Abstract
G-protein-coupled receptors (GPCRs), also known as seven transmembrane receptors (7-TMRs), are the largest protein receptor superfamily in the body. These receptors and their ligands direct a diverse array of physiological responses, and hence have broad relevance to numerous diseases. As a result, they have generated considerable interest in the pharmaceutical industry as drug targets. Recently, GPCRs have been demonstrated to elicit signals through interaction with the scaffolding proteins, β-arrestins-1 and 2, independent of heterotrimeric G-protein coupling. This review discusses several known G-protein-independent, β-arrestin-dependent pathways and their potential physiological and pharmacological significance. The emergence of G-protein-independent signalling changes the way in which GPCR signalling is evaluated, from a cell biological to a pharmaceutical perspective and raises the possibility for the development of pathway specific therapeutics.
Keywords: G-protein, arrestins, G-protein-independent, GPCR, 7-TMR, heterotrimeric G-protein, Gα
Introduction
G-protein-coupled receptors (GPCRs), also known as seven transmembrane receptors (7-TMRs), are the largest protein receptor superfamily in the body. These receptors and their ligands direct a diverse array of physiological responses, and hence have broad relevance to numerous diseases. As a result, they have generated considerable interest in the pharmaceutical industry as drug targets. Therapeutics targeting GPCRs include agonists, partial agonists and antagonists, based on a two-state model of receptor activation and the concept that activation is dependent on association with heterotrimeric G-proteins. Different subclasses of Gα proteins, such as Gαs, Gαi, Gαq and Gα12, signal through distinct pathways involving second messenger molecules such as cAMP, inositol triphosphate (IP3), diacylglycerol, intracellular Ca2+ and RhoA GTPases. Recent studies suggest that signalling through these receptors is far more diverse than originally thought, as a single GPCR can couple to multiple G-proteins, as well as signal through other adaptor proteins, independent of G-protein coupling. There are a number of parameters that can affect the pathway activated by a given GPCR. First, they can adopt multiple ‘active' states, making predictions based on the two-state model of receptor activation incomplete. Second, receptor dimers appear to exhibit distinct pharmacology with respect to activation, signalling and internalization. Third, receptor clustering in membrane microdomains, such as lipid rafts, may affect G-protein coupling and trafficking. The emergence of these paradigm shifts is of vital importance to the development of drugs targeting GPCRs, and highlights the importance of evaluating multiple signalling pathways when screening specific agonists, partial agonists and antagonists.
The first evidence that there was more to GPCR signalling than meets the eye came from studies showing promiscuous coupling of a single 7-TMR to multiple G-proteins. A number of receptors can couple to multiple heterotrimeric G-proteins to elicit specific signals, often in a cell-type-specific, agonist-specific or dose-dependent fashion. For example, β2-adrenergic receptor can couple to both Gαs and Gαi; the latter appears to occur only after phosphorylation of the receptor by PKA (Daaka et al., 1997). Another example is protease-activated receptor (PAR)-1, a member of a novel class of GPCRs that are activated by proteolytic cleavage of their N terminus, which unveils a six-codon tethered ligand sequence. PAR-1 is a receptor for thrombin, and can couple to Gαi/o, Gαq and Gα12/13 (Vanhauwe et al., 2002; Marinissen et al., 2003); coupling to Gαi appears to be favoured by proteolytic activation, while coupling to Gαq is favoured by activation with soluble peptides mimicking the tethered ligand (McLaughlin et al., 2005). These studies demonstrating promiscuous coupling suggested that assays measuring the generation of specific early intermediates such as cAMP (for Gαs) or intracellular Ca2+ and IP3 accumulation (for Gαq) were no longer sufficient to quantify receptor activation, and pointed to the importance of evaluating multiple signalling pathways. Global increases in G-protein coupling in response to agonist addition can still be assessed by quantifying membrane-associated GTPase activity. However, to complicate matters further, over the last few years it has become clear that GPCRs can signal without even coupling to a G-protein (Smith and Luttrell, 2006a).
A primary transducer of G-protein-independent signalling appears to be the β-arrestin family. These proteins, β-arrestin-1 and β-arrestin-2, were originally identified as terminators of heterotrimeric G-protein coupling and mediators of endocytosis, but were later shown to serve as scaffolds linking receptor activation to a variety of signalling cascades (Luttrell and Lefkowitz, 2002). There is now a large body of work demonstrating that various parameters such as agonist dose, agonist structure, receptor clustering and perhaps the prevalence of downstream signalling components can switch the signal from a G-protein-dependent to G-protein-independent one (Violin and Lefkowitz, 2007). Muddying the waters even further, β-arrestins sometimes work in opposition and other times in synergy with the G-protein signal. In other cases, β-arrestins and G-proteins can activate the same downstream enzyme, but through different mechanisms, leading to distinct cellular outcomes. This review covers studies demonstrating various β-arrestin-dependent signalling pathways identified for different GPCRs and their relationship with heterotrimeric G-protein signalling.
β-arrestins as transducers of G-protein- independent signals
β-arrestins were first identified as mediators rather than simply terminators of GPCR signalling in a paper by Daaka et al. (1998), demonstrating that β2-adrenergic receptor (β2AR)-induced activation of mitogen-activated protein kinases (MAPKs) was inhibited by transfection of dominant-negative mutants of β-arrestin. Shortly thereafter, formation of src/β-arrestin complexes in response to β2AR activation was demonstrated (Luttrell et al., 1999), leading to the idea that β-arrestins can serve as scaffolds to link receptors to downstream signalling pathways such as MAPK. Subsequently, similar scaffolds downstream of PAR-2, type I angiotensin II receptor (AT1R), neurokinin-1 receptor, vasopressin 2 receptor (V2R), parathyroid hormone receptor (PTH1R), CXCR4 and CCR7 were identified (Luttrell et al., 1997; DeFea et al., 2000a, 2000b; Sun et al., 2002; Tohgo et al., 2002, 2003; Ge et al., 2004; Kohout et al., 2004; Caunt et al., 2006). The formation of these signalling complexes is important for subcellular targeting, leading to the hypothesis that β-arrestins can exert spatial control over signalling pathways. The requirement for β-arrestins in the activation of a variety of downstream signalling pathways, including MAPKs, the small GTPase RhoA, Ral GDP dissociation factor and the actin filament severing protein cofilin, as well as the inhibition of other molecules such as NF-kB and LIMK, has now been confirmed using dominant-negative mutants, siRNA knockdown and genetic deletion (McDonald et al., 2000; Kohout et al., 2001; Luttrell et al., 2001; Bhattacharya et al., 2002; Shenoy and Lefkowitz, 2003; Ge et al., 2004; Barnes et al., 2005; Beaulieu et al., 2005; Ren et al., 2005; Kumar et al., 2007; Zoudilova et al., 2007). β-arrestins can also act as both inhibitors and activators of some signalling pathways; in some cases the two β-arrestins antagonize each other. For example, β-arrestin-1 can both facilitate and inhibit the phosphoinositide-3-kinase (PI3K) pathway (Povsic et al., 2003; Beaulieu et al., 2005; Wang and DeFea, 2006). In the case of β2AR-, V2R- and AT1R-induced MAPK activation, one β-arrestin stimulates, while the other inhibits signalling. Despite these rather confusing aspects to β-arrestin signalling, it is becoming increasingly clear that they can elicit some of their inhibitory and stimulatory signals through direct interaction with receptors and their downstream targets.
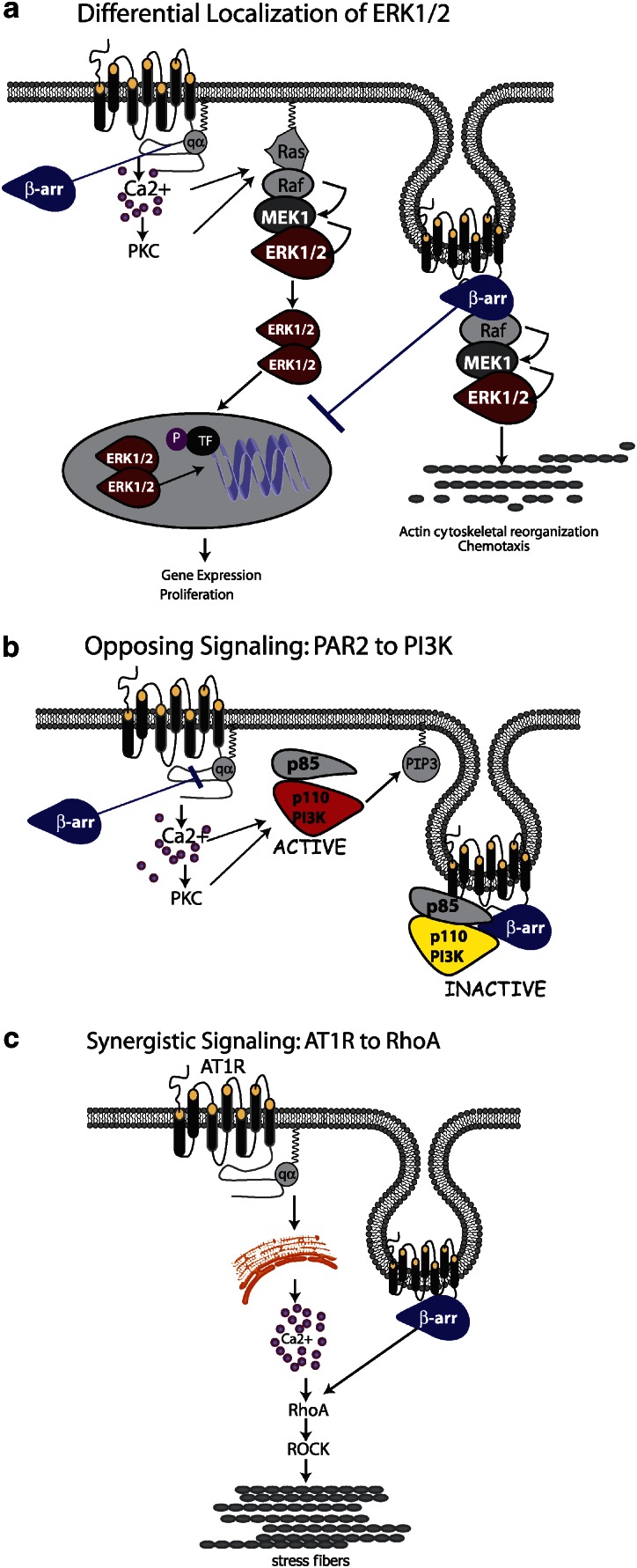
Dissecting the role of β-arrestins as dual terminators and facilitators of signalling has become increasingly confusing, as new and diverse functions unfold. In this review, I will present three examples of β-arrestin-dependent, G-protein-independent signalling (diagrammed in Figure 1). First, a single receptor can trigger activation of the same protein through G-protein- and β-arrestin-dependent pathways to elicit spatially distinct signals and sometimes opposing responses (for example, MAPKs). Second, β-arrestin-dependent pathways can synergize with G-protein-dependent pathways to elicit an integrated response (for example, RhoA). Third, β-arrestins and G-proteins can send opposing signals to the same downstream target (for example, PI3K). This can occur through localized activation and inhibition of distinct upstream regulators.
Figure 1.
Types of β-arrestin-dependent, G-protein-independent signals. (a) Differential localization of ERK1/2. β-Arrestins can activate ERK1/2 downstream of numerous GPCRs and effectively ‘steal' the kinases away the G-protein-dependent pathway. In the case of PAR-2 and AT1R, signalling through Gαq leads to mobilization of intracellular Ca2+, activation of conventional PKCs and Ras-dependent activation of the MAPK module (Raf-1, MEK1/2 and ERK1/2). The activated ERKs translocate to the nucleus where they phosphorylate transcription factors (TF) leading to gene expression and proliferation. When activated through β-arrestins, the entire MAPK module is scaffolded onto the β-arrestin-bound receptor, forming an ‘endosomal scaffold' that promotes prolonged activation of ERK1/2 at the membrane or within the cytosol preventing the transcriptional and proliferative effects and promoting cytoskeletal reorganization and chemotaxis. (b) Opposing signalling: PAR-2 to PI3K. PAR-2 can both activate and inhibit PI3K in a cell-type-specific fashion, depending on the expression level of β-arrestins. Activation of the classical Gaq/Ca2+ pathway leads to increased activity of the p110 catalytic subunit of PI3K, leading to generation of PIP3. Recruitment of β-arrestins leads to the formation of a unique endosomal scaffold containing the regulatory (p85) and catalytic PI3K subunits. Binding to β-arrestins directly inhibits PI3K activity. (c) Synergistic signalling: AT1R and RhoA. AT1R activates RhoA, leading to stress fibre formation, through activation of the RhoA effector ROCK. Both β-arrestin-dependent recruitment to the receptor and coupling to Gαq are required for RhoA activation and subsequent stress fibre formation, indicating a convergence of the two pathways at the level of RhoA. AT1R, type I angiotensin II receptor; GPCR, G-protein-coupled receptor; MAPK, mitogen-activated protein kinase; PAR, protease-activated receptor.
That β-arrestins could mediate signalling, independent of heterotrimeric G-proteins, was demonstrated using a novel agonist of the AT1R, dubbed SII. SII was incapable of promoting G-protein coupling (as assessed by membrane GTPase activity), could still activate MAPK through a β-arrestin-2-dependent pathway. Likewise, an AT1R mutant (AT1-i2m), deficient in G-protein recruitment, also activated MAPK in response to angiotensin II (AngII) (Wei et al., 2003). Subsequent studies by others have confirmed the existence of AT1R-mediated G-protein-independent ERK1/2 activation (Feng et al., 2005; Yee et al., 2006; Aplin et al., 2007; Szidonya et al., 2007). Over the past few years, numerous receptors have been added to the list of 7-TMRs capable of eliciting G-protein-independent signals, including PAR-2, metatrobic glutamate receptor, β2AR, PTHR and dopamine D2 receptor (D2R). Most of these studies have assayed MAPK activation as a readout for G-protein-independent signalling; however, β-arrestins are capable of triggering activation of other pathways (for example cofilin) and inhibiting others (for example PI3K, LIMK, Akt), independent of prior G-protein coupling (Beaulieu et al., 2005; Wang and DeFea, 2006; Wang et al., 2007; Zoudilova et al., 2007).
β-arrestin-dependent MAPK activation: differential pathways, localization and responses
By far the most well-characterized role for β-arrestin-dependent signalling is in the regulation of MAPKs; ERK1/2 (p42/44MAPK), p38MAPK and Jnk can all be activated by β-arrestin-dependent signals (Shenoy and Lefkowitz, 2003). In many cases, β-arrestin-dependent activation leads to cytosolic sequestration of the activated enzymes (DeFea et al., 2000b; McDonald et al., 2000; Tohgo et al., 2002; Ahn et al., 2004; Sneddon and Friedman, 2007), although they can also facilitate nuclear translocation (DeFea et al., 2000a). While their ability to facilitate endocytosis may play a role in their ability to activate MAPKs (Daaka et al., 1998; DeFea et al., 2000b), for some receptors, β-arrestin-dependent ERK1/2 activation can proceed even in the absence of internalization (Pierce et al., 2000; de Gortízar et al., 2006). Studies using an inducible heterodimerization strategy to target β-arrestin to vasopressin receptor, independent of receptor activity, suggested that membrane localization of scaffolded Raf, MEK1/2 and ERK1/2 by β-arrestin was sufficient to trigger activation (Terrillon and Bouvier, 2004). For some receptors, β-arrestins scaffold tyrosine kinases such as Src, thus providing a link to classical Ras-dependent pathways of ERK1/2 activation. In other studies, expression of a stable GPCR/β-arrestin chimera resulted in agonist-independent ERK1/2 activation. In that study, the β-arrestin-dependent ERK1/2 activation required PKC and PKA activity, suggesting that β-arrestins scaffold and activate a number of enzymes linked to MAPK activity, independent of agonist-induced receptor activation. These proteins can be distinct from those activated by G-protein-dependent pathways; alternatively, β-arrestins can effectively ‘steal' proteins from the G-protein pathway to direct alternate processes. Discussed below are well-characterized MAPK pathways downstream of various GPCRs, and the possible pharmacological significance of β-arrestin-dependent signalling.
Adrenergic receptors
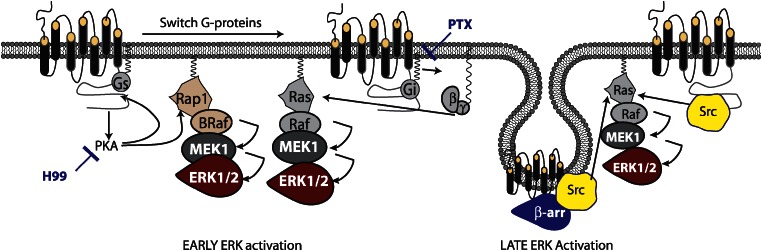
β-arrestins have been implicated in many aspects of adrenergic receptor signalling; as adrenergic antagonists are a standard method for treating heart failure, understanding the variety of mechanisms by which these receptors signal is of utmost importance. The role of β-arrestins in β2AR-directed MAPK activation appears to vary depending on the cell type, agonist dose and time course. As mentioned previously, β2AR can couple to both Gαs and Gαi proteins; early studies suggested that both coupling to Gαi and β-arrestin-dependent Src recruitment directed ERK1/2 (aka p42/44MAPK) activation (Daaka et al., 1997, 1998; Luttrell et al., 1999). In a study by Shenoy et al. (2005), careful dissection of the β2AR MAPK pathway revealed that in HEK293 cells, early ERK1/2 activation (between 0 and 5 min) is reduced by treatment with pertussis toxin or the PKA inhibitor H-99, while late ERK1/2 activation (between 5 and 60 min) is inhibited by knockdown of either β-arrestin-1 or β-arrestin-2. These studies are consistent with others demonstrating that some inverse agonists of β2AR can activate β-arrestin-dependent ERK1/2 independent of cAMP production (Azzi et al., 2003). Other studies suggest that ubiquitinylation of β-arrestin can stabilize receptor/β-arrestin/ERK complexes leading to their sequestration at the plasma membrane; whereas, under other conditions, β-arrestin might facilitate nuclear translocation of the active kinases (Kobayashi et al., 2005; Shenoy et al., 2007). Together, these data suggested a model where receptor coupling to both Gαs and Gαi mediates the early rise in phospho-MAPK levels by different mechanisms, while β-arrestins direct the prolonged MAPK phosphorylation by multiple mechanisms including scaffolding of Src (Figure 2).
Figure 2.
Model of ERK1/2 activation by β2AR: contribution of G-protein- and β-arrestin-dependent signals. β2AR promotes several temporally distinct phases of ERK1/2 activation, each dependent on a different signalling moiety. Activation of PKA through coupling to Gαs and subsequent cAMP generation leads to activation of the small GTPase Rap1, which activates B-raf. B-Raf can then activate MEK1 and ERK1/2. PKA also phosphorylates the receptor leading to Gαi coupling, the release of free active Gβγ subunits and activation of Ras. Both of these pathways are rapid and transient, within minutes of receptor activation. Recruitment of β-arrestin allows scaffolding and activation of Src and subsequent Ras-dependent ERK1/2 activation. At high ligand concentrations, Src can bind directly to the receptor, bypassing the requirement for β-arrestins in Src activation.
β2 Adrenergic receptor may have yet another mechanism for activation of ERK1/2. Other studies demonstrate that G-protein-independent activation of MAPK by β2AR can involve direct interaction with and activation of Src by the receptor without requiring β-arrestins (Huang et al., 2004; Sun et al., 2007). In these studies, the G-protein-independent pathway was observed only at high agonist concentrations (>10 μM), leading the authors to invoke a model of dose-dependent switching from G-protein-dependent to G-protein-independent signalling. Both sets of studies demonstrate Src-dependent, G-protein-independent signalling at high agonist doses. They are also consistent with reports demonstrating that heterologous activation of Src by insulin receptor leads to internalization of β2AR and raise the possibility that G-protein-independent signals emanating from GPCRs might be triggered through cross talk with other receptors. The discrepancies observed in the apparent requirement for β-arrestins between some of these studies could reflect differences in transfected versus endogenous receptor concentrations, cell-line-specific differences or perhaps differences in the contribution from β1AR.
Studies on the α2 adrenergic receptor (α2AR) suggest a similar requirement for β-arrestin in ERK1/2 activation (Pierce et al., 2000; Wang et al., 2006). In one of these studies, Src inhibitors blocked ERK1/2 activation in wild-type, but not β-arrestin-deficient cells, suggesting that β-arrestins act as a molecular switch, to direct src-dependent ERK1/2 activity (Wang et al., 2006). An over-arching hypothesis arising from these studies is that adrenergic receptors utilize multiple pathways to activate ERK1/2, some being independent of classic heterotrimeric G-protein-dependent coupling. The G-protein-independent pathway involves transactivation of tyrosine kinases such as Src, for which β-arrestin can serve as an adaptor to facilitate its activation. Studies on other receptors (discussed below) demonstrate that different pathways of ERK1/2 activation can result in distinct cellular outcomes. Thus, antagonists of adrenergic receptors that appear to inhibit activation as determined by either G-protein coupling or ERK1/2 activation may affect only a subset of receptor functions.
Angiotensin II receptor and protease-activated receptor-2
Shortly after the initial observations that β-arrestins were required for β2AR-induced ERK1/2 activation, additional studies emerged demonstrating β-arrestin-dependent ERK1/2 activation downstream of PAR-2 and AT1R (DeFea et al., 2000b; Luttrell et al., 2001). Both receptors signal through Gαq to elicit Ca2+-dependent nuclear ERK1/2 and through β-arrestins (β-arrestin-2 in the case of AT1R and both β-arrestin-1 and β-arrestin-2 in the case of PAR-2) to promote cytosolic/membrane ERK1/2 activity (DeFea et al., 2000b; Tohgo et al., 2002; Ge et al., 2003; Stalheim et al., 2005; Smith and Luttrell, 2006b; Kumar et al., 2007). Both receptors trigger formation of stable endosomal complexes independent of, and temporally and spatially distinct from, G-protein-activated ERK1/2 (DeFea et al., 2000b; Wei et al., 2003; Kumar et al., 2007). The two receptors differ somewhat in their signalling pathways, as the β-arrestin-dependent AngII pathway is PKC-independent, whereas both the G-protein- and β-arrestin-dependent PAR-2 pathways require PKC (DeFea et al., 2000b; Wei et al., 2003; Ahn et al., 2004). In summary, ERK1/2 activation by these two receptors can occur by either G-protein-dependent or β-arrestin-dependent pathways and the two pathways are not inter-dependent. In the case of both the receptors, the β-arrestin-dependent pathway ‘opposes' the G-protein-dependent pathway by sequestering the activated kinases away from the nucleus along with their upstream activators, thus preventing their transcriptional and mitogenic effects while prolonging their activation in non-nuclear compartments (Figure 1a) (DeFea et al., 2000b; Tohgo et al., 2002).
As mentioned above, AT1R was the first GPCR shown to elicit G-protein-independent β-arrestin signalling, and this has been confirmed in numerous other cell types (Wei et al., 2003; Kim et al., 2005; Szidonya et al., 2007; Tabata et al., 2007). This receptor system is an ideal example of the significance of alternate signalling pathways and its multiple mechanisms for activation of MAPK have been well characterized. AT1R is a key regulator of blood pressure and inhibitors of its agonist, AngII, as well as inverse agonists of the receptor itself are used to treat cardiac disease (de la Sierra, 2006; Voors, 2007; Ruef et al., 2007; Westermann et al., 2007). Pointing to the pharmacological significance of G-protein-independent AT1R signalling, a recent in vivo study confirmed β-arrestin-dependent ERK1/2 activation by the G-protein-independent SII agonist in cardiac myocytes and perfused hearts (Rajagopal et al., 2006; Tabata et al., 2007). Another study in mice overexpressing either wt AT1R or the mutant AT1R (AT1-i2m) demonstrated that AngII stimulated cardiac hypertrophy and bradycardia in the AT1-i2M mice, but tachycardia in the wt mice. These effects correlated with the increased cytosolic versus nuclear localization of activated ERK1/2 (Tohgo et al., 2002; Zhai et al., 2005).
In addition to its role in cardiovascular pathology, AT1R may be involved in inflammation as well. For example, AngII contributes to immune cell recruitment into tissue in response to inflammatory signals, and plays a role in proliferation during tissue repair (Ruiz-Ortega et al., 2006; Price et al., 2007; Voors, 2007; Westermann et al., 2007). A study by Hunton et al. (2005) demonstrated that AT1R-induced chemotaxis was Gαq/Gαi-independent and β-arrestin-2-dependent, as both the SII peptide and AngII promoted chemotaxis that was sensitive to siRNA knockdown of β-arrestin-2, but not knockdown of Gαq or pertussis toxin pretreatment. These studies raise the question of whether more specific AT1R antagonists that inhibit only one pathway might be developed. Conversely, it is possible that specific inhibition of AngII-induced Gαq signalling might enhance β-arrestin-dependent chemotaxis, an effect that may or may not be desirable in a therapeutic.
Like AT1R, PAR-2 has been implicated in a number of inflammatory disorders as well as tumour cell metastasis (Cocks and Moffatt, 2001; Fiorucci et al., 2001; Cenac et al., 2002; Ge et al., 2004; Morris et al., 2006). Current studies indicate that PAR-2 may have both protective and pathogenic effects in inflammatory diseases such as asthma and colitis, depending on the disease model, the administration of PAR-2 agonist and the physiological readout (Cocks et al., 1999; Cocks and Moffatt, 2001; Fiorucci et al., 2001; Schmidlin and Bunnett, 2001; Cenac et al., 2002; Ge et al., 2004; Ebeling et al., 2005; Morris et al., 2006). As a result, both agonists and antagonists of PAR-2 have been posed as therapeutics for the same diseases. Most of these drugs are screened using the Gαq-mediated IP3 generation or Ca2+ mobilization as readouts of receptor activation; however PAR-2 can direct a number of G-protein-independent events in a cell-type-specific manner (Ge et al., 2003, 2004; Wang and DeFea, 2006; Kumar et al., 2007; Zoudilova et al., 2007). The major proinflammatory phenotypes associated with PAR-2 activation are immune cell recruitment and increased cytokine production; whereas studies demonstrating PAR-2-induced smooth muscle cell relaxation and bronchodilation suggest a protective role. The latter two responses are dependent on epithelial-derived prostaglandin production, which is thought to be a Gαq-dependent event involving nuclear ERK1/2-induced COX2 gene transcription. In contrast, PAR-2-evoked chemotaxis is dependent on β-arrestins, and pseudopodial sequestration of ERK1/2. Thus, these seemingly paradoxical findings may be explained by G-protein versus β-arrestin-dependent signalling to ERK1/2, leading to responses associated with either protective or inflammatory events. Consistent with this idea, PAR-2 can promote opposing signals at the molecular level, in a manner dependent on β-arrestin expression (discussed in the next section). Furthermore, constitutive activation of β-arrestin-dependent ERK1/2 by PAR-2 was shown to promote migration of metastatic breast carcinoma cells, implying that β-arrestins might contribute to the metastatic potential of some cancers (Kamath et al., 2001; Ge et al., 2004; Morris et al., 2006). As was mentioned for AT1R, the elucidation of these distinct signalling pathways may eventually lead to the development of pathway-specific drugs.
Parathyroid hormone receptor
Type I PTHR is highly expressed in kidney and bone where it plays a role in regulating Ca2+ homoeostasis and bone remodelling. Intermittent exposure to PTH promotes bone formation whereas persistent exposure promotes bone resorption; thus, it exerts both anabolic and catabolic effects on bone. Classical PTHR signalling involves activation of both Gαs and Gαq, and it has been shown to activate ERK1/2 by multiple pathways in different cell types through distinct PKC- and PKA-dependent pathways, as well as transactivation of epidermal growth factor receptor. A recent study by Gesty-Palmer et al. (2006) demonstrated for the first time that PTHR-induced ERK1/2 activation involves separable PKA, PKC and β-arrestin-dependent components. Inhibition of PKA significantly reduced early ERK1/2 activation (at 2–5 min), while inhibition of PKC mildly inhibited late ERK1/2 activation. A Gαs-specific PTHR agonist (PTHNBR) promoted transient PKA-dependent ERK1/2 activation. Conversely, an inverse agonist of PTHR (PTH1A), which had no effect on cAMP generation or Ca2+ mobilization, was able to elicit prolonged ERK1/2 activation that was insensitive to PKC or PKA inhibition. siRNA knockdown of β-arrestin-1 and β-arrestin-2 eliminated ERK1/2 activation between 5 and 30 min of PTH or PTH1A treatment, but had no effect on PTH-NBR-induced ERK1/2 activation, suggesting that β-arrestins mediate ERK1/2 activation independent of Gαs or Gαq engagement. Interestingly, PTH1A promoted β-arrestin recruitment to the receptor, despite its ability to simultaneously inhibit Gαs-mediated cAMP generation. These studies support the theory of ‘ligand-directed signalling', whereby an antagonist of one signalling pathway might promote another through the same receptor. Whether different pathways are linked to anabolic versus catabolic effects, or are preferentially activated by intermittent versus persistent PTH exposure is not yet clear; however, recent in vivo studies point to the physiological significance of β-arrestin-dependent signalling. In two studies, overall bone mass was shown to be decreased in β-arrestin-2−/− mice. Surprisingly, however, bone formation in response to intermittent PTH treatment, was decreased in male β-arrestin-2 knockout mice, but increased in female β-arrestin-2−/− mice (Ferrari et al., 2005; de Gortízar et al., 2006). The different responses may reflect the Gαs suppressing and MAPK-potentiating effects of β-arrestins, suggesting that additional hormonal influences may affect pathway-specific PTH activities.
Other examples of GPCRs inducing G-protein-independent MAPK activation
The V2R, a Gαs-coupled GPCR, plays an important role in controlling salt and water homoeostasis in renal tubules and regulation of blood pressure by promoting vasoconstriction. V2R, like AT1R and PAR-2, promotes the formation of stable receptor/β-arrestin/ERK1/2 complexes and β-arrestins are required for the prolonged ERK1/2 activation (Charest and Bouvier, 2003; Tohgo et al., 2003). More recently, Charest et al. demonstrated that downregulation of Gαs with cytotoxin increased vasopressin-induced cAMP generation but inhibited prolonged ERK1/2 activation. Inhibition of Gαi/o, Gαq or Gβγ had no effect on vasopressin-induced ERK1/2 activation. In contrast, expression of dominant-negative β-arrestin-1 and treatment with wortmannin and src family kinase inhibitors inhibited ERK1/2 activation, suggesting that V2R activates ERK1/2 via a β-arrestin-dependent, G-protein-independent pathway (Charest et al., 2007). In a study by Ren et al. (2005), early but not prolonged ERK1/2 activation by vasopressin was sensitive to inhibitors of PKA, a downstream target of Gαs. Taken together, these studies suggest that V2R activates ERK1/2 by at least two pathways; a transient, PKA-dependent one and a prolonged β-arrestin-dependent one. From a physiological perspective, these studies are important, as the vasoconstrictive effects of vasopressin may be mediated by the ERK1/2 pathway, while regulation of sodium absorption and potassium secretion are thought to be cAMP/PKA-dependent (Streefkerk et al., 2004; Knoers, 2005). Vasopressin receptor antagonists have been developed as therapeutics for treatment of hyponatremia (an electrolyte imbalance resulting from low sodium or water intoxication) and heart disease (Yamamura et al., 1998; Gheorghiade et al., 2004). These recent studies suggesting vasopressin receptors might utilize distinct pathways to direct vascular and renal effects raise the possibility that distinct effects of vasopressin could be specifically inhibited. Furthermore, they point to the importance of evaluating multiple pathways, as inhibition of one might increase the other.
A number of neuronal receptors that appear require β-arrestins for downstream signals. Of these, the μ-opioid receptor (MOR) and metabotropic glutamate receptor (mGluR1) may exhibit G-protein-independent, β-arrestin-dependent ERK1/2 activation. In addition, the D2R signalling regulates PKB (aka AkT), a downstream target of PI3K via a G-protein-independent pathway, and is discussed in a later section.
μ-opioid receptors, typically couple to Gαo to mediate changes in K+ and Ca2+ channel activity as well as activation of ERK1/2. Several studies suggest a role for G-protein-independent, β-arrestin-dependent activation of ERK1/2 by these receptors, which may occur in an agonist-specific manner. MOR agonists of the enkephalin family promote recruitment of β-arrestin-2 to the receptor and subsequent ERK1/2 activation whereas, morphine does not, although both triggered Gαi coupling (Clark et al., 2004; Macey et al., 2006). ERK1/2 activation was abolished after siRNA knockdown of β-arrestins and in mice lacking the G-protein receptor kinase-3. Other studies have demonstrated the existence of heterodimers of MOR and δ-opioid receptors that function as a distinct signalling unit from their homodimeric counterparts. In a study by Rozenfeld and Devi (2007), cells expressing both receptors constitutively recruited β-arrestin-2 to the plasma membrane and subsequent treatment with the MOR agonist (DAMGO) resulted in two waves of ERK1/2 activation. In cells expressing MOR alone, only the early phase of ERK1/2 activation was observed, which was dependent on PKC (presumably G-protein-dependent), while transfection with β-arrestin-2 siRNA abolished the later phase. Consistent with what was reported for other receptors, the β-arrestin-activated ERK1/2 was cytosolic whereas the PKC-activated ERK1/2 was primarily nuclear. Simultaneous administration of MOR and δ-opioid receptors agonists disrupted the heterodimers, leading to activation G-protein-dependent pathways and abolition of the prolonged, cytosolic MAPK activity. The role of β-arrestin-2 in opioid-associated responses is not entirely understood. Its classic role as a G-protein uncoupler may be important for development of tolerance, as β-arrestin-2−/− mice exhibit enhanced morphine analgesia and decreased tolerance that is partially reversed by PKC. Whether β-arrestin-dependent MAPK mediates responses other than those associated with pain, or plays an additional role in enhancing MOR-induced analgesia is not clear. Certainly, the apparent ability of different opioid receptor agonists to preferentially activate β-arrestin versus G-protein pathways merits further investigation.
Adenosine receptors constitute a widely distributed family of receptors that regulate a number of CNS behavioural activities. The mGluR1 is a Gαq-coupled receptor that can form complexes with Gαi/o-coupled adenosine receptors A1R and A2AR in Purkinje cells. A recent study by Tabata et al. (2007) suggested that A1R inhibited mGluR1-coupled inward current, independent of Gαs, Gαi and Gαq proteins, in response to adenosine through heterodimer formation. While the role of β-arrestins in this G-protein-independent signalling pathway was not addressed, in other studies, mGluR1 was demonstrated to exhibit β-arrestin-dependent ERK1/2 activation, while internalization was β-arrestin-independent (Iacovelli et al., 2003). Thus, A1R/mGluR1 heterodimers may preferentially signal to the β-arrestin-dependent pathway, which in turn might inhibit inward current.
Opposing β-arrestin and G-protein signals: PI3K, cofilin and NF-κB pathways
While both G-protein- and β-arrestin-dependent pathways can lead to MAPK activation, recent studies have revealed examples of a single GPCR simultaneously activating and inhibiting the same enzyme through G-protein- and β-arrestin-dependent pathways. A number of these examples involve β-arrestin-dependent regulation of proteins involved in chemotaxis (Sun et al., 2002; Hunton et al., 2005; Wang and DeFea, 2006; DeFea, 2007; Zoudilova et al., 2007). For example, in a cell line that expresses relatively low levels of endogenous β-arrestins, activation of PAR-2 leads to increased PI3K activity; this is abolished by Gαq knockdown or intracellular Ca2+ chelation. (PAR-2-induced MAPK activation is discussed in a previous section). In contrast, in a cell line with high levels of endogenous β-arrestins, PAR-2 activation reduces baseline PI3K activity by twofold. Expression of β-arrestins in the former cell line abolishes the PAR-2-stimulated PI3K activity and knockdown of β-arrestins in the latter cell line uncovers a PAR-2-stimulated increase in PI3K activity. Inhibition of PI3K by β-arrestins is attained through direct interaction with the enzyme in response to PAR-2 activation (Wang and DeFea, 2006; Wang et al., 2007) (Figure 1b).
Consistent with β-arrestin-dependent PI3K inactivation, β-arrestin-2 plays an important role in the inactivation of the ser/thr kinase Akt (a downstream target of PI3K) by D2R. D2R is a Gαi-coupled receptor, but some of its effects involve dephosphorylation and inactivation of Akt through activation of the phosphatase PP2A. A recent study demonstrated that β-arrestin-2 scaffolds Akt with its inactivating phosphatase, and that D2R-induced Akt dephosphorylation requires β-arrestin-2 (Beaulieu et al., 2005). Dopamine receptors and their signalling pathways are the molecular targets of many antidepressants and antipsychotics; the antipsychotic haloperidol inhibits D2R signalling to Gαi but increases AkT phosphorylation. Some other drugs, such as lithium, appear to act through inhibition of Akt (Beaulieu et al., 2007), suggesting that some existing antidepressants may already selectively target the β-arrestin-dependent pathway. Thus, the emergence of β-arrestin-dependent Akt inactivation as a separable D2R-mediated pathway raises the possibility of the development of more specific antidepressant/antipsychotic drugs.
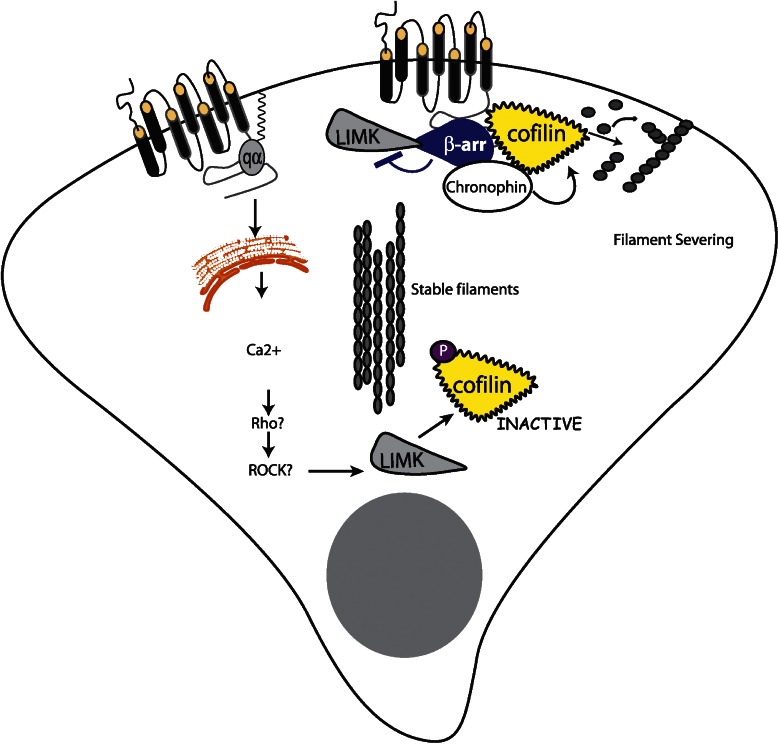
Similar findings were reported for regulation of the cofilin pathway. Cofilin is an actin filament severing protein essential for chemotaxis. Its activity is typically tightly controlled, being activated by dephosphorylation at the leading edge of migrating cells and maintained in an inactive state by LIMK-mediated phosphorylation at the back. In the presence of β-arrestins, PAR-2 inhibits LIMK and activates cofilin; in their absence, PAR-2 stimulates LIMK activity and does not activate cofilin. β-arrestins also associate with cofilin, LIMK and the recently identified cofilin phosphatase, chronophin, suggesting a distinct scaffolding complex for regulating this pathway (Zoudilova et al., 2007) (Figure 3). This β-arrestin-dependent regulation of cofilin is likely involved in chemotaxis downstream of other GPCRs, as it was recently identified in a proteomics screen as a β-arrestin-binding partner in response to AngII treatment (Xiao et al., 2007).
Figure 3.
Localized activation and inactivation of cofilin by PAR-2. PAR-2 can activate cofilin, an actin filament-severing protein important for chemotaxis, through recruitment of β-arrestins and subsequent binding of cofilin, its activating phosphatase (chronophin) and its inhibitory kinase (LIMK). β-arrestin inhibits LIMK activity, preventing it from dephosphorylating and inactivating cofilin. The net result is increased cofilin activity at the leading edge, which is important for turnover of filaments during cell migration. Simultaneously, through coupling to Gαq, PAR-2 can activate LIMK, presumably in regions of the cell where filament stability is essential, leading to localized inhibition of cofilin. PAR, protease-activated receptor.
While they may initially appear convoluted, these findings are entirely consistent with the ability of PAR-2 to promote directed cell migration. For a cell to migrate towards a chemotactic signal, it must first polarize and begin to form a leading edge in the direction of the agonist. This process involves severing of existing actin filaments paired with polymerization of new ones; to maintain forward motion, cooperation between cofilin-induced severing and actin polymerization continues within the leading edge. PI3K also plays an important role in chemotaxis, as it can regulate acidic phospholipid levels and activate downstream pathways involved in actin filament stability. Importantly, the same processes must be inhibited in other parts of the cell. Thus, it is logical for a single receptor to be capable of stimulation and inhibition of proteins involved in chemotaxis in a spatially regulated fashion (DeFea, 2007). As discussed earlier, PAR-2 is a popular therapeutic target; both agonists and antagonists have been posed for the treatment of various diseases. As PAR-2-induced cell migration is dependent on β-arrestins (Ge et al., 2003), and plays a role in the migration of some metastatic tumour cells (Kamath et al., 2001; Ge et al., 2004; Morris et al., 2006) as well as inflammation (Schmidlin and Bunnett, 2001), the possibility that antagonizing PAR-2-induced Gαq coupling could increase cell migration is an important consideration.
β2 adrenergic receptor has been shown to activate and inhibit NF-κB, a transcription factor that plays a major role in expression of genes involved in innate immune responses, cell proliferation and differentiation (Chandrasekar et al., 2004; Gao et al., 2004; Parameswaran et al., 2006). In its inactive state, NF-κB is bound to an inhibitory protein IFκB. A number of inflammatory signals result in phosphorylation of IFκB, promoting dissociation from and activation of NF-κB. In a study of transfected cells by Gao et al. (2004), it was demonstrated that the N terminus of β-arrestin-2 directly interacts with IFκB and prevents its degradation, leading to inhibition of NF-κB activity. Interaction of IFκB with β-arrestin-2 was enhanced by activation of β2AR, and this appeared to antagonize NF-κB activation and subsequent transcriptional activity in response to inflammatory mediators such as TNFα. An inhibitory role for β-arrestin-2 in NF-κB activity was corroborated by a study demonstrating enhanced lipopolysaccharide-induced NF-κB activation after β-arrestin-2 knockdown (Parameswaran et al., 2006). In contrast, a study in isolated mouse myocardium and cardiac-derived endothelial cells showed β2AR-induced activation of NF-κB and subsequent transcriptional activity (Chandrasekar et al., 2004). Thus, it is possible that β2AR can have both pro- and anti-inflammatory effects, in a manner dependent on the expression of β-arrestins. Furthermore, selective activation of β-arrestin-dependent β2AR signalling might have therapeutic potential as an anti-inflammatory agent.
Synergistic G-protein and β-arrestin-dependent signals
While most of the examples of β-arrestin-dependent signalling have been G-protein-independent, a study by Barnes et al. (2005) suggested that activation of the small GTPase RhoA by AT1R, and subsequent stress fibre formation, required input from Gαq- and β-arrestin-1-dependent pathways. RhoA can be activated by PI3K through PIP3-mediated activation of Rho-specific guanine exchange factors and by Gα12/13 proteins. It plays an important role in the formation and maintenance of stress fibres, in part through activating LIMK (which prevents filament severing) and activating myosin light-chain kinase (which promotes myosin binding and cell contraction). Localized activation of RhoA is important for cell attachment, and is often spatially distinct from activation of cofilin- and actin-polymerizing factors. Interestingly, β-arrestin-2, which is essential for AT1R-mediated chemotaxis, was not required for RhoA activity. It is likely that successful AT1R-mediated chemotaxis in vivo requires the integration of β-arrestin-2-dependent migratory signals (discussed above) with Gαq/β-arrestin-1-dependent cell attachment signals (through RhoA) (Figure 1c). A similar integrated signalling network between activation and inhibition of LIMK may also be involved in successful PAR-2-induced chemotaxis (discussed above) in vivo.
While there is increasing evidence for β-arrestin-dependent, G-protein-independent pathways of ERK1/2 activation, some receptors appear to utilize β-arrestin in a manner that is synergistic with G-protein activation. One such example is the ghrelin receptor growth hormone secretagogue receptor type 1a, expressed in both neuronal and non-neuronal tissue, is a Gαq/Gαi-coupled receptor that promotes the release of growth hormone, prolactin and corticotrophin from the anterior pituitary, and ERK1/2-depenent proliferation in peripheral tissues. A recent study by Camina et al. suggested that GHS-R1a utilizes three pathways to activate ERK1/2, all of which converge at the level of Src activation: Gαi-dependent activation free βγ subunits, Gαq-dependent activation via PKC and Ca2+, and β-arrestin-dependent scaffolding (Camina et al., 2007). Temporally, all three pathways are overlaid; however, differences in subcellular localization were not examined. The idea of convergent β-arrestin and Gαq-dependent pathways for ERK1/2 activation has been suggested for other receptors, such as neurokinin-1 receptor, which promotes proliferation via a β-arrestin- and G-protein-dependent ERK1/2 activation.
Concluding remarks
Clearly, for many GPCRs, β-arrestins constitute a separable signalling arm from the classical heterotrimeric G-proteins, in addition to functioning as terminators of G-protein/receptor coupling. While in some instances, β-arrestin signalling may functionally oppose G-protein signalling at multiple levels, in other cases, it may serve to prolong the same signal by sequestration of activated enzymes. In the latter case, selective agonists or antagonists that do not promote β-arrestin recruitment may allow for transient, but not prolonged, signals. Conversely, in the former example, selective activation or inhibition of G-protein- or β-arrestin-dependent pathways may allow for more selective regulation of GPCR-mediated events. What determines whether β-arrestins terminate or facilitate a signal, or whether they synergize with or oppose the G-protein signal? Recent evidence suggests that some ligands will preferentially activate β-arrestin-dependent signalling pathways (reviewed by Violin and Lefkowitz, 2007). Other possible factors that determine which pathway is activated include agonist concentration (Sun et al., 2007), the phosphorylation state of the receptor (Ren et al., 2005), receptor palmitoylation (Charest and Bouvier, 2003) and the stabilization of specific activation state conformations (Azzi et al., 2003; Yee et al., 2006). Modification of β-arrestins themselves by ubiquitinylation or phosphorylation can affect the stability of β-arrestin complexes and potentially the efficiency of their signalling (Lin et al., 1999; Shenoy et al., 2007). Furthermore, the availability of downstream effectors, such as β-arrestins or specific heterotrimeric G-proteins can vary in a cell-type-specific fashion, leading to distinct signalling events (Wang and DeFea, 2006). GPCRs remain one of the primary therapeutic targets for numerous diseases. In addition, there are other GPCR-interacting proteins that might mediate G-protein-independent signals (Brzostowski and Kimmel, 2001; Sun et al., 2007). From a pharmacological standpoint, the existence of G-protein-independent pathways alters the way in which agonists, antagonists and inverse agonists must be selected, while simultaneously opening the door for the development of more pathway-specific therapeutics (Table 1).
Table 1. β-arrestin-dependent, G-protein-independent signalling by G-protein-coupled receptors.
| GPCR | β-arrestin-dependent activity | G-protein-independent? | Sequestration | Response | Reference | |
|---|---|---|---|---|---|---|
| B2AR | ERK1/2 | ↑ | Yes | Membrane/nucleus | ?? Transcription | Luttrell et al. (1999); Kobayashi et al. (2005); Shenoy et al. (2005) |
| NF-kB | ↓ | ?? | Cytosol | Inhibition of inflammation | Chandrasekar et al. (2004); Gao et al. (2004); Parameswaran et al. (2006) | |
| A2AR | Src | ↑ | Yes | ?? | ?? | Pierce et al. (2000) |
| ERK1/2 | ↑ | Yes | Wang et al. (2006) | |||
| AT1R | ERK1/2 (β2) | ↑ | Yes | Cytosol | Inhibition of transcription | Tohgo et al. (2002, 2003); Ahn et al. (2004); Szidonya et al. (2007) |
| RhoA (β1) | ↑ | No | Cytosol | Stress fibre formation | Barnes et al. (2005) | |
| Jnk | ↑ | ? | Cytosol | ? | McDonald et al. (2000) | |
| PAR-2 | ERK1/2 | ↑ | Yes | Cytosol/membrane | Chemotaxis, inhibits proliferation | DeFea et al. (2000a, 2000b); Ge et al. (2003, 2004); Kumar et al. (2007) |
| Cofilin | ↑ | Yes | Leading edge | Filament severing, chemotaxis | Zoudilova et al. (2007) | |
| LIMK | ↓ | Yes | Membrane | Filament stability | Zoudilova et al. (2007) | |
| PI3K | ↓ | Yes | Membrane/leading edge | Chemotaxis, other functions? | Wang et al. (2006, 2007) | |
| PTHR | ERK1/2 | ↑ | Yes | ?? | Bone remodelling? | Ferrari et al. (2005); de Gortízar et al. (2006); Gesty-Palmer et al. (2006) |
| V2R | ERK1/2 | ↑ | Yes | Cytosol | ?? | Charest and Bouvier (2003); Togho et al. (2003); Charest et al. (2007) |
| CXCR4 | p38MAPK | ↑ | ? | ? | Chemotaxis | Sun et al. (2002) |
| D2R | AkT | ↓ | Yes? | Cytosol | Motor control/mood | Beaulieu et al. (2005) |
| MOR | ERK1/2 (β2) | ↑ | ?? | Cytosol | ? | Clark et al. (2004); Macey et al. (2006); Rozenfeld and Devi (2007) |
| mGLUR1 | ERK1/2 | ↑ | Yes | ? | ↓Inward current | Tabata et al. (2007) |
| GHS-R1a | ERK1/2 | ↑ | No | Nucleus | Proliferation | Camina et al. (2007) |
Acknowledgments
KD is supported by National Institutes of Health, R01GM066151 and the Division of Biomedical Sciences, University of California.
Glossary
- β2AR
β2 adrenergic receptor
- AngII
angiotensin II
- AT1R
type I angiotensin II receptor
- COX2
cyclooxygenase 2
- D2R
dopamine-D2 receptor
- DAMGO
d-Ala2-NMePhe4-Gly-ol-enkephalin
- GPCR
G-protein-coupled receptor
- HEK
human embryonic kidney
- MAPKs
mitogen-activated protein kinases
- mGluR1
metabotropic glutamate receptor
- MOR
μ-opioid receptor
- PAR
protease-activated receptor
- PI3K
phosphoinositide-3-kinase
- PTHR
parathyroid hormone receptor
- 7-TMRs
seven transmembrane receptors
- V2R
vasopressin 2 receptor
Footnotes
Conflict of interest
The author states no conflict of interest
References
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of {beta}-arrestin and G Protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- Aplin M, Christensen GL, Schneider M, Heydorn A, Gammeltoft S, Kjolbye AL, et al. The angiotensin type 1 receptor activates extracellular signal-regulated kinases 1 and 2 by G protein-dependent and -independent pathways in cardiac myocytes and langendorff-perfused hearts. Basic & Clinical Pharmacology & Toxicology. 2007;100:289–295. doi: 10.1111/j.1742-7843.2007.00063.x. [DOI] [PubMed] [Google Scholar]
- Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, et al. Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. The Akt–GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M, Anborgh PH, Babwah AV, Dale LB, Dobransky T, Benovic JL, et al. Beta-arrestins regulate a Ral-GDS Ral effector pathway that mediates cytoskeletal reorganization. Nat Cell Biol. 2002;4:547–555. doi: 10.1038/ncb821. [DOI] [PubMed] [Google Scholar]
- Brzostowski JA, Kimmel AR. Signaling at zero G: G-protein-independent functions for 7-TM receptors. Trends Biochem Sci. 2001;26:291–297. doi: 10.1016/s0968-0004(01)01804-7. [DOI] [PubMed] [Google Scholar]
- Camina JP, Lodeiro M, Ischenko O, Martini AC, Casanueva FF. Stimulation by ghrelin of p42/p44 mitogen-activated protein kinase through the GHS-R1a receptor: role of G-proteins and beta-arrestins. J Cell Physiol. 2007;213:187–200. doi: 10.1002/jcp.21109. [DOI] [PubMed] [Google Scholar]
- Caunt CJ, Finch AR, Sedgley KR, Oakley L, Luttrell LM, McArdle CA. Arrestin-mediated ERK activation by gonadotropin-releasing hormone receptors: receptor-specific activation mechanisms and compartmentalization. J Biol Chem. 2006;281:2701–2710. doi: 10.1074/jbc.M507242200. [DOI] [PubMed] [Google Scholar]
- Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, et al. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar B, Marelli-Berg FM, Tone M, Bysani S, Prabhu SD, Murray DR. [beta]-Adrenergic stimulation induces interleukin-18 expression via [beta]2-AR, PI3K, Akt, IKK, and NF-[kappa]B. Biochem Biophys Res Commun. 2004;319:304–311. doi: 10.1016/j.bbrc.2004.04.185. [DOI] [PubMed] [Google Scholar]
- Charest PG, Bouvier M. Palmitoylation of the V2 vasopressin receptor carboxyl tail enhances {beta}-arrestin recruitment leading to efficient receptor endocytosis and ERK1/2 activation. J Biol Chem. 2003;278:41541–41551. doi: 10.1074/jbc.M306589200. [DOI] [PubMed] [Google Scholar]
- Charest PG, Oligny-Longpre G, Bonin H, Azzi M, Bouvier M. The V2 vasopressin receptor stimulates ERK1/2 activity independently of heterotrimeric G protein signalling. Cell Signal. 2007;19:32–41. doi: 10.1016/j.cellsig.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Neubig RR, Traynor JR. Endogenous regulator of G protein signaling proteins suppress G{alpha}o-dependent, {micro}-opioid agonist-mediated adenylyl cyclase supersensitization. J Pharmacol Exp Ther. 2004;310:215–222. doi: 10.1124/jpet.103.064824. [DOI] [PubMed] [Google Scholar]
- Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, et al. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- Cocks TM, Moffatt JD. Protease-activated receptor-2 (PAR2) in the airways. Pulm Pharmacol Ther. 2001;14:183–191. doi: 10.1006/pupt.2001.0285. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, et al. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- de Gortízar A, Alonso V, Alvarez-Arroyo M, Esbrit P. Transient exposure to PTHrP (107–139) exerts anabolic effects through vascular endothelial growth factor receptor 2 in human osteoblastic cells in vitro. Calcif Tissue Int. 2006;79:360–369. doi: 10.1007/s00223-006-0099-y. [DOI] [PubMed] [Google Scholar]
- de la Sierra A. Angiotensin receptor blockers in hypertension and cardiovascular diseases. Cardiovasc Hematol Agents Med Chem. 2006;4:67–73. doi: 10.2174/187152506775268839. [DOI] [PubMed] [Google Scholar]
- DeFea KA. Stop that cell! beta-arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol. 2007;69:535–560. doi: 10.1146/annurev.physiol.69.022405.154804. [DOI] [PubMed] [Google Scholar]
- DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Dery O, Bunnett NW. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta-arrestin-dependent scaffolding complex. Proc Natl Acad Sci USA. 2000a;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. Beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000b;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling C, Forsythe P, Ng J, Gordon JR, Hollenberg M, Vliagoftis H. Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. J Allergy Clin Immunol. 2005;115:623–630. doi: 10.1016/j.jaci.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Feng YH, Ding Y, Ren S, Zhou L, Xu C, Karnik SS. Unconventional homologous internalization of the angiotensin II type-1 receptor induced by G-protein-independent signals. Hypertension. 2005;46:419–425. doi: 10.1161/01.HYP.0000172621.68061.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari SL, Pierroz DD, Glatt V, Goddard DS, Bianchi EN, Lin FT, et al. Bone response to intermittent parathyroid hormone is altered in mice null for {beta}-Arrestin2. Endocrinology. 2005;146:1854–1862. doi: 10.1210/en.2004-1282. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Mencarelli A, Palazzetti B, Distrutti E, Vergnolle N, Hollenberg MD, et al. Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc Natl Acad Sci USA. 2001;98:13936–13941. doi: 10.1073/pnas.241377298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, et al. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- Ge L, Ly Y, Hollenberg M, DeFea K. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem. 2003;278:34418–34426. doi: 10.1074/jbc.M300573200. [DOI] [PubMed] [Google Scholar]
- Ge L, Shenoy SK, Lefkowitz RJ, DeFea KA. Constitutive protease-activated-receptor-2 mediated migration of MDA MB-231 breast cancer cells requires both beta-arrestin-1 and 2. J Biol Chem. 2004;279:55419–55424. doi: 10.1074/jbc.M410312200. [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, et al. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- Gheorghiade M, Gattis WA, O'Connor CM, Adams KF, Jr, Elkayam U, Barbagelata A, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA. 2004;291:1963–1971. doi: 10.1001/jama.291.16.1963. [DOI] [PubMed] [Google Scholar]
- Huang J, Sun Y, Huang XY. Distinct roles for Src tyrosine kinase in beta2-adrenergic receptor signaling to MAPK and in receptor internalization. J Biol Chem. 2004;279:21637–21642. doi: 10.1074/jbc.M400956200. [DOI] [PubMed] [Google Scholar]
- Hunton DL, Barnes WG, Kim J, Ren XR, Violin JD, Reiter E, et al. {beta}-arrestin 2-dependent angiotensin II type 1A receptor-mediated pathway of chemotaxis. Mol Pharmacol. 2005;67:1229–1236. doi: 10.1124/mol.104.006270. [DOI] [PubMed] [Google Scholar]
- Iacovelli L, Salvatore L, Capobianco L, Picascia A, Barletta E, Storto M, et al. Role of G protein-coupled receptor kinase 4 and beta-arrestin 1 in agonist-stimulated metabotropic glutamate receptor 1 internalization and activation of mitogen-activated protein kinases. J Biol Chem. 2003;278:12433–12442. doi: 10.1074/jbc.M203992200. [DOI] [PubMed] [Google Scholar]
- Kamath L, Meydani A, Foss F, Kuliopulos A. Signaling from protease-activated receptor-1 inhibits migration and invasion of breast cancer cells. Cancer Res. 2001;61:5933–5940. [PubMed] [Google Scholar]
- Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, et al. Functional antagonism of different G protein-coupled receptor kinases for {beta}-arrestin-mediated angiotensin II receptor signaling. PNAS. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoers NVAM. Hyperactive vasopressin receptors and disturbed water homeostasis. N Engl J Med. 2005;352:1847–1850. doi: 10.1056/NEJMp058006. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Narita Y, Nishida M, Kurose H. [beta]-Arrestin2 enhances [beta]2-adrenergic receptor-mediated nuclear translocation of ERK. Cell Signal. 2005;17:1248–1253. doi: 10.1016/j.cellsig.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. Beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking 13. Proc Natl Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout TA, Nicholas SL, Perry SJ, Reinhart G, Junger S, Struthers RS. Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J Biol Chem. 2004;279:23214–23222. doi: 10.1074/jbc.M402125200. [DOI] [PubMed] [Google Scholar]
- Kumar P, Lau CS, Mathur M, Wang P, DeFea KA. Differential effects of beta-arrestins on the internalization, desensitization and ERK1/2 activation downstream of protease activated receptor-2. Am J Physiol Cell Physiol. 2007;293:C346–C357. doi: 10.1152/ajpcell.00010.2007. [DOI] [PubMed] [Google Scholar]
- Lin FT, Miller WE, Luttrell LM, Lefkowitz RJ. Feedback regulation of beta-arrestin1 function by extracellular signal-regulated kinases. J Biol Chem. 1999;274:15971–15974. doi: 10.1074/jbc.274.23.15971. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, et al. Activation and targeting of extracellular signal-regulated kinases by beta -arrestin scaffolds. Proc Natl Acad Sci USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, van Biesen T, Hawes BE, Koch WJ, Krueger KM, Touhara K, et al. G-protein-coupled receptors and their regulation: activation of the MAP kinase signaling pathway by G-protein-coupled receptors. Adv Second Messenger Phosphoprotein Res. 1997;31:263–277. [PubMed] [Google Scholar]
- Macey TA, Lowe JD, Chavkin C. Mu opioid receptor activation of ERK1/2 is GRK3 and arrestin dependent in striatal neurons. J Biol Chem. 2006;281:34515–34524. doi: 10.1074/jbc.M604278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinissen MJ, Servitja JM, Offermanns S, Simon MI, Gutkind JS. Thrombin protease-activated receptor-1 signals through Gq- and G13-initiated MAPK cascades regulating c-jun expression to induce cell transformation. J Biol Chem. 2003;278:46814–46825. doi: 10.1074/jbc.M305709200. [DOI] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, et al. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- McLaughlin JN, Shen L, Holinstat M, Brooks JD, Dibenedetto E, Hamm HE. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005;280:25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res. 2006;66:307–314. doi: 10.1158/0008-5472.CAN-05-1735. [DOI] [PubMed] [Google Scholar]
- Parameswaran N, Pao CS, Leonhard KS, Kang DS, Kratz M, Ley SC, et al. Arrestin-2 and G protein-coupled receptor kinase 5 interact with NFkappaB1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. J Biol Chem. 2006;281:34159–34170. doi: 10.1074/jbc.M605376200. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Maudsley S, Daaka Y, Luttrell LM, Lefkowitz RJ. Role of endocytosis in the activation of the extracellular signal-regulated kinase cascade by sequestering and nonsequestering G protein-coupled receptors. Proc Natl Acad Sci USA. 2000;97:1489–1494. doi: 10.1073/pnas.97.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povsic TJ, Kohout TA, Lefkowitz RJ. {beta}-Arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem. 2003;278:51334–51339. doi: 10.1074/jbc.M309968200. [DOI] [PubMed] [Google Scholar]
- Price A, Lockhart JC, Ferrell WR, Gsell W, McLean S, Sturrock RD. Angiotensin II type 1 receptor as a novel therapeutic target in rheumatoid arthritis: in vivo analyses in rodent models of arthritis and ex vivo analyses in human inflammatory synovitis. Arthritis Rheum. 2007;56:441–447. doi: 10.1002/art.22335. [DOI] [PubMed] [Google Scholar]
- Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, et al. beta-Arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. PNAS. 2006;103:16284–16289. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: {beta}-arrestin2-mediated ERK activation by {micro}-{delta} opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruef J, Browatzki M, Pfeiffer CA, Schmidt J, Kranzhofer R. Angiotensin II promotes the inflammatory response to CD40 ligation via TRAF-2. Vasc Med. 2007;12:23–27. doi: 10.1177/1358863X07076766. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Ruperez M, Esteban V, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, et al. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006;21:16–20. doi: 10.1093/ndt/gfi265. [DOI] [PubMed] [Google Scholar]
- Schmidlin F, Bunnett NW. Protease-activated receptors: how proteases signal to cells. Curr Opin Pharmacol. 2001;1:575–582. doi: 10.1016/s1471-4892(01)00099-6. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Barak LS, Xiao K, Ahn S, Berthouze M, Shukla AK, et al. Ubiquitination of beta-arrestin links 7-transmembrane receptor endocytosis and ERK activation. J Biol Chem. 2007;282:29549–29562. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, et al. Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta 2 adrenergic receptor. J Biol Chem. 2005;271:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J. 2003;375:503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, Luttrell LM. Signal switching, crosstalk, and arrestin scaffolds: novel G protein-coupled receptor signaling in cardiovascular disease. Hypertension. 2006a;48:173–179. doi: 10.1161/01.HYP.0000232641.84521.92. [DOI] [PubMed] [Google Scholar]
- Smith NJ, Luttrell LM. Signal switching, crosstalk, and arrestin scaffolds: novel G protein-coupled receptor signaling in cardiovascular disease. Hypertension. 2006b;48:173–179. doi: 10.1161/01.HYP.0000232641.84521.92. [DOI] [PubMed] [Google Scholar]
- Sneddon WB, Friedman PA. {beta}-Arrestin-dependent parathyroid hormone-stimulated extracellular signal-regulated kinase activation and parathyroid hormone type 1 receptor internalization. Endocrinology. 2007;148:4073–4079. doi: 10.1210/en.2007-0343. [DOI] [PubMed] [Google Scholar]
- Stalheim L, Ding Y, Gullapalli A, Paing MM, Wolfe BL, Morris DR, et al. Multiple independent functions of arrestins in the regulation of protease-activated receptor-2 signaling and trafficking. Mol Pharmacol. 2005;67:78–87. doi: 10.1124/mol.104.006072. [DOI] [PubMed] [Google Scholar]
- Streefkerk JO, Hoogaars WMH, Christoffels VM, Sand C, Pfaffendorf M, Peters SLM, et al. Vasopressin-induced vasoconstriction is dependent on MAPKerk1/2 phosphorylation. Fundam Clin Pharmacol. 2004;18:45–50. doi: 10.1046/j.1472-8206.2003.00221.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- Sun Y, Huang J, Xiang Y, Bastepe M, Juppner H, Kobilka BK, et al. Dosage-dependent switch from G protein-coupled to G protein-independent signaling by a GPCR. EMBO J. 2007;26:53–64. doi: 10.1038/sj.emboj.7601502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szidonya L, Supeki K, Karip E, Turu G, Varnai P, Clark AJL, et al. AT1 receptor blocker-insensitive mutant AT1A angiotensin receptors reveal the presence of G protein-independent signaling in C9 cells. Biochem Pharmacol. 2007;73:1582–1592. doi: 10.1016/j.bcp.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Tabata T, Kawakami D, Hashimoto K, Kassai H, Yoshida T, Hashimotodani Y, et al. G protein-independent neuromodulatory action of adenosine on metabotropic glutamate signalling in mouse cerebellar Purkinje cells. J Physiol. 2007;581:693–708. doi: 10.1113/jphysiol.2007.129866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M. Receptor activity-independent recruitment of betaarrestin2 reveals specific signalling modes. EMBO J. 2004;23:3950–3961. doi: 10.1038/sj.emboj.7600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, Oakley RH, et al. The stability of the G protein-coupled receptor-beta -Arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278:6258. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. Beta-arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- Vanhauwe JF, Thomas TO, Minshall RD, Tiruppathi C, Li A, Gilchrist A, et al. Thrombin receptors activate Go proteins in endothelial cells to regulate intracellular calcium and cell shape changes. J Biol Chem. 2002;277:34143–34149. doi: 10.1074/jbc.M204477200. [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. [beta]-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Voors AA. Vascular benefits of angiotensin receptor blockers. Expert Opin Investig Drugs. 2007;16:987–997. doi: 10.1517/13543784.16.7.987. [DOI] [PubMed] [Google Scholar]
- Wang P, DeFea K. Protease-activated-receptor-2 simultaneously directs beta-arrestin-dependent inhibition and Gαq-dependent activation of PI3K. Biochemistry. 2006;45:9374–9385. doi: 10.1021/bi0602617. [DOI] [PubMed] [Google Scholar]
- Wang P, Kumar P, Wang C, DeFea K. Differential regulation of Class IA Phosphatidylinositol 3-Kinase catalytic subunits p110a and β by protease-activated-receptor-2 and β-arrestins. Biochem J. 2007;408:221–230. doi: 10.1042/BJ20070483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Lu R, Zhao J, Limbird LE. Arrestin serves as a molecular switch, linking endogenous alpha2-adrenergic receptor to SRC-dependent, but not SRC-independent, ERK activation. J Biol Chem. 2006;281:25948–25955. doi: 10.1074/jbc.M605415200. [DOI] [PubMed] [Google Scholar]
- Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, et al. Independent {beta}-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. PNAS. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann D, Rutschow S, Jager S, Linderer A, Anker S, Riad A, et al. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes. 2007;56:641–646. doi: 10.2337/db06-1163. [DOI] [PubMed] [Google Scholar]
- Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, et al. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, Yamashita T, et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther. 1998;287:860–867. [PubMed] [Google Scholar]
- Yee DK, Suzuki A, Luo L, Fluharty SJ. Identification of structural determinants for G protein-independent activation of mitogen-activated protein kinases in the seventh transmembrane domain of the angiotensin II Type 1 receptor. Mol Endocrinol. 2006;20:1924–1934. doi: 10.1210/me.2006-0018. [DOI] [PubMed] [Google Scholar]
- Zhai P, Yamamoto M, Galeotti J, Liu J, Masurekar M, Thaisz J, et al. Cardiac-specific overexpression of AT1 receptor mutant lacking G{alpha}q/G{alpha}i coupling causes hypertrophy and bradycardia in transgenic mice. J Clin Invest. 2005;115:3045–3056. doi: 10.1172/JCI25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. Beta-arrestin-dependent regulation of the Cofilin pathway downstream of protease-activated receptor-2. J Biol Chem. 2007;282:20634–20646. doi: 10.1074/jbc.M701391200. [DOI] [PubMed] [Google Scholar]