butyl hydroxylamine, a hydrolysis product of á-phenyl-N-t-butyl nitrone, is more potent in delaying senescence in human lung fibroblasts. J Biol Chem. 2000;275:6741–6748. doi: 10.1074/jbc.275.10.6741. [DOI] [PubMed] [Google Scholar]
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, et al. Tumor necrosis factor-alpha—a mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Barone FC, Parsons AA. Therapeutic potential of anti-inflammatory drugs in focal stroke. Exp Opin Invest Drugs. 2000;9:2281–2306. doi: 10.1517/13543784.9.10.2281. [DOI] [PubMed] [Google Scholar]
- Belayev LL, Liu YT, Zhao WZ, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke, marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–560. doi: 10.1161/01.str.32.2.553. [DOI] [PubMed] [Google Scholar]
- Bringmann P, Gruber D, Liese A, Toschi L, Kratzchmar J, Schleuning WD, et al. Structural features mediating fibrin selectivity of vampire bat plasminogen activators. J Biol Chem. 1995;270:25596–25603. doi: 10.1074/jbc.270.43.25596. [DOI] [PubMed] [Google Scholar]
- Brinker G, Franke C, Hoehn M, Uhlenkuken U, Hossmann KA. Thrombolysis of cerebral clot embolism in rat: effect of treatment delay. NeuroReport. 1999a;10:3269–3272. doi: 10.1097/00001756-199911080-00004. [DOI] [PubMed] [Google Scholar]
- Brinker G, Pillekamp F, Hossmann KA. Brain hemorrhages after rt-PA treatment of embolic stroke in spontaneously hypertensive rats. NeuroReport. 1999b;10:1943–1946. doi: 10.1097/00001756-199906230-00027. [DOI] [PubMed] [Google Scholar]
- Camerlingo M, Salvi P, Belloni G, Gamba T, Cesana BM, Mamoli A. Intravenous heparin started within the first 3 hours after onset of symptoms as a treatment for acute nonlacunar hemispheric cerebral infarctions. Stroke. 2005;36:2415–2420. doi: 10.1161/01.STR.0000185730.50480.e7. [DOI] [PubMed] [Google Scholar]
- Carney S. What do you call 500 scientists coming together to address a productivity gap? Answer: a start. Drug Discov Today. 2005;10:1025–1029. doi: 10.1016/S1359-6446(05)03544-0. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signalling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, Liu LS, et al. Indications for early aspirin use in acute ischemic stroke. Stroke. 2000;31:1240–1249. doi: 10.1161/01.str.31.6.1240. [DOI] [PubMed] [Google Scholar]
- Chenard BL, Bordner J, Butler TW, Chambers LK, Collins MA, De Costa DL, et al. 1S,2S)-1-(4-hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: a potent new neuroprotectant which blocks N-methyl-D-aspartate responses. J Med Chem. 1995;38:3138–3145. doi: 10.1021/jm00016a017. [DOI] [PubMed] [Google Scholar]
- Culot M, Rentfel M, Dehouck M-P, Lundquist S, Cecchelli R.2006NXY-059, a free radical-trapping neuroprotectant protects the blood brain barrier (BBB) in ischaemic conditions in vitro Soc for Neurosciabstract no67913 [Google Scholar]
- Dávalos A, Castillo J, Alvarez-Sabin J, Secades JJ, Mercadal J, Lopez S, et al. Oral citicoline in acute ischemic stroke: an individual patient data pooling analysis of clinical trials. Stroke. 2002;33:2850–2857. doi: 10.1161/01.str.0000038691.03334.71. [DOI] [PubMed] [Google Scholar]
- Davis M, Mendelow AD, Perry RH, Chambers IR, James OF. Experimental stroke and neuroprotection in the ageing rat brain. Stroke. 1995;26:1072–1078. doi: 10.1161/01.str.26.6.1072. [DOI] [PubMed] [Google Scholar]
- Dehouck M-P, Cecchelli R, Green AR, Renftel M, Lundquist S. In vitro blood-brain barrier permeability and cerebral endothelial cell uptake of the neuroprotective nitrone compound NXY-059 in normoxic, hypoxic and ischemic conditions. Brain Res. 2002;955:229–235. doi: 10.1016/s0006-8993(02)03469-8. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Stroke and neurovascular protection. N Engl J Med. 2006;354:553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- Di Fabio R, Cugola D, Donati A, Feriani G, Gaviraghi E, Ratti E, et al. Identification and pharmacological characterization of GV 150526, a novel glycine antagonist as a potent neuroprotective agent. Drugs Future. 1998;23:61–69. [Google Scholar]
- Di X, Bullock R, Watson J, Fatouros P, Chenard B, White F, et al. Effect of CP101,606, a novel NR2B subunit antagonist of the N-methyl-D-aspartate receptor, on the volume of ischaemic brain damage and cytotoxic brain edema after middle cerebral artery occlusion in the feline brain. Stroke. 1997;28:2244–2251. doi: 10.1161/01.str.28.11.2244. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Davis SM. Stroke drug development. Usually, but not always, animal models. Stroke. 2005;36:2326. doi: 10.1161/01.STR.0000179042.06535.2f. [DOI] [PubMed] [Google Scholar]
- Editorial Tackling the global burden of stroke. Lancet Neurol. 2005;4:689. doi: 10.1016/S1474-4422(05)70202-7. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Ellsworth JL, Garcia R, Yu J, Kindy MS. Fibroblast growth factor-18 reduced infarct volumes and behavioural deficits after transient occlusion of the middle cerebral artery in rats. Stroke. 2003;34:1507–1512. doi: 10.1161/01.STR.0000071760.66720.5F. [DOI] [PubMed] [Google Scholar]
- Emsley HCA, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiat. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein GZ, Zaleska MM, Krams M, Wang X, Day M, Rutkowski JL, et al. 2007Missing steps on the STAIR case: a translational perspective on the development of NXY-059 for the treatment of acute ischemic stroke J Cereb Blood Flow Metabdoi: 10.1038/sj.jcbfm.9600516 [DOI] [PubMed]
- Fisher M, Tatlisumak T. Use of animal models has not contributed to development of acute stroke therapies. Con Stroke. 2005;36:2324–2325. doi: 10.1161/01.STR.0000179039.76922.e8. [DOI] [PubMed] [Google Scholar]
- Friedman JE, Resnitsky D, Rudich H, Gilead C, Schatz G, Hanig R, et al. The lipophilic transition modulator DP-b99 attenuates oxidative stress-induced neuronal death. Soc Neurosci Abstract. 2004;1019:12. [Google Scholar]
- Gido G, Cronberg T, Wieloch T. The effect of α-phenyl-tert-butyl nitrone (PBN) on free radical formation in transient focal ischemia measured by microdialysis and 3,4-dihydroxybenzoate formation. Acta Physiol Scand. 2000;168:277–285. doi: 10.1046/j.1365-201x.2000.00657.x. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Becker DA, Busto R, Belayev A, Zhang YB, Khoutorova L, et al. Stilbazulenyl nitrone, a novel antioxidant, is highly neuroprotective in focal ischemia. Ann Neurol. 2003;54:330–342. doi: 10.1002/ana.10659. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Hill MD, Palesch YY, Rykborst KJ, Tamariz D.2005The ALIAS phase I trial: a dose-escalation and safety study of albumin for acute ischemic stroke Stroke 36420(abstract 13). [Google Scholar]
- Green AR, Ashwood T. Free radical trapping as a therapeutic approach to neuroprotection in stroke: experimental and clinical studies with NXY-059 and free radical scavengers. Curr Drug Targets-CNS & Neurol Dis. 2005;4:109–118. doi: 10.2174/1568007053544156. [DOI] [PubMed] [Google Scholar]
- Green AR, Ashwood T, Odergren T, Jackson DM. Nitrones as neuroprotective agents in cerebral ischemia, with particular reference in NXY-059. Pharmacol Ther. 2003b;100:195–214. doi: 10.1016/j.pharmthera.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Green AR, Cross AJ. Techniques for examining neuroprotective drugs in vivo. Int Rev Neurobiol. 1997;40:47–68. doi: 10.1016/s0074-7742(08)60715-1. [DOI] [PubMed] [Google Scholar]
- Green AR, Lanbeck-Vallen K, Ashwood T, Lundquist S, Lindstrom Böö E, Jonasson H, et al. Brain penetration of the novel free radical trapping neuroprotectant NXY-059 in rats subjected to permanent focal ischemia. Brain Res. 2006;1072:224–226. doi: 10.1016/j.brainres.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Green AR, Odergren T, Ashwood T. Animal models of stroke: do they have value for discovering neuroprotective agents. Trends Pharmacol Sci. 2003a;24:402–408. doi: 10.1016/S0165-6147(03)00192-5. [DOI] [PubMed] [Google Scholar]
- Green AR, Shuaib A. Therapeutic strategies for the treatment of stroke. Drug Discov Today. 2006;11:681–693. doi: 10.1016/j.drudis.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, et al. The Desmoteplase in Acute Ischaemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Fieschi C, Vonkummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischemic stroke (ECASS II) Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- Hainsworth AH, Bhuiyan N, Green AR.2007The nitrone NXY-059 is without cytoprotective effect on SNP-induced cell death in N1E-115 neuroblastoma cells in vitro J Cereb Blood Flow Metabdoi: 10.1038/sj.jcbfm.9600517 [DOI] [PubMed]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nature Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Heldmann U, Thored P, Claasen JH, Arvidsson A, Kokaia Z, Lindvall O. TNF-alpha antibody infusion impairs survival of stroke-generated neuroblasts in adult rat brain. Exp Neurol. 2005;196:204–208. doi: 10.1016/j.expneurol.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Hoyte L, Barber PA, Buchan AM, Hill MD. The rise and fall of NMDA antagonists for ischemic stroke. Curr Mol Med. 2004;4:131–136. doi: 10.2174/1566524043479248. [DOI] [PubMed] [Google Scholar]
- Huh PW, Belayev L, Zhao WZ, Busto R, Saul I, Ginsberg MD. The effect of high-dose albumin therapy on local cerebral perfusion after transient focal cerebral ischaemia in rats. Brain Re. 1998;804:105–113. doi: 10.1016/s0006-8993(98)00674-x. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Roussel S, Pinard E, Seylaz J. Reduction in infarct volume by magnesium after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1991;11:1025–1030. doi: 10.1038/jcbfm.1991.170. [DOI] [PubMed] [Google Scholar]
- Kakihana M, Fukuda N, Suno M, Nagaoka A. Effects of CDP-choline on neurologic deficits and cerebral glucose metabolism in a rat model of cerebral ischemia. Stroke. 1988;19:217–222. doi: 10.1161/01.str.19.2.217. [DOI] [PubMed] [Google Scholar]
- Kamei K, Maeda N, Ogino R, Koyama M, Nakajima M, Tatsuoka T, et al. New 5-HT1A receptor agonists possessing 1,4-benzoxazepine scaffold exhibit highly potent anti-ischaemic effects. Bioorg Med Chem Letts. 2001;11:595–598. doi: 10.1016/s0960-894x(01)00008-7. [DOI] [PubMed] [Google Scholar]
- Kaste M. Use of animal models has not contributed to development of acute stroke therapies. Pro Stroke. 2005;36:2323–2324. doi: 10.1161/01.STR.0000179037.82647.48. [DOI] [PubMed] [Google Scholar]
- Kaur J, Zhao ZG, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator. J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- Koch S, Concha M, Wazzan T, Romano JG, Forteza A. High dose serum albumin for the treatment of acute ischemic stroke - a safety study. Neocrit Care. 2004;1:335–341. doi: 10.1385/NCC:1:3:335. [DOI] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Kundrotiene J, Cebers G, Wagner A, Liljequist S. The NMDA NR2B subunit selective receptor antagonist CP-101,606 enhances the functional recovery and reduces brain damage after cortical compression-induced brain ischemia. J Neurotrauma. 2004;21:83–93. doi: 10.1089/089771504772695977. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Tsuchidate R, Smith M-L, Maples KR, Siesjö BK. Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1999;19:778–787. doi: 10.1097/00004647-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Song DH, Wei JD, Zivin JA. Neuroprotective effects of the spin trap agent disodium-[(tert-butylimino)methyl]benzene-1,3-disulfonate N-oxide (Generic NXY-059) in rabbit small clot embolic stroke model. Stroke. 2002;33:1411–1415. doi: 10.1161/01.str.0000015346.00054.8b. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–3039. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]
- Lees KR, Ashwood T, Odergren T. Response to letter by Proctor and Tamborello. Stroke. 2007;38:e110. [Google Scholar]
- Lees KR, Barer D, Ford GA, Hacke W, Kostulas V, Sharma AK, et al. Tolerability of NXY-059 at higher target concentrations in patients with acute stroke. Stroke. 2003;34:482–487. doi: 10.1161/01.str.0000053032.14223.81. [DOI] [PubMed] [Google Scholar]
- Lees KR, Green AR, Odergren T. Comparison of neuroprotective data for NXY-059 in animal models with STAIR criteria. Cerebrovasc Dis. 2001;11 suppl 14:77. [Google Scholar]
- Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, et al. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354:588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- Ley JJ, Vigdorchik A, Belayev L, Zhao WZ, Busto R, Khoutorova L, et al. Stilbazulenyl nitrone, a second-generation azulenyl nitrone antioxidant, confers enduring neuroprotection in experimental focal cerebral ischemia in the rat: neurobehavior, histopathology, and pharmacokinetics. J Pharmacol Exp Ther. 2005;313:1090–1100. doi: 10.1124/jpet.105.083386. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Samson A, Bladin C, Schleuning WD, Medcalf RL. Vampire bat salivary plasminogen activator (desmoteplase)—a unique fibrinolytic enzyme that does not promote neurodegeneration. Stroke. 2003;34:537–543. doi: 10.1161/01.str.0000049764.49162.76. [DOI] [PubMed] [Google Scholar]
- Lin J-Y, Chung SY, Lin MC, Cheng FC. Effects of magnesium sulphate on energy metabolites and glutamate in the cortex during focal ischemia and reperfusion in the gerbil monitored by a dual-probe microdialysis technique. Life Sci. 2002;71:803–811. doi: 10.1016/s0024-3205(02)01738-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Belayev L, Zhao W, Busto R, Belayev A, Ginsberg MD. Neuroprotective effect of treatment with human albumin in permanent focal cerebral ischemia: histopathology and cortical perfusion studies. Eur J Pharmacol. 2001;428:193–201. doi: 10.1016/s0014-2999(01)01255-9. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nature Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lopez-Atalaya JP, Roussel BD, Ali C, Maubert E, Petersen KU, Berezowski V, et al. Recombinant Desmodus rotundus salivary plasminogen activator crosses the blood-brain barrier through a low-density lipoprotein receptor-related protein-dependent mechanism without exerting neurotoxic effects. Stroke. 2007;38:1036–1043. doi: 10.1161/01.STR.0000258100.04923.84. [DOI] [PubMed] [Google Scholar]
- Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering F, Zhang Y. Therapeutic potential of TACE inhibitors in stroke. Curr Drug Targets-CNS & Neurol Dis. 2005;4:161–168. doi: 10.2174/1568007053544147. [DOI] [PubMed] [Google Scholar]
- Lu M, Krams M, Zhang L, Zhang ZG, Chopp M. Assessing combination treatments for acute stroke: preclinical experiences. Behav Brain Res. 2005;162:165–172. doi: 10.1016/j.bbr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Lu XM, Yang X, Chen R, Almassian B, Jiang Z, Ghanbari HR, et al. 2004A novel neuroprotectant, PAN-811, decreases infarct volume following transient focal ischemia in rats Soc Neurosciabstract3432 [Google Scholar]
- Maples KR, Ma F, Zhang YK. Comparison of the radical trapping ability of PBN, S-PBN and NXY-059. Free Rad Res. 2001;34:417–426. doi: 10.1080/10715760100300351. [DOI] [PubMed] [Google Scholar]
- Marinov MB, Harbaugh KS, Hoopes PJ, Pikus HJ, Harbaugh RE. Neuroprotective effects of pre-ischemia intra-arterial magnesium sulfate in reversible cerebral ischemia. J Neurosurg. 1996;85:117–124. doi: 10.3171/jns.1996.85.1.0117. [DOI] [PubMed] [Google Scholar]
- Marshall JWB, Cummings RM, Bowes LJ, Ridley RM, Green AR. Functional and histological evidence for the protective effect of NXY-059 in a primate model of stroke when given 4 hours after occlusion. Stroke. 2003;34:2228–2233. doi: 10.1161/01.STR.0000087790.79851.A8. [DOI] [PubMed] [Google Scholar]
- Marshall JWB, Duffin KJ, Green AR, Ridley RM. NXY-059, a free radical trapping agent, substantially attenuates the functional disability induced by stroke in a primate species. Stroke. 2001;32:190–198. doi: 10.1161/01.str.32.1.190. [DOI] [PubMed] [Google Scholar]
- Marshall JWB, Ridley RM. Assessment of functional impairment following permanent middle cerebral artery occlusion in a non-human primate species. Neurodegeneration. 1996;5:275–286. doi: 10.1006/neur.1996.0036. [DOI] [PubMed] [Google Scholar]
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signalling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- Masuda S, Nagao M, Takahata K, Konishi Y, Gallyas F, Jr, Tabira T, et al. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J Biol Chem. 1993;268:11208–11216. [PubMed] [Google Scholar]
- Mauler F, Horváth E. Neuroprotective efficacy of repinotan HCl, a 5-HT1A receptor agonist, in animal models of stroke and traumatic brain injury. J Cereb Blood Flow Metab. 2005;25:451–459. doi: 10.1038/sj.jcbfm.9600038. [DOI] [PubMed] [Google Scholar]
- Menniti F. CP-101,606, a potent neuroprotectant selective for forebrain neurons. Eur J Pharmacol. 1997;331:117–126. doi: 10.1016/s0014-2999(97)10092-9. [DOI] [PubMed] [Google Scholar]
- Mohr JP, Gautier JC, Hier D, Stein RW.1986Middle cerebral arteryIn: Barnett HJM, Stein BM, Mohr JP, Yatsu FM (eds)Stroke, Vol 1: Pathophysiology, Diagnosis and Management Churchill Livingstone: New York; 377–450. [Google Scholar]
- Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke. Emerging pharmacological, mechanical and imaging strategies. Stroke. 2005;36:2311–2320. doi: 10.1161/01.STR.0000182100.65262.46. [DOI] [PubMed] [Google Scholar]
- Montoney M, Gardell SJ, Marder VJ. Comparison of the bleeding potential of vampire bat salivary plasminogen activator versus tissue plasminogen activator in an experimental rabbit model. Circulation. 1995;91:1540–1544. doi: 10.1161/01.cir.91.5.1540. [DOI] [PubMed] [Google Scholar]
- Muir KW, Grosset DG. Neuroprotection for acute stroke, making clinical trials work. Stroke. 1999;30:180–182. doi: 10.1161/01.str.30.1.180. [DOI] [PubMed] [Google Scholar]
- Muir KW, Lees KR, Ford I, Davis S. Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial): randomised controlled trial. Lancet. 2004;363:439–445. doi: 10.1016/S0140-6736(04)15490-1. [DOI] [PubMed] [Google Scholar]
- Mutch NJ, Moore NR, Mattsson C, Jonasson H, Green AR, Booth NA.2007The use of the Chandler loop to examine the interaction potential of NXY-059 on the thrombolytic properties of rtPA on human thrombi in vitro Br J Pharmacoldoi: 10.1038/sj.bjp.0707543 [DOI] [PMC free article] [PubMed]
- NINDS t-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Noguchi N, Yoshida Y, Kaneda H, Yamamoto Y, Niki E. Action of ebselen as an antioxidant against lipid peroxidation. Biochem Pharmacol. 1992;44:39–44. doi: 10.1016/0006-2952(92)90035-h. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Payne KA, Huybrechts KF, Caro JJ, Green TJC, Klittich WS. Long term cost-of-illness in stroke. Pharmacoeconomics. 2002;20:813–825. doi: 10.2165/00019053-200220120-00002. [DOI] [PubMed] [Google Scholar]
- Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. Br Med J. 2007;334:197–200. doi: 10.1136/bmj.39048.407928.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Regan MH. A potentially critical role of phospholipases in central nervous system ischemic, traumatic and neurodegenerative disorders. Brain Res Revs. 2004;44:13–47. doi: 10.1016/j.brainresrev.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Proctor PH, Tamborello LP. SAINT-1 worked, but the neuroprotectant is not NXY-059. Stroke. 2007;38:e109. doi: 10.1161/STROKEAHA.107.489161. [DOI] [PubMed] [Google Scholar]
- Rao AM, Hatcher JF, Dempsey RJ. CDP-choline: neuroprotection in transient forebrain ischemia of gerbils. J Neurosci Res. 1999;58:697–705. [PubMed] [Google Scholar]
- Reddrop C, Moldrich RX, Beart PM, Farso M, Liberatore GT, Howells D, et al. Vampire bat salivary plasminogen activator (desmoteplase) inhibits tissue-type plasminogen activator-induced potentiation of excitotoxic injury. Stroke. 2005;36:1241–1246. doi: 10.1161/01.STR.0000166050.84056.48. [DOI] [PubMed] [Google Scholar]
- Relton JK, Rothwell NJ. Involvement of interleukin-1 and lipocortin-1 in ischemic brain damage. Cerebrovasc Brain Metab Rev. 1992;5:178–198. [PubMed] [Google Scholar]
- Ren JM, Finkelstein SP. Growth factor treatment of stroke. Curr Drug Targets-CNS & Neurol Dis. 2005;4:121–125. doi: 10.2174/1568007053544101. [DOI] [PubMed] [Google Scholar]
- Resnick L, Fennell M. Targeting JNK3 for the treatment of neurodegenerative disorders. Drug Discov Today. 2004;9:932–939. doi: 10.1016/S1359-6446(04)03251-9. [DOI] [PubMed] [Google Scholar]
- Ringelstein EB, Biniek R, Weiller C, Ammeling B, Nolte PN, Thron A. Type and extent of hemispheric brain infarctions and clinical outcome in early and delayed middle cerebral artery recanalization. Neurology. 1992;42:289–298. doi: 10.1212/wnl.42.2.289. [DOI] [PubMed] [Google Scholar]
- Rosenberg G, Angel I, Kozak A. Clinical pharmacology of DP-b99 in healthy volunteers: First administration to humans. Br J Clin Pharmacol. 2005;60:7–16. doi: 10.1111/j.1365-2125.2005.02378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco RL, DeRosa JT, Haley EC, Levin B, Ordronneau P, Phillips SJ, et al. Glycine antagonist in neuroprotection for patients with acute stroke: GAIN Americas: a randomized controlled trial. JAMA. 2001;285:1719–1728. doi: 10.1001/jama.285.13.1719. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salom JB, Perez-Asensio FJ, Burguete MC, Marin N, Pitarch C, Torregrosa G, et al. Single-dose ebselen does not afford sustained neuroprotection to rats subjected to severe focal cerebral ischemia. Eur J Pharmacol. 2004;495:55–62. doi: 10.1016/j.ejphar.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Sassoli PM, Emmell EL, Tam SH, Trikha M, Zhou Z, Jordan RE, et al. 7E3 f(ab')2 an effective antagonist of rat αIIbβ3 and αvβ3, blocks in vivo thrombus formation and in vitro angiogenesis. Thromb Haemostasis. 2001;85:896–902. [PubMed] [Google Scholar]
- Saver JL. Time is brain—quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- Savitz SI. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: a need for more rigorous testing of neuroprotective agents in animal models of stroke. Neurology. 2007;205:20–25. doi: 10.1016/j.expneurol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Schaller BJ. Influence of age on stroke and preconditioning-induced ischemic tolerance in brain. Exp Neurol. 2007;205:9–19. doi: 10.1016/j.expneurol.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Schleuning WD. Vampire bat plasminogen activator DSPA-α1 (desmoteplase): a thrombolytic drug optimised by natural selection. Haemostasis. 2001;31:118–122. doi: 10.1159/000048054. [DOI] [PubMed] [Google Scholar]
- Schmid R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ. Neuroprotective effects of combination therapy with tirilazad and magnesium in rats subjected to reversible focal cerebral ischaemia. Neurosurgery. 1999;44:163–171. doi: 10.1097/00006123-199901000-00100. [DOI] [PubMed] [Google Scholar]
- Sherman DG. Antithrombotic and hypofibrinogenetic therapy in acute ischemic stroke: What is the next step. Cerebrovasc Dis. 2004;17 Suppl 1:138–143. doi: 10.1159/000074806. [DOI] [PubMed] [Google Scholar]
- Sherman DG, Atkinson RP, Chippendale T, Levin KA, Ng K, Futrell N, et al. Intravenous ancrod for treatment of acute ischemic stroke: the STAT study: a randomised controlled trial. Stroke treatment with ancrod trial. JAMA. 2000;283:2395–2403. doi: 10.1001/jama.283.18.2395. [DOI] [PubMed] [Google Scholar]
- Shuaib A. Neuroprotection - STAIR-way to the future. Cerebrovasc Dis. 2006;22 Suppl 1:10–17. [Google Scholar]
- Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Yang Y, Li Q. Evaluating the efficacy of citicoline in embolic ischemic stroke in rats: neuroprotective effects when used alone or in combination with urokinase. Exp Neurol. 2000;161:733–739. doi: 10.1006/exnr.1999.7314. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Yang Y, Nakada MT, Li Q, Yang T. Glycoprotein IIb/IIIa antagonist, murine 7E3 f(ab')2 and tissue plasminogen activator in focal ischemia: Evaluation of efficacy and risk of hemorrhage with combination therapy. J Cereb Blood Flow Metab. 2002;22:215–222. doi: 10.1097/00004647-200202000-00009. [DOI] [PubMed] [Google Scholar]
- Sobrado M, Lopez MG, Carceller F, Garcia AG, Roda JM. Combined nimodipine and citicoline reduce infarct size, attenuate apoptosis and increase bcl-2 expression after focal cerebral ischemia. Neuroscience. 2003;118:107–113. doi: 10.1016/s0306-4522(02)00912-0. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable Recommendations for standards regarding pre-clinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable II 2001Stroke Therapy Academic Industry Roundtable II (STAIR-II) Stroke 321598–1606.11441207 [Google Scholar]
- Sydserff SG, Borelli AR, Green AR, Cross AJ. Effect of NXY-059 on infarct volume after transient or permanent middle cerebral artery occlusion in the rat; studies on dose, plasma concentration and therapeutic time window. Br J Pharmacol. 2002;135:103–112. doi: 10.1038/sj.bjp.0704449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SH, Sassoli PM, Jordan RE, Nakada MT. Abciximab (ReoPro, chimeric 7E3 Fab) demonstrates equivalent affinity and functional blockade of glycoprotein IIb/IIIa and αvβ3 integrins. Circulation. 1998;98:1085–1091. doi: 10.1161/01.cir.98.11.1085. [DOI] [PubMed] [Google Scholar]
- Tateishi N, Mori T, Kagamiishi Y, Satoh S, Katsube N, Morikawa E, et al. Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part II: Suppression of astrocytic activation by a novel agent (R)-(-)-2-propyloctanoic acid (ONO-2506) leads to mitigation of delayed infarct expansion and early improvement of neurologic deficits. J Cereb Blood Flow Metab. 2002;22:723–734. doi: 10.1097/00004647-200206000-00011. [DOI] [PubMed] [Google Scholar]
- Torup L. Neuroprotection with or without erythropoiesis, sometimes less is more. Br J Pharmacol. 2007;151:1141–1142. doi: 10.1038/sj.bjp.0707287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traystman RJ. Animal models of focal and global cerebral ischemia. Ilar J. 2003;44:85–95. doi: 10.1093/ilar.44.2.85. [DOI] [PubMed] [Google Scholar]
- Trovarelli G, de Medio GE, Dorman RV, Piccinin GL, Horrocks LA, Porcellati G. Effect of cytidine diphosphate choline (CDP-choline) on ischemia-induced alterations of brain lipid in the gerbil. Neurochem Res. 1981;6:821–833. doi: 10.1007/BF00965041. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Jauch EC. Extending the window for acute ischaemic stroke treatment: thrombolytics plus CNS protective therapies. Exp Neurol. 2004;188:195–199. doi: 10.1016/j.expneurol.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Wang CX, Shuaib A. NXY-059: a neuroprotective agent in acute stroke. Int J Clin Practice. 2004;58:964–969. doi: 10.1111/j.1368-5031.2004.00245.x. [DOI] [PubMed] [Google Scholar]
- Wang CX, Shuaib A. NMDA/NR2B selective antagonists in the treatment of ischaemic brain injury. Curr Drug Targets-CNS & Neurol Dis. 2005;4:143–151. doi: 10.2174/1568007053544183. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang ZG, Rhodes K, Renzi M, Zhang RL, Kapke A, et al. Post-ischaemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of cerebral ischaemia. Br J Pharmacol. 2007;151:1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Warlow CP, Counsell C. Systematic review of evidence on thrombolytic therapy for acute ischaemic stroke. Lancet. 1997;350:607–614. doi: 10.1016/s0140-6736(97)03022-5. [DOI] [PubMed] [Google Scholar]
- Weiser T. AMPA receptor agonists for the treatment of stroke. treatment of ischaemic brain injury. Curr Drug Targets-CNS & Neurol Dis. 2005;4:153–159. doi: 10.2174/1568007053544129. [DOI] [PubMed] [Google Scholar]
- Williams HE, Claybourn M, Green AR. Investigating the free radical trapping ability of NXY-059, S-PBN and PBN. Free Radical Res. 2007;41:1047–1052. doi: 10.1080/10715760701557161. [DOI] [PubMed] [Google Scholar]
- Xue D, Slivka A, Buchan AM. Tirilazad reduces cortical infarction after transient but not permanent focal cerebral ischemia in rats. Stroke. 1992;23:894–899. doi: 10.1161/01.str.23.6.894. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li Q, Yang T, Hussain M, Shuaib A. Reduced brain infarct volume and improved neurological outcome by inhibition of the NR2B subunit of NMDA receptors by using CP101,606-27 alone and in combination with rt-PA in a thromboembolic stroke model in rats. J Neurosurg. 2003;98:397–403. doi: 10.3171/jns.2003.98.2.0397. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li Q, Ahmad F, Shuaib A. Survival and histological evaluation of therapeutic window of post-ischemia treatment with magnesium sulphate in embolic stroke model of rat. Neurosci Letts. 2000;285:119–122. doi: 10.1016/s0304-3940(00)01048-x. [DOI] [PubMed] [Google Scholar]
- Yurkewicz L, Weaver J, Bullock MR, Marshall LF. The effect of the selective NMDA receptor antagonist traxprodil in the treatment of traumatic brain injury. J Neurotrauma. 2005;22:1428–1443. doi: 10.1089/neu.2005.22.1428. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Zhang C, Zhang RL, Chopp M. Intravenous administration of a GPIIb/IIIa receptor antagonist extends the therapeutic window of intra-arterial tenecteplase-tissue plasminogen activator in a rat stroke model. Stroke. 2004;35:2890–2895. doi: 10.1161/01.STR.0000147963.68238.da. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Zhang R, Morris D, Lu M, Coller BS, et al. Adjuvant treatment with a glycoprotein IIb/IIIa receptor inhibitor increases the therapeutic window for low-dose tissue plasminogen activator administration in a rat model of embolic stroke. Circulation. 2003a;107:2837–2843. doi: 10.1161/01.CIR.0000068374.57764.EB. [DOI] [PubMed] [Google Scholar]
- Zhang L-F, Yang J, Hong Z, Yuan GG, Zhou BF, Zhao LC, et al. Proportion of different subtypes of stroke in China. Stroke. 2003b;34:2091–2096. doi: 10.1161/01.STR.0000087149.42294.8C. [DOI] [PubMed] [Google Scholar]
- Zhao ZG, Cheng MS, Maples KR, Ma JY, Buchan AM. NXY-059, a novel free radical trapping compound, reduces cortical infarction after permanent focal cerebral ischemia in the rat. Brain Res. 2001;909:46–50. doi: 10.1016/s0006-8993(01)02618-x. [DOI] [PubMed] [Google Scholar]