Abstract
A study of rabbit tear protein expression in a dry eye rabbit model was performed to determine if a pattern in expressed proteins could be identified. The uniqueness of the model allows the comparison of normal (control) eye tear protein expression with surgically induced dry eye tear protein expression in individual animals. The sensitivity of the method allows for single eye analysis. One-dimensional mini-gel electrophoresis of the tear proteins did not show substantial differences between band patterns of the normal versus the dry eye, but was used to assess the molecular weight ranges of the major proteins. Specific assignments of some of the predominant proteins were obtained by tandem mass spectrometry (MS) which showed that the lower molecular weight lipid-binding proteins (approximately 10 kDa to 36 kDa) constitute a considerable amount of the observed protein, followed in lesser quantities by the transferrins which have higher molecular weights ranging from 70 kDa to 85 kDa. Enhancement of matrix-assisted laser desorption/ionization time-of-flight (MALDI-ToF) MS linear mode analysis of intact proteins in tear fluid was demonstrated through the use of wax-coated MALDI plates and spot washing. MALDI-ToF MS analysis of the expressed tear proteins illustrates that differences between normal eye tear and dry eye tear protein content are manifested in changes in the lower molecular weight lipid-binding proteins such as lipophilin which exhibits an increase in concentration in the dry eye, and β-2 microglobulin which undergoes a decrease.
Keywords: Proteomics, Dry eye, Wax coat, Lipophilin, Microglobulin
Introduction
The thin ocular tear film is a complex mixture of components that serves to maintain the health of the eye's outer corneal epithelium. Extensive study has been conducted on the tear film, particularly on the protein components that constitute the film [1-13]. In general, the tear film structure is divided into three layers: the innermost glycocalix-mucin layer adjacent to the cornea, an intermediate aqueous-mucin layer [14, 15], and an outermost lipid layer [16]. Proteins are found predominantly in the aqueous portion of the tear film. The primary source of tear proteins is the main (orbital) lacrimal gland [17]. Extensive studies have been performed on human and rabbit tears, with fewer on dog [18] and rat tears [19]. A primary goal of these studies has been protein quantification and identification, in addition to protein expression studies that may provide insight into physiological changes that have resulted in dry eye symptoms.
Protein studies have employed techniques such as 1-dimensional [20] and 2-dimensional [21] sodium dodecyl sulfate polyacrylamide gel electrophoresis (1D and 2D SDS PAGE), capillary electrophoresis [22], liquid chromatography (LC) [23, 24], immunochemistry [25-27], chip-based micro-fluidic techniques [28], and thin-layer chromatography [29]. Recent advances in mass spectrometry (MS)-based proteomics approaches to protein characterization and identification are proving to be extremely valuable in the study of tear proteins. The employed mass spectrometric methods include gas chromatography-MS [29], liquid chromatography/electrospray ionization MS [30], and matrix-assisted laser desorption ionization-time of flight MS [31, 32]. Capillary electrophoresis/electrospray ionization-MS has also been applied to the analysis of proteins in whole human blood [33].
Profiles, identification, and quantification have been reported for both normal tear protein content, and protein content for dry eye syndrome (both human and rabbit). Reported total protein concentrations for rabbit include 12.6 μg/μL in unstimulated tears [34], and 40.0±5.3 μg/μL in lacrimal gland fluid at flow rates <2 μL/min [35]. For normal human tears, total protein concentrations have been reported as 8.1 μg/μL in stimulated tears and 22.7 μg/μLin unstimulated tears [36], 8±2.25 g/L from gentle stimulation [24], and from 6.05±1.58 mg/mL to 11.48±2.32 mg/mL (difference depending upon which calculation method was used) for tears collected by yawn reflex [37].
Human tears contain numerous types of proteins including serum-based proteins such as IgA and IgG, antibacteriolytic components such as lysozyme, transferrin, and albumin, and lipid-binding proteins such as lipocalin and the lipophilins. Fung et al. [32] recently reported the identification of approximately 500 proteins in normal human basal tear fluid. Rabbit tears contain many of the same types of proteins as are contained in human tears. Early studies of rabbit tears by Boonstra et al. [26] reported the identification of transferrin in rabbit tear, but not lactoferrin, leading the authors to conclude that rabbit tear may be substantially different from human tear. Recently, however, Zhou et al. [30] reported the presence of lactoferrin in rabbit tear, demonstrating that differences in techniques and methods may influence findings. Nonetheless, it is clear that there are similarities in the types of protein contained in human and rabbit tears.
More than 12 million people in the USA alone have been clinically diagnosed with some form of Keratoconjunctivitis sicca (KCS), also known as “dry eye”. Although the development of the dry eye condition has been associated with a number of physiological and environmental conditions such as aging, hormonal imbalance, disease, surgery, smoke, and/or low humidity plus heat [38], the precise underlying causes are often unknown, and no practical methodology for evaluating the composition of tears from such patients exists. In the past 25 years, many advances have been made in diagnosing, determining causes, and treating the dry eye condition [39]. The National Eye Institute (NEI) defines KCS as a disorder of the ocular tear film layer usually caused by a tear deficiency, or excessive evaporation of the aqueous layer, resulting in damage of the ocular surface and discomfort [38]. Various forms of dry eye may have similar physiological origins, perhaps from the breakdown of the eye tear layer, or they may be due to hormone imbalances, as demonstrated in a hormone replacement study by Schaumberg et al. [40]or to Sjogren's syndrome [41]. In human dry eye studies, the analytical evaluation is performed by determining tear volume, osmolality, and break-up time, as well as the extent of corneal surface damage. Additionally, compositional evaluation of tear samples has been undertaken by various instrumental approaches. However, without knowing the underlying cause of the dry eye condition, it is difficult to correlate a specific change in composition to a precise physiological origin. Animal models employing artificially induced dry eye provide the ability to study the effects of specific physiological changes on tear film composition. Studies have been performed on rabbits where the dry eye condition has been artificially induced through sensory denervation of the lacrimal gland [17, 42] or through corneal wounding [43]. In the current study, the rabbit dry eye model was produced through the surgical removal of the main and accessory lacrimal glands from one of the rabbit's eyes, while the other eye remained untouched as the contralateral control. This surgically induced dry eye is a non-evaporative condition where the total tear volume becomes reduced.
Experimental
Chemicals
Methanol and chloroform solvents were of HPLC/spectroscopy grade, the acetic acid was reagent grade, and all were purchased from EM Science (Darmstadt, Germany). All other chemicals were of analytical-reagent grade, and all standards and chemicals were used as received without further purification. Distilled and deionized water (Milli-Q water system, Millipore Inc., Bedford, MA) was used throughout standard and sample preparations.
Rabbit tear samples
The rabbit dry eye model was created in seven female New Zealand white rabbits by two sequential surgical procedures. First, the main lacrimal gland was removed from the experimental eye; the contralateral eye was used as control. Approximately 4 weeks later, the accessory lacrimal gland was removed from the experimental eye. At the same time, the nictitating membranes were removed from both eyes to facilitate measurement of the tear break-up time. Tear break-up time (TBUT, measured by using a slit lamp with a Tearscope) was then measured daily. When the TBUT of the surgical eye was half or less that of the control eye, the surgical eye was considered to be ‘dry’. Baseline tears (representing tears produced under non-irritating conditions) were collected daily from both the normal and surgically induced dry eyes using 5-μL silanated microcapillary pipettes. The usual volume obtained from a normal eye was 3 μL, whereas only 1 μL was typically obtained from a dry eye. The tear samples were immediately placed in Eppendorf tubes and preserved at −70 °C. Tears were stored in vials containing a week's collection of a single eye's tears.
Tear delipidation and protein isolation
The lipids were extracted from tear samples with a simple two-phase (chloroform/water) extraction technique; the resulting aqueous phase contained the tear proteins and the chloroform phase contained the lipids [44, 45]. Briefly, 1 week's tears collected from one eye were diluted to 300 μL. The aqueous tear solution was then extracted three times with 500 μL of chloroform.
1D gel electrophoresis
One-dimensional gel electrophoresis separation was performed employing a Mini-PROTEAN electrophoresis module assembly (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer's guidelines using a 1-mm-thick 12% acrylamide gel. 0.0625 M Tris-HCl, 2% SDS, 10% glycerol (v/v), 0.1 M dithiothreitol (DTT), and 0.01% bromophenol blue (Amersham Biosciences, Piscataway, NJ) at pH 6.8 were used for separation (at constant 200 V) and staining. A low molecular weight (LMW) calibration kit (Amersham Biosciences) contained the following proteins (Fig. 1, lanes 1 and 6): α-lactalbumin at 14.4 kDa, trypsin inhibitor at 20.1 kDa, ovalbumin at 45.0 kDa, albumin at 66.0 kDa, and phosphorylase b at 97.0 kDa. Lanes 2 and 4 are 1:1 sample/dye, whereas lanes 3 and 5 are 1:3 sample/dye. The gel was de-stained in a solution of 5% methanol plus 5% acetic acid (v/v), and stored in a solution of this same composition.
Fig. 1.
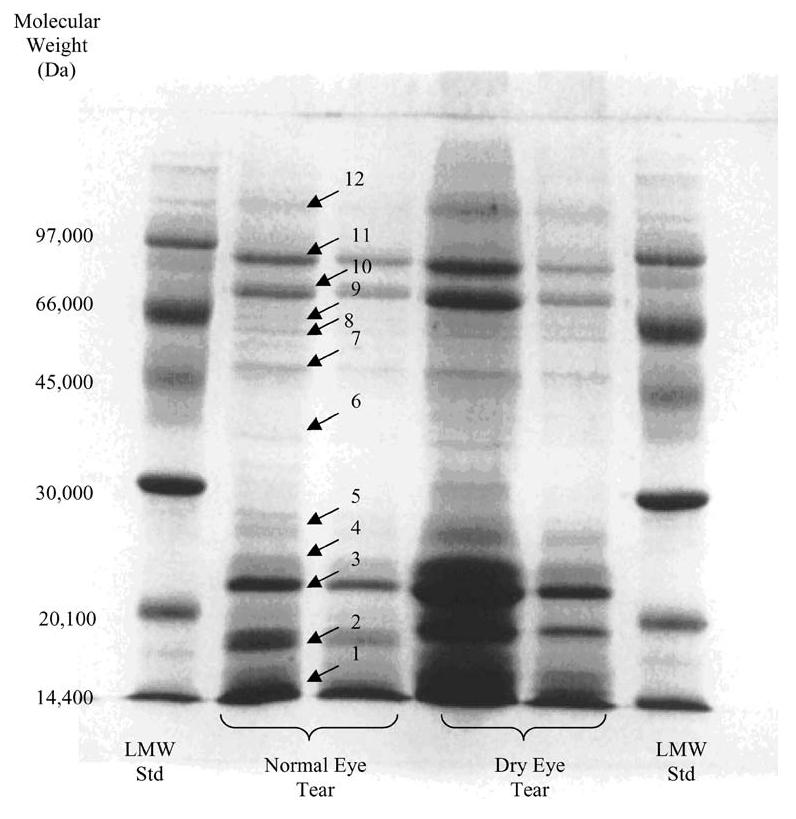
1D SDS-PAGE (1-mm-thick gel with 12% acrylamide) results of protein separation in rabbit normal eye tear versus the dry eye model tear. Lanes 1 and 6 low molecular weight (LMW) standard; lanes 2 and 4 1:1 mixtures of sample and dye; lanes 3 and 5 1:3 mixtures of sample and dye
In-gel reduction, alkylation, digestion, and extraction of peptides
The protein bands destined for matrix-assisted laser desorption/ionization time-of-flight (MALDI-ToF) mass spectrometry analyses were subjected to in-gel digestion. Briefly, the bands of interest were excised from the gel, chopped into 1-mm cubes, and washed/centrifuged three times with 100–150 μL of distilled, deionized water. The gels were then dehydrated for 15 min in 100 μL of acetonitrile (ACN). The gels were dried in an Eppendorf Vacufuge Concentrator 5301 (Brinkman Instruments, Inc.), and reduction was then performed in 50 μL of freshly prepared 10 mM DTT in 100 mM NH4HCO3. The gels were incubated for 60 min at 56 °C, after which the liquid was removed. The gels were again dehydrated in 100 μL of acetonitrile, dried, and alkylation (45 min, room temp., dark) was performed by the addition of 50 μL of freshly prepared 55 mM iodoacetamide in 100 mM NH4HCO3. After liquid removal, the gels were washed with 100 μL of 100 mM NH4HCO3; 100 μL of acetonitrile was added and the solution was vortexed for 20 min before removing the liquid. The gels were rehydrated with 10–20 μL of fresh 50 mM NH4HCO3, 5 mM CaCl2, and 12 ng/μL trypsin, then digested for 40 min at 4 °C, then overnight at 37 °C. The digestion solution was collected and the gels were incubated for 15 min in 30 μL 20 mM NH4HCO3 at 37 °C. The supernatent was then collected, and gels were incubated 3 times for 15 min in 30 μL of a 60% acetonitrile/5% formic acid solution. The supernatants were pooled and the volume was reduced. For MALDI-ToF mass spectrometry analysis, the peptides were desalted using ZipTipC18 (Millipore, Bredford, MA) according to the manufacturer's method. The sample was loaded and dispensed for 10 cycles, washed with 3×10 μL of 0.1% trifluoracetic acid (TFA), and then eluted with 1 to 4 μL of 50% acetonitrile, 0.1% TFA, into a 0.5-mL Eppendorf tube by drawing and expelling the elution solution 10 times.
MALDI-ToF MS and MALDI-PSD-ToF MS
MALDI-ToF mass spectra were acquired using an Applied Biosystems Voyager Elite r-ToF with delayed extraction (Applied Biosystems, Inc., Framingham, MA). The laser power was set to just above the threshold energy required to obtain desorption/ionization. Each mass spectrum is comprised of an average of 150 to 200 traces. For peptide analysis, an acceleration voltage of 20 kV was employed and delayed extraction was used with a 225-ns delay time. For matrix, saturated α-cyano-4-hydroxycinnamic acid (α-CHCA) was used in 50:50 H2O/ACN with 0.1% TFA. After mixing in a 1:1 ratio with sample, 1 μL was spotted onto the MALDI target plate and allowed to air-dry. For protein analysis, an acceleration voltage of 25 kV was employed; delayed extraction was again used, this time with a 750-ns delay time. The matrix for protein analysis consisted of saturated 3,5-dimethoxy-4-hydroxycinnamic acid (sinapinic acid) in 30:70 ACN/H2O with 0.1% TFA. Comparison of tear proteins was made by diluting the tear fluid in a 50:1 ratio of matrix to tear, then spotting the mixture directly onto the wax-coated MALDI plate; dried spots were washed with 10 mM diammonium citrate. For wax coating, the cleaned MALDI plates were swabbed in smooth strokes with a Q-tip that had been dipped into a 50 mg/mL paraffin wax chloroform solution; the plate was allowed to air-dry before spotting.
ES triple quadrupole MS/MS
Tandem mass spectra were acquired using a “triple quadrupole” (quadrupole-hexapole-quadrupole) mass spectrometer (Quattro II, Micromass, Inc., Manchester, UK) equipped with an electrospray interface operating at flow rates of 2–4 μL/min. Nitrogen was used as both nebulizing and drying gas. The ES capillary voltage was set at 2.5 to 3.5 kV and cone voltages between 30 and 40 V were employed. Argon (ca. 3.0×10−4 mBar, measured externally to the central hexapole collision cell) was used as collision gas, and the hexapole offset energy values ranged from 30 to 60 eV for collision-induced dissociation (CID) experiments. MassLynx software (Micromass) was used to acquire, process, and store mass spectral data.
Results and discussion
Tear total protein and preliminary separation
Quantification of total tear protein was determined by the Bradford Assay method (Sigma-Aldrich, St. Louis, MO) which has a high tolerance for salts and contaminants other than detergents. The dissolved protein is complexed with the dye Brilliant Blue G which causes a shift in the dye's absorbance maximum from 465 nm to 595 nm. Absorbance is directly proportional to protein concentration in a linear working range of 0.1–1.4 mg/mL. Table 1 presents a tabulation of total tear protein results for normal versus dry eye tear for seven different rabbits. In comparing Table 1 averages derived from the Bradford Assay method employing a standard calibration curve, a 14% reduction in total tear protein was found in the dry eye state relative to the normal eye, but, as the standard deviation (Std dev) indicates, animal-to-animal variability was too high to call this difference statistically significant.
Table 1.
Quantification of tear proteins in normal (control) eye tears and dry eye tears
| Sample ID | Control eye (mg/mL) | Dry eye (mg/mL) |
|---|---|---|
| SJ-71 (06-01-04) | 1.69 | 3.24 |
| SJ-72 (06-01-04) | 4.42 | 3.45 |
| SJ-73 (06-01-04) | 6.95 | 7.94 |
| SJ-75 (10-09-03) | 3.33 | NAa |
| SJ-75 (06-01-04) | 7.58 | 5.44 |
| SJ-76 (10-13-03) | NAa | 3.23 |
| SJ-76 (06-01-04) | 4.70 | 1.43 |
| Average | 4.78 | 4.12 |
| Std dev | 2.21 | 2.26 |
NA not analyzed
The extracted proteins from the normal (control) eye rabbit tear sample and from the dry eye model rabbit tear sample were separated for approximate molecular weight determination. Figure 1 shows the results of the 1-dimensional electrophoresis for the low molecular weight standard, the normal (control) eye rabbit tear, and the dry eye model rabbit tear, where well over 40 distinct bands are observed.
Optimization of MALDI-ToF MS for protein analyses
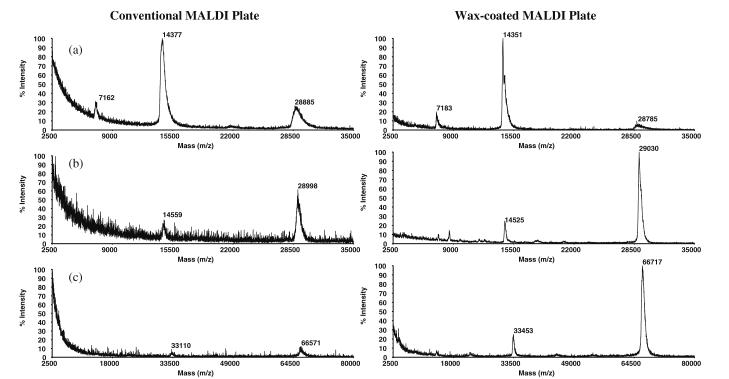
In the recent literature, there have been numerous accounts of incremental improvements in sample pretreatment prior to MALDI-ToF MS analyses of peptides [46]. These include the addition of monobasic ammonium phosphate as a matrix enhancement and post-crystallization washing with dibasic ammonium citrate, both resulting in suppression of metal adduct and matrix cluster formation [47]. Wax coating of MALDI plates prior to sample spotting has also been reported as a way of improving response in peptide analysis [48]. We have evaluated these optimization steps and incorporated certain aspects of them into the acquisition of the presently reported results. For the analysis of peptides, wax coating of the MALDI plates (see “MALDI-ToF MS and MALDI-PSD-ToF MS” section in the “Experimental” for wax coating procedure) was observed to decrease the chemical noise in MALDI-ToF spectra and increase the resolution of the peaks. These features were even more dramatically manifested when applying the wax coating to MALDI plates in preparation for protein analysis in the linear mode, e.g., employing aqueous 1 mg/mL lysozyme (Fig. 2a). Here, the signal-to-noise (S/N) ratio of MH+ (m/z 14.4 kDa) increased from 71 to 154 (uncoated vs. coated), whereas the S/N ratio increased from 19 to 31 for doubly protonated lysozyme at m/z 7.2 kDa. A decrease in the signal of the proton-bound dimer at m/z 28.8 kDa is also observed in Fig. 2a (right spectrum). For 1 mg/mL carbonic anhydrase (Fig. 2b), wax-coated plates afforded a S/N ratio increase from 12 to 135 for MH+ at m/z 29.0 kDa, and from 7 to 32 for (M+2H)2+ at m/z 14.5 kDa. Lastly, for BSA (Fig. 2c) the S/N ratio increased from 10 for the 33-kDa peak (+2 charge state), and 15 for the 66-kDa peak (MH+) to 39 and 158, respectively. In general, the S/N ratio is observed to have an approximate three- to tenfold increase for protein determinations when using wax-coated plates.
Fig. 2.
MALDI-ToF mass spectra of a left aqueous 1 mg/mL lysozyme (14.4 kDa) standard yielding a S/N ratio of 71 acquired using a conventional, non-coated MALDI plate; right lysozyme protein standard spectrum acquired using a wax-coated plate giving a S/N of 154 and a decrease in dimerization at m/z 28.8 kDa; b left aqueous 1 mg/mL carbonic anhydrase (29.0 kDa) standard with a S/N of 12 acquired using a non-coated plate; right carbonic anhydrase protein standard acquired using a wax-coated plate giving a S/N of 135; c left 1 mg/mL BSA (33.2 kDa and 66.4 kDa) standard acquired using a non-coated plate. The S/N for the 33-kDa peak is 10, whereas that for the 66-kDa peak is 15; right BSA spectrum acquired using wax-coated plate (S/N for the 33-kDa peak is 39 and S/N for the 66-kDa peak is 158)
MALDI-ToF MS of rabbit tear protein
Studies were performed to compare the tear protein of the normal eye versus the dry eye model for individual rabbits by depositing a 50:1 ratio of matrix/tear fluid and acquiring MALDI-ToF mass spectra in the linear mode. A combination of desalting and employment of wax-coated target plates allowed for single eye analyses of tear proteins by MALDI-ToF-MS.
Sample preparation
Tear samples contain considerable amounts of salts, therefore, the matrix/sample spots were washed with a 10 mM diammonium citrate (aqueous) solution, which was noted to enhance the response of the proteins as a result of salt removal. Figure 3 shows the enhancement effect observed for the tear protein spectrum when employing the wax-coated MALDI plates. Figure 3a is the MALDI-ToF spectrum acquired using an uncoated plate; Fig. 3b is the analogous spectrum acquired using the wax-coated MALDI plate, illustrating a clear enhancement in the MALDI-ToF MS spectrum of the tear proteins.
Fig. 3.
MALDI-ToF mass spectral response for a normal eye rabbit tear protein sample: tear protein spectrum acquired using a conventional plate (a) and a wax-coated plate (b)
Protein assignments
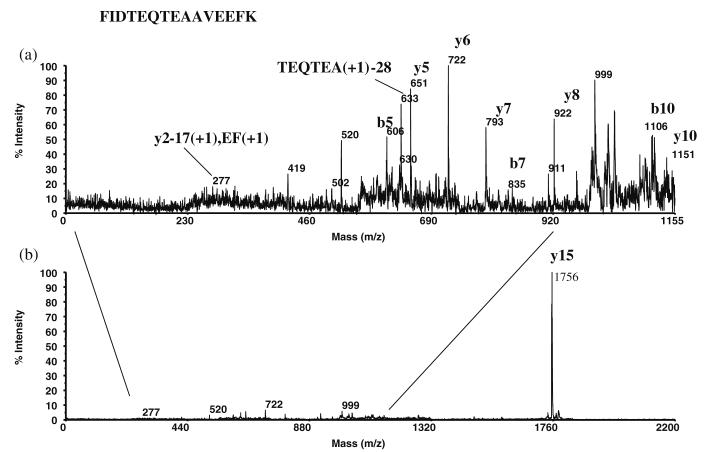
Figure 4 shows MALDI-ToF mass spectra of rabbit tear fluid proteins from a single rabbit with the dry eye condition induced in one eye only. Figure 4a is the mass spectrum of the left (normal) eye of the rabbit, whereas Figure 4b is the spectrum of the right (dry) eye of the rabbit. The large peaks at 11.3 kDa and 18.7 kDa have been assigned as β-2 microglobulin and lipocalin, respectively. It appears that the likely candidates for the 14.4-kDa protein are cystatin and/or lysozyme. The 16.5-kDa protein may be a heterodimer of lipophilins A (7,575 Da) and C (8,855 Da) [49, 50], and the 10.2-kDa protein is probably the lipophilin CL2 protein. Figure 5 shows the post-source decay (PSD) spectrum of an m/z 1,756 tryptic digest peptide obtained from band 1 in the 1D SDS-PAGE gel, identified as the lipophilin CL2 protein at 10,456 Da by database matching. The 1D SDS-PAGE separation of the tear proteins (Fig. 1) shows that the majority of the proteins are in the lower molecular weight range (approximately 10 kDa to 25 kDa, bands 1–4), followed by the transferrins of bands 10 and 11 (70–85 kDa). The low molecular weight proteins appear to be primarily comprised of the lipid-binding proteins such as the lipophilins, the apolipoproteins, and lipocalin. In addition, previous work done in our laboratory has shown that the major lipids in the tear film are mono and diacylglycerols [51], while other known tear lipids such as cholesterol, the wax esters, and the phosphorylated lipids, were detected in much lower quantities. It would seem likely then that the lipid-binding proteins in the tear film are the fatty acid chain-type binding proteins. For example, the human tear lipocalin (also known as tear-specific pre-albumin) is found to bind in vitro to retinol and fatty acids, but not to cholesterol, while tear fluid apolipoprotein D (apoD) does bind to cholesterol and the phosphorylated lipids [52] (apoD would be expected to be in the vicinity of bands 6 and 8, but these appear in low intensities).
Fig. 4.
MALDI-ToF mass spectra of a normal eye tear proteins and b dry eye tear proteins. The latter shows an increase in the 10.2-kDa lipophilin CL2 protein, and a decrease in both the 14.4-kDa cystatin/lysozyme protein(s) and the 16.5-kDa lipophilin protein
Fig. 5.
PSD spectrum from an m/z 1,756 precursor peptide obtained from a tryptic digest of band 1 in the 1D SDS-PAGE gel, identified as the lipophilin CL2 protein at 10.5 kDa: a blow up of region below m/z 1,155; b full PSD spectrum
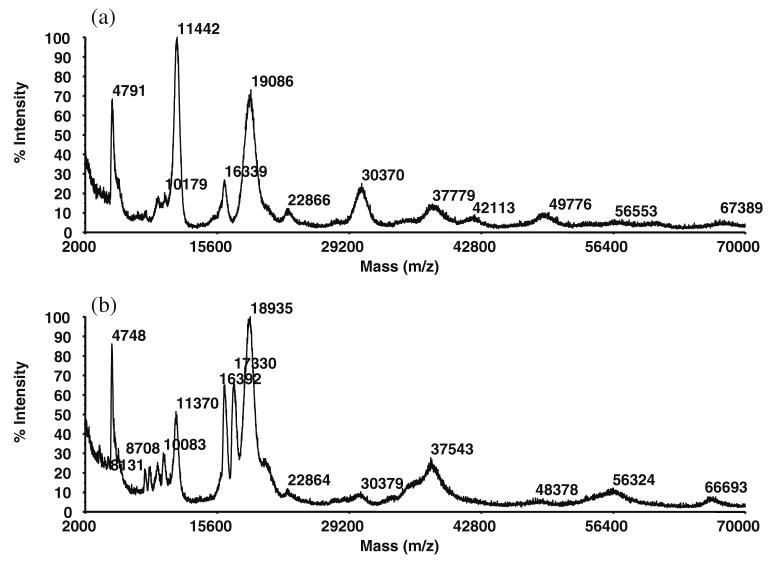
A second set of MALDI-ToF mass spectra obtained from a second rabbit that had the dry eye condition surgically induced in the right eye only is presented in Fig. 6, where the normal eye spectrum is Fig. 6a, and the dry eye model spectrum is Fig. 6b. While some of the same peaks that were observed in Fig. 4 also appear in Fig. 6, there are evident differences when comparing the two data sets obtained for the normal eyes, as well as when comparing the two data sets for dry eyes (a comparison of normal versus dry eyes appears below). Notably, the major peaks at 11.4 and 18.9 kDa (previously observed in Fig. 4, and assigned as β-2 microglobulin and lipocalin, respectively) appear again in Fig. 6 in very high intensities. In addition, peaks at 16.4 kDa are again observed (previously assigned as heterodimers of lipophilins), as are the 10.2 kDa previously assigned lipophilins; conversely, the 14.4-kDa peak (assigned as cystatin and/or lysozme, Fig. 4a) is no longer visible in Fig. 6. Some new peaks also appear in medium to high intensities, such as those at 30.4 kDa and 17.3 kDa; these are assigned as apoD monomer and lipophilin CL, respectively.
Fig. 6.
MALDI-ToF mass spectra of a normal eye tear proteins and b dry eye tear proteins. The latter shows an increase in the 17.3-kDa lipophilin CL protein, and a decrease in both the 11.4-kDa β-2 microglobulin protein and the 30.4-kDa apoD protein
In total, tear fluid samples from six rabbits with the surgically induced dry eye condition in the right eye were examined. Of the six, five showed a predominant MALDI-ToF peak at m/z 18.7±0.2 kDa (see Figs. 3, 4, and 6) that has been assigned as lipocalin. Tear lipocalin (also known as tear-specific pre-albumin) constitutes approximately 15–33% of the total tear protein in human tears [29, 53] and approximately 18–20% in rabbit tears [17, 22]. In a recent study by Azzarolo et al. [54] tear lipocalin was identified as one of the major proteins in rabbit lacrimal fluids. Tear lipocalin readily binds to lipids, and it is thought to play a major role in tear stability, thus providing an anchoring effect between the tear film lipid layer and the tear aqueous region. The molecular weight of the rabbit tear lipocalin was reported as ca. 18 kDa [54]. The slightly higher molecular weight 18.7±0.2 kDa protein observed in our MALDI-ToF mass spectra may be a holo form of this lipocalin identified by Azzarolo et al. [54]; however, a detailed investigation of this major peak at 18.7±0.2 kDa has not been performed.
ES triple quadrupole MS/MS of digested peptides
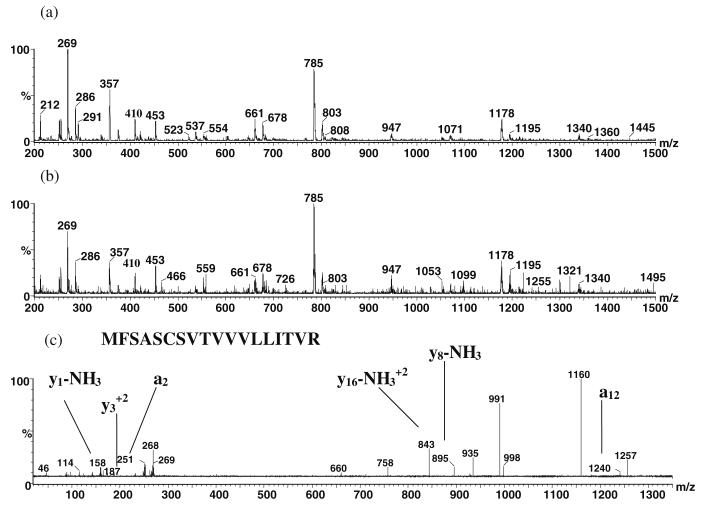
As a further aid in the identification of the tear proteins, electrospray (ES) triple quadrupole tandem MS was performed on the most abundant peptides from an in-solution digestion of the tear proteins. The lipid fraction was first removed from the tear fluid following extraction (see “Tear delipidation and protein isolation” in the “Experimental” section). The aqueous tear protein extract was then cleaned using a ProteoPrep protein precipitation kit (Sigma, St. Louis, MO) to isolate the proteins and wash off the tear salts. In-solution digestion was then performed on the proteins, and the resulting peptides were infused into the electrospray source using a syringe pump. Figure 7a is the electrospray single stage mass spectrum of the normal eye sample illustrating the most abundant peptides. Figure 7b is the ES single stage mass spectrum of the dry eye peptides. The most abundant peptides were subjected to collision-induced dissociation, and the resultant product ion spectra were run through the Protein Prospector database searching algorithm to identify likely proteins. As can be seen in Fig. 7a and b, many of the same abundant peptides (e.g., m/z 357, 453, 661, 678, 785, 803, 947, 1,178, and 1,195) are observed in both spectra. Figure 7c is a product ion spectrum from collision-induced dissociation of the m/z 661 (+3) peptide (from normal eye tear). The protein was identified as the T-cell receptor alpha chain V region RL-5 precursor with a molecular weight of 14,856 Da based upon the appearance of product ions corresponding to the y1–NH3 peak at m/z 158, the peak at m/z 187, the a2 peak at m/z 251, the (y16 – NH3)2+ at m/z 843, the y8–NH3 peak at m/z 895, and the a12 peak at m/z 1,240. This is an immunoglobulin, antigen receptor-type protein involved in antimicrobial activity; however, the significance of its presence in tears is unknown.
Fig. 7.
a ES single stage mass spectrum of the normal eye peptides. b ES single stage mass spectrum of the dry eye peptides. c ES-MS/MS product ion spectrum of the m/z 661.3 (+3) peptide precursor (from normal eye tear)
The total number of peaks presumed to arise from proteins in normal eye MALDI-ToF mass spectra was 63, whereas a total of 66 peaks were observed in the dry eye spectra. The majority of these proteins remain unidentified at the present time. Tables 2 and 3 present summaries of approximate molecular weights and tentative assignments of proteins that were repeatedly observed in the MALDI-ToF spectra of the normal and dry eye tears of the six replicate rabbits; comparative differences in the major proteins observed in the normal vs. dry eye model spectra are shown in Table 2, and these will be discussed in a separate section below. The assignments in Tables 2 and 3 are based upon MALDI-ToF PSD analysis, ES-MS/MS analysis, MALDI-ToF fingerprint analysis, and molecular weight information from MALDI-ToF mass spectra, in combination with literature reports and MS database searching, as denoted by footnotes for each assigned protein.
Table 2.
Protein peaks found in normal and dry eye rabbit tears by MALDI-TOF MS and ES MS/MS with tentative assignments
| Average molecular weight (kDa)a |
Protein ID from literature and mass spectrometry |
% Difference in S/N from normal to dryb |
% Difference in response from normal to dryc |
|---|---|---|---|
| 10.1±0.06 | Lipophilind | 99±42 | 108±32 |
| 11.4±0.09 | β-2 Microglobulind | −82±33 | −59±17 |
| 14.4±0.13 | Cystatin/lysozymed | 120±156 | 178±21 |
| 16.4±0.07 | Heterodimer of lipophilins A (7,575 Da) and C (8,855 Da)d | −17±29 | −48±12 |
| 17.3±0.08 | Lipophilin CLd | 30±17 | 39±87 |
| 18.7±0.23 | Lipocalin (tear-specific pre-albumin)d | 8±33 | 13±91 |
| 30.2±0.57 | Apolipoprotein D monomerd | −51±43 | −33±43 |
| 37.6±0.29 | Unidentified | 18±49 | 29±94 |
Average plus or minus one standard deviation
Calculated as ((S/N dry–S/N normal)/S/N normal)×100% for each individual rabbit; shown as avg ± std dev
Calculated as ((peak height dry–peak height normal)/peak height normal)×100% for each individual rabbit; shown as avg ± std dev
Assignment based upon molecular weight information from MALDI-ToF mass spectra in combination with literature reports and MS database (Protein Prospector) searching
Table 3.
Assignments of other abundant proteins found in both normal and dry eye rabbit tears (no quantification performed)
| Accession number | Protein molecular weight (Da) |
Protein ID | Sequence or number of peptides matched |
MOWSE score |
|---|---|---|---|---|
| AF308618a | 10,456 | Lipophilin CL2 | FIDTEQTEAAVEEFK | |
| 136487b | 14,856 | T-cell receptor alpha chain V region RL-5 precursor | MFSASCSVTVVVLLITVR | |
| P42069c | 25,307 | T-cell-specific surface glycoprotein CD28 precursor | 4 | 138 |
| P17666b | 55,721 | Cytochrome P450 2C14 | NLSPINK | |
| P21195c | 56,808 | Protein disulfide isomerase precursor | 7 | 62 |
| P49065c | 68,915 | Serum albumin precursor | 14 | 1.9×106 |
Identification determined by MALDI-ToF PSD analysis
Identification determined by ES-MS/MS analysis
Identification determined by MALDI-ToF fingerprint analysis
Comparison of normal vs. dry eye model tears
Tear fluid analyses performed in this study, like many analyses of biological fluids originating from mammalian sources, suffer from a high degree of variability from one animal replicate to the next. Ascribing the origin of differences in excretion content in replicate samples to specific metabolic causality is a complex endeavor. An advantage of our study relative to past studies, however, is that the “pooling” of samples from multiple animal replicates has been avoided, i.e., in the current study, each sample comes from one eye of a single animal. Single eye tears collected daily during one week were combined prior to analysis, hence, day-to-day variability within the week could not be assessed. When protein quantities were calculated as the means of the six animal replicates ± standard deviations, variability in results obtained from individual rabbits would not allow the demonstration of a significant difference between normal versus surgically induced dry eye tears. Some distinctions between normal and dry eye samples did become apparent, however, when differences between the left (normal) and right (dry eye) of individual rabbits were compared; thus, variability is reduced when the difference between left and right eyes is assessed for each replicate rabbit. Table 2 lists the percentage change in results for normal vs. dry eye samples calculated in two ways for each individual rabbit (six replicates) and reported as the average percentage difference ± standard deviation; positive values indicate an increase in the dry eye relative to the normal, whereas negative numbers indicate a decrease. The third column lists the average percentage change in signal-to-noise ratio for each detected protein, whereas the fourth column lists the average percentage change in background subtracted peak heights for each protein as detailed in the Table 2 footnotes. By comparing left eye versus right eye data for each replicate rabbit and then averaging the results for the six replicates, two clear trends emerge that are evident by either calculation method. The first is that the 10.1-kDa protein, assigned as lipophilin, undergoes a significant increase in the dry eye relative to the normal. The second clear trend is that the 11.4-kDa protein, assigned as β-2 microglobulin, exhibits a significant decrease in the dry eye relative to the normal. The observation of such statistically significant changes in normal versus dry eyes represents a first step toward identifying potential disease state markers.
Conclusion
Wax coating of MALDI plates significantly enhances the response of tear proteins in linear mode time-of-flight MS analysis. In combination with spot washing to reduce salt suppression effects, single eye tear analyses were performed at high sensitivities. One-dimensional SDS-PAGE gel analysis showed that the predominant proteins present in both the normal eye and the dry eye model are the lower molecular weight proteins (approximately 10–30 kDa) and the transferrins at 70–85 kDa. The MALDI-ToF MS linear mode analysis of the intact tear proteins indicated that expression differences exist primarily in the low molecular weight range of the lipid-binding proteins. Substantial differences were observed in the dry eye vs. normal tear protein MALDI-ToF MS spectra that indicated an increased expression of a 10.1±0.06 kDa protein assigned as lipophilin, and a decreased expression of an 11.4±0.09 kDa protein assigned as β-2 microglobulin. These findings suggest that MALDI-ToF mass spectra may be used to identify disease state markers, and may find application in the diagnosis of the dry eye condition.
Acknowledgements
Financial support was provided by the Louisiana Board of Regents through grants HEF(2001-06)-08 and LEQSF (2001-04)-RD-B-11, and from the National Eye Institute through NEI/R03 EY014021, and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Contributor Information
Bryan M. Ham, Department of Chemistry, University of New Orleans, 2000 Lakeshore Drive, New Orleans, LA 70148, USA
Jean T. Jacob, Department of Ophthalmology, Louisiana State University Health Sciences Center, 2020 Gravier Street, Suite B, New Orleans, LA 70112, USA
Richard B. Cole, Department of Chemistry, University of New Orleans, 2000 Lakeshore Drive, New Orleans, LA 70148, USA
References
- 1.Holly FJ, Lemp MA. Int Ophthalmol Clin. 1973;13:29. doi: 10.1097/00004397-197301310-00008. [DOI] [PubMed] [Google Scholar]
- 2.Ellison SA, Jacobson M, Levine MJ. Lacrimal and salivary proteins. Immunol eye, workshop 3: immunological aspects of ocular disease: infection, inflammation, and allergy; 1980; 1981. Meeting Date. [Google Scholar]
- 3.Frey WH, DeSota-Johnson D, Hoffman C, McCall JT. Am J Ophthalmol. 1981;92:559. doi: 10.1016/0002-9394(81)90651-6. [DOI] [PubMed] [Google Scholar]
- 4.Holly FJ, Hong BS. Am J Optom Physiol Opt. 1982;59:43. doi: 10.1097/00006324-198201000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Gachon AM, Richard J, Dastugue B. Curr Eye Res. 1982;2:301. doi: 10.3109/02713688209000774. [DOI] [PubMed] [Google Scholar]
- 6.Suarez JC. Ophthalmologica (Basel) 1986;193:75. doi: 10.1159/000309680. [DOI] [PubMed] [Google Scholar]
- 7.Kuizenga A, Stolwijk TR, van Agtmaal EJ, van Haeringen NJ, Kijlstra A. Curr Eye Res. 1990;9:997. doi: 10.3109/02713689009069936. [DOI] [PubMed] [Google Scholar]
- 8.Gachon AMF. Human lacrimal gland secretes proteins belonging to the group of hydrophobic molecule transporters. In: Sullivan DA, editor. Lacrimal gland, tear film, and dry eye syndromes. Plenum; New York: 1994. p. 205. [DOI] [PubMed] [Google Scholar]
- 9.Baguet J, Claudon-Eyl V, Sommer F, Chevallier P. CLAO J. 1995;21:114. [PubMed] [Google Scholar]
- 10.Asrani AC, Lumsden AJ, Kumar R, Laurie GW. Gene cloning of BM180, a lacrimal gland enriched basement membrane protein with a role in stimulated secretion. In: Sullivan DA, Dartt DA, Meneray MA, editors. Lacrimal gland, tear film, and dry eye syndromes 2. Plenum; New York: 1998. p. 49. [DOI] [PubMed] [Google Scholar]
- 11.Lehrer RI, Xu G, Abduragimov A, Dinh NN, Qu XD, Martin D, Glasgow BJ. FEBS Lett. 1998;432:163. doi: 10.1016/s0014-5793(98)00852-7. [DOI] [PubMed] [Google Scholar]
- 12.Schoenwald RD, Vidvauns S, Wurster DE, Barfknecht CF. The role of tear proteins in tear film stability in the dry eye patient and in the rabbit. In: Sullivan DA, Dartt DA, Meneray MA, editors. Lacrimal gland, tear film, and dry eye syndromes 2. Plenum; New York: 1998. p. 391. [Google Scholar]
- 13.Boptom VE, Willcox MDP, Millar TJ. Clin Exp Ophthalmol. 2000;28:208. doi: 10.1046/j.1442-9071.2000.00285.x. [DOI] [PubMed] [Google Scholar]
- 14.Moore JC, Tiffany JM. Exp Eye Res. 1981;33:203. doi: 10.1016/s0014-4835(81)80069-3. [DOI] [PubMed] [Google Scholar]
- 15.Carrington SD, Hicks SJ, Corfield AP, Kaswan RL, Packer N, Bolis S, Morris CA. Structural analysis of secreted ocular mucins in canine dry eye. In: Sullivan DA, Dartt DA, Meneray MA, editors. Lacrimal gland, tear film, and dry eye syndromes 2. Plenum; New York: 1998. p. 253. [DOI] [PubMed] [Google Scholar]
- 16.McCulley JP, Shine WE. Biosci Rep. 2001;21:407. doi: 10.1023/a:1017987608937. [DOI] [PubMed] [Google Scholar]
- 17.Salvatore MF, Pedroza L, Beuerman RW. Curr Eye Res. 1999;18:455. doi: 10.1076/ceyr.18.6.455.5270. [DOI] [PubMed] [Google Scholar]
- 18.Barrera R, Jimenez A, Lopez R, Mane MC, Rodriguez JF, Molleda JM. Am J Vet Res. 1992;53:454. [PubMed] [Google Scholar]
- 19.Kondo Y. Exp Anim. 1994;43:1. [Google Scholar]
- 20.Berta A. Graefe Arch Clin Exp Ophthalmol. 1982;219:95. doi: 10.1007/BF02173448. [DOI] [PubMed] [Google Scholar]
- 21.Joo W, Lee D, Kim C. Biosci Biotechnol Biochem. 2003;67:1574. doi: 10.1271/bbb.67.1574. [DOI] [PubMed] [Google Scholar]
- 22.Varnell RJ, Maitchouk DY, Beuerman RW, Salvatore MF, Carlton JE, Haag AM. J Cap Elec. 1997;1:1. [PubMed] [Google Scholar]
- 23.Boonstra A, Kijlstra A. Curr Eye Res. 1984;3:1461. doi: 10.3109/02713688409000842. [DOI] [PubMed] [Google Scholar]
- 24.Wollensak G, Mur E, Mayr A, Baier G, Gottinger W, Stoffler G. Graefe Arch Clin Exp Ophthalmol. 1990;228:78–82. doi: 10.1007/BF02764296. [DOI] [PubMed] [Google Scholar]
- 25.Gachon AM, Verrelle P, Betail G, Dastugue B. Exp Eye Res. 1979;29:539. doi: 10.1016/0014-4835(79)90154-4. [DOI] [PubMed] [Google Scholar]
- 26.Boonstra A, Kijlstra A. Exp Eye Res. 1984;38:561. doi: 10.1016/0014-4835(84)90174-x. [DOI] [PubMed] [Google Scholar]
- 27.Inada K, Baba H, Okamura R. Jpn J Ophthalmol. 1982;26:314. [PubMed] [Google Scholar]
- 28.Schmut O, Horwath-Winter J, Zenker A, Trummer G. Graefe Arch Clin Exp Ophthalmol. 2002;240:900. doi: 10.1007/s00417-002-0537-0. [DOI] [PubMed] [Google Scholar]
- 29.Glasgow BJ, Abduragimov AR, Farahbakhsh ZT, Faull KF, Hubbell WL. Curr Eye Res. 1995;14:363. doi: 10.3109/02713689508999934. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, Beuerman RW, Barathi A, Tan D. Rapid Commun Mass Spectrum. 2003;17:401. doi: 10.1002/rcm.925. [DOI] [PubMed] [Google Scholar]
- 31.Mulvenna I, Stapleton F, Hains PG, Cengiz A, Tan M, Walsh B, Holden B. Clin Exp Ophthalmol. 2000;28:205. doi: 10.1046/j.1442-9071.2000.00288.x. [DOI] [PubMed] [Google Scholar]
- 32.Fung K, Morris C, Duncan M. Mass spectrometric techniques applied to the analysis of human tears: a focus on the peptide and protein constituents. In: Sullivan DA, Calmenson SA, Stern ME, Dartt DA, editors. Lacrimal gland, tear film, and dry eye syndromes 3. Kluwer Academic/Plenum; New York: 2002. p. 601. [DOI] [PubMed] [Google Scholar]
- 33.Cao P, Moini M. J Am Soc Mass Spectrom. 1998;9:1081. doi: 10.1016/S1044-0305(98)00081-6. [DOI] [PubMed] [Google Scholar]
- 34.Bonavida B, Sapse AT, Sercarz EE. Invest Ophthalmol. 1968;7:435. [PubMed] [Google Scholar]
- 35.Dartt LA, Botelho SY. Invest Ophthalmol Vis Sci. 1979;18:1207. [PubMed] [Google Scholar]
- 36.Dohlman CH, Friend J, Kalevar V, Yogoda D, Balazs E. Exp Eye Res. 1976;22:359. doi: 10.1016/0014-4835(76)90228-1. [DOI] [PubMed] [Google Scholar]
- 37.Ng V, Cho P, To C. Graefe Arch Clin Exp Ophthalmol. 2000;238:738. doi: 10.1007/s004170000140. [DOI] [PubMed] [Google Scholar]
- 38.Lemp MA. CLAO J. 1995;21:221. [PubMed] [Google Scholar]
- 39.Pflugfelder SC, Solomon A, Stern ME. Cornea. 2000;19:644. doi: 10.1097/00003226-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Schaumberg DA, Buring JE, Sullivan DA, Dana MR. JAMA. 2001;286:2114. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 41.Ohashi Y, Ishida R, Kojima T, Goto E, Matsumoto Y, Watanabe K, Ishida N, Nakat K, Takeuchi T, Tsubota K. Am J Ophthalmol. 2003;136:291. doi: 10.1016/s0002-9394(03)00203-4. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen DH, Beuerman RW, Meneray MA, Maitchouk D. Sensory denervation leads to deregulated protein synthesis in the lacrimal gland. In: Sullivan DA, Dartt DA, Meneray MA, editors. Lacrimal gland, tear film, and dry eye syndromes 2. Plenum; New York: 1998. p. 55. [DOI] [PubMed] [Google Scholar]
- 43.Varnell RJ, Maitchouk DM, Beuerman RW, Carlton JE, Haag A. Small-volume analysis of rabbit tears and effects of a corneal wound on tear protein spectra. In: Sullivan DA, Dartt DA, Meneray MA, editors. Lacrimal gland, tear film, and dry eye syndromes 2. Plenum; New York: 1998. p. 659. [DOI] [PubMed] [Google Scholar]
- 44.Folch J, Lees M, Stanly GHS. J Biol Chem. 1957;226:497. [PubMed] [Google Scholar]
- 45.Bligh EG, Dyer WJ. Can J Biochem Physiol. 1959;37:911. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 46.Shevchenko A, Wilm M, Vorm O, Mann M. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 47.Smirnov IP, Zhu X, Taylor T, Huang Y, Ross P, Papayanopoulos IA, Martin SA, Pappin DJ. Anal Chem. 2004;76:2958. doi: 10.1021/ac035331j. [DOI] [PubMed] [Google Scholar]
- 48.Terry DE, Umstot E, Desiderio DM. J Am Soc Mass Spectrom. 2004;15:784. doi: 10.1016/j.jasms.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Lehrer RI, Xu G, Abduragimov A, Dinh NN, Qu XD, Martin D, Glasgow BJ. FEBS Lett. 1998;432:163. doi: 10.1016/s0014-5793(98)00852-7. [DOI] [PubMed] [Google Scholar]
- 50.Lehrer RI, Nguyen T, Zhao C, Ha CX, Glasgow BJ. Ann N Y Acad Sci. 2000;923:59. doi: 10.1111/j.1749-6632.2000.tb05519.x. [DOI] [PubMed] [Google Scholar]
- 51.Ham BM, Jacob JT, Keese MM, Cole RB. J Mass Spectrom. 2004;39:1321. doi: 10.1002/jms.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holzfeind P, Merschak P, Dieplinger H, Bernhard R. Exp Eye Res. 1995;61:495. doi: 10.1016/s0014-4835(05)80145-9. [DOI] [PubMed] [Google Scholar]
- 53.Azen EA. Biochem Genet. 1976;14:225. doi: 10.1007/BF00484762. [DOI] [PubMed] [Google Scholar]
- 54.Azzarolo AM, Brew K, Kota S, Ponomareva O, Schwartz J, Zylberberg C. Comp Biochem Physiol, B. 2004;138:111. doi: 10.1016/j.cbpc.2004.02.012. [DOI] [PubMed] [Google Scholar]