Abstract
This study demonstrates that endogenously produced interferon γ (IFN-γ) forms the basis of a tumor surveillance system that controls development of both chemically induced and spontaneously arising tumors in mice. Compared with wild-type mice, mice lacking sensitivity to either IFN-γ (i.e., IFN-γ receptor-deficient mice) or all IFN family members (i.e., Stat1-deficient mice) developed tumors more rapidly and with greater frequency when challenged with different doses of the chemical carcinogen methylcholanthrene. In addition, IFN-γ-insensitive mice developed tumors more rapidly than wild-type mice when bred onto a background deficient in the p53 tumor-suppressor gene. IFN-γ-insensitive p53−/− mice also developed a broader spectrum of tumors compared with mice lacking p53 alone. Using tumor cells derived from methylcholanthrene-treated IFN-γ-insensitive mice, we found IFN-γ’s actions to be mediated at least partly through its direct effects on the tumor cell leading to enhanced tumor cell immunogenicity. The importance and generality of this system is evidenced by the finding that certain types of human tumors become selectively unresponsive to IFN-γ. Thus, IFN-γ forms the basis of an extrinsic tumor-suppressor mechanism in immunocompetent hosts.
Interferon γ (IFN-γ) is a pleiotropic cytokine that plays a central role in promoting innate and adaptive mechanisms of host defense (1, 2). It is now well recognized that IFN-γ exerts its biologic effects by interacting with an IFN-γ receptor that is ubiquitously expressed on nearly all cells (3). Functionally active IFN-γ receptors consist of two distinct subunits: a 90-kDa receptor α chain (IFNGR1) and a 62-kDa receptor β chain (IFNGR2). Recent work from several laboratories has established that most, if not all, IFN-γ responses in cells result from the ligand-induced coupling of the activated IFN-γ receptor complex to particular components of the JAK-STAT signaling pathway (3–6). IFN-γ signaling requires three specific JAK-STAT pathway components: the protein tyrosine kinases Jak1 and Jak2 and the transcription factor Stat1.
By using mice that lack IFN-γ, either of the IFN-γ receptor subunits, or any of the three proximal JAK-STAT signaling proteins, it has been demonstrated that any event that leads to a disruption of IFN-γ signaling results in a catastrophic ablation of innate immunity, rendering the host highly susceptible to infection by a variety of microbial pathogens and certain viruses (5–10). These findings recently have been generalized to humans by the discovery of individuals with inactivating mutations in the IFN-γ receptor complex who die early in life from uncontrolled mycobacterial infections (11–13). Thus, the physiologic role of IFN-γ in promoting host resistance to infectious organisms is unequivocal.
In contrast, the role that IFN-γ plays in the development of host antitumor responses is less well established. Four years ago, we defined a novel role for IFN-γ in this process by showing that it functioned to enhance the immunogenicity of certain tumor cells (14). Using a series of murine fibrosarcoma cell lines that were engineered to be either sensitive or insensitive to IFN-γ, rejection of transplanted tumor cells was shown to depend on the endogenous production of IFN-γ by the host and the generation of tumor-specific T cells. More important, this study showed that the tumor cell itself was the major target of IFN-γ’s actions and that IFN-γ functioned in this model to promote the detection and/or elimination of preformed tumor cells in naive or immune syngeneic hosts.
Although this study clearly identified a role for IFN-γ in promoting rejection of transplantable tumors, it did not address the critical question of whether IFN-γ also participates in the development of host responses to nascent transformed cells, i.e., whether it is involved in promoting tumor surveillance against primary tumors. The availability of mice that lack either the IFN-γ receptor α chain (8) or Stat1 (5) and therefore are insensitive to IFN-γ in all tissues has allowed us to study IFN-γ’s participation in controlling tumor formation by using genetic- and carcinogen-dependent tumorigenesis models. Herein, we report that lack of IFN-γ sensitivity predisposes a murine host to enhanced tumor development. IFN-γ insensitivity at the level of the developing tumor cell appears to be a key determinant, dictating whether it forms a progressively growing tumor. In addition, we report the characterization of human tumors that naturally have developed inactivating genetic mutations in different IFN-γ signaling proteins, rendering them insensitive to IFN-γ. These results thus establish a central role for IFN-γ in tumor surveillance and suggest that certain tumors may lose IFN-γ sensitivity as a mechanism to evade immune detection.
MATERIALS AND METHODS
Reagents.
Purified cytokines were obtained as indicated: murine tumor necrosis factor α (MuTNFα) and murine IFN-γ (MuIFN-γ) from Genentech, recombinant human IFN-α (rHuIFN-αA-D) from Michael Brunda of Hoffman–La Roche, and murine interleukin 12 (MuIL-12) from Stanley Wolf, Genetics Institute (Cambridge, MA). Recombinant MuIL-1α and H22, a neutralizing hamster mAb specific for MuIFN-γ, were produced as described (15, 16). The W6/32 mAb specific for human major histocompatibility complex (MHC) class I was provided by Thalachallour Mohanakumar (Washington Univ. School of Medicine). 3-Methylcholanthrene (MCA), peanut oil, and G418 were purchased from Sigma.
Mice.
129/Sv/Ev mice and 129/Sv/Ev strain IFN-γR−/− and Stat1−/− mice were described previously (5, 8). SCID (severe combined immunodeficient) mice were kindly provided by E. R. Unanue and H. Virgin (Washington Univ. School of Medicine). Mice heterozygous for the p53 tumor-suppressor gene (p53+/− mice) bred onto the 129/Sv/J background and mice with a homozygous deletion of the RAG1 gene were obtained from The Jackson Laboratory. 129/Sv/Ev × p53−/−, IFN-γR−/− × p53−/−, and Stat1−/− × p53−/− mice were generated from the same p53+/− male mouse. Genotyping of mice was performed by PCR as described previously (19).
MCA Tumor Induction.
Groups of mice were injected subcutaneously in the flank with MCA diluted in 0.1 ml of peanut oil. Mice were observed weekly for tumor development over the course of 130–165 days. Tumors larger than 5 mm and showing progressive growth were counted as positive.
Tumor Cell Lines.
Murine primary MCA-induced tumors were surgically excised and passaged two times in SCID mice and once in IFN-γR−/− mice. The passaged tumors were minced, trypsin-treated for 5 min at 37°C, and maintained by culture in RPMI 1640 medium supplemented with 10% FCS, 1% glutamine, 1 mM sodium pyruvate, 10 mM nonessential amino acids, 50 units/ml penicillin, 50 μg/ml streptomycin, and 5 × 10−5 M 2-mercaptoethanol (R-10 medium). Human tumor lines, obtained from the human tumor cell bank at the Ludwig Institute for Cancer Research, New York Branch, Memorial Sloan–Kettering Cancer Institute, New York, were maintained by culture in R-10 medium.
Expression Vectors.
cDNAs encoding murine Jak1 and Jak2, kindly provided by S. Nagata (Osaka Bioscience Institute, Osaka) were cloned into the SRα.EMCV.neo expression vector between the XhoI and SacI sites. Expression plasmids containing the cDNAs for human and murine IFN-γR α and β chains have been described previously (17, 18).
DNA Transfection.
Murine tumor cell lines (5 × 106 cells) were transfected with 25 μg of expression plasmid by electroporation, selected by culture in G418, and cloned by limiting dilution as described (17). Human tumors were stably transfected by using an identical technique.
Analysis of IFN-γ Sensitivity by Enhanced MHC Class I Surface Expression.
Responsiveness of human tumor cells to IFN was assessed after culture of the cells in the presence of buffer, rHuIFN-γ [1,000 international reference units (IRU)/ml], or rHuIFN-αA/D (1,000 units/ml) for 72 hr followed by quantitation by fluorescence-activated cell sorter of MHC class I antigen expression as described previously (17).
Electrophoretic Mobility-Shift Assay.
Tumor cells, resuspended in 1 ml of PBS containing 10% FCS, were treated with either PBS or rHuIFN-γ (10,000 IRU)/ml] for 5 min at 37°C. DNA-binding activity in 5 μg of nuclear extracts was quantitated by electrophoretic mobility-shift assay using a 32P-labeled γ-IFN response region probe as described (20).
Proliferation Assays.
Tumor cells plated at a density of 1.25 × 104 cells per ml were incubated with different combinations of IFN-γ (5,000 units/ml), IFN-α (1,000 units/ml), TNFα (10 ng/ml), IL-1 (10 ng/ml), and IL-12 (50 units/ml) for 24 hr. Proliferation was assessed by thymidine incorporation (15).
RESULTS
Increased Incidence of MCA-Induced Tumors in IFN-γ-Insensitive Mice.
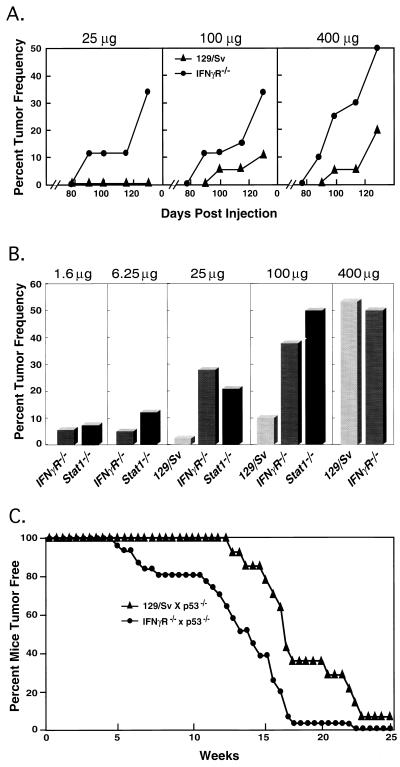
Groups of IFN-γ receptor α chain knockout mice (IFN-γR−/−), which were derived on a pure 129/Sv/Ev genetic background, as well as wild-type inbred 129/Sv/Ev controls were injected subcutaneously with three different doses of MCA, and tumor development was monitored for a period of 130 days (Fig. 1A). This mouse background was chosen specifically for these experiments because 129/Sv/Ev mice are relatively resistant to the tumorigenic actions of MCA and the pure background of the mice ensured that we would be able to study the in vivo growth of the resulting tumors by using tumor transplantation approaches. At all doses examined, IFN-γR−/− mice generated tumors more frequently and at earlier times than the control mice. At the end of the observation period, a high percentage of IFN-γR−/− mice treated with 100 and 25 μg MCA developed tumors whereas only limited or no tumor development was noted in the corresponding groups of wild-type mice.
Figure 1.
IFN-γ-insensitive mice demonstrate an increased susceptibility to development of spontaneous and chemically induced tumors. (A) Groups of 15–20 129/Sv/Ev wild-type mice (▴) and syngeneic IFN-γR−/− mice (•) were injected with a single dose of methylcholanthrene, and tumor development was quantitated for 130 days. (B) Groups of wild-type, IFN-γR−/−, and Stat1−/− mice were injected with MCA, and tumor development was monitored for 165 days. Values represent the composite of four independent experiments. (C) Spontaneous tumor development in IFN-γR−/− × p53−/− (•) and 129/Sv/Ev × p53−/− mice (▴). The difference in average tumor development times between 129/Sv/Ev × p53−/− (18.5 weeks) and IFN-γR−/− × p53−/− (13.7 weeks) is statistically significant by the Wilcoxon rank sum test (P = 0.001).
To explore the magnitude of increased sensitivity of IFN-γ-unresponsive mice to MCA, the experiment was repeated four times by using an extended MCA dose range, a longer observation period (165 versus 130 days), and two different types of IFN-γ-insensitive mice: IFN-γR−/− mice and Stat1−/− mice (Fig. 1B). Similar to the previous experiment, wild-type 129/Sv/Ev mice were relatively resistant to MCA. At an MCA dose of 400 μg, 53.4% (93/174) of wild-type mice developed tumors and tumor induction fell rapidly as the dose of MCA was lowered such that only 2.4% (1/42) of the wild-type group developed tumors when challenged with 25 μg MCA. In contrast, IFN-γ-unresponsive mice were significantly more sensitive to tumor induction by MCA and no differences were noted between mice that lacked sensitivity to IFN-γ alone (IFN-γR−/− mice) versus all forms of IFN (Stat1−/− mice). At a dose of 100 μg MCA, 39% (14/36) of IFN-γR−/− mice and 50% (10/20) of Stat1−/− mice developed tumors. More important, 28% (14/50) of IFN-γR−/− mice and 21% (5/24) of Stat1−/− mice developed tumors when challenged with 25 μg MCA, and tumor induction was detected in groups of these mice exposed to even lower doses of carcinogen. A histologic comparison of the tumors that developed in IFN-γ-responsive and IFN-γ-unresponsive mice indicated that MCA induced histologically indistinguishable fibrosarcomas in the three mouse strains. Thus, these experiments demonstrate that IFN-γ plays a critical role in providing the host with a mechanism to eliminate chemically induced, nascently transformed cells.
Increased Incidence of p53-Regulated Tumors in IFN-γ-Insensitive Mice.
To examine whether IFN-γ plays a similar role in preventing the incidence of spontaneous tumors, we monitored tumor development in IFN-γ-sensitive and IFN-γ-insensitive mice that lacked the p53 tumor-suppressor gene (Fig. 1C). IFN-γ-sensitive, p53−/− × 129/Sv/Ev mice (n = 14) formed tumors with a mean time to tumor detection of 18.5 weeks, a result that is consistent with published data (19, 21, 22). In contrast, p53−/− × IFN-γR−/− double knockout mice (n = 31) formed tumors significantly more rapidly with a mean time until tumor detection of 13.7 weeks (P < 0.001 as determined by the Wilcoxon rank sum test). A similar acceleration in time to tumor development was also observed in p53−/− × Stat1−/− mice (n = 13, data not shown).
Single and double knockout mice also displayed differences in the types of tumors that formed. In agreement with the reports of others, all of the IFN-γ-sensitive p53−/− × 129/Sv/Ev mice developed either thymomas or lymphocytic lymphosarcomas, although one member of this group developed a second nonlymphoid tumor. In contrast, 35% of the p53−/− × IFN-γR−/− mice and 38% of the p53−/− × Stat1−/− mice developed nonlymphoid tumors that included teratomas, hemangiomas, and chondrosarcomas without developing lymphoid tumors. Taken together these results demonstrate that IFN-γ plays a central role in promoting tumor surveillance toward both chemically induced tumors and tumors that form because of genetic defects in intrinsic mechanisms of tumor suppression.
Tumors Derived from IFN-γ-Insensitive Mice Grow Progressively in IFN-γ-Sensitive Hosts.
Because IFN-γR−/− and Stat1−/− mice are unresponsive to IFN-γ in all tissues, the aforementioned results did not define whether increased tumor formation was a result of the lack of IFN-γ responsiveness by tumor cells or by host immune cells. If cells of the host immune system are the major target of IFN-γ, then the tumors that developed in IFN-γ-insensitive mice would have been produced in the absence of a strong antitumor-selective pressure. These cells therefore would be expected to be highly immunogenic in wild-type mice and thus should not establish progressively growing tumors when transplanted into IFN-γ-responsive, immunocompetent animals. Conversely, if insensitivity to IFN-γ at the level of the transformed cell results in tumors that cannot be recognized and/or eliminated by the immune system, then tumor cells produced in IFN-γR−/− mice should grow equally well in both IFN-γ-sensitive and IFN-γ-insensitive immunocompetent hosts.
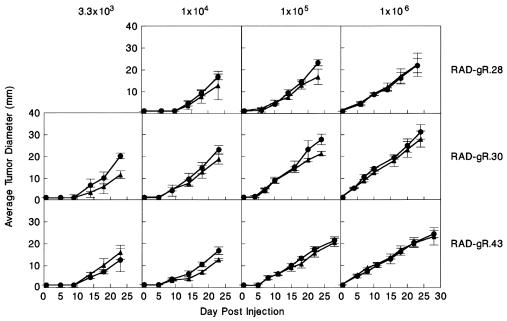
To distinguish between these two possibilities, tumor cells from individual MCA-treated IFN-γR−/− mice (denoted RAD-gR tumor cell lines) were transplanted into syngeneic wild-type and IFN-γR−/− mice and tumor growth was quantitated. Seven RAD-gR tumor cell lines (RAD-gR.14, 21, 26, 27, 28, 30, and 43) were studied in detail and were found to grow equally well in either naive IFN-γ-sensitive or IFN-γ-insensitive mice when injected at a dose of 1 × 106 cells per mouse (data not shown). To rule out the possibility that an inoculum of 1 × 106 RAD-gR cells was sufficiently large to mask minor tumor growth differences in IFN-γR−/− and 129/Sv/Ev mice, we performed dose-response experiments on three representative RAD-gR cell lines (RAD-gR.28, 30, and 43) (Fig. 2). No differences were noted in the growth kinetics of each tumor line in either the IFN-γ-sensitive or IFN-γ-insensitive mouse strains. Thus, IFN-γ-insensitive tumors grow progressively in mice regardless of whether IFN-γ responsiveness is present or absent in the host cell compartment.
Figure 2.
RAD-gR tumors demonstrate equivalent growth kinetics in IFN-γR−/− and 129/Sv/Ev mice. Three representative IFN-γR−/− tumor lines were injected subcutaneously into naive IFN-γR−/− (•) and 129/Sv/Ev (▴) mice at the indicated doses. Tumor growth kinetics were monitored by measuring the diameter of the tumor masses and are represented as an average ± SE of four to five mice per group.
Reconstitution of IFN-γ Responsiveness in Tumor Cells Derived From IFN-γR−/− Mice Leads to Tumor Rejection Through a Process That Requires IFN-γ and Adaptive Immunity.
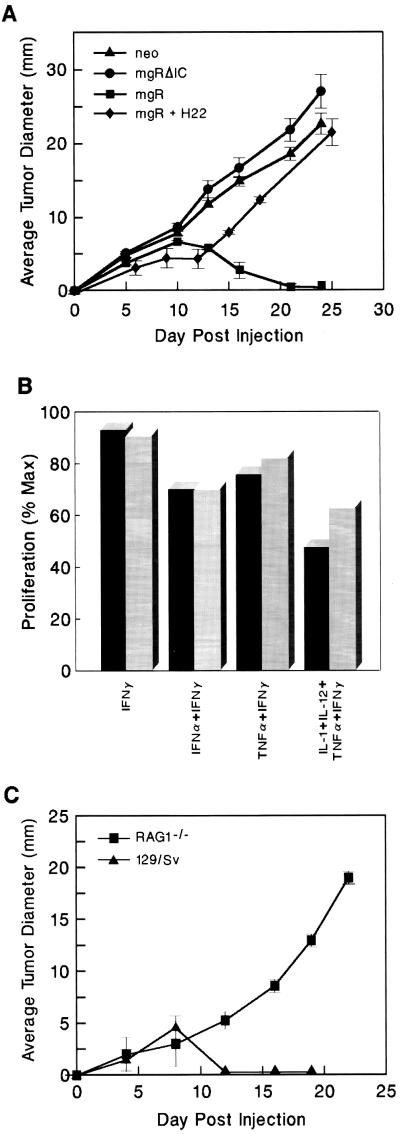
To directly examine whether IFN-γ sensitivity at the level of the tumor was the critical factor determining tumor growth in vivo, we reconstituted IFN-γ responsiveness (as assessed by monitoring IFN-γ-dependent enhancement of MHC class I protein expression) in a representative RAD-gR tumor cell line (RAD-gR.28) by stably transfecting it with an expression plasmid encoding the full-length wild-type IFN-γR α chain (to produce the RAD-gR.28.mgR cell line). As controls RAD-gR.28 cells were stably transfected with either empty vector (RAD-gR.28.neo) or a plasmid encoding a functionally inactive IFN-γR α chain intracellular domain truncation mutant (RAD-gR.28.mgR.ΔIC). Growth of each transfected tumor cell line then was monitored in naive IFN-γ-sensitive 129/Sv/Ev mice. Both of the IFN-γ-insensitive tumor cell lines formed progressively growing tumors in wild-type mice (Fig. 3A). In contrast, the functionally reconstituted RAD-gR.28.mgR cell line was rejected in wild-type mice, initially producing a small cellular mass that was eliminated in every animal by day 12–15. Importantly, rejection of RAD-gR.28.mgR cells was inhibited in wild-type mice that had been pretreated with a neutralizing mAb to IFN-γ (H22), indicating that RAD-gR.28.mgR has the capacity to generate progressively growing tumors in the absence of IFN-γ. Taken together these results demonstrate that IFN-γ sensitivity by the tumor is required for the development of an efficient antitumor response.
Figure 3.
Growth of reconstituted and unreconstituted RAD-gR.28 cells in wild-type and immunodeficient mice. (A) Rejection of reconstituted RAD-gR.28.mgR in 129/Sv/Ev mice. IFN-γ-insensitive RAD-gR.neo (▴) or RAD-gR.mgRΔIC (•) tumor cells and IFN-γ-sensitive RAD-gR.28.mgR (▪) cells were injected subcutaneously at a dose of 106 cells per animal into 129/Sv/Ev mice. RAD-gR.28.mgR cells were also injected subcutaneously into 129/Sv/Ev mice that had been pretreated on days −1, +2, and +5 with i.p. injections of 250 μg of a neutralizing mAb to murine IFN-γ (⧫) or saline (data not shown). (B) RAD-gR.28.mgR is resistant to the antiproliferative actions of IFN-γ. Triplicate cultures of RAD-gR.28.neo and RAD-gR.28.mgR cells were incubated for 24 hr with different combinations of IFN-γ (5,000 units/ml), IFN-α (1,000 units/ml), TNFα (10 ng/ml), IL-1 (10 ng/ml), and IL-12 (50 units/ml), and proliferation was assessed by monitoring 3H-labeled thymidine incorporation. Values are expressed as the percentage of incorporation compared with untreated cells. (C) RAG1−/− mice cannot reject reconstituted, IFN-γ-sensitive RAD-gR.28.mgR cells. RAD-gR.28.mgR cells were injected subcutaneously at a dose of 106 cells per mouse into either 129/Sv/Ev (▴) or RAG1−/− (▪) mice. Values represent the average ± SE tumor diameter of four to five mice per group.
Having identified the tumor cell as the major target of IFN-γ’s actions, we next sought to define the mechanism underlying the rejection process. IFN-γ can induce an antiproliferative state in many cell types including certain tumor cells. To examine whether IFN-γ’s antiproliferative actions were involved in mediating the regressive growth phenotype of reconstituted RAD-gR.28.mgR cells, the proliferation of these cells as well as mock-transfected RAD-gR.28.neo controls was determined after in vitro treatment of the cells with high concentrations of IFN-γ either alone or in combination with other cytokines. Although some of the cytokine combinations resulted in a reduction of tumor cell proliferation, no substantial differences were noted between IFN-γ-sensitive and IFN-γ-insensitive tumor cell lines (Fig. 3B). Thus, whereas certain cytokines such as IFN-α and TNFα indeed can effect the growth of these sarcomas in vitro, IFN-γ does not.
Therefore we considered the second possibility, that IFN-γ functioned to enhance tumor cell immunogenicity and thereby promoted development of tumor-specific immune responses. To test this possibility, we examined whether T cells were required for rejection of the reconstituted, IFN-γ-sensitive RAD-gR.28.mgR tumor cell line. Whereas the IFN-γ-sensitive tumor cells were rejected in syngeneic wild-type mice, they grew in a progressive manner in RAG1−/− mice that lack T and B lymphocytes (Fig. 3C). As expected, IFN-γ-insensitive RAD-gR.28.neo control cells grew progressively in both wild-type and RAG1−/− mice, forming 17-mm tumors after 20 days of in vivo growth (data not shown). Thus, lymphocytes play an obligate role in controlling the growth of reconstituted RAD-gR.28.mgR tumor cells in vivo.
Identification of Human Tumor Cells That Lack Sensitivity to IFN-γ.
The data obtained from the murine models demonstrate that endogenously produced IFN-γ forms the basis of a tumor surveillance system in mice that is effective in eliminating nascently transformed cells. This observation raises the question of whether naturally occurring tumors can develop a state of IFN-γ unresponsiveness to escape immune detection and/or elimination. To investigate this possibility as well as to generalize our findings to human tumors, we examined IFN-γ responsiveness in a variety of human tumor cell lines. These cells were assayed by fluorescence-activated cell sorter for their ability to enhance surface expression of MHC class I and by electrophoretic mobility-shift assay for the capacity to form Stat1-dependent DNA-binding complexes after treatment with IFN-γ. Analysis of 33 melanoma and 17 lung tumor cell lines classified as nonadenocarcinoma revealed that approximately 33% of each group showed a quantitative reduction in IFN-γ sensitivity (data not shown). However, analysis of 17 human lung adenocarcinoma cell lines revealed that four tumor lines (SK-LC-2, SK-LC-7, SK-LC-19, and CALU-5) were totally unresponsive to IFN-γ either when examined for initiation of IFN-γ signaling or development of an IFN-γ biologic response (Table 1).
Table 1.
Analysis of human lung adenocarcinoma tumor lines for IFN-γ sensitivity
| Tumor line | MHC class I enhancement, MCS
|
IFN-γ Stat activation by EMSA analysis | Cellular defect | |
|---|---|---|---|---|
| IFN-γ | IFN-α | |||
| SK-LC-1 | 16.9 | 18.7 | ND | |
| SK-LC-2 | 0.0 | 16.7 | − | Inactive Jak2 |
| SK-LC-4 | 59.5 | 36.7 | + | |
| SK-LC-7 | 0.0 | 37.2 | − | Lacks IFN-γR α chain |
| SK-LC-9 | 49.3 | 53.4 | + | |
| SK-LC-10 | 31.7 | 22.6 | ND | |
| SK-LC-11 | 25.3 | 13.8 | ND | |
| SK-LC-12 | 30.4 | 12.7 | ND | |
| SK-LC-15 | 91.3 | 79.6 | + | |
| SK-LC-16 | 27.0 | 10.9 | + | |
| SK-LC-19 | 0.0 | 25.1 | − | Abnormal phosphorylated Jak2 |
| SK-LC-20 | 25.6 | 25.9 | + | |
| SK-LU-1 | 47.1 | 16.7 | + | |
| A457 | 27.7 | 27.1 | ND | |
| A549 | 36.9 | 21.8 | ND | |
| CALU-3 | 30.4 | 17.0 | ND | |
| CALU-5 | 0.0 | 0.0 | − | Lacks Jak1 protein |
| Total | 4/17 (23.5%) | 1/17 | ||
Four of 17 human lung adenocarcinoma tumor cell lines examined lack sensitivity to IFN-γ. Human lung adenocarcinoma cell lines were cultured in the presence of buffer, human IFN-γ (1,000 IRU/ml), or human IFN-αA/D (1,000 units/ml) for 72 hr followed by quantitation of MHC class I surface expression by flow cytometry by using a murine polyclonal antibody specific for framework regions of human MHC class I. Data represent the mean channel shift (MCS) between stimulated and unstimulated samples. To assess IFN-γ-dependent Stat1 activation, cells were stimulated with either PBS or human IFN-γ and subjected to electrophoretic mobility-shift assay (EMSA) using a 32P-labeled GRR probe. All cell lines were of human origin and displayed aneuploidy. In addition, SK-LC-1, -2, -7, -10, -12, and -19 and CALU-5 were tested for expression of the F19 cell surface marker (32) by using a rosetting assay and were found to be negative, thereby ruling out the possibility that the cells were of fibroblast origin. ND, not determined.
All of the lung adenocarcinomas also were tested for the ability to up-regulate MHC class I in response to stimulation with IFN-α. IFN-α signal transduction shares two intracellular signaling molecules with IFN-γ. Thus, if IFN-γ insensitivity were the result of mutations that randomly occur in multiple signal transduction pathways, then a similar number of tumors with insensitivity to IFN-α should be identified. None of the tumors demonstrated a selective lack of IFN-α sensitivity. However, one tumor (CALU-5) lacked sensitivity to both IFN-α and IFN-γ (Table 1). Thus, at least certain types of human tumors show a tendency to develop a selective insensitivity to IFN-γ, a phenotype that may provide the developing tumor with a growth advantage in vivo.
Identification of the IFN-γ Signaling Protein Defect in IFN-γ-Insensitive Human Lung Adenocarcinomas.
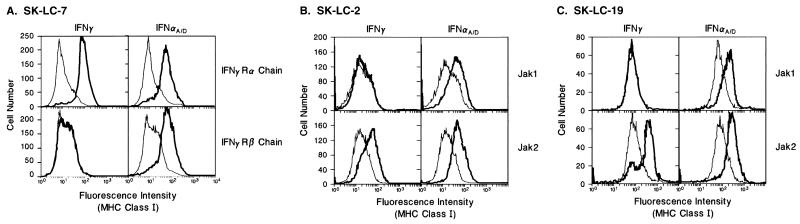
Of note, all of the tumors that failed to develop a biologic response to IFN-γ also failed to initiate IFN-γ signaling. This observation localized the biochemical lesions present in these tumors to the five proteins responsible for initiation of IFN-γ signaling [i.e., the IFN-γ receptor α and β subunits, Jak1, Jak2, and Stat1 (3)]. By examining expression of these five signaling proteins in each of the IFN-γ-insensitive tumor cell lines and by stably transfecting each tumor line with individual expression plasmids encoding these proteins, we have identified the defective signaling component in each cell line. SK-LC-7 lacks expression of the IFN-γR α chain and was reconstituted only after expression of this protein (Fig. 4A). Similarly, SK-LC-2 and SK-LC-19 produce abnormal Jak2 proteins, and their ability to respond to IFN-γ could only be restored after expression of wild-type Jak2 (Fig. 4 B and C). The fourth IFN-γ-insensitive tumor cell line, CALU-5, could not be transfected. However, Western blot analysis revealed that it lacks expression of Jak1 (data not shown). The insensitivity of this cell line to both IFN-γ and IFN-α thus is consistent with the absence of Jak1, one of two JAK-STAT pathway components that are used in common by both the IFN-γ and IFN-α receptor systems.
Figure 4.
IFN-γ responsiveness can be restored to SK-LC-7 by enforced expression of the IFN-γR α chain and to SK-LC-2 and SK-LC-19 by expression of Jak2. The ability of cells to enhance expression of MHC class I proteins is measured after 72 hr of incubation with 1,000 IRU/ml of either HuIFN-γ (Left) or HuIFN-αA/D (Right) (thick lines) or buffer (thin lines). (A) SK-LC-7 cells transfected with expression plasmids encoding either the HuIFN-γR α chain (Upper) or HuIFN-γR β chain (Lower). (B) SK-LC-2 cells transfected with plasmids encoding either Jak1 (Upper) or Jak2 (Lower). (C) SK-LC-19 cells transfected with plasmids encoding Jak1 (Upper) or Jak2 (Lower).
DISCUSSION
This study shows that IFN-γ plays a central role in providing an immunocompetent host with a mechanism of tumor surveillance. This system is operative for both chemically induced and spontaneously arising tumors. Thus it appears to represent a generalized mechanism for controlling the development of at least certain primary neoplasms in mice and humans.
Our results indicate that the key target of IFN-γ’s actions is the transformed cell itself and that IFN-γ is acting to enhance the recognition of the transformed cell by the immune system. Our hypothesis predicts that immune recognition of a transformed cell that secondarily acquires a defect in the IFN-γ signaling pathway will be decreased. Thus this cell may go undetected in an immunocompetent host and eventually develop into a progressively growing tumor that may have an extremely aggressive clinical course. This concept is supported by the finding of naturally occurring human tumors that have developed a permanent and selective IFN-γ insensitivity. Thus identification of IFN-γ-insensitive tumors may have prognostic and/or therapeutic relevance.
The concept that the immune system plays an important role in the elimination of transformed cells was conceived originally in 1909 by Paul Ehrlich, who suggested that cancer would occur at high frequency if host defenses did not prevent the outgrowth of continuously arising transformed cells (23). Almost 50 years later, this hypothesis was revived by Thomas (24) and further elaborated by Burnet (25), who proposed that T cells would function as the major effectors in this system. This refinement resulted in the coining of the term “immune surveillance” (25), which embodied the concept that the immune system was responsible for elimination of spontaneously arising tumors. However, the theory of immune surveillance against tumors of nonviral origin came under strong attack in the 1970s when Stutman (26, 27) noted that nude mice, which, at the time, were thought to be completely devoid of T cells, failed to display increased sensitivity to tumor induction by chemical carcinogens and did not develop spontaneous tumors at higher frequency compared with normal mice.
Recent insights into the nature of tumor antigens and the mechanisms by which they are recognized by the immune system have led to a renewed interest in resolving this important issue (28–31). Based on the extensive amount of information now available concerning IFN-γ biology (1–3), it is known that this cytokine plays a key role in promoting antigen processing and presentation via both the MHC class I and class II pathways. Thus it can be speculated that IFN-γ, by inducing in tumor cells specific cellular proteins involved in antigen processing/presentation, may force the transformed cell to up-regulate expression of the appropriate antigenic peptide(s), leading to its recognition and elimination by the immune system. IFN-γ, therefore, plays a central role in promoting tumor surveillance, and this system may in fact be the basis of immune surveillance.
Although our data suggest that IFN-γ may effect its tumor surveillance functions through mechanisms involving adaptive immunity, this mechanism does not preclude other actions of IFN-γ that also could promote destruction of transformed cells. IFN-γ may activate, recruit, or enhance the production of cells such as NK cells, macrophages, and neutrophils that may promote innate antitumor responses. Moreover, IFN-γ may indeed have antimetabolic or antiproliferative effects on certain types of tumor cells. Finally, IFN-γ may promote elimination of transformed cells either directly or indirectly through nonimmune mechanisms such as those involving angiostatic actions that restrict tumor growth by interfering with the development of a blood supply to the growing tumor. Thus, although our results unequivocally demonstrate the existence of an IFN-γ tumor surveillance process, the molecular basis of this system remains to be elucidated.
The data presented in this report support the concept that IFN-γ’s actions on tumor cells serve an important tumor-suppressor function. Unlike traditional intrinsic tumor-suppressor factors, such as p53, which inhibit transformation, IFN-γ works extrinsically together with the adaptive immune response and potentially with other host effector systems to control the progression of transformed cells into successfully established tumors. In sum, our studies add insights into the initial events of host antitumor responses and emphasize the importance of IFN-γ in providing the naive host with an effective mechanism of tumor surveillance.
Acknowledgments
The authors are grateful to Marie La Regina for veterinary pathology and murine tumor typing services. The authors thank Osami Kanagawa and Nils Jacobson (Department of Pathology, Washington University School of Medicine) for helpful discussions. This work was supported by an M.D./Ph.D. fellowship awarded by the American Life and Health Insurance Fund (D.H.K.) and Grant CA43059 from the National Institutes of Health (R.D.S.).
ABBREVIATIONS
- IFN-γ
interferon γ
- TNFα
tumor necrosis factor α
- IL
interleukin
- MCA
3-methylcholanthrene
- MHC
major histocompatibility complex
References
- 1.Farrar M A, Schreiber R D. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 2.Boehm U, Klamp T, Groot M, Howard J C. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 3.Bach E A, Aguet M, Schreiber R D. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 4.Darnell J E, Jr, Kerr I M, Stark G R. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 5.Meraz M A, White J M, Sheehan K C F, Bach E A, Rodig S J, Dighe A S, Kaplan D H, Riley J K, Greenlund A C, Campbell D, et al. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 6.Durbin J E, Hackenmiller R, Simon M C, Levy D E. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 7.Dalton D K, Pitts-meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 8.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R, Aguet M. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 9.Rodig S J, Meraz M A, White J M, Lampe P A, Riley J K, Arthur C D, King K L, Sheehan K C F, Yin L, Pennica D, Johnson E M, Schreiber R D. Cell. 1998;93:373–383. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 10.Parganas E, Wang D, Stravopodis D, Topham D J, Marine J C, Teglund S, Vanin E, Bodner S, Colamonici O R, van Deursen J M, Grosveld G, Ihle J N. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 11.Newport M J, Huxley C M, Huston S, Hawrylowicz C M, Oostra B A, Williamson R, Levin M. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 12.Jouanguy E, Altare F, Lamhamedi S, Revy P, Newport M, Levin M, Blanche S, Fischer A, Casanova J-L. N Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 13.Pierre-Audigier C, Jouanguy E, Lamhamedi S, Altare F, Rauzier J, Vincent V, Canioni D, Emile J-F, Fischer A, Blanche S, Gaillard J-L, Casanova J-L. Clin Infect Dis. 1997;24:982–984. doi: 10.1093/clinids/24.5.982. [DOI] [PubMed] [Google Scholar]
- 14.Dighe A S, Richards E, Old L J, Schreiber R D. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 15.Rogers H W, Sheehan K C F, Brunt L M, Dower S K, Unanue E R, Schreiber R D. Proc Natl Acad Sci USA. 1992;89:1011–1015. doi: 10.1073/pnas.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schreiber R D, Hicks L J, Celada A, Buchmeier N A, Gray P W. J Immunol. 1985;134:1609–1618. [PubMed] [Google Scholar]
- 17.Farrar M A, Fernandez-Luna J, Schreiber R D. J Biol Chem. 1991;266:19626–19635. [PubMed] [Google Scholar]
- 18.Bach E A, Tanner J W, Marsters S A, Ashkenazi A, Aguet M, Shaw A S, Schreiber R D. Mol Cell Biol. 1996;16:3214–3221. doi: 10.1128/mcb.16.6.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacks T, Ramington L, Williams B O, Schmitt E M, Halachmi S, Bronson R T, Weinberg R A. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 20.Weber-Nordt R M, Riley J K, Greenlund A C, Moore K W, Darnell J E, Schreiber R D. J Biol Chem. 1996;271:27954–27961. doi: 10.1074/jbc.271.44.27954. [DOI] [PubMed] [Google Scholar]
- 21.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Nature (London) 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 22.Harvey M, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A, Donehower L A. Nat Genet. 1993;5:225–229. doi: 10.1038/ng1193-225. [DOI] [PubMed] [Google Scholar]
- 23.Ehrlich, P. (1909) Ned. Tijdschr. Geneeskd. 5 (Pt. 1), 273–290.
- 24.Thomas L. Discussion of Cellular and Humoral Aspects of Hypersensitivity States. New York: Hoeber–Harper; 1959. pp. 529–532. [Google Scholar]
- 25.Burnet F M. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 26.Stutman O. J Natl Cancer Inst. 1979;2:353–358. [PubMed] [Google Scholar]
- 27.Stutman O. Science. 1970;183:534–536. doi: 10.1126/science.183.4124.534. [DOI] [PubMed] [Google Scholar]
- 28.Boon T, Cerottini J C, Van den Eynde B, van der Bruggen P, Van Pel A. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 29.Urban J L, Schreiber H. Annu Rev Immunol. 1992;10:617–644. doi: 10.1146/annurev.iy.10.040192.003153. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber H, Ward P L, Rowley D A, Strauss H J. Annu Rev Immunol. 1988;6:465–483. doi: 10.1146/annurev.iy.06.040188.002341. [DOI] [PubMed] [Google Scholar]
- 31.van den Broek M F, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutza W K, Melief C J M, Zinkernagel R M, Hengartner H. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scanlan M J, Mohan R B K, Calvo B, Garin-Chesa P, Sanz-Moncasi M P, Healey J H, Old L J, Rettig W J. Proc Natl Acad Sci USA. 1994;91:5657–5661. doi: 10.1073/pnas.91.12.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]