Abstract
Background & Aims
The functional involvement of the endocannabinoid system in modulation of pancreatic inflammation, such as acute pancreatitis, has not been studied to date. Moreover, the therapeutic potential of cannabinoids in pancreatitis has not been addressed.
Methods
We quantified endocannabinoid levels and expression of cannabinoid receptors 1 and 2 (CB1 and CB2) in pancreas biopsies from patients and mice with acute pancreatitis. Functional studies were performed in mice using pharmacological interventions. Histological examination, serological, and molecular analyses (lipase, myeloperoxidase, cytokines, and chemokines) were performed to assess disease pathology and inflammation. Pain resulting from pancreatitis was studied as abdominal hypersensitivity to punctate von Frey stimuli. Behavioral analyses in the open-field, light-dark, and catalepsy tests were performed to judge cannabinoid-induced central side effects.
Results
Patients with acute pancreatitis showed an up-regulation of cannabinoid receptors and elevated levels of endocannabinoids in the pancreas. HU210, a synthetic agonist at CB1 and CB2, abolished abdominal pain associated with pancreatitis and also reduced inflammation and decreased tissue pathology in mice without producing central, adverse effects. Antagonists at CB1- and CB2-receptors were effective in reversing HU210-induced antinociception, whereas a combination of CB1- and CB2-antagonists was required to block the anti-inflammatory effects of HU210 in pancreatitis.
Conclusions
In humans, acute pancreatitis is associated with up-regulation of ligands as well as receptors of the endocannabinoid system in the pancreas. Furthermore, our results suggest a therapeutic potential for cannabinoids in abolishing pain associated with acute pancreatitis and in partially reducing inflammation and disease pathology in the absence of adverse side effects.
Acute pancreatitis (AP) is a potentially lethal disorder involving inflammation, cell death, and complex neuroimmune interactions. Pain management in acute pancreatitis represents a major clinical challenge and influences the clinical outcome of the disease.1,2 Understanding pathophysiological mechanisms underlying morbidity and pain associated with pancreatitis is therefore a prerequisite toward the design of novel therapeutic approaches. Only very recently, aided by the establishment of disease models, focus has been placed on understanding nociceptive mechanisms in pancreatitis.1–5 For example, abdominal hyperalgesia observed upon cerulein-induced murine pancreatitis was recently found to closely represent the pain syndrome seen in human disease.3–5 Involvement of peripheral nociceptive neurons5 as well as central neurons in the spinal cord6 and rostroventral medulla3 has been demonstrated in pain associated with pancreatitis. In addition to mediating and modulating pancreatic pain, neural innervation of the pancreas plays an important role in edema, inflammatory responses, and vascular processes in pancreatitis and significantly affects disease initiation and progression.6
Recently, the endocannabinoid system has been identified as a major regulator of physiological and pathological processes, such as pain, inflammation, cell growth, cell death, and as a regulator of diverse gastrointestinal functions, such as intestinal motility and secretion.7 Although cannabinoid-induced analgesia was initially primarily attributed to the activation of cannabinoid receptor 1 (CB1) in the nervous system, later studies demonstrated a contribution of cannabinoid receptor 2 (CB2), localized peripherally on immune cells as well as in the nervous system.7–9 A complex interplay between endogenously released cannabinoids, such as anandamide or 2-arachidonoylglycerol, and their receptors both on inflammatory cells and neurons is involved in modulation of inflammatory pain.7 However, the significance of the endocannabinoid system and of exogenously applied cannabinoids has not been adequately addressed in the context of visceral inflammatory pain. In this article, we demonstrate the in vivo significance and therapeutic potential of cannabinoids in inflammation and pain associated with pancreatitis using human specimens and mouse models as test systems.
Materials and Methods
Patients and Tissue Sampling
Tissue samples were collected from patients following pancreatic resections for acute pancreatitis (n = 19). Normal pancreatic tissue samples were obtained through an organ donor procurement program whenever there was no suitable recipient for pancreas transplantation (n = 10). Pancreatic tissues were immediately snap frozen at −80°C or formalin fixed and paraffin embedded. Local ethical committees approved the use of human tissue for the analysis (University of Heidelberg, Germany and the University of Berne, Switzerland), and written informed consent was obtained.
Measurement of Endocannabinoids
For endocannabinoid measurements, pancreatic tissue specimens from acute pancreatitis were dissected, and necrotic parts were removed prior to analysis. Levels of anandamide (AEA), 1-arachidonoylglycerol (1-AG), and 2-arachidonoylglycerol (2-AG) in frozen human pancreas samples were determined by liquid chromatography/mass spectrometry as described previously.10
Cerulein-Model for Pancreatitis and Pharmacological Treatment
All animal procedures were performed according to local ethical guidelines. Cerulein (50 μg/kg/injection in saline (Takus, Pharmacia, Karlsruhe, Germany) or saline (control) was administered intraperitoneally 10 times at hourly intervals to age- and gender-matched C57BL/6J mice. The synthetic cannabinoid, HU210 (0.05 mg/kg in saline), the CB1-specific antagonist (AM251; 3 mg/kg intraperitoneally), and the CB2-specific antagonist (AM630; 1 mg/kg) (all from Tocris Bioscience, Ellisville, MD) were administered subcutaneously11 either alone or in combinations (information to follow) at 30 minutes before and 4 hours after the first injection of cerulein or saline. AM251 and AM630 were dissolved in one drop of Tween-20 (Roth, Karlsruhe, Germany) in 3 mL 2.5% di-methylsulfoxide in saline,11 and control animals were given the vehicle only. In total, 10 groups of animals were used: (1) intraperitoneal saline (control, n = 8); (2) intraperitoneal saline + subcutaneous HU210 (n = 6); (3) intraperitoneal cerulein (n = 8); (4) intraperitoneal cerulein + subcutaneous HU210 (n = 8); (5) intraperitoneal cerulein + subcutaneous HU210 + subcutaneous AM251 (n = 8); (6) cerulein + subcutaneous HU210 + subcutaneous AM630 (n = 6); (7) cerulein + subcutaneous HU210 + subcutaneous AM251 + subcutaneous AM630 (n = 8); (8) cerulein + subcutaneous AM251 (n = 8); (9) cerulein + subcutaneous AM630 (n = 8); and (10) cerulein + subcutaneous AM251 + subcutaneous AM630 (n = 8).
Behavioral Testing
Behavioral measurements were carried out in awake, unrestrained mice in a blinded manner using standardized methods.12 Frequency of abdominal nocifensive responses (licking of the abdomen, abdominal and/or whole-body withdrawal) to graded punctate abdominal pressure was analyzed using von Frey filaments (0.008g to 0.6g). Withdrawal frequency was calculated as the mean number of withdrawals out of 10 applications of the respective filament at 10-second intervals. As described previously, potential central side effects of HU210 were evaluated in the open-field test,12 light/dark test,12 and catalepsy tests.13
Immunohistochemical Analysis and Immunoblotting
Immunohistochemistry on paraffin sections of human pancreas was performed using rabbit anti-CB1 and anti-CB2 antibodies (Cayman Chemical, Ann Arbor, MI) as described previously.14 Specificity controls included preadsorption of the primary antibody (1 μg) with the corresponding blocking peptide (1 μg) for 1 hour at 37°C. Immunoblotting of normal human pancreas and acute pancreatitis tissue lysates was performed using rabbit anti-CB1 and anti-CB2 antibodies (2.5 μg/mL and 2.0 μg/mL, respectively) ± corresponding blocking peptide preincubated for 1 hour at 37°C,– as described previously.14
Histology Scoring, Serum Lipase, Pancreatic Myeloperoxidase, Interleukin-6, and KC1 Levels
Following behavioral analyses, mice were anesthetized, and mixed arteriovenous blood and pancreata were collected. The wet weight/dry weight ratio of mouse pancreata was determined as described previously.15,16 Whole blood glucose levels were determined using an Accucheckcomfort device (Roche, Mannheim, Germany). Serum lipase levels were measured in the central clinical laboratories (Heidelberg University) according to locally defined guidelines. Histological grading was performed on hematoxylin-eosin–stained paraffin sections (5 μm) by a clinical pathologist unaware of the experimental design using modified criteria, which have been described in detail previously.6 Total histological score (0 –3) was expressed as the sum of edema, neutrophil infiltration, and tissue necrosis scores. Myeloperoxidase (MPO) and interleukin-6 (IL-6) levels were quantified using a mouse MPO-ELISA kit or a mouse IL-6 ELISA kit on tissue homogenates according to the manufacturers’ instructions (HyCult Biotechnology, Uden, the Netherlands or BD Biosciences, San Diego, CA). Total RNA was isolated using an RNeasy mini kit and replacing RLT buffer with Trizol (Qiagen, Hilden, Germany). Quantitative real-time reverse-transcription polymerase chain reaction was performed as described previously.14 Mouse KC1 mRNA was amplified by using the primers sense: 5′-TAG TAG AAG GGT GTT GTG CGA AA-3′and antisense: 5′-CGA GCG AGA CGA GAC CAG GAG-3′.
Statistical Analysis
Results are expressed as mean ± SEM. The Mann–Whitney U test and analysis of variance for random measures followed by Bonferroni’s post hoc test were used to evaluate statistical significance. Significance was established at P < .05.
Results
Regulation of CB1, CB2, and Endocannabinoids in Human Acute Pancreatitis
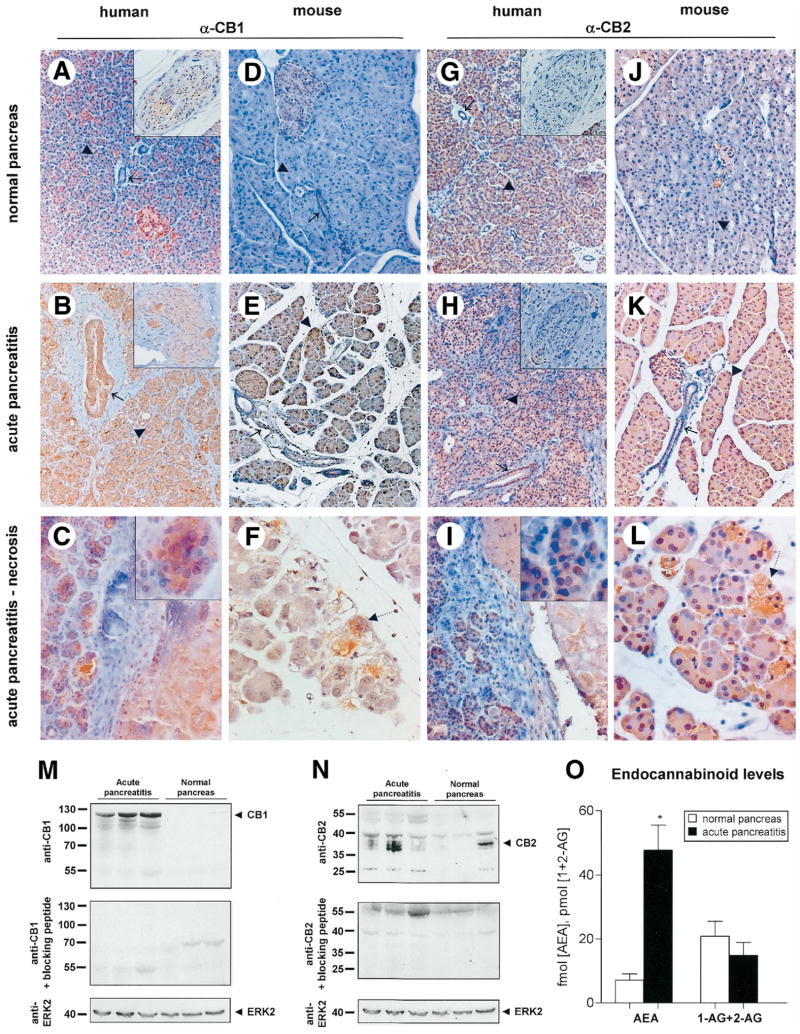
Immunohistochemical analyses of normal human pancreata revealed weak immunoreactivity for CB1 and CB2 in pancreatic acini (arrowheads), nerves (insets), blood vessels, and duct cells (arrows) (Figure 1A and G). Vital pancreatic parenchyma in acute pancreatitis specimens displayed a striking increase in immunostaining of CB1 in ducts and a moderate increase in nerves (insets) and acini as compared to normal pancreas (Figure 1B), whereas acinar and ductal CB2 appeared to be increased to a lesser extent than CB1 (Figure 1H). Acinar cells in the vicinity of severe necrosis in human acute pancreatitis specimens revealed intense cytoplasmic CB1 and CB2 immunoreactivity (Figure 1C and I; magnified in respective insets). Furthermore, acinar cell necrosis in cerulein-induced acute pancreatitis showed a strong immunoreactivity for both CB1 and CB2 (dotted arrows, Figure 1F and L).
Figure 1.
Induction of the endocannabinoid system in acute pancreatitis. Immunohistochemical detection of CB1 and CB2 is shown in human pancreas (A–C and G–I) or mouse pancreas (D–F and J–L) derived either from control subjects (A, D, G, and J) or subjects with acute pancreatitis (B and C, E and F, H and I, K and L). (C, F, I and L) Areas of necrosis within human and mouse acute pancreatitis are indicated by dotted lines. In normal human pancreas (A) CB1-immunoreactivity in acinar cells (▶; magnified in inset C), ducts (→), and nerves (magnified in insets), was weak but strong in acute pancreatitis (B, C). (D–F) Similarly, pancreas demonstrated increased immunoreactivity for CB1 after induction of acute pancreatitis with cerulein (E, F) over normal mouse pancreas (D). Intense CB1 immunostaining is observed on acinar cell necrosis (dotted arrow; F). (G–I) Moderate staining for CB2 is seen in normal human pancreas (G) with a slight increase in acute pancreatitis (H, I) in acinar cells (▶; magnified in inset I), ducts (→), and nerves (magnified in insets). Upon induction of acute pancreatitis in mice, there is a pronounced increase in CB2 immunoreactivity over expression levels in normal mouse pancreas (J–L). This is particularly present within acinar cell necrosis (dotted arrow; L). Original magnification: 40x (A and B, D and E, G and H, J and K) or 80x (F and L; insets in A–C and G–I) objective. (M, N) Immunoblot analysis of pancreas samples derived from acute pancreatitis (lanes 1–3) and control human donors (lanes 4–6) with antibodies recognizing CB1 (M) or CB2 (N). (▶) indicates anti-CB1 (at approximately 128 kDa) and anti-CB2-immunoreactive bands (at 38 and 26 kDa), which were abolished by preadsorption with the respective blocking peptide (lower lanes). Anti-ERK2 was used as an equal loading control. (O) In humans, pancreatic concentrations of the endocannabinoid anandamide (AEA) are higher in acute pancreatitis (black bars) than in normal pancreas (white bars; P < .05), whereas levels of combined 1- plus 2-arachidonoylglycerol (1 - AG + 2 - AG) are unchanged.
To evaluate significance of the cannabinoid system in acute pancreatitis, we used the well-established mouse model of cerulein-induced acute pancreatitis.6 Importantly, following cerulein-induced pancreatitis, the mouse pancreas demonstrated similar changes in CB1 and CB2 immunoreactivity (Figure 1D–F and J–L), as described in the preceding paragraph for the human disease. Following preadsorption with the corresponding blocking peptide, antibodies against CB1 or CB2 failed to show any appreciable staining on human or mouse pancreas (Supplementary Figure 1). (See supplementary figure online at www.gastrojournal.org.)
Western blot analysis on human pancreas using the anti-CB1 antibody revealed a prominent up-regulation of a 125-kDa band (which was abolished in preadsorption controls) in acute pancreatitis (Figure 1M), thereby corroborating our observations from immunohistochemistry experiments. Furthermore, Western blotting using the anti-CB2 antibody confirmed a weak-to-moderate up-regulation of approximately 28 kDa and 36 kDa bands corresponding to CB2 (which were abolished in preadsorption controls) in acute pancreatitis (Figure 1N).
We then addressed whether levels of endocannabinoids are up-regulated in pancreatitis concomitant with changes in expression of cannabinoid receptors. Several endocannabinoids, such as anandamide (AEA), 1- and 2-arachidonoylglycerol (1-AG, 2-AG) were detectable in human pancreas tissues, whereas oleoylethanolamide was not detectable (Figure 1O). Interestingly, anandamide was increased 6.6-fold in biopsy material from patients with acute pancreatitis over normal human pancreas (P = .002). In contrast, levels of 1-AG and 2-AG remained unchanged (Figure 1O; P = .93).
Functional Significance of the Endocannabinoid System in Acute Pancreatitis
To evaluate the potential functional relevance of increased endocannabinoid release and up-regulation of cannabinoid receptors in human disease, we performed experiments with CB1/CB2 antagonists in the cerulein model of acute pancreatitis in mice. Consistent with previous reports,6,17 treatment of mice with cerulein led to a steep increase in serum levels of lipase, tissue MPO, IL-6, and KC1 mRNA expression (Figure 2A and B), pancreatic wet weight/dry weight ratio (Figure 2A), and to characteristic histological changes associated with acute pancreatitis (Figure 1E and K). AM251 and AM630 were used as specific antagonists to block CB1 and CB2, respectively. Neither disease pathology nor serological and inflammatory markers (serum lipase, pancreatic tissue MPO and IL-6 levels, wet/dry weight ratio) were affected by treatment with CB receptor antagonists (Figure 2A and B). However, with combined application of AM251 and AM630, there was a trend for a further increase in serum lipase levels and pancreatic MPO levels, which did not reach statistical significance (Figure 2A). Surprisingly, administration of AM630 alone or in combination with AM251 reduced KC1 mRNA levels (P < .05; Figure 2B).
Figure 2.
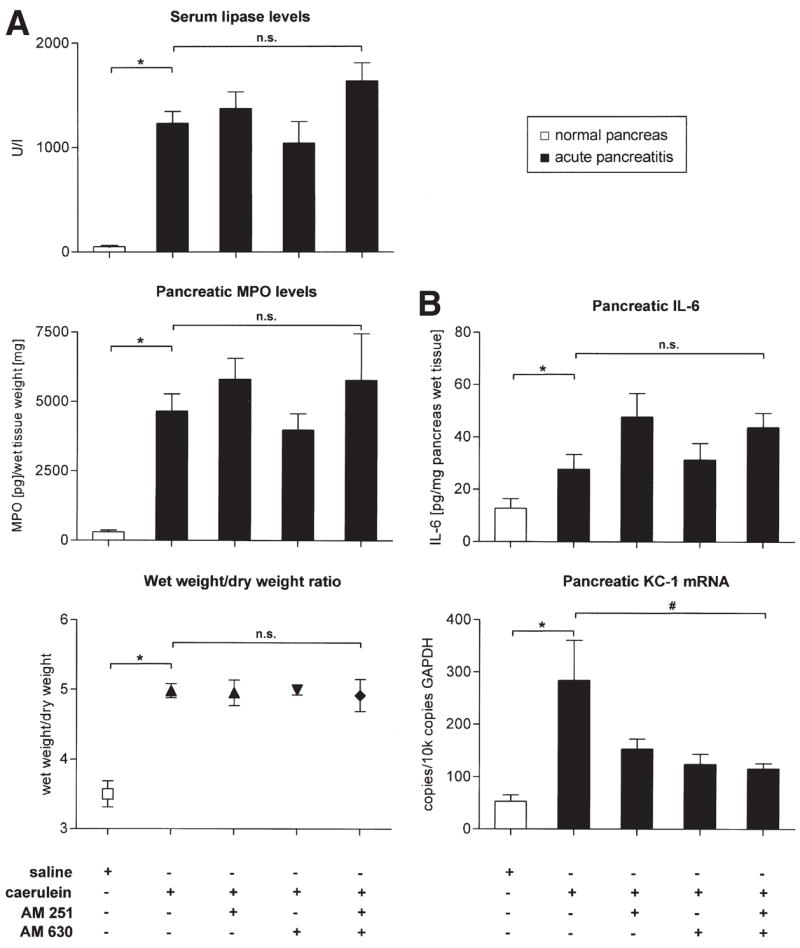
Levels of serological, molecular, and inflammatory markers in acute pancreatitis and effects of CB1/CB2 antagonists. (A) Levels of serum lipase, pancreatic MPO, and the pancreatic wet weight/dry weight ratio are increased in mice following cerulein-induced pancreatitis (black bars) over control animals (white bars; * P < .05). (B) Pancreatic levels of IL-6 protein and KC1 mRNA (normalized to GAPDH expression levels) rise significantly following induction of acute pancreatitis. Treatment with cannabinoid receptor antagonists AM251 or AM630 either alone or in combination did not change levels of these parameters, except pancreatic KC1 mRNA expression (P < .05). * and # represent P < .05 as compared with the saline group or the cerulein group, respectively. (*) Mann–Whitney U test. (#) Analysis of variance followed by post hoc Bonferroni’s multiple comparison test. n.s. = statistically not significant.
Because disease pathology was not significantly worsened by application of CB1/CB2 antagonists, we addressed effects on pancreatitis-induced pain. Abdominal pain is a cardinal feature of acute pancreatitis. Here, we assessed pancreatitis-induced pain by studying nocifensive reactions to acute, punctuate stimuli applied to the abdomen of mice. Mice with acute pancreatitis demonstrated a significant increase in the response frequency to forces of 0.008g, 0.02g, 0.04g, or 0.07g (P < .01, respectively; Figure 3A). These results show that acute pancreatitis is accompanied by marked mechanical hyperalgesia and allodynia. When cannabinoid receptor antagonists were administered to animals with acute pancreatitis, the frequency of withdrawal was increased in response to forces of 0.008g, 0.07g, and 0.16g for AM251 alone (P < .05; Figure 3B), 0.07g and 0.16g for AM630 alone (P < .01; Figure 3C), or 0.008g, 0.07g, and 0.16g for AM251 in combination with AM630 (P < .01; Figure 3D). These results suggest that the endocannabinoid system tonically inhibits pain associated with pancreatitis.
Figure 3.
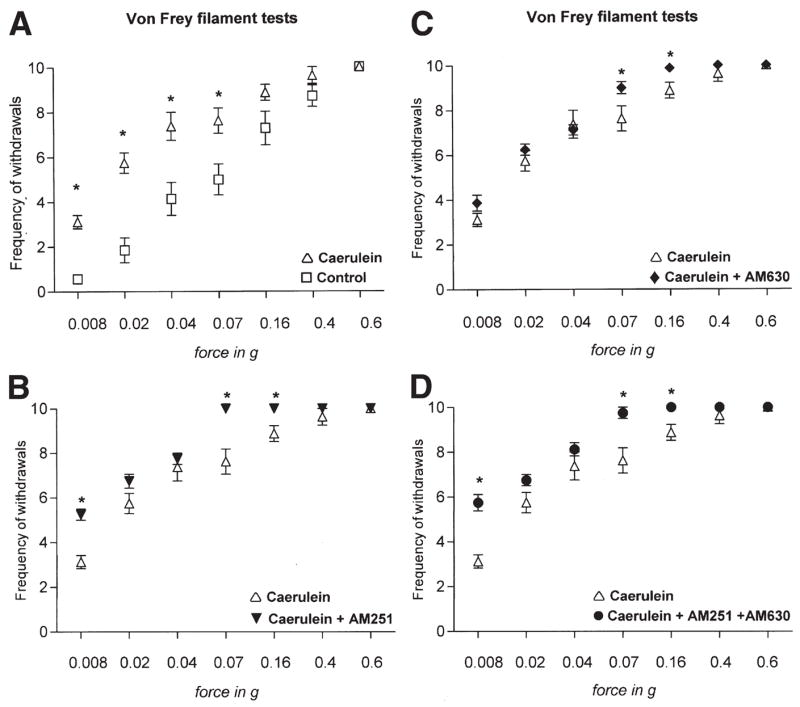
Analysis of abdominal pain thresholds to graded pressure applied via von Frey filaments in saline- or cerulein-treated mice. Y-axes show frequency of withdrawals to von Frey filaments over 10 applications. (A) Cerulein-induced pancreatitis leads to hyperalgesia (increased response frequency to a force of 0.02g to 0.16g) and allodynia (increased response frequency to 0.008g) over saline-injected mice (control). (B–D) AM251, AM630, and a combination of both increase response thresholds significantly at some filament forces. Data are shown as mean ± SEM. *P < .05; analysis of variance with post hoc Bonferroni’s multiple comparison test.
Cannabinoid Treatment Reduces Inflammation and Disease Pathology in Acute Pancreatitis
We then addressed whether an augmentation of the endocannabinoid system via exogenously applied cannabinoids could be beneficial in acute pancreatitis. Cannabinoid receptors were activated by treatment with HU210, a synthetic agonist at CB1 and CB2.11 Intraperitoneal application of HU210 led to a reduction of the levels of various serological and inflammatory markers, such as serum lipase (P < .015; Figure 4A), pancreatic MPO (P = .001; Figure 4A), pancreatic IL-6 levels (P = .02; Figure 4B), as well as pancreatic KC1 mRNA (P = .08; Figure 4B). Furthermore, HU210 significantly reduced the pancreatic edema as judged by pancreas wet weight/dry weight ratio (P = .007; Figure 4A). Pancreatitis-associated tissue histopathology was ameliorated with HU210, with a 40% reduction in overall histology score of edema, neutrophil infiltration, and acinar cell necrosis (P < .05; data not shown). The incidence of acinar cell necrosis was lower in HU210-treated mice (0% to 0.5% overall necrosis) than in the saline-treated group with acute pancreatitis (2% overall necrosis). To assess whether pancreatitis or cannabinoids impact upon glucose metabolism, we assessed serum glucose levels in mice with acute pancreatitis. Surprisingly, glucose levels were significantly lower in animals with cerulein-treated mice compared with saline-treated mice (P = .0002, data not shown). However, glucose levels remained unaffected by HU210 treatment in these mice (P = .38; data not shown).
Figure 4.
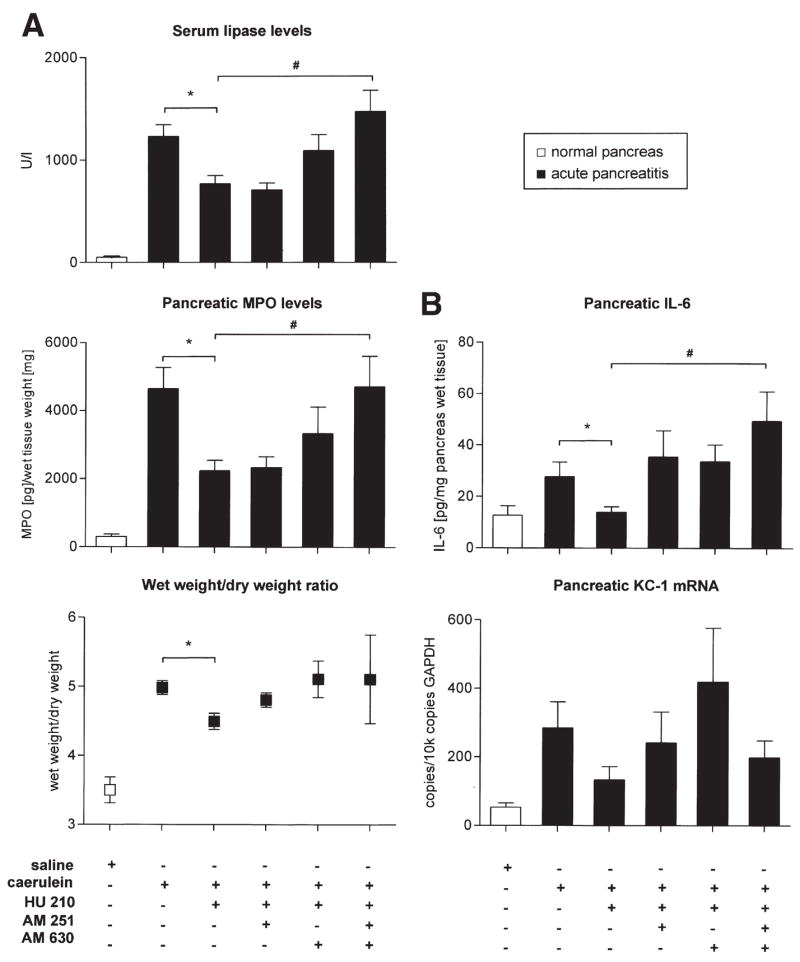
Nature and receptor mechanisms of effects of a cannabinoid agonist (HU210) on serological, molecular, and inflammatory markers in acute pancreatitis. Subcutaneous treatment with HU210 (0.05 mg/kg intraperitoneally) led to decreased serum lipase (P = .015), pancreatic MPO levels, and pancreatic IL-6 levels (P = .001 and P = .02, respectively; A, B), which was reversed completely by a combination of AM251 and AM630 (#P < .05). Pancreatic edema (as judged by wet weight/dry weight ratio) was significantly reduced by HU210 (P = .007) with a tendency toward reversal by CB receptor antagonists (P > .05). Data are shown as mean ± SEM. *,#P < .05; Mann–Whitney U test. (*) analysis of variance with post hoc Bonferroni’s multiple comparison test (#).
The Anti-Inflammatory Effects of HU210 in Pancreatitis Are Mediated by CB1 and CB2
We then performed pharmacological interventions in order to study mechanisms mediating the beneficial effects of HU210 on inflammation associated with pancreatitis. Treatment with either AM251 or AM630 did not significantly alter the anti-inflammatory effects of HU210, as judged by serum levels of lipase, pancreatic MPO, IL-6, and KC1 mRNA as well as the pancreatic wet/dry weight ratio (Figure 4A and B). In contrast, a combination of AM251 and AM630 fully reversed the beneficial effects of HU210 on pancreatitis-induced inflammatory changes as judged by increased serum lipase, pancreatic MPO, and IL-6 levels (P < .05, respectively; Figure 4A and B). Both AM251 and AM630 worsened the pancreatitis histology score by 20% and 24%, respectively, when compared with animals receiving HU210 alone.
CB1 and CB2 Mediate Cannabinoid-Induced Analgesia in Pancreatitis
One of the most important aims of this study was to test whether treatment with exogenous cannabinoids can alleviate pain associated with pancreatitis. After cerulein administration, mice treated with HU210 developed hyperalgesia and allodynia to abdominal pressure to a much lesser extent as compared to mice with mock treatment. At all pressures tested (from 0.008g to 0.6g), withdrawal latencies were significantly decreased in the presence of HU210 (Figure 5A; P < .001). Administration of HU210 to control mice in the absence of pancreatitis did not affect nociceptive responses (Figure 5B; P > .05).
Figure 5.
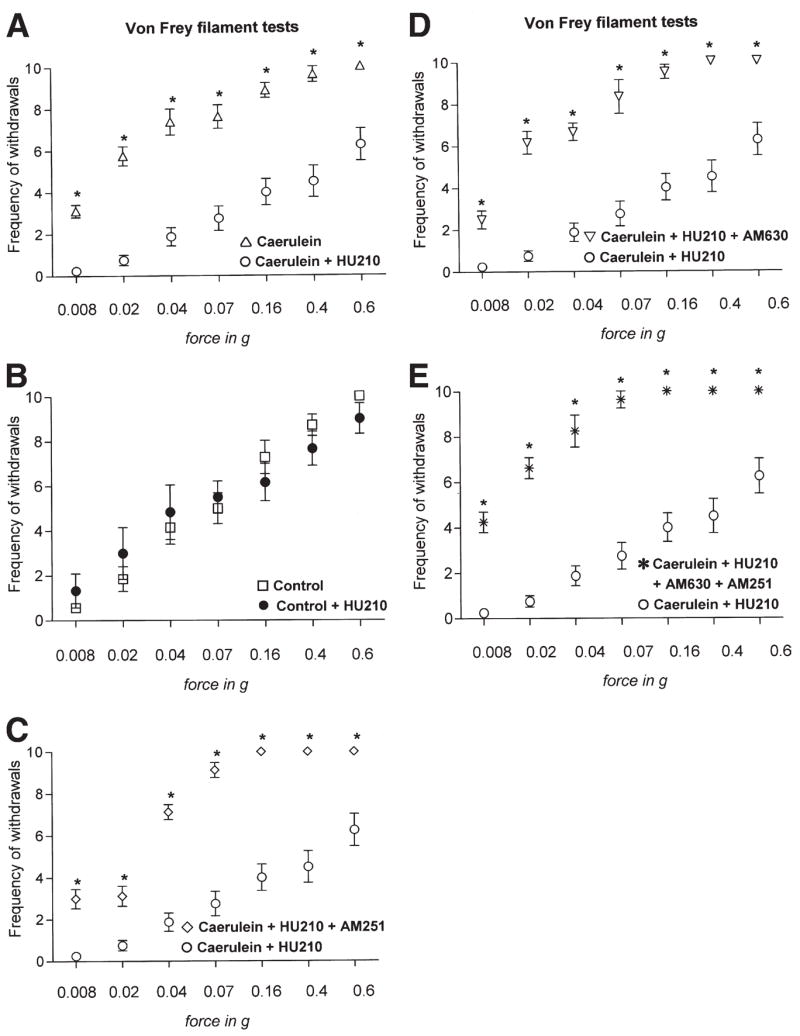
Nature and receptor mechanisms of effects of a cannabinoid agonist (HU210) on abdominal pain thresholds to graded pressure applied via von Frey filaments in cerulein- or saline-treated mice. Y-axes show frequency of withdrawals to von Frey filaments over 10 applications. (A) HU210 (0.05 mg/kg intraperitoneally) completely reversed cerulein-induced hyperalgesia (increased response frequency to a force of 0.02g to 0.16g) and allodynia (increased response frequency to 0.008g) and produced analgesia. (B) In control mice (saline-injected), HU210 treatment did not affect nociceptive responses by itself. (C–E) Pretreatment with AM251, with AM630, or a combination of both blocked HU210-induced analgesia in cerulein-injected mice. Data are shown as mean ± SEM. *P < .05; analysis of variance with post-hoc Bonferroni’s multiple comparison test.
Furthermore, we observed that administration of either AM251 or AM630 concurrently with HU210 significantly reduced the beneficial effects of HU210 on pancreatitis-associated abdominal hypersensitivity (Figure 5C and D; P < .05). Similar effects were seen with a combination of AM251 and AM630 together with HU210 (Figure 5E; P < .01). These data indicate that both CB1 and CB2 are required for the antinociceptive effects of HU210 in pancreatitis.
Absence of Central Side Effects on CB Receptor Antagonist and HU210 Treatment in Acute Pancreatitis
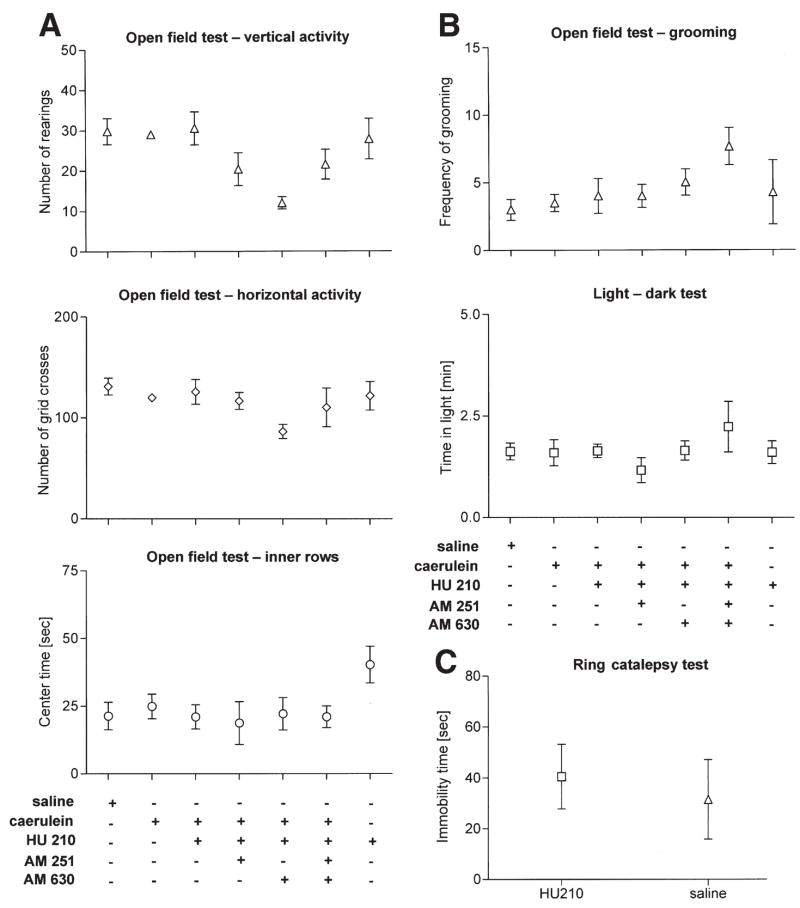
Side effects such as sedation, psychotropic effects, and motor dysfunction can limit the use of cannabinoids to alleviate pain. It was therefore important to ensure that these parameters do not confound our analysis of the antinociceptive effects of HU210 and were not influenced by CB receptor antagonist treatment. Mice with acute pancreatitis did not demonstrate any overall impairment of locomotor functions following HU210 administration, as judged via vertical and horizontal activity in the open field test compared with mice receiving saline injections (Figure 6A). Furthermore, time spent in the center of the open field (Figure 6A) and grooming (Figure 6B) remained unchanged upon HU210 treatment, suggesting that, at the dose used here, HU210 did not affect anxiety levels in mice, which was further confirmed in the light-dark test (Figure 6B). Similarly, combined administration of HU210 with antagonists at CB1 or CB2 did not have any effect on motor performance or affective behavior (Figure 6A and B). Furthermore, administration of AM251 and AM630 did not significantly alter behavior in these tests (Supplementary Figure 2A and B) (See supplementary figures online at www.gastrojournal.org.). Catalepsy is another classical behavior associated with cannabinoids, which is believed to be mediated by receptors in the central nervous system.13 In the ring-catalepsy test, we observed that the dose of HU210 used in this study failed to induce freezing behavior in mice (Figure 6C). Taken together, these results show that the dose of HU210, which abrogated pancreatitis-induced abdominal pain in our mouse model, did not produce side effects such as catalepsy, inhibition of activity, impaired ambulation, or psychotropic effects.
Figure 6.
Effects of HU210 (0.05 mg/kg intraperitoneally) on locomotor activity, anxiety, and grooming in mice with acute pancreatitis. (A) In the open-field test, the numbers of rearings (vertical activity, A), of grid crosses (horizontal activity, A), and of time in inner rows (center time, A) is not affected by HU210. (B) HU210 treatment does not affect the frequency of grooming in the open-field test and time spent in the dark chamber in the light-dark test for evaluation of anxiety. Co-administration of AM251 or AM630 has no significant effects in any of the previously mentioned tests. (C) HU210 (0.05 mg/kg) did not induce freezing behavior in the ring catalepsy test.
Discussion
The most important finding of this study is that activation of cannabinoid receptors is beneficial against abdominal pain as well as disease pathology of cerulein-induced acute pancreatitis. Pain management is a key cornerstone in the conventional therapy of acute pancreatitis and still represents a major clinical challenge. In-sufficient pain control results in high respiratory rates, reduced lung function, and inadequate ingestion, factors that significantly delay recovery and may worsen the course of the disease.1,2 The use of opioids as a standard therapy regimen is effective but frequently results in severe and difficult-to-handle side effects, such as nausea, vomiting, decreased gastrointestinal motility, and adynamia, which overlap with the symptoms of pancreatitis.2 Nonsteroidal analgesics lack these side effects but do not provide adequate pain relief. We demonstrate here that a cannabinoid completely blocked abdominal pain in a state of cerulein-induced acute pancreatitis. Importantly, this beneficial effect was elicited following systemic delivery of HU210 at a low dose lacking central side effects, suggesting that HU210 abolished pancreatitis-induced abdominal pain via peripheral mechanisms. Consistent with this appraisal, we could demonstrate that cannabinoid-induced analgesia was not accompanied by effects such as hindrance of activity, motor dysfunction, sedation, or catalepsy, which are typically associated with activation of cannabinoid receptors in the central nervous system.
Because lower-abdominal tactile hypersensitivity after pancreatic inflammation essentially represents secondary hyperalgesia and allodynia (phenomena that require sensitization of central synapses), the antinociceptive effects we observed likely occur as a result of cannabinoid-induced decrease in nociceptor excitability and consequently, reduced central sensitization. Consistent with peripheral antinociceptive effects of cannabinoids, CB1 localized on peripheral nociceptive endings has been shown to negatively modulate excitability of nociceptors and inhibit release of peptides,18 thereby countering neurogenic inflammation. Interestingly, neurogenic inflammation has been postulated to be a key mechanism in the pathophysiology of acute pancreatitis.19 In addition to CB1, we also observed a CB2-component to the antinociceptive effects of HU210 in cerulein-induced pancreatitis. Although a functional contribution of CB2 expressed on nerves is possible, the anti-inflammatory effects of CB2 could also indirectly account for its antinociceptive role. Our anatomical data from human specimens reveal the presence and up-regulation of both CB1 and CB2 in neural, as well as non-neural cells in the pancreas. Future studies are therefore required to pinpoint the precise loci and mechanisms of cannabinoid-induced anti-nociception in acute pancreatitis.
Another key feature of acute pancreatitis is local inflammation resulting from acinar cell injury, which, if significant and unchecked, can lead to a systemic inflammatory response syndrome, thereby initiating a potentially fatal stage of the disease.20 The probable benefits of anti-inflammatory therapies in acute pancreatitis have been hypothesized previously.20 In this study, we found that a low dose of HU210, which is devoid of central side effects, does exert moderate anti-inflammatory effects in the pancreas and partially reduces cerulein-induced disease pathology. This finding is consistent with a recent study11 demonstrating that HU210 exerts local anti-inflammatory effects in a model of experimental colitis in mice, suggesting that cannabinoids are generally beneficial in visceral inflammatory disorders. Whereas CB1 receptors were found to play a key role in the study by Massa et al, we observed that the moderate anti-inflammatory effects achieved via low-dose HU210 in acute pancreatitis largely require both CB1 and CB2 activation. Another interesting aspect of the present study is the finding that anandamide as well as cannabinoid receptors are up-regulated in human pancreatitis. We observed that blockade of CB1 and CB2 led to a significant worsening of pancreatitis-induced pain but produced only a nonsignificant trend toward exacerbation of cerulein-induced disease pathology. These data suggest that in our experimental conditions, an induction of the endocannabinoid system during acute pancreatitis primarily represents an endogenous protective mechanism against pancreatic pain.
Recently, Matsuda et al21 reported prolonged survival in rats upon AM251 treatment in a model of tauro-cholate-induced necrotizing pancreatitis. Because effects of cannabinoid agonists were not reported in the study by Matsuda et al and cerulein-induced pancreatitis in mice is far less severe, non-necrotizing, and does not lead to animal death, it is difficult to directly compare the outcome of the study by Matsuda et al21 with the results described in this paper. Our results are more in line with a recent study reporting a protective role for the endogenous cannabinoid system against colonic inflammation in a mouse model of experimental colitis.9,11,22 Consistent with the above, we now show that acute pancreatitis, a visceral inflammatory disease in humans, is associated with an activation of the endocannabinoid system. Because management of visceral inflammatory diseases should ideally include antinociceptive23 as well as anti-inflammatory components, our results lay a basis for testing the therapeutic value of cannabinoids as supplements to conventional analgesic therapy.
Acknowledgments
Supported by a KFG107 grant from the Deutsche Forschungsge-meinschaft (DFG) to R.K., by a DFG grant SE1095, as well as a Postdoctoral Research Program grant from the University of Heidelberg (to C.W.M.), and in part by Intramural Research Program of the NIH/NIAAA (#1Z01AA000375-01) to P.P. The authors are indebted to Judith Harvey-White for help with endocannabinoid measurements and Brunhilde Bentzinger, Monika Meinhardt, and Sylvia Schaller for excellent technical assistance.
Abbreviations used in this paper
- 1-/2-AG
1-/2-arachidonoylglycerol
- AEA
anandamide
- AP
acute pancreatitis
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
Appendix
Supplementary Data
Supplementary data associated with this article can be found, in the online version, at zdoi:10.1053/j.gastro.2007.02.035.
References
- 1.Mayerle J, Hlouschek V, Lerch MM. Current management of acute pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2005;2:473–483. doi: 10.1038/ncpgasthep0293. [DOI] [PubMed] [Google Scholar]
- 2.Toouli J, Brooke-Smith M, Bassi C, Carr-Locke D, Telford J, Freeny P, Imrie C, Tandon R. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002;17(Suppl):S15–39. doi: 10.1046/j.1440-1746.17.s1.2.x. [DOI] [PubMed] [Google Scholar]
- 3.Vera-Portocarrero LP, Yie JX, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006;130:2155–2164. doi: 10.1053/j.gastro.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Kawabata A, Matsunami M, Tsutsumi M, Ishiki T, Fukushima O, Sekiguchi F, Kawao N, Minami T, Kanke T, Saito N. Suppression of pancreatitis-related allodynia/hyperalgesia by proteinase-activated receptor-2 in mice. Br J Pharmacol. 2006;148:54–60. doi: 10.1038/sj.bjp.0706708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winston JH, Toma H, Shenoy M, He ZJ, Zou L, Xiao SY, Micci MA, Pasricha PJ. Acute pancreatitis results in referred mechanical hypersensitivity and neuropeptide up-regulation that can be suppressed by the protein kinase inhibitor k252a. J Pain. 2003;4:329–337. doi: 10.1016/s1526-5900(03)00636-9. [DOI] [PubMed] [Google Scholar]
- 6.Nathan JD, Peng RY, Wang Y, McVey DC, Vigna SR, Liddle RA. Primary sensory neurons: a common final pathway for inflammation in experimental pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G938–946. doi: 10.1152/ajpgi.00105.2002. [DOI] [PubMed] [Google Scholar]
- 7.Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med. 2005;83:944–954. doi: 10.1007/s00109-005-0698-5. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacher P, Batkai S, Kunos G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, Ferri GL, Sibaev A, Storr M, Lutz B. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tappe A, Kuner R. Regulation of motor performance and striatal function by synaptic scaffolding proteins of the Homer1 family. Proc Natl Acad Sci U S A. 2006;103:774–779. doi: 10.1073/pnas.0505900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pertwee RG. The ring test: a quantitative method for assessing the “cataleptic” effect of cannabis in mice. Br J Pharmacol. 1972;46:753–763. doi: 10.1111/j.1476-5381.1972.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erkan M, Kleeff J, Esposito I, Giese T, Ketterer K, Buchler MW, Giese NA, Friess H. Loss of BNIP3 expression is a late event in pancreatic cancer contributing to chemoresistance and worsened prognosis. Oncogene. 2005;24:4421–4432. doi: 10.1038/sj.onc.1208642. [DOI] [PubMed] [Google Scholar]
- 15.Frossard JL, Kwak B, Chanson M, Morel P, Hadengue A, Mach F. Cd40 ligand-deficient mice are protected against cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2001;121:184–194. doi: 10.1053/gast.2001.25483. [DOI] [PubMed] [Google Scholar]
- 16.Lupia E, Goffi A, De Giuli P, Azzolino O, Bosco O, Patrucco E, Vivaldo MC, Ricca M, Wymann MP, Hirsch E, Montrucchio G, Emanuelli G. Ablation of phosphoinositide 3-kinase-gamma reduces the severity of acute pancreatitis. Am J Pathol. 2004;165:2003–2011. doi: 10.1016/s0002-9440(10)63251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatia M, Saluja AK, Singh VP, Frossard JL, Lee HS, Bhagat L, Gerard C, Steer ML. Complement factor C5a exerts an anti-inflammatory effect in acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2001;280:G974–978. doi: 10.1152/ajpgi.2001.280.5.G974. [DOI] [PubMed] [Google Scholar]
- 18.Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–9. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- 19.Liddle RA, Nathan JD. Neurogenic inflammation and pancreatitis. Pancreatology. 2004;4:551–559. doi: 10.1159/000082180. discussion 559–560. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–144. doi: 10.1159/000085265. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda K, Mikami Y, Takeda K, Fukuyama S, Egawa S, Sunamura M, Maruyama I, Matsuno S. The cannabinoid 1 receptor antagonist, AM251, prolongs the survival of rats with severe acute pancreatitis. Tohoku J Exp Med. 2005;207:99–107. doi: 10.1620/tjem.207.99. [DOI] [PubMed] [Google Scholar]
- 22.Kunos G, Pacher P. Cannabinoids cool the intestine. Nat Med. 2004;10:678–679. doi: 10.1038/nm0704-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebhart GFJJ. Bonica Lecture--2000: Physiology, pathophysiology, and pharmacology of visceral pain. Reg Anesth Pain Med. 2000;25:632–638. doi: 10.1053/rapm.2000.18187. [DOI] [PubMed] [Google Scholar]