Abstract
The effect of small unilamellar phospholipid vesicles on the acid-catalyzed dissociation of nitric oxide from diazeniumdiolate ions, R1R2N[N(O)NO]-, [1: R1 = H2N(CH2)3-, R2 = H2N(CH2)3NH(CH2)4-; 2: R1 = R2 = H2N(CH2)3-; 3: R1 = n-butyl-, R2 = n-butyl-NH2+(CH2)6-; 4: R1 = R2 = n Pr-] has been examined at pH 7.4 and 37 °C. NO release was catalyzed by anionic liposomes (DPPG, DOPG, DMPS, POPS and DOPA) and by mixed phosphatidylglycerol/phosphatidylcholine (DPPG/DPPC and DOPG/DPPC) covesicles, while cationic liposomes derived from 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) and the zwitterionic liposome DMPC did not significantly affect the dissociation rates of the substrates examined. Enhancement of the dissociation rate constant in DPPG liposome media (0.010M phosphate buffer, pH 7.4, 37 °C) at 10 mM phosphoglycerol levels, ranged from 37 for 1 to 1.2 for the anionic diazeniumdiolate 4, while DOPA effected the greatest rate enhancement, achieving 49-fold rate increases with 1 under similar conditions. The observed catalysis decreases with increase in the bulk concentration of electrolytes in the reaction media. Quantitative analysis of catalytic effects has been obtained through the application of pseudophase kinetic models and equilibrium binding constants at different liposome interfaces are compared. The stoichiometry of nitric oxide release from 1 and 2 in DPPG/DPPC liposome media has been obtained through oxyhemoglobin assay. DPPG = 1,2-dipalmitoyl-sn-glycero-3-[phospho-rac-(1-glycerol)], DOPG = 1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)], DMPS = 1,2-diacyl-sn-glycero-3-[phospho-L-serine], POPS= 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-L-serine], DOPA = 1,2-dioleoyl-sn-glycero-3-phosphate; DPPC = 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, DMPC = 1,2-dimyristoyl-sn-glycero-3-phosphocholine, DOTAP = 1,2-dioleoyl-3-trimethylammonium-propane.
Keywords: Diazeniumdiolates, nitric oxide, phospholipid liposomes, catalysis
Introduction
Because of the widespread use of diazeniumdiolates for targeting NO at specific biological sites and cell types [1,2], there is an interest in the nature of their interactions with complex environments encountered biologically and how such interactions influence their NO release rates [3-5]. Since both unilamellar and multilamellar phospholipid vesicles have been used extensively as models for bilayer membranes in studies of a variety of membrane-mediated processes, we have employed phospholipid liposomes as biomimetic media to explore the effect of different lipid interfaces and bilayer structures on diazeniumdiolate reactivity. Previously we have used charge neutral phosphatidylcholine liposomes to promote the association of diazeniumdiolates that have significant hydrophobic structure with the liposome pseudo-phase so as to explore factors that may be influencing diazeniumdiolate reactivity in lipophilic media [4]. In the present study, the effectiveness of different anionic and cationic phospholipid vesicles in binding and catalyzing NO release from diazeniumdiolate ions has been compared, and we have examined in more detail the influences that anionic liposomes, their phospholipid head groups, bilayer structures and the salt concentrations present in their reaction media, have on reaction rates. This study furthers our understanding of medium effects on diazeniumdiolate reactivity, particularly factors influencing their reaction rates at interfaces.
Although phosphatidylcholines are the predominant lipids in most cell membranes, negatively charged phospholipids are important components of bacterial cell membranes and of pulmonary surfactants. The electrostatic potential generated by negatively charged phospholipids is a key property of bilayer membranes and the interaction between the anionic membrane and electrolytes plays an important role in regulating the concentration of protons, cations and other positively charged substrates at the membrane surface. Interest in anionic diazeniumdiolates in phospholipid media extends also to their application as nitric oxide sources for the relief of pulmonary hypertension [6-10] since they have been successfully employed as pulmonary vasodilators in porcine animal models [11] and one has been administered to a human patient with acute respiratory distress syndrome [12]. The present study provides fundamental information on the binding and reactivity of diazeniumdiolate substrates in phospholipid media, particularly negatively charged interfaces of phosphoglycerol, phosphatidylserine and phosphatidic acid bilayers and phosphatidylcholine-phosphoglycerol aggregates that have important roles in multiphase transitions involved in lung surfactant function [13].
Experimental Procedures
Materials
Zwitterionic diazeniumdiolates 1-3 were prepared, as described previously by treating the parent polyamine in CH3CN with NO at 60-80 psi for 24 h [14]. The product was isolated by filtration, washed with CH3CN and ether and dried under vacuum. Compound 4 was similarly prepared by the high pressure reaction of NO with di-n-propylamine [15]. The initial dialkylammonium salt obtained was converted to the sodium salt using sodium methoxide. Purities were checked by the ∼250 nm UV spectral band and by proton NMR. All phospholipids were obtained from Avanti Polar Lipids (Alabaster, Alabama). Sephadex G-25 (PD-10) desalting columns were obtained from Amersham Biosciences and sodium hydrosulfite (dithionite) and dried human hemoglobin (Hb) were purchased from Sigma.
Rate measurements
Rate constants were measured spectrophotometrically by following changes in absorption of the diazeniumdiolate chromophore at 250 nm, using a Hewlett Packard 8453 Diode Array UV-visible spectrophotometer. Substrate concentrations of 100 μM were employed in all cases. Phosphate (Na2HPO4.7H2O/NaH2PO4.H2O), Tris-HCl and PBS (9.57 mM phosphate, 137 mM NaCl, 2.7 mM KCl) buffers were employed to maintain constant pH 7.4 in the kinetic runs. pH values were checked against standard buffer solutions using an Accumet Research AR15 pH meter (Fisher).
Preparation of vesicle solutions
Unilamellar phospholipid vesicles were prepared from chloroform solutions of the phospholipids [16]. Lipid films were deposited on the walls of a round-bottom flask by evaporation of the solvent under a N2 flux followed by removal of trace solvent under high vacuum. The dry lipid films were hydrated in pH 7.4 phosphate buffer solutions by agitation in a rotary evaporator for 45 minutes. The resulting homogeneous vesicle suspensions were sonicated with a Branson 2510 thermostated bath-sonicator for one hour to obtain transparent liposome solutions. During hydration and sonication, solutions were maintained above the liquid crystalline phase transition temperature of the lipid. The desired lipid concentrations for kinetic runs were obtained by appropriate dilution of the liposome stock solutions with buffer. Liposome solutions were typically used within 2 hours of their preparation, and good reproducibility of kinetic data was obtained from different liposome preparations.
Oxyhemoglobin assay of NO release
Nitric oxide was determined using the standard oxyhemoglobin assay method [17]. Oxyhemoglobin (HbO2) was prepared by dissolving 10 mg of hemoglobin (Hb) with gentle stirring in 1 ml of phosphate buffer (0.01 M pH= 7.4). The hemoglobin solution was then treated with 1 mg of sodium hydrosulfite to ensure complete reduction of the hemoglobin before oxidizing it completely to HbO2 by blowing O2 gas gently on the surface with stirring. Completion (within a few minutes) was signaled by the bright red color of the solution. Purification was achieved by passing the solution through a Sephadex G-25 (PD-10) desalting column before use in the kinetic experiments. The concentration of the Hb stock solution was determined after dilution with buffer from its absorption at 415 nm (ε = 131 mM-1 cm-1). The HbO2 stock was kept on ice and O2 gas was gently blown over the surface of the HbO2 before each run to maintain its concentration at optimal level.
Liposome Size Analysis
The sizes of the liposomes employed in the study were determined by dynamic light scattering (photon correlation spectroscopy) using a Beckman-Coulter N5 Sub-Micron particle size analyzer. Measurements were made at 37°C at a measurement angle of 90°. Liposome size was determined both in the absence of substrate and after diazeniumdiolate was added and reaction allowed to go to completion, which typically took from 20 min – 90 min. Values reported are mean values of three measurements from the same sample.
Results
Catalysis in anionic liposome media
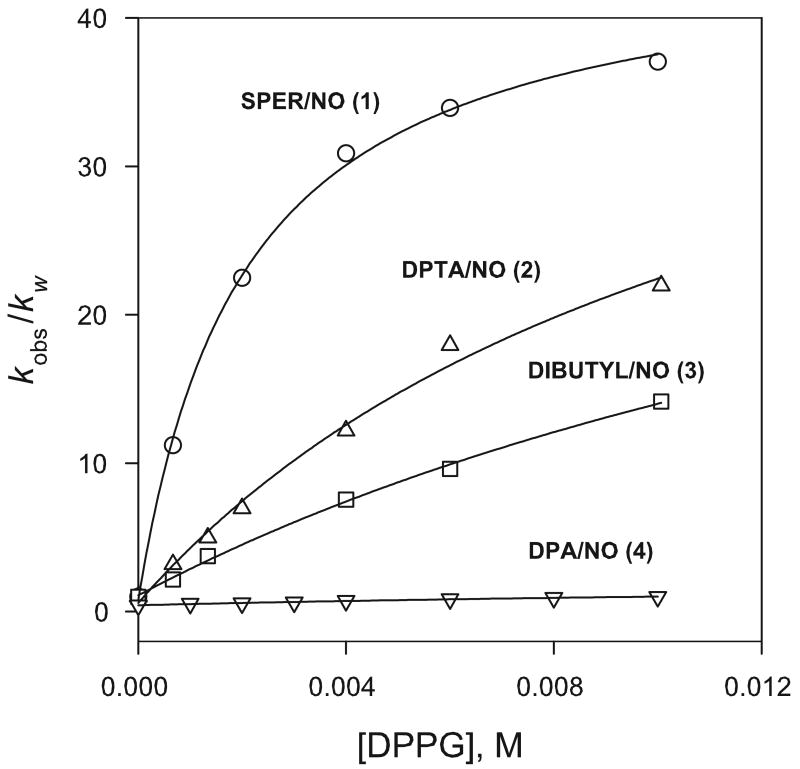
Initial studies examined dissociation rates of the spermine-derived 1 in phosphate buffered DPPG liposome solutions at pH 7.4 (0.010 M phosphate) and 37 °C. Reactions followed first-order behavior over several half-lives and yielded rate constants (kobs) that increased with liposome concentration and displayed a dependence on lipid concentration that approached rate saturation at relatively low lipid levels. When the dissociation rates of a variety of diazeniumdiolate substrates were compared in DPPG liposome media, the rate enhancements observed were found to depend quite strongly on the structure of the diazeniumdiolate employed. Figure 3 shows catalytic factors, kobs/kw, the ratio of the rate constant in the buffered liposome solution (kobs) to that in the buffered solution alone (kw), plotted as a function of lipid concentration for zwitterionic substrates 1-3 and the anionic substrate 4. The extent of the catalysis observed for the different substrates can be compared through kobs/kw values at 10 mM phosphoglycerol levels, which ranged from 37 for 1 to 1.2 for 4.
Figure 3.
Effect of DPPG liposome concentration on dissociation rates of 1 – 4 in 0.010 M phosphate buffer at pH 7.4 and 37 °C. [Diazeniumdiolate] = 100 μM.
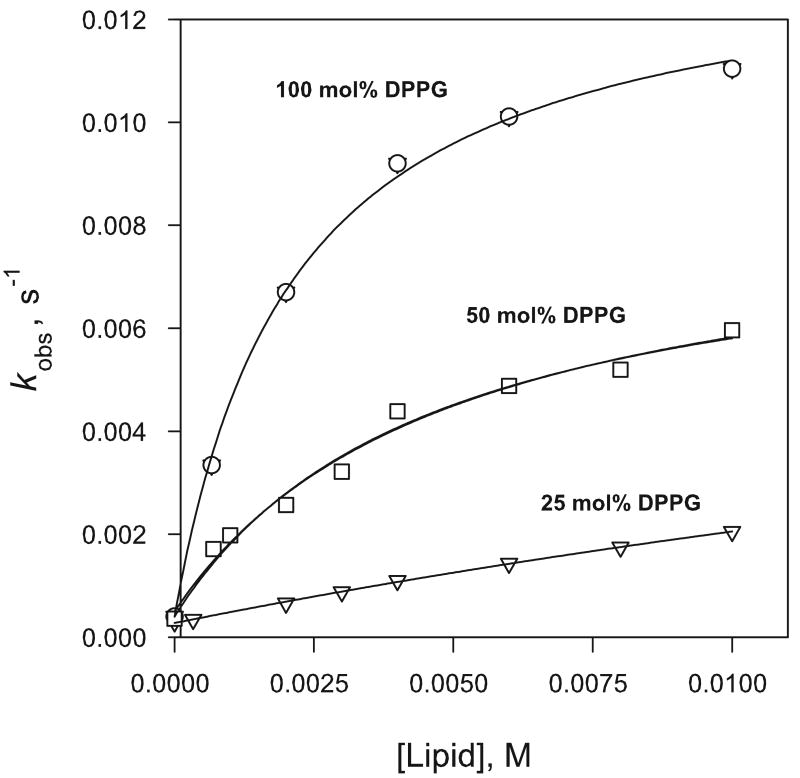
The effect of varying the negative charge density at the liposome interface on the dissociation rate of 1, was examined through rate data obtained in the presence of covesicles prepared from lipid mixtures comprised of DPPG and the charge-neutral phosphatidylcholine DPPC in which the DPPG/DPPC mole ratio was varied at constant total amphiphile ([DPPC] + [DPPG]) concentration. The dissociation rate increased with increase in the anionic phosphoglycerol present in the covesicles and the larger number of anionic binding sites at the liposome surface. Catalysis observed in 100 % DPPG, 50/50 mol % DPPG/DPPC and 25/75 mol % DPPG/DPPC is compared in Figure 4.
Figure 4.
Effect of mixed DPPC/DPPG liposome composition on dissociation rate of 0.10 mM 1 in 0.010 M phosphate buffer at pH 7.4 and 37 °C.
To explore the effect of the anionic head group on substrate binding, rate data was obtained for 1 in 0.010 M phosphate buffer in the presence of liposomes prepared from the phosphatidylserine, DMPS, that has an amino acid zwitterion replacing the terminal glycerol of DPPG, and from the phosphatidic acid salt DOPA, that derives its negative charge from a terminal phosphate rather than from a bridging phosphate in the glycerophosphate acyl chain. Like DPPG, both had a marked catalytic effect on the dissociation rate of 1. The kobs-[lipid] rate profiles obtained in the presence of DMPS liposomes showed rate acceleration to be slightly less than with DPPG (data not shown) whereas significantly greater catalysis was found with DOPA whose rate profiles exhibited rate saturation at much lower lipid levels than was apparent with the other anionic liposomes.
Effect of Salt Concentration on Reactivity in Anionic Liposome Media
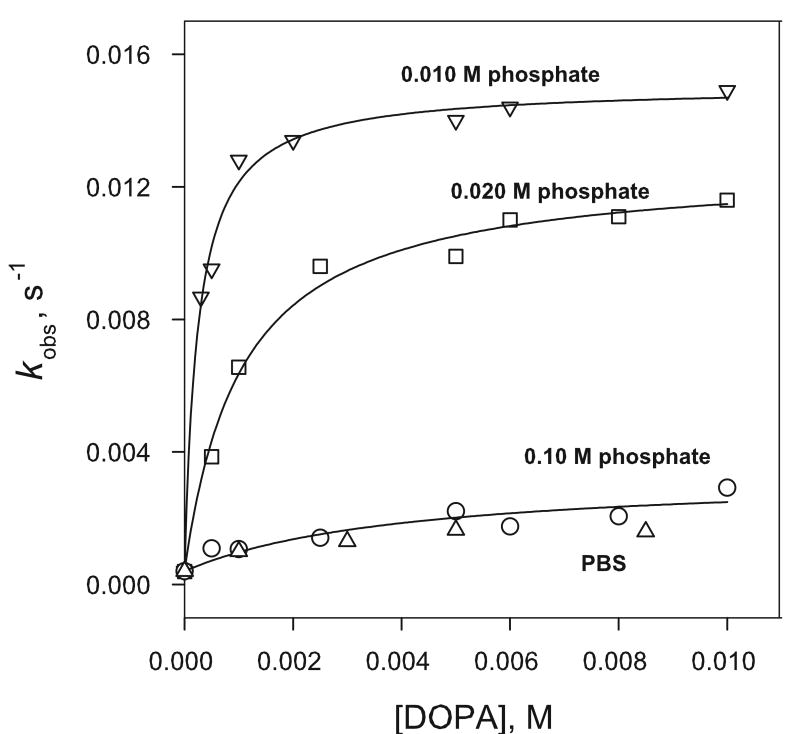
Our initial findings strongly suggested that the catalysis of diazeniumdiolate dissociation rates in anionic liposome media is dependent on substrate binding through electrostatic interactions with the negatively charged liposome surface. For charged liposome systems, the magnitude of the liposome surface potential is expected to decrease with increase in the bulk concentration of electrolytes, and any catalytic effects arising from substrate binding would be expected to be less marked in solutions with higher salt concentrations. Such an effect has been demonstrated in rate data obtained for 1 in phosphate buffered DOPA solutions by employing different concentrations of phosphate buffer to maintain the pH at 7.4 Rate profiles obtained in DOPA solutions with 0.010 M, 0.020 M, 0.10 M total phosphate and phosphate buffered saline (PBS) containing 0.01 M phosphate, 137 mM NaCl and 2.7 mM KCl are compared in Figure 6. Measured dissociation rates decreased with increase in the buffer concentration at each liposome concentration examined. Similar trends were observed for the catalysis of 1 by other anionic liposomes (data not shown), where the catalysis was also strongly reduced at the higher salt concentrations.
Figure 6.
Effect of phosphate buffer and salt concentration on the dissociation rate of 1 in DOPA liposome media at pH 7.4 and 37 °C. PBS (Δ) has 1.47 mM KH2PO4, 8.10 mM Na2HPO4, 2.7 mM KCl and 137 mM NaCl. [1] = 100 μM.
Reaction in unsaturated DOPG and POPS media
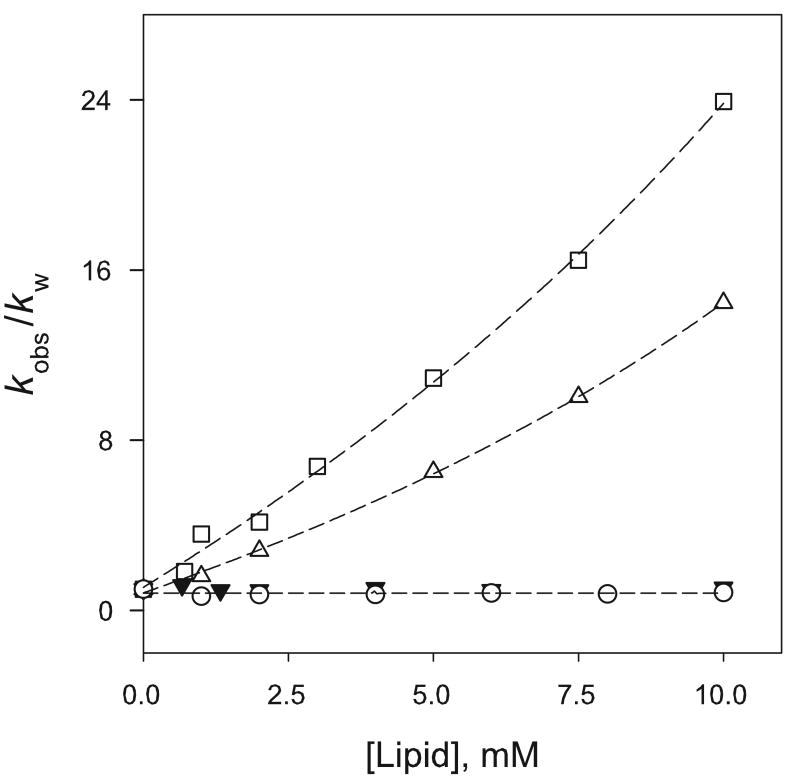
To probe possible differences in behavior that might arise from the physical properties of unsaturated lipids, rate data was obtained in the presence of liposomes prepared from the unsaturated lipids 1,2-dioleoyl phosphoglycerol, DOPG, and the phosphatidylserine, POPS. The rate profiles obtained with 1, in the presence of both DOPG and 50/50 DOPG/DPPC covesicles (data not shown), closely resembled those observed with DPPG and corresponding DPPG/DPPC mixtures and there were no apparent changes in reaction rate arising from the increased fluidity of the unsaturated bilayer. When a comparison was made of the effect of unsaturated DOPG and POPS liposomes, on the dissociation rate of the more hydrophobic dibutyl-substrate, 3, although both effectively catalyzed NO dissociation, their dissociation rates at the higher lipid concentrations employed did not approach the rate saturation typically observed with other anionic lipids. In Figure 5, the effect of POPS and DOPG liposomes on the dissociation rates of 3 is compared with that of the cationic DOTAP and phosphatidylcholine DMPC liposome media.
Figure 5.
Comparison of the effect of DOPG (□), POPS (Δ), DMPC (○) and DOTAP (▼) liposomes on the dissociation rate of 3 in 0.010 M phosphate or 0.050 M Tris-HCl buffer (for DOTAP) at pH 7.4 and 37 °C. [3] = 100 μM
Pseudo-phase kinetic model
Quantitative analysis of the catalysis mediated by anionic liposomes has been obtained through a general pseudophase kinetic model (eq 2), widely used for reactions catalyzed by surfactant micelles and synthetic vesicles, in which the diazeniumdiolate substrate (S) is partitioned between the aqueous and liposome pseudophase
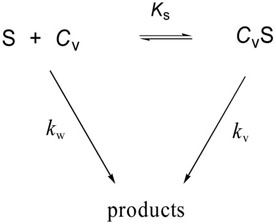 |
while reacting simultaneously in both [18]. For such a two-state model, individual values of kv and Ks are obtained by a non-linear regression fit of kobs-[CV] data to eq 2, where kv
| (2) |
is the first-order rate constant for reaction in the liposome phase, Ks is the association constant for substrate binding and CV is the concentration of the sonicated lipid. The value of kw, the pseudo-first order rate constant for reaction in the bulk aqueous phase, is taken to be that measured in pH 7.4 phosphate buffered solutions in the absence of lipid. The model provides a relative measure of the binding of diazeniumdiolate substrates at different vesicle interfaces although it is limited in some instances by our inability to follow reactions to rate saturation conditions due to the relatively low liposome concentrations available. The rate and equilibrium parameters for reactions of 1 – 4, provided by the model, are summarized in Table 1.
Table 1.
Rate and Equilibrium Parameters from Liposome-Mediated Catalysis of Diazeniumdiolate Ions in 0.010 M Phosphate Buffered Solutions at pH 7.4 and 37 °C.
| Substrate | Reaction Medium | kv/kw | KS (M-1) |
|---|---|---|---|
| 1 | DPPG | 37 | 477 ± 46 |
| DPPG/DPPC
50/50 mol% |
21 | 201 ± 54 | |
| DPPG/DPPC
25/75 mol% |
17 | 22 ± 5 | |
| 1 | DOPG | 48 | 406 ± 105 |
| 2 | DPPG | 40 | 136 ± 107 |
| 3 | DPPG | 5.6 | 31 ± 5 |
| 1 | DMPS | 55 | 358 ± 135 |
| DOPA | 38 | 4040 ± 520 | |
| DOPA b | 32 | 947 ± 166 | |
| DOPA c | 8.4 | 245 ± 240 |
0.020 M phosphate.
0.10 M phosphate.
Rate Data in Cationic Liposome Media
Cationic liposomes have found wide application in liposome-mediated gene transfer transfection studies due to their ability to bind anionic DNA and form complexes with high affinity for cell membranes. The liposome formulations most frequently used have contained DOTAP usually mixed with a “helper lipid” such as the phosphatidylethanolamine DOPE to increase the transfection potency. In our study, we looked at reactions of the polar 1, the more hydrophobic 3 and the anionic substrate 4 in the presence of cationic liposomes prepared from DOTAP. The dissociation rates of all three substrates were unaffected by the presence of the cationic liposomes and no significant difference was noted in their behavior when compared to that found in the Tris-buffered solutions alone. Data are summarized in Table 2.
Table 2.
Dependence of First-Order Rate Constants for Dissociation of 1 - 3 on DOTAP Concentration in 0.050 M Tris-HCl Buffer at pH 7.5 and 37.0 °C a
| 1, SPER/NO | 4, DPA/NO | 3, DIBUTYL/NO | |||
|---|---|---|---|---|---|
| [DOTAP] (M) | 104 × kobs (s−1) | [DOTAP] (M) | 102 × kobs(s−1) | [DOTAP] (M) | 103 × kobs (s−1) |
| 0 | 3.50 | 0 | 1.42 | 0 | 2.26 |
| 0.001 | 3.54 | 0.0002 | 1.37 | 0.001 | 1.51 |
| 0.002 | 3.57 | 0.0004 | 1.40 | 0.002 | 1.67 |
| 0.003 | 3.95 | 0.001 | 1.30 | 0.004 | 1.70 |
| 0.004 | 3.91 | 0.003 | 1.36 | 0.006 | 1.85 |
| 0.006 | 4.23 | 0.005 | 1.30 | 0.008 | 1.74 |
| 0.007 | 4.35 | 0.007 | 1.29 | 0.01 | 1.90 |
| 0.009 | 4.51 | ||||
| 0.01 | 4.23 | ||||
[Diazeniumdiolate] = 100 μM.
Measurement of NO Release from 2 in DPPG/DPPC Liposome Media by Oxyhemoglobin Assay
The stoichiometry of NO release from 1 and 2 in liposome media was determined by the standard oxyhemoglobin assay method using the NO-mediated conversion of oxyhemoglobin to methemoglobin (HbO2 + NO → MetHb + NO3- : k2 = 3.7 × 107 M-1s-1), which is ∼ 26 times faster than its reaction with O2 at saturated oxygen concentrations [19]. Concentrations of NO were calculated from absorbance changes at 401 nm using the literature value of 49 mM-1cm-1 for the difference between the molar extinction coefficient of metHb and HbO2 at that wavelength, Δε401(metHb-oxyHb) [17]. The yields of NO calculated by difference spectral measurements are summarized in Table 3. The mean experimental values for Δ[NO]/Δ[Diazeniumdiolate] determined for 1 and 2 were 1.47 ± 0.23 and 1.69 ± 0.24, respectively, in 0.010M phosphate buffered 50/50 DPPC/DPPG media at 37 °C. The data indicate somewhat lower yields of NO in the liposome media than the 2.0 value expected for diazeniumdiolate dissociation reactions. The low precision in the data may be tied, in part, to the experimental method requiring measurement of absolute absorbance values after blanking background HbO2 and liposome absorbance.
Table 3.
Yields of NO (ENO = mole of NO released / mole of substrate) during reaction of 1 and 2 in 0.010M phosphate buffered 50/50 DPPC/DPPG media at 37 °C. [NO] = ΔAbs401/Δε401 obtained from difference spectra using HbO2 as reference. Δε401 = 49 mM-1cm-1.
| [Lipid]T μM | [1], μM | [NO] Released μM | ENO |
|---|---|---|---|
| 0 | 2.0 | 3.65 | 1.82 |
| 8.0 | 2.0 | 2.63 | 1.32 |
| 20 | 2.0 | 2.53 | 1.27 |
|
| |||
| [Lipid]T μM | [2] μM | [NO] Released μM | ENO |
|
| |||
| 0 | 2.0 | 4.27 | 2.13 |
| 8.0 | 2.0 | 3.14 | 1.57 |
| 20 | 2.0 | 3.55 | 1.78 |
| 100 | 2.0 | 2.41 | 1.20 |
| 500 | 2.0 | 3.51 | 1.76 |
Liposome Size Analysis by Photon Correlation Spectroscopy and Atomic Force Microscopy Imaging
Size analysis of the liposomes prepared in the study, as determined by photon correlation spectroscopy (PCS) immediately after preparation, revealed a mean vesicle diameter of 43.6 ± 0.29, 30.6 ± 0.69, 56.3 ± 0.31, and 38.8 ± 0.20 nm for DPPC, DPPG, DOPG and DOTAP vesicles, respectively. Some measurements of vesicle size were initiated after adding diazeniumdiolate substrate and allowing its dissociation reaction to go to completion. These showed similar values, indicating that the vesicle size did not significantly change during the course of the reaction. Data are summarized in Table 4. The vesicle sizes reported here are typical of those reported for small unilamellar liposomes prepared by bath sonication [20].
Table 4.
Mean diameters of liposomes determined by dynamic light scattering at 37°C.
| Liposome | Mean Diameter (nm) a |
|---|---|
| DPPC b | 43.6 ± 0.29 |
| DPPG b | 30.6 ± 0.69 |
| DOPC b | 56.3 ± 0.31 |
| DOTAP c | 38.8 ± 0.20 |
| DOTAP c,d | 39.1 ± 0.21 |
Mean values of three repeat measurements.
0.010 M phosphate buffer.
0.050 M Tris.HCl buffer.
Measurement initiated after completion of reaction.
Discussion
We have shown that the acid-catalyzed dissociation of nitric oxide from diazeniumdiolate ions is subject to catalysis by small unilamellar vesicles prepared from anionic lipids with the catalytic activity arising from an increase in the local concentration of the diazeniumdiolate substrates and hydrogen ions at the negatively charged liposome interface. Substrate binding occurs through electrostatic interaction of the positively charged nitrogen centers in the diazeniumdiolate substrates with the negatively charged vesicle surface, as indicated by the good correlation that exists between the values of the binding constants obtained for 1-3 in DPPG media and the positive polarity of the individual diazeniumdiolate substrates that have three, two and one cationic nitrogen centers, respectively. A predominantly ionic association is further supported by the small rate acceleration (< 2-fold at 10 mM DPPG) that was found with the anionic substrate, 4, that lacks any cationic nitrogen centers beyond that carrying the -N2O2- group and by the reduced catalysis that is observed on decreasing the anionic lipid content of mixed DPPC/DPPG covesicles. Although KS values may reflect some additional hydrophobic contributions to liposome binding, they appear to be minor for the substrates involved.
Binding constants for 1 in DPPG and DOPG liposome media are of comparable magnitude and appear to reflect only the electrostatic interaction generated by the monoanionic surface charge of the lipids involved. The slightly lower binding constant obtained for 1 in DMPS media could be due to steric factors associated with the phosphatidylserine head group or repulsion of the cationic nitrogen sites in 1 by the protonated serine amino group at the surface. pKa values of the functional groups in the phosphatidylserine head group indicate an overall -1 charge for DMPS at physiological pH similar to DPPG [21, 22].
Since the vesicle bilayer provides both internal and external interfaces as potential reaction sites, several outcomes are possible for reactions in vesicle-containing solutions depending on reagent distribution between the different binding sites [23]. A kinetic distinction between endovesicular and exovesicular reactions can result from differences in reactivity at the inner and outer surfaces and by rate limiting permeation of the reactants through the membrane. Intrinsic differences in binding and reactivity at the inner and outer interfaces have been shown to arise from differences in the reaction media at the two locations and from the effect of vesicle size and vesicle curvature on the pKa of both the lipid head groups and the bound substrate [24, 25]. For the acid-catalyzed dissociation reactions in our study, the significant positive charges carried by the zwitterionic substrates at pH 7.4, would be expected to inhibit transmembrane diffusion and we found no evidence of biphasic kinetic behavior which generally characterizes transmembrane-limiting rate processes or modulation of chemical reactivity by internal surfaces of vesicular membranes.
An interesting possibility (suggested by reviewer 2) is nitrosamine formation during our liposome-catalyzed reactions due to the high solubility of our reaction products, NO and the free amine, in the lipid bilayer following substrate dissociation at the liposome-solution interface. Rate enhancements up to 800-fold have been reported for the nitrosation of dialkylamines by nitrite at pH 3.5 in the presence of cationic and neutral micelles [26], where reaction is mediated by N2O3. We observed no spectral evidence for nitrosamine formation at pH 7.4 and no significant differences were noted in UV spectral scans (λ 200-400 nm) obtained for diazeniumdiolate dissociation reactions in liposome media and aqueous buffer.
In contrast to the behavior of 1, the more hydrophobic substrate 3 showed rate profiles in unsaturated DOPG and POPS media that suggested rate acceleration rather than rate saturation at the higher lipid concentrations employed. Aggregation of unsaturated POPS and DOPG liposomes, induced by interaction of the hydrophobic hexane-dibutyl structure of 3 with the unsaturated lipid bilayer, resulting in enhanced binding and catalysis by the larger phospholipid aggregates formed, may be responsible, at least in part, for the kinetic behavior displayed. Aggregation and fusion of phosphatidylserine and phosphatidylglycerol liposomes, induced by a variety of reagents, has been previously reported [27-29].
Although phosphatidylcholine liposomes derived from DPPC have been shown to effect weak catalysis of diazeniumdiolate dissociation rates [4], the hydrophobic substrate 3 was found to be unaffected by the smaller DMPC liposomes. There is possibly insufficient flexibility in the phosphatidylcholine head group of the smaller DMPC lipid to permit significant ionic interaction between the phosphate negative charge and cationic diazeniumdiolate centers. The mild catalysis found with DPPC may also result from slightly stronger hydrophobic association between 3 and the choline methyl groups at the surface.
The larger KS value obtained with liposomes prepared from the phosphatidic acid salt, DOPA is consistent with the stronger electrostatic interaction expected from the higher negative charge of the DOPA head group at pH 7.4. The pKa values reported for the first and second dissociations of the terminal phosphate in the phosphatidic acid salt predict a net charge of ∼1.7 for DOPA at pH 7.4 [24, 30] compared to the -1 charge of the phosphate in DPPG (pKa = ∼1.1).
The sensitivity displayed by the measured binding constants in anionic liposome media, to the concentration of electrolytes in the bulk aqueous solution, is consistent with changes in the reduction in the liposome surface charge density resulting from the effect of added salts on the diffuse double layer, as predicted by Guoy-Chapman-Stern models [31]. Although at typical ionic salt levels encountered near most cell membranes, substrate binding and its effect on NO release rates is expected to be small, at lipid bilayers encountered in certain “hydrophobic” protein or lipid environments more significant changes in diazeniumdiolate rate behavior may be possible.
Cationic DOTAP vesicles did not influence the acid-catalyzed dissociation rates of any of the diazeniumdiolate substrates examined consistent with reaction being confined to the aqueous phase due to the repulsion of H+aq ions from the cationic surface. The absence of any rate inhibition in the presence of DOTAP vesicles further suggests that electrostatic attraction between the cationic surface and the negative charge of the diazeniumdiolate functional group, that would partition the diazeniumdiolate out of the aqueous phase, is outweighed by the repulsion of the positive centers by the cationic interface.
The limited oxyhemoglobin assay study of nitric oxide released in liposome media has found yields of NO lower than the expected 2.0 mol of NO/mol of substrate. Although the lower amounts of NO detected could be due to the partial release or formation of alternative nitrogen oxide species such as NO- or NO2, nitroxyl formation would be unlikely for the secondary amine-derived substrates involved and significant oxidation would be unexpected at the concentration levels employed. Keynes et al have reported that NO delivered via a diazeniumdiolate was consumed by reaction with ingredients, Hepes buffer and riboflavin, present in a tissue-culture medium [32]. A mechanism involving formation of superoxide radical (O2 ▪-) through oxidation of Hepes followed by the rapid reaction of superoxide with NO to form peroxynitrite was proposed. It was suggested that riboflavin (in the light) provided an additional source of superoxide. Since both Hepes and riboflavin have hydroxyl functional groups similar to those present in the glycerol moiety of DPPG, a similar reaction may be responsible for the low NO yields we have found in DPPG media. It should be mentioned that, although not considered by the previous authors, an additional quantity of NO may be lost if these strongly oxidizing conditions result in partial oxidation of the hydroxyls themselves to form small amounts of aldehyde- and/or ketone-containing impurities. Reaction of NO with carbanions formed by abstraction of the acidic protons alpha to carbonyl groups (the Traube reaction) has been known for more than a century [33].
Previous experimental and theoretical studies have shown that diazeniumdiolate dissociation reactions are acid-catalyzed with equilibrium protonation of the N(1) amine nitrogen triggering the decomposition of the [N(O)NO]- functional group [34, 35]. Although there is strong evidence that the enhanced NO dissociation rates in anionic liposome media are due to the concentration of the reactants, H+(aq) ions and substrate, at the vesicle's aqueous interface, we have explored the possibility that the catalysis of NO release in liposome media may also be due, at least in part, to an increase in the intrinsic diazeniumdiolate dissociation rate constant in the polarizing environment of the negatively charged vesicle interface. This has been achieved through application of the pseudo-phase ion exchange (PIE) model, previously described [3, 36], that compares the intrinsic second-order rate constant, k2w (= kw/[H+]w) in the aqueous phase with that estimated for the vesicle-bound substrate, k2v (= kv/[H+]v), where [H+]w and [H+]v are the local molarities of hydrogen ions expressed with respect to the volumes of the aqueous and vesicle pseudophase, respectively. In examining data obtained for the reaction of 1 in DPPG liposome media, we used V = 0.78 M-1 for the effective volume of the DPPG pseudo-phase, calculated by multiplying the lipid partial specific volume by its molecular weight [37] and the literature values, KH/Na = 1, for the equilibrium constant for exchange between bound Na+ counter ions and H+ ions in the interfacial region, β = 0.78 for the fraction of the vesicle surface covered by Na+ ions [25, 38]. [Na+]v/[Na+]w was estimated assuming [Na+]v = β [DPPG] and [Na+]w = (1 - β) [DPPG] + [Na+]buffer. When data for 1 in DPPG media was applied to the PIE model, best fit values for k2v yielded k2v/ k2w = 0.64. Since estimated k2v values that are 2-3 fold smaller than k2w has been a common finding for many micellar- and vesicle-catalyzed reactions [18], the relatively close agreement between k2v and k2w in this study does not support a significant change in the intrinsic dissociation rate constant in the polarizing microenvironment of the liposome interface.
In summary, the information obtained in this study adds to our understanding of local environmental factors influencing diazeniumdiolate reactivity, particularly those existing near negatively charged aqueous interfaces of phospholipid bilayer membranes. Such membranes are important components of bacterial cell membranes and of pulmonary surfactants. Of significance to the application of diazeniumdiolates as NO sources in vivo is the finding that the strongest binding and greatest catalysis of NO release occurs with polyamine-derived zwitterionic substrates having multiple protonated nitrogen sites. Brilli et al have shown that the positive polarity of cationic tertiary amine sites in diazeniumdiolate can be utilized to restrict the movement of diazeniumdiolates across transmucosal membranes, thereby enabling them to be more selective pulmonary vasodilators by inhibiting systemic hypertension [6,8]. Previous studies involving inhaled NO gas for the treatment of acute lung injury in both animals and children have also found a therapeutic synergy when NO exposure was combined with replacement surfactant therapy [11] and there is interest in the effectiveness of soluble NO donors when co-administered with surfactant. To the extent that the present study provides fundamental information on the interaction of diazeniumdiolates with anionic lipid interfaces prevalent in lung surfactant, particularly aqueous interfaces of phosphatidylcholine, phosphatidylglycerol and phosphatidylserine bilayers which are involved in the monolayer-to-multilayer transitions that are important to lung surfactant function, information obtained may be of relevance to the use of diazeniumdiolates as pulmonary vasodilators in vivo.
Figure 1.
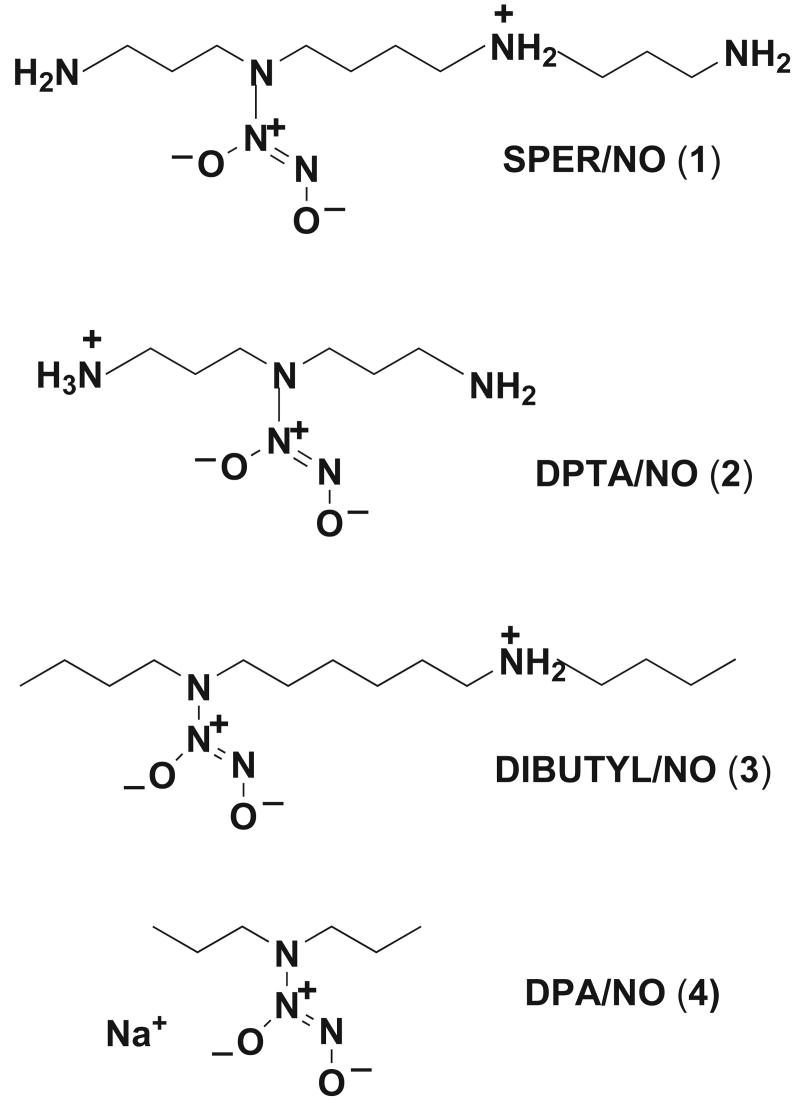
Diazeniumdiolates, R1R2N[N(O)NO]-, employed in the study. [1, SPER/NO: R1 = H2N(CH2)3-, R2 = H2N(CH2)3NH(CH2)4-; 2, DPTA/NO: R1 = R2 = H2N(CH2)3-; 3, DIBUTYL/NO: R1 = n-butyl-, R2 = n-butyl-NH2+(CH2)6-; 4, DPA/NO: R1 = R2 = n Pr-]
Figure 2.
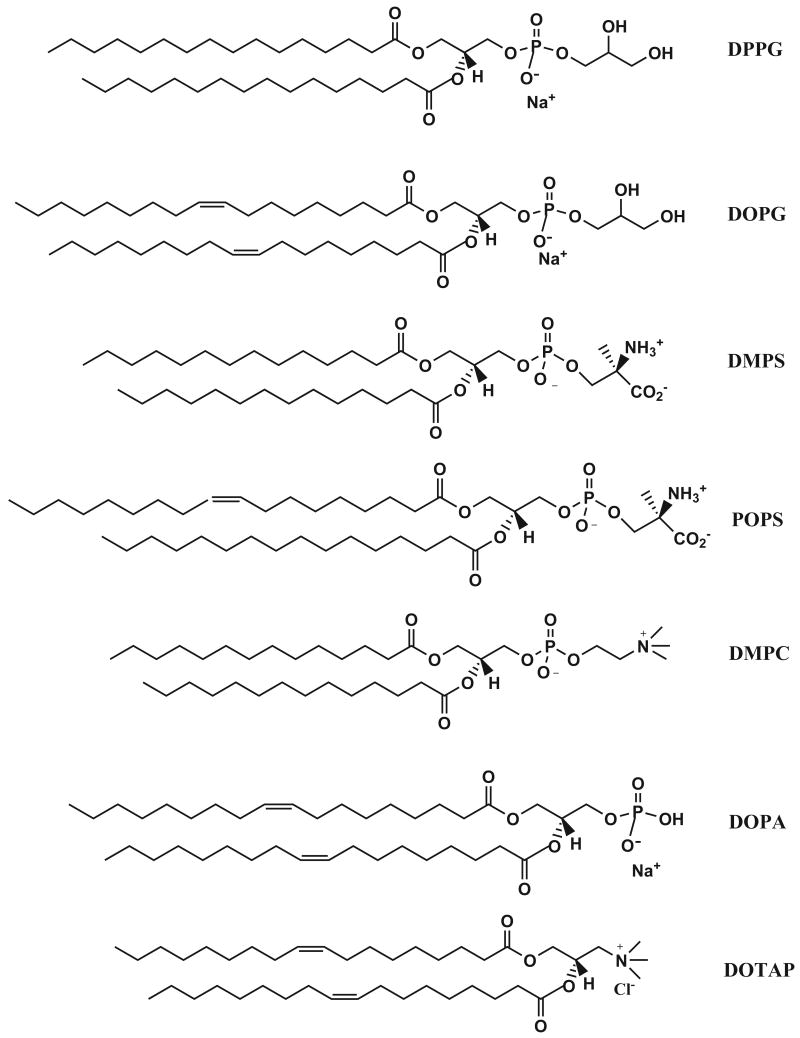
Lipids employed in the study. DPPG = 1,2-dipalmitoyl-sn-glycero-3-[phospho-rac-(1-glycerol)], DOPG = 1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)], DMPS = 1,2-diacyl-sn-glycero-3-[phospho-L-serine], POPS= 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-L-serine], DOPA = 1,2-dioleoyl-sn-glycero-3-phosphate; DPPC = 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, DMPC = 1,2-dimyristoyl-sn-glycero-3-phosphocholine, DOTAP = 1,2-dioleoyl-3-trimethylammonium-propane.
Acknowledgments
Support of this work by the National Institutes of Health (Grant number R15-HL078750-01) and by the Jeffress Foundation is gratefully acknowledged. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health under Contract No. NO1-CO-12400. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keefer LK, Nims RW, Davies KM, Wink DA. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: Convenient nitric oxide dosage forms. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 2.Keefer LK. Progress towards clinical applications of the nitric oxide-releasing diazeniumdiolates. Annu Rev Pharmacol Toxicol. 2003;43:587–607. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- 3.Price SE, Jappar D, Lorenzo P, Saavedra JE, Hrabie JA, Davies KM. Micellar catalysis of nitric oxide dissociation from diazeniumdiolates. Langmuir. 2003;19:2096–2102. [Google Scholar]
- 4.Dinh BT, Dove K, Jappar D, Hrabie JA, Davies KM. Effect of hydrophobic structure on the catalysis of nitric oxide release from zwitterionic diazeniumdiolates in surfactant and liposome media. Nitric Oxide. 2005;13:204–209. doi: 10.1016/j.niox.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Batchelor MM, Reoma SL, Fleser PS, Paul S, Nuthakki VK, Callahan RE, Shanley CJ, Politis JK, Elmore J, Mertz SI, Meyerhoff ME. More lipophilic dialkylamine-based diazeniumdiolates: Synthesis, characterization, and application in preparing thromboresistant nitric oxide release polymeric materials. J Med Chem. 2003;46:5153–5161. doi: 10.1021/jm030286t. [DOI] [PubMed] [Google Scholar]
- 6.Brilli R, Krafte-Jacobs B, Smith DJ, Roselle D, Passerini D, Vromen A, Moore L, Szabo C, Salzman A. Intratracheal instillation of a novel NO/nucleophile selectively reduces pulmonary hypertension. J Appl Physiol. 1997;83:1968–1975. doi: 10.1152/jappl.1997.83.6.1968. [DOI] [PubMed] [Google Scholar]
- 7.Brilli RJ, Krafte-Jacobs B, Smith DJ, Passerini D, Moore L, Ballard ET. Aerosolization of novel nitric oxide donors selectively reduces pulmonary hypertension. Crit Care Med. 1998;26:1390–1396. doi: 10.1097/00003246-199808000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs B, Brilli RJ, Moore L, Ballard E, Smith D. Areosolized soluble nitric oxide donor improves oxygenation and pulmonary hypertension in acute lung injury. Am J Crit Care Med. 1998;158:1536–1542. doi: 10.1164/ajrccm.158.5.9802114. [DOI] [PubMed] [Google Scholar]
- 9.Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Eng J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 10.Schreber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Eng J Med. 2003;349:2099–2107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs BR, Smith DJ, Zingarelli B, Passerini DJ, Ballard ET, Brilli RJ. Soluble nitric oxide donor and surfactant improve oxygenation and pulmonary hypertension in porcine lung injury. Nitric Oxide. 2000;4:412–422. doi: 10.1006/niox.2000.0292. [DOI] [PubMed] [Google Scholar]
- 12.Lam CF, Van Heerden PV, Sviri S, Roberts BL, Ilett KF. The effects of inhalation of a novel nitric oxide donor, DETA/NO, in a patient with severe hypoxaemia due to acute respiratory distress syndrome. Anaesth Intensive Care. 2002;30:472–476. doi: 10.1177/0310057X0203000413. [DOI] [PubMed] [Google Scholar]
- 13.Notter RH. Basic Science and Clinical Applications. Vol. 149 Marcel Dekker; New York: 2000. Lung Surfactants. [Google Scholar]
- 14.Hrabie JA, Klose JR, Wink DA, Keefer LK. New nitric oxide-releasing zwitterions derived from polyamines. J Org Chem. 1993;58:1472–1476. [Google Scholar]
- 15.Drago RS, Karstetter BR. The Reaction of Nitrogen(II) Oxide with Various Primary and Secondary Amines. J Am Chem Soc. 1961;83:1819–1822. [Google Scholar]
- 16.New RC. Liposomes: A Practical Approach. Oxford University Press; New York: 1990. pp. 33–16. [Google Scholar]
- 17.Feelisch M, Kubitzek D, Werringloer J. The oxyhemoglobin assay. In: Feelisch M, Stamler JS, editors. Methods in Nitric Oxide Research. John Wiley; 1996. pp. 455–478. [Google Scholar]
- 18.Bunton CA, Nome F, Quina FH, Romsted LS. Ionic binding and reactivity at charged aqueous interfaces. Acc Chem Res. 1991;24:357–364. [Google Scholar]
- 19.Doyle MP, Hoekstra JW. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 20.Jones MN, Chapman D. Micelles, Monolayers and Biomembranes. Wiley-Liss; 1995. pp. 117–121. [Google Scholar]
- 21.Tsui FC, Ojcius DM, Hubbell WL. The intrinsic pKa values for phosphatidylserine and phosphatidylethanolamine in phosphatidylcholine host bilayers. Biophys J. 1986;49:459–468. doi: 10.1016/S0006-3495(86)83655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapovalov ME, Mohwald VL, Brezesinski G. Ionization state and structure of 1,2-dipalmitoylphosphatidylglycerol monolayers at the liquid/air interface. J Phys Chem B. 2006;110:919–26. doi: 10.1021/jp0555697. [DOI] [PubMed] [Google Scholar]
- 23.Chaimovich H, Cuccovia IM. Quantitative analysis of reagent distribution and reaction rates in vesicles. Progr Colloid Polym Sci. 1997;103:67–77. [Google Scholar]
- 24.Swairjo MA, Seaton BA, Roberts MF. Effect of vesicle composition and curvature on the dissociation of phosphatidic acid in small unilamellar vesicles - a 31P-NMR study. Biochim Biophys Acta. 1994;1191:354–61. doi: 10.1016/0005-2736(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 25.Kawamuro MK, Chaimovich H, Abuin EB, Lissi EA, Cuccovia IM. Evidence that the effects of synthetic amphiphile vesicles on reaction rates depend on vesicle size. J Phys Chem. 1991;95:1458–1463. [Google Scholar]
- 26.Okun JD, Archer MC. Kinetics of nitrosamine formation in the presence of micelle-forming surfactants. J Natl Cancer Inst. 1977;58:409–411. doi: 10.1093/jnci/58.2.409. [DOI] [PubMed] [Google Scholar]
- 27.Walter A, Siegel D. Divalent cation-induced lipid mixing between phosphatidylserine liposomes studied by stopped-flow fluorescence measurements: Effects of temperature, comparison of barium and calcium, and perturbation by DPX. Biochemistry. 1993;32:3271–81. doi: 10.1021/bi00064a009. [DOI] [PubMed] [Google Scholar]
- 28.Ohki S, Duzgunes N, Leonards K. Phospholipid vesicle aggregation: Effect of monovalent and divalent ions. Biochemistry. 1982;21:2127–33. doi: 10.1021/bi00538a022. [DOI] [PubMed] [Google Scholar]
- 29.Lee G, Pollard HB. Highly sensitive and stable phosphatidylserine liposome aggregation assay for annexins. Anal Biochem. 1997;252:160–164. doi: 10.1006/abio.1997.2311. [DOI] [PubMed] [Google Scholar]
- 30.Moncelli MR, Becucci L. The intrinsic pKa values for phosphatidic acid in monolayers deposited on mercury electrodes. J Electroanal Chem. 1995;385:183–189. [Google Scholar]
- 31.McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- 32.Keynes RG, Griffiths C, Garthwaite J. Superoxide-dependent consumption of nitric oxide in biological media may confound in vitro experiments. Biochem J. 2003;369:399–406. doi: 10.1042/BJ20020933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hrabie JA, Keefer LK. Chemistry of the Nitric Oxide-Releasing Diazeniumdiolate (“Nitrosohydroxylamine”) Functional Group and Its Oxygen-Substituted Derivatives. Chem Rev. 2002;102:1135–1154. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 34.Davies KM, Wink DA, Saavedra JE, Keefer LK. Chemistry of Diazeniumdiolates. 2. Kinetics and mechanisms of dissociation to nitric oxide in a aqueous solution. J Am Chem Soc. 2001;123:5473–5481. doi: 10.1021/ja002899q. [DOI] [PubMed] [Google Scholar]
- 35.Dutton AS, Fukuto JM, Houk KN. The mechanism of NO formation from decomposition of dialkylamino diazeniumdiolates: Density functional theory and CBS-QB3 predictions. Inorg Chem. 2004;43:1039–1045. doi: 10.1021/ic0349609. [DOI] [PubMed] [Google Scholar]
- 36.Quina FH, Chaimovich H. Ion exchange in micellar solutions. 1. Conceptual framework for ion exchange in micellar solution. J Phys Chem. 1979;83:1844–1850. [Google Scholar]
- 37.Newman GC, Huang CH. Structural studies on phosphatidylcholine-cholesterol mixed vesicles. Biochemistry. 1975;14:3363–3370. doi: 10.1021/bi00686a012. [DOI] [PubMed] [Google Scholar]
- 38.Cuccovia IM, Feitosa E, Chaimovich H, Sepulveda L, Reed W. Size, electrophoretic mobility, and ion dissociation of vesicles prepared with synthetic amphiphiles. J Phys Chem. 1990;94:3722–3725. [Google Scholar]