Abstract
Objective
Nitric oxide (NO) has been shown to inhibit neointimal hyperplasia following arterial interventions in several animal models. However, to date NO-based therapies have not been used in the clinical arena. Our objective was to combine nanofiber delivery vehicles with NO chemistry to create a novel, more potent NO-releasing therapy that can be used clinically. Thus, the aim of this study was to evaluate perivascular application of spontaneously self-assembling NO-releasing nanofiber gels, and our hypothesis was that this application will prevent neointimal hyperplasia.
Methods
Gels consisted of a peptide amphiphile, heparin, and a diazeniumdiolate NO donor (1-[N-(3-Aminopropyl)-N-(3-ammoniopropyl]diazen-1-ium-1,2-diolate [DPTA/NO] or disodium 1-[(2-Carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate [PROLI/NO]). Nitric oxide release from the gels was evaluated by the Griess reaction, and scanning electron microscopy confirmed nanofiber formation. In vitro, vascular smooth muscle cell (VSMC) proliferation and cell death were assessed by 3H-thymidine incorporation and Guava PCA. For the in vivo work, gels were modified by reducing free water content. Neointimal hyperplasia following peri-adventitial gel application was evaluated using the rat carotid artery injury model at 14 days (n=6 per group). Inflammation and proliferation were examined in vivo with immunofluorescent staining against CD45, ED1 and Ki67 at 3 days (n=2 per group), and graded by blinded observers. Endothelialization was assessed by Evans blue injection at 7 days (n=3 per group).
Results
Both DPTA/NO and PROLI/NO, in combination with the peptide amphiphile and heparin, formed nanofiber gels and released NO for 4 days. In vitro, DPTA/NO inhibited VSMC proliferation and induced cell death to a greater extent than PROLI/NO. However, the DPTA/NO nanofiber gel only reduced neointimal hyperplasia by 45% (intima/media [I/M] area ratio 0.45±0.07) whereas the PROLI/NO nanofiber gel reduced neointimal hyperplasia by 77% (I/M area ratio 0.19±0.03, P<.05) versus control (injury alone I/M area ratio 0.83±0.07; P<.05). Both DPTA/NO and PROLI/NO nanofiber gels significantly inhibited proliferation in vivo (1.06±0.30, 0.19±0.11 vs injury alone, 2.02±0.20, P<.05), yet had minimal effect on apoptosis. Only the PROLI/NO nanofiber gel inhibited inflammation (monocytes and leukocytes). Both NO-releasing nanofiber gels stimulated re-endothelialization.
Conclusions
Perivascular application of NO-releasing self-assembling nanofiber gels is an effective and simple therapy to prevent neointimal hyperplasia following arterial injury. Our study demonstrates that the PROLI/NO nanofiber gel most effectively prevents neointimal hyperplasia and resulted in less inflammation than the DPTA/NO nanofiber gel. This therapy has great clinical potential to prevent neointimal hyperplasia following open vascular interventions in patients.
CLINICAL RELEVANCE
Atherosclerosis affects over 71 million Americans, many of whom require arterial intervention. Unfortunately, treatment modalities often fail secondary to the development of neointimal hyperplasia, necessitating re-intervention. It is well established that nitric oxide (NO) inhibits neointimal hyperplasia, but no NO-based therapies have been clinically applied due to various concerns. In this study, we demonstrate inhibition of neointimal hyperplasia in the rat carotid artery balloon-injury model using local application of a gel made from a combination of NO and self-assembling nanofibers. By inhibiting neointimal hyperplasia and subsequent restenosis following arterial interventions, we aim to improve long-term patency rates and reduce the number of repeat interventions.
INTRODUCTION
Atherosclerosis is prevalent in all developed nations and is the leading cause of death and disability in the United States. Deaths due to cardiovascular disease account for nearly 2,400 deaths per day, and it is estimated that $432 billion was spent last year alone in the United States on cardiovascular disease, with a significant portion being attributed to the cost of repeat interventions.1 While current treatment modalities improve blood flow, their long-term success is limited due to restenosis secondary to neointimal hyperplasia.
Nitric oxide (NO) has been shown to possess many different vasoprotective properties.2 These include inhibition of platelet aggregation,3 leukocyte chemotaxis,4 vascular smooth muscle cell (VSMC) proliferation and migration,5, 6 and endothelial cell apoptosis.7 Additionally, NO stimulates endothelial cell proliferation8 and is a potent vasodilator.9 These properties have led investigators to study the efficacy of NO-based therapies to inhibit the development of neointimal hyperplasia in small and large animal models of arterial injury, vein bypass grafting, and prosthetic bypass grafting.10-30 While each of these therapies has demonstrated varying degrees of success, none have been introduced to the clinical arena.
Our goal is to create a potent NO-releasing therapy that can be used clinically to inhibit neointimal hyperplasia by combining nanotechnology with NO chemistry. The therapy should be simple to use, safe, and free from any side effects. Diazeniumdiolates are a class of NO donors that release NO spontaneously when placed in an aqueous environment.31 These compounds release two moles of NO per mole of compound at varying rates depending on the specific diazeniumdiolate.31 For our delivery vehicle, we used a peptide amphiphile molecule, which consists of a hydrophobic, fatty acid segment and a hydrophilic peptide segment containing a sequence that binds to negatively-charged biopolymers, such as glycosoaminoglycan heparin sulfate [Figure 1B].32, 33 When placed in an aqueous environment, aggregation of the hydrophobic segments induces spontaneous assembly of the molecules into long nanofibers. The addition of heparin or other negatively-charged molecules promotes nanofiber growth and networking, forming a macroscopic gel [Figure 1C]. The nanofiber gels are biocompatible and serve as an elegant delivery vehicle for the adventitial application of NO. Therefore, the aim of this study was to examine the effect of NO-releasing nanofiber gels on the development of neointimal hyperplasia following arterial injury and our hypothesis was that our NO-based therapy would prevent neointimal hyperplasia.
Figure 1.
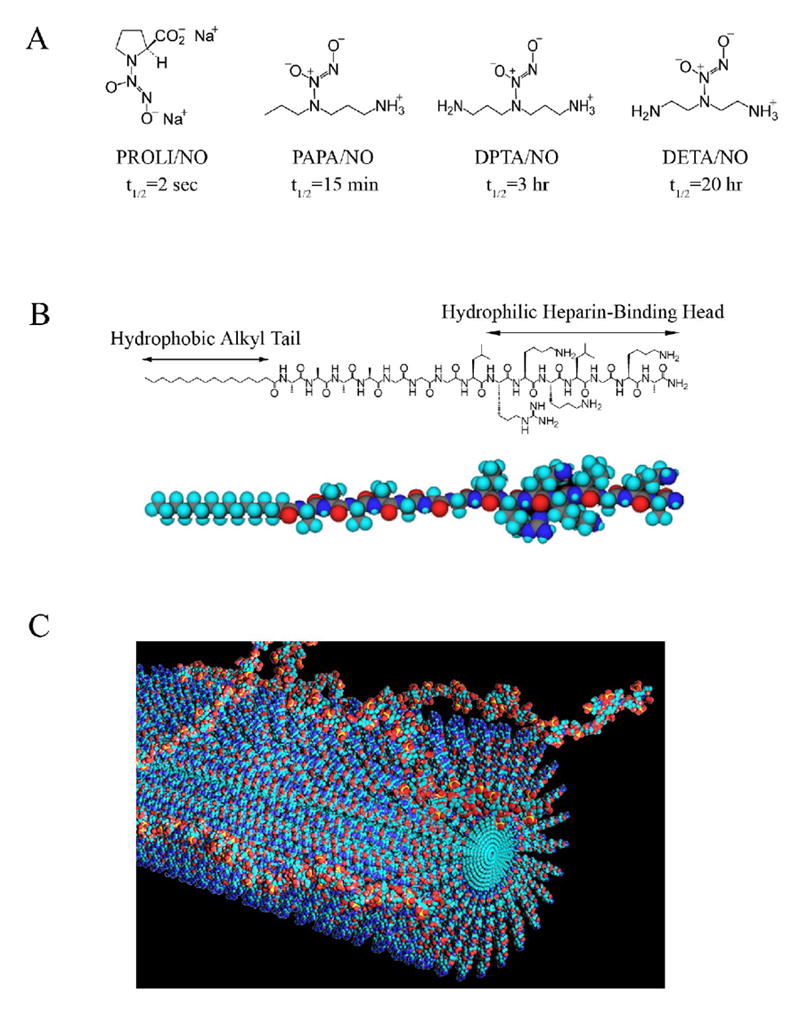
Our approach to inhibiting neointimal hyperplasia utilizes two components: A) diazeniumdiolate NO-donors, PROLI/NO, PAPA/NO, DPTA/NO and DETA/NO (half-lives listed are for physiologic conditions, 37°C and pH 7.4) and B) heparin-binding peptide amphiphile; C) The peptide amphiphiles spontaneously form nanofibers in aqueous solutions when exposed to heparin as depicted in this drawing.
METHODS
Nitric oxide-releasing nanofiber gels
The diazeniumdiolates evaluated in this study include disodium 1-[(2-carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate (PROLI/NO), 1-[N-(3-ammoniopropyl)-N-(n-propyl)amino]diazen-1-ium-1,2-diolate (PAPA/NO), 1-[N-(3-aminopropyl)-N-(3-ammoniopropyl]diazen-1-ium-1,2-diolate (DPTA/NO), 1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA/NO) [Figure 1A]. These were chosen based on their NO release rates as well as their safety profiles.31, 34 Gels were made by mixing equal volumes of the following in order: peptide amphiphile (synthesized as previously described32 and purified by high performance liquid chromatography) solution in ultrapure water (3 wt %); 1 mol/L diazeniumdiolate (prepared as previously described35, 36) in phosphate buffered saline (PBS); and heparin in PBS (2 wt %, heparin sodium salt, Sigma, St Louis, MO). To determine NO production from each of the gels, 100 μL of gel was submerged in 500 μL of fresh PBS and maintained at 37°C. Every 24 hours, the supernatant was removed to measure nitrite release (an indirect determinant of NO production) by the Griess reaction.37 Another 500 μL of fresh PBS was then added each day, and the cycle was repeated until the gels no longer released NO.
For in vivo experiments, the gel recipe was modified to reduce free-water content. PROLI/NO or DPTA/NO (10 mg) was dissolved directly in 100 μL of 3 wt % heparin (in PBS, pH 12.1 and 10.4, respectively, room temperature). This solution was added to 100 μL of 4.5 wt % peptide amphiphile (pH 7.4, room temperature) in deionized water. The final solution was placed on a glass slide to gel ex vivo for 10 minutes prior to application. The final pH for the PROLI/NO and DPTA/NO nanofiber gels was 10.87 and 10.27, respectively. The control gel was made up of 100 μL of 3wt% heparin and 100 μL of 4.5wt% peptide amphiphile; it did not contain either PROLI/NO or DPTA/NO.
Scanning electron microscopy
Gels were dehydrated in a graded ethanol series and dried by the critical point method in a Samdri 790A critical point drying apparatus (Tousimis Research Corporation, Rockville, MD). The dried samples were sputter-coated with 3 nm of gold/palladium using a Cressington Sputter Coater (Cressington Scientific Instruments, Watford, England) before imaging with a Hitachi S-4800 scanning electron microscope (Hitachi Kokusai Electric America, Woodbury, NY).
Cell culture
Vascular smooth muscle cells were isolated and cultured from the abdominal aorta of Sprague-Dawley rats (Harlan, Indianapolis, IN) using the explant method38 and maintained as previously described.37
Proliferation assay
Vascular smooth muscle cells plated in 12-well plates (4 × 104 cells/well) were growth-arrested for 24 hours with starvation media (containing no FBS). Cells were then exposed to media containing one of the two NO donors (PROLI/NO or DPTA/NO powder) in the presence of tritiated (3H) thymidine (5 μCi/mL, PerkinElmer, Wellesley, MA) for an additional 24 hours. 3H-thymidine incorporation into trichloroacetic acid–precipitated DNA was quantified by scintillation counting.
Cell death assay
Vascular smooth muscle cells plated in 6-well plates (1 × 105 cells/well) were growth-arrested for 24 hours with starvation media, after which they were exposed to media with one of the two NO donors (PROLI/NO or DPTA/NO powder) for 24 hours. Cells were trypsinized, collected, pelleted, and re-suspended in PBS (250 μL). This suspension (40 μL) was added to 160 μL of Guava ViaCount Reagent and cell death was assessed using Guava PCA (Guava Technologies, Hayward, CA).
Animal surgery
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication 85-23, 1996) and approved by the Northwestern University Animal Care and Use Committee. Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 350-400 gm underwent carotid artery balloon injury as previously described.20, 37 After injury and restoration of flow, one of the NO-eluting nanofiber gels or control therapies (200 μL) was applied to the external surface of the injured common carotid artery and the neck incision was closed. Rats were sacrificed at 14-day (n=6 per group), 7-day (n=3 per group) and 3-day (n=2 per group) time points.
Tissue processing
Carotid arteries were harvested following in-situ perfusion-fixation with PBS (250 mL) and 2% paraformaldehyde (500 mL). Tissue was processed as previously described.20
Morphometric analysis
Carotid arteries harvested at 14 days were examined histologically for evidence of neointimal hyperplasia using routine hematoxylin-eosin (H&E) staining. A modified Verhoeff von Gieson stain was used to evaluate elastin and collagen. Digital images were collected with light microscopy using an Olympus BHT microscope (Melville, NY) with 4X, 10X and 40X objectives. Six evenly-spaced sections through each injured carotid artery were morphometrically analyzed. Lumen area, Intimal area (I), medial area (M), and external elastic lamina circumference were measured (arbitrary units) using ImageJ software (National Institutes of Health, Bethesda, MD).
Immunohistochemistry
Carotid arteries harvested at 3 days were examined for evidence of inflammation and proliferation using immunofluorescent staining. Sections fixed with 2% paraformaldehyde or acetone were permeabilized with Triton-X100 in PBS. Sections were then blocked with goat serum (Sigma, St Louis, MO) in 0.5% bovine serum albumin (BSA; Vector, Burlingame, CA). Primary antibody in BSA was applied for 1 hour: anti-ED1 (macrophage, 1:500; Serotec, Raleigh, NC), anti-CD45 (lymphocyte, 1:500; BD Pharmingen, San Diego, CA) or anti-Ki67 (1:100; BD Biosciences, San Jose, CA). Secondary antibody in BSA was applied for 1 hour (goat anti-mouse Alexa Fluor 555, 1:3000; Invitrogen, Carlsbad, CA). Coverslips were affixed with gelvatol. From each animal, six sections from the area of injury were stained. Eight independent blinded observers qualitatively graded the inflammation and proliferation in each treatment group on a scale of 0-3. Digital images were acquired using a Zeiss LSM-510 microscope (Hallbergmoos, Germany) at 40X.
Apoptosis was evaluated in carotid arteries harvested at 3 and 14 days by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) using a commercial system according to manufacturer instructions (DeadEnd Colorimetric TUNEL system; Promega, Madison, WI).
Endothelialization
Rat carotid arteries were harvested at 7 days. Thirty minutes prior to sacrifice, rats received an intravenous njection of Evans blue dye (0.5 mL of 0.5%, Sigma). Carotid arteries were procured following in-situ perfusion with 500 mL of PBS and photographed. Blue staining indicated area of increased endothelial permeability. Images were qualitatively analyzed by two independent observers.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Differences between multiple groups were analyzed using one-way analysis of variance (ANOVA) with the Student-Newman-Keuls post hoc test for all pairwise comparisons (SigmaStat; SPSS, Chicago, IL). Statistical significance was assumed when P<.05.
RESULTS
Nitric oxide release from nanofiber gels
Initially, the diazeniumdiolate nanofiber gels tested included PROLI/NO, PAPA/NO, DPTA/NO, and DETA/NO. When combined with peptide amphiphile and heparin, DETA/NO and PAPA/NO did not form a consistent gel; instead, the majority of the product remained liquid. Therefore, we chose to proceed using the PROLI/NO and DPTA/NO nanofiber gels only.
To determine the amount and duration of NO release from the gels (100 μL), Griess reactions were conducted. Nitrite release was observed for 4 days from both the PROLI/NO and DPTA/NO nanofiber gels, with the majority of nitrite release occurring in the first 2 days [Figure 2A]. The PROLI/NO nanofiber gel appeared to have steady nitrite release for the initial 2 days, whereas the DPTA/NO nanofiber gel had similar release on Day 1 compared to the PROLI/NO nanofiber gel, but on Day 2 the nitrite release doubled. Both PROLI/NO and DPTA/NO nanofiber gels had a significant decline in NO release on Day 3 and 4, and by Day 5 there was no difference when compared to control gels. Scanning electron microscopy conducted on the nanofiber, PROLI/NO nanofiber, and DPTA/NO nanofiber gels revealed distinct nanofiber formation [Figures 2B-D].
Figure 2.
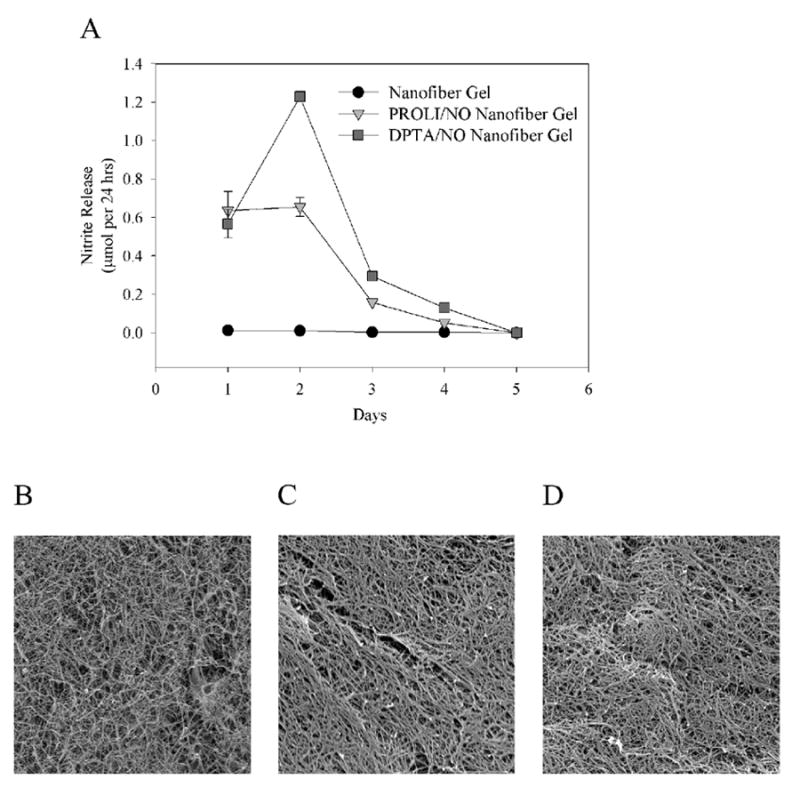
A) Nitrite release from nanofiber, PROLI/NO nanofiber and DPTA/NO nanofiber gels in vitro, determined using the Griess reaction, n=2 per group. Data are representative of 3 separate experiments. Scanning electron microscopy images (4000x magnification) of the B) nanofiber gel, C) PROLI/NO nanofiber gel, and D) DPTA/NO nanofiber gel.
PROLI/NO and DPTA/NO inhibit VSMC proliferation in vitro
In an effort to characterize the effects of PROLI/NO and DPTA/NO on proliferation of VSMC, an in vitro 3H-thymidine proliferation assay was conducted [Figure 3A-B]. The highest concentration of PROLI/NO (1000 μM) inhibited VSMC proliferation by 32% (P<.05). While there was a trend toward less proliferation with lower concentrations of PROLI/NO (125-500 μM), there was no statistically significant effect versus control. In contrast, the longer-acting NO donor, DPTA/NO, induced a concentration-dependent inhibition of VSMC proliferation (125-1000 μM; P<.05). Furthermore, the antiproliferative effect of DPTA/NO was more potent than PROLI/NO, as DPTA/NO inhibited VSMC proliferation by 51-78% (P<.05).
Figure 3.
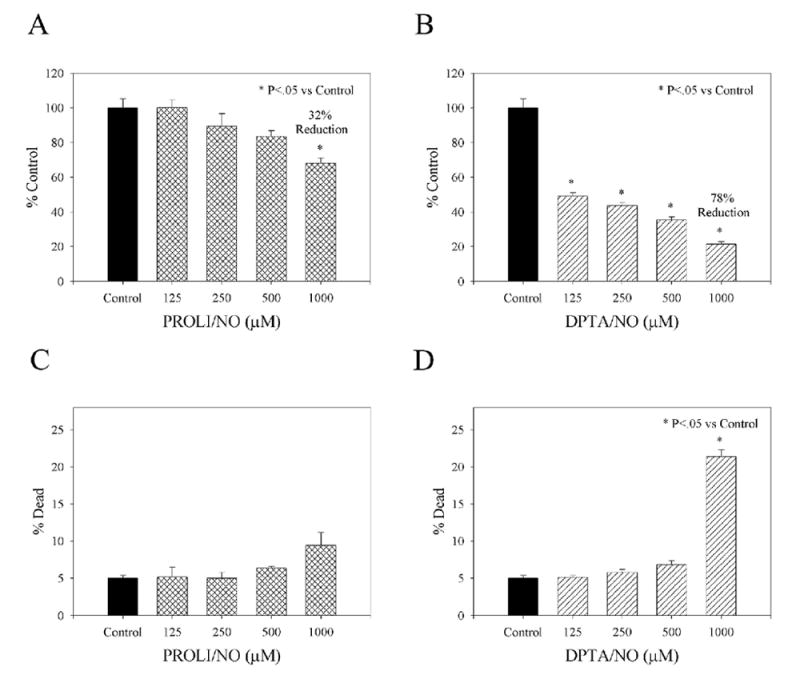
Effect of PROLI/NO and DPTA/NO on vascular smooth muscle cell proliferation (A, B) and cell death (C, D) in vitro. Proliferation and cell death were quantified using tritiated thymidine and Guava PCA, respectively, n=3 per group. Data are representative of 3 separate experiments.
PROLI/NO and DPTA/NO induce minimal VSMC death in vitro
To determine if PROLI/NO and DPTA/NO induce VSMC death in vitro, the Guava PCA system was implemented [Figure 3C-D]. While not statistically significant, there was a trend towards increased cell death with increasing concentrations of PROLI/NO (125-1000 μM). At lower concentrations, DPTA/NO (125-500 μM) did not induce statistically significant VSMC death. However, at the highest concentration evaluated, DPTA/NO (1000 μM) induced 21.4±0.9% cell death in VSMC (P<.05).
Nitric oxide-releasing nanofibers inhibit neointimal hyperplasia in the rat carotid artery injury model
Because of the multiple-day NO-release, and the ability of PROLI/NO and DPTA/NO to form gels with peptide amphiphile and heparin, we proceeded to examine the in vivo effect of gel application on neointimal hyperplasia following rat carotid artery balloon injury [Figure 4]. Importantly, the gel was applied to the adventitial surface of the carotid artery only. Balloon injury produced reproducible neointimal hyperplasia at 14 days [Table 1 and Figure 4A]. With the application of the nanofiber control gel, there was no statistically significant difference versus injury alone for either the intimal area (3.20±0.34 vs 3.63±0.35, respectively) or the I/M area ratio (0.69±0.07 vs 0.83±0.07, respectively). However, with application of PROLI/NO and DPTA/NO nanofiber gels, there was significant inhibition of neointimal hyperplasia. The PROLI/NO nanofiber gel showed an 80% reduction of intimal area (0.71±0.14 vs injury alone, P<.05) and a 77% reduction of I/M area ratio (0.19±0.03 vs injury alone, P<.05). While not as impressive, the DPTA/NO nanofiber gel demonstrated modest inhibition of neointimal hyperplasia: 40% reduction of intimal area (2.19±0.34 vs injury alone, P<.05) and 45% reduction of I/M area ratio (0.45±0.07 vs injury alone, P<.05). There was a statistically significant difference in inhibition of neointimal hyperplasia between PROLI/NO and DPTA/NO nanofiber gels (P<.05). There was no statistically significant difference between the medial areas for injury alone, nanofiber control gel and DPTA/NO nanofiber gel groups; however, the PROLI/NO nanofiber gel treatment resulted in a 15% medial area reduction versus injury alone (P<.05). There was no difference in the circumference of the external elastic lamina between the groups. Therefore, it appears that our NO-nanofiber gels had very little effect on adaptive vascular remodeling while having a large effect on inhibiting the development of neointimal hyperplasia.
Figure 4.
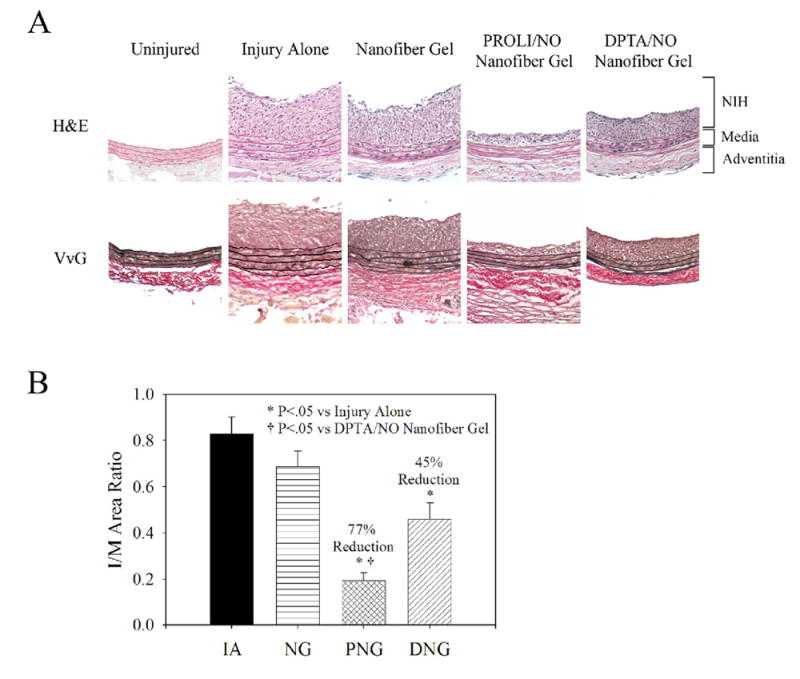
Rat carotid artery sections from uninjured, injury alone, nanofiber gel, PROLI/NO nanofiber gel, and DPTA/NO nanofiber gel animals sacrificed at 14 days (n=6 per group). A) Representative sections (100x magnification) from each group using routine Hematoxylin and Eosin (H&E) stain and Verhoff-van Gieson (VvG) stain. Graphical representation of B) Intima/Media (I/M) area ratio. Morphometric analysis conducted on 6 sections per rat. Units are arbitrary. IA=injury alone, NG=nanofiber gel, PNG=PROLI/NO nanofiber gel, and DNG=DPTA/NO nanofiber gel.
Table I.
Morphometric analysis of carotid arteries 14 days after balloon injury.
| Treatment Group | Circumference | Lumen Area | Intimal Area | Medial Area | I/M Area Ratio | I/(I+M) |
|---|---|---|---|---|---|---|
| Injury Alone‡ | 1.64 ± 0.03 | 8.53 ± 0.77 | 3.63 ± 0.35 | 4.37 ± 0.17 | 0.83 ± 0.07 | 0.42 ± 0.02 |
| Nanofiber Gel‡ | 1.64 ± 0.02 | 12.00 ± 0.31* | 3.20 ± 0.34 | 4.56 ± 0.16 | 0.69 ± 0.07 | 0.38 ± 0.02 |
| PROLI/NO Nanofiber Gel‡ | 1.68 ± 0.02 | 15.57 ± 0.43* | 0.71 ± 0.14*† | 3.70 ± 0.17*† | 0.19 ± 0.03*† | 0.14 ± 0.02* |
| DPTA/NO Nanofiber Gel‡ | 1.68 ± 0.03 | 12.75 ± 0.66* | 2.19 ± 0.34* | 4.59 ± 0.17 | 0.45 ± 0.07* | 0.26 ± 0.03* |
P<.05 versus Injury Alone
P<.05 versus DPTA/NO Nanofiber Gel
n=6 rats, 6 sections per rat
Nitric oxide-releasing nanofiber gels inhibit inflammation and proliferation in vivo
To characterize the effect of the PROLI/NO and DPTA/NO nanofiber gels on neointimal hyperplasia at a cellular level, we conducted immunohistochemical staining for markers of inflammation and proliferation at 3 days [Figure 5]. Similar numbers of monocytes (anti-ED1) were seen in the injury alone, nanofiber and DPTA/NO groups. However, in the PROLI/NO group, there was a reduction in the observed mononuclear infiltrate versus injury alone. When examining the CD45-positive leukocytes, the injury alone and DPTA/NO groups appeared to have similar leukocyte infiltration. However, nanofiber and PROLI/NO nanofiber groups had fewer leukocytes when compared to injury alone. To summarize, the DPTA/NO nanofiber gel appeared to have no effect on inflammation when compared to injury alone, whereas the PROLI/NO nanofiber gel appeared to inhibit inflammation following arterial injury.
Figure 5.
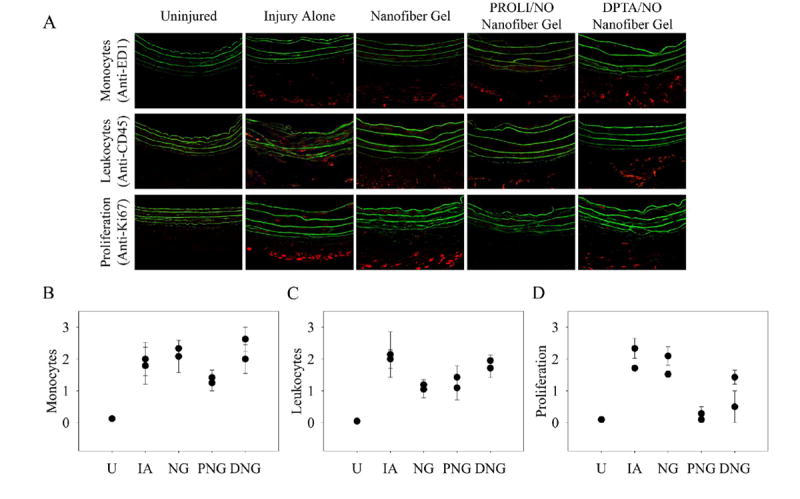
Immunofluorescence staining for markers of inflammation and proliferation. A) Representative sections (400x magnification) from uninjured, injury alone, nanofiber gel, PROLI/NO nanofiber gel, and DPTA/NO nanofiber gel treatment groups (n=2 per group, 3 sections per animal). Positive staining is indicated by red and elastic lamina is green. Graphical representation of scoring by 8 blinded observers (scale 0-3) for B) monocytes (anti-ED1), C) leukocytes (anti-CD45) and D) proliferation (anti-Ki67). Each data point represents scoring for a particular animal. U=uninjured, IA=injury alone, NG=nanofiber gel, PNG=PROLI/NO nanofiber gel, and DNG=DPTA/NO nanofiber gel.
Next we determined the effect of the nanofiber gels on cellular proliferation (anti-Ki67) in vivo. Injury alone exhibited the most proliferation, and similar levels were observed with the nanofiber gel; the majority of proliferating cells appeared to be in the adventitia. Both PROLI/NO and DPTA/NO nanofiber gels demonstrated an inhibition of cellular proliferation in vivo, and the effect appeared to be greatest on the adventitial cells; however, the PROLI/NO nanofiber gel seemed to inhibit proliferation dramatically, whereas the DPTA/NO nanofiber gel inhibited proliferation modestly. This pattern mirrored the effect on neointimal hyperplasia.
Nitric oxide-releasing nanofiber gels induce little apoptosis in vivo
We sought to determine whether the NO-releasing nanofiber gels limited neointimal hyperplasia by inducing apoptosis in vivo. Sections stained using TUNEL did not reveal any distinct patterns, and showed little apoptosis among the different treatment groups [data not shown]. These data are fairly consistent with the in vitro data.
Nitric oxide-releasing nanofiber gels stimulate reestablishment of the endothelial barrier following arterial injury
To determine the effect of the NO-releasing nanofiber gels on the endothelium following balloon injury, animals were injected with Evans blue dye pre-mortem; arterial segments with increased endothelial permeability stained blue [Figure 7]. By qualitative analysis, untreated carotid arteries and arteries treated with nanofiber gel exhibited the greatest amount of blue staining. Arteries treated with PROLI/NO nanofiber gel demonstrated a very small amount of blue staining, and those treated with DPTA/NO nanofiber gel had little to no staining, suggesting little endothelial permeability by 7 days. These data suggest that both NO-releasing nanofiber gels stimulate reestablishment of an intact endothelial layer.
DISCUSSION
Neointimal hyperplasia develops following arterial injury and endothelial denudation through a series of events that includes platelet adherence and aggregation, leukocyte chemotaxis, and VSMC and fibroblast proliferation and migration.39-42 This cascade often results in luminal narrowing and restenosis. We examined the effects of two NO-releasing nanofiber gels on neointimal hyperplasia in an attempt to develop a simple-to-use, potent, NO-based clinical therapy.
An unexpected result from this research is that in vitro, PROLI/NO had little effect, whereas DPTA/NO demonstrated both inhibition of VSMC proliferation, and induction of cell death. The NO-eluting nanofiber gels created using these two diazeniumdiolates exhibited prolonged nitrite release (a decomposition product of NO) over 4 days, indicating either an increased half-life for the NO donor in the nanofiber gel environment by shielding it from hydrogen ions, or prolonged retention of NO itself within the gel. NO retention within the hydrophobic cores of the nanofiber would be expected, given NO’s low dipole moment. Furthermore, Moller et al demonstrated NO partitioning to the hydrophobic phase of biological membranes.43 While the exact disposition of NO and its diazeniumdiolate precursor in the nanofiber gels will require further study, an enhanced therapeutic effect of NO in this novel delivery vehicle is evident.
Several investigators have successfully demonstrated inhibition of neointimal hyperplasia using NO-based approaches in animal models.10-30 These approaches include inhalational NO therapy,10 L-arginine supplementation,11-13 systemic administration of NO donors,14 NOS gene therapy,15-21 and local delivery of NO donors, both intraluminal and perivascular.22-27, 30 However, none of these therapies have been implemented clinically due to various concerns, including lack of significant and durable effect, systemic side effects, safety concerns, and complicated delivery schemes. Our novel therapy utilizes periadventitial delivery. Because NO is freely diffusible, it penetrates all layers of the vascular wall. Thus, adventitial delivery effectively reduces neointimal hyperplasia by inhibiting both adventitial fibroblasts and medial VSMC. Another advantage of localized therapy is that the NO delivery is concentrated at the site of injury. This avoids systemic side effects, such as hypotension, coagulopathy and headaches. Kown et al demonstrated that L-arginine polymer-treated vein grafts exhibited 43% less neointimal hyperplasia in a rabbit vein bypass model.30 Kaul et al applied a biodegradable polymer (polylactic-polyglycolic acid) containing SPER/NO (2.5% w/w) to the periadventitial surface of balloon-injured rat ileofemoral arteries; this resulted in a 69% reduction in intimal area.26 Chaux et al used the same biodegradable polymer with SPER/NO in a rabbit jugular vein grafting model. At 28 days there was a 41% reduction in intimal area versus control.24 Two studies from Dr. West’s group utilized polyethylene glycol (PEG)-based hydrogels covalently modified with S-nitrosocysteine groups.44, 45 Following rat carotid artery balloon injury, the hydrogels were applied to the periadventitial arterial surface. Photopolymeraization of the hydrogels was conducted with a UV light, and NO release was observed for approximately 24 hours. In arteries harvested at 14 days, a 75% inhibition of neointimal hyperplasia was seen. Thus, these studies demonstrate, similar to our study, that perivascular NO application is effective in reducing neointimal hyperplasia.
Our NO-eluting nanofiber therapy has several additional attractive features. The first is the use of customizable nanostructures formed from peptide amphiphiles as a delivery vehicle. These molecules spontaneously assemble into nanofiber gels under physiological conditions, and similar nanofibers support growth and differentiation of various cell types in vitro.46, 47 The spontaneous formation is important as it requires no additional activators, such as UV light. Thus, our approach is simple to use. In addition, peptide amphiphile nanofibers have been used as bioactive coatings for tissue engineering implants,48 and preliminary testing in murine models has revealed no large-scale immune response to the nanofibers.49 Recently, Rajangam et al reported a peptide amphiphile molecule that was designed to bind heparin.32 Heparin-binding nanofibers gels were shown in vitro and in vivo to bind and control the bioavailability of various growth factors (VEGF and FGF-2),32 presenting another potential therapeutic axis for inhibiting neointimal hyperplasia. In vivo testing of this molecule in other animal models is underway, and thus far has revealed no significant biocompatibility issues (unpublished data). The other component of our approach utilizes diazeniumdiolates. Diazeniumdiolates are advantageous because of their predictable, spontaneous NO release in an aqueous environment, and they can be tailored to a wide range of NO release rates.31 Thus, our unique approach to marry these two entities, peptide amphiphiles and the diazeniumdiolates, has several beneficial therapeutic qualities. Additionally, an important physical characteristic of our therapy is that the peptide amphiphile and heparin solutions are stable when refrigerated. When combined with the NO-heparin solution, the self-assembling nature of the peptide amphiphile system allows the nanofibers to form a gel nearly instantly, and, thus, requires very little preparation time. Lastly, when the therapy is applied to the injured area, the gel adheres, ensuring that the treatment reaches the targeted location as accurately as possible.
An interesting finding is the opposing results from the in vitro and in vivo studies. DPTA/NO more effectively inhibited VSMC proliferation and induced VSMC cell death in vitro, yet the PROLI/NO nanofiber gel more successfully inhibited neointimal hyperplasia in vivo. This may be occurring for several reasons. The PROLI/NO powder has a very short half-life and therefore, in vitro, its effect may not be sustained enough to exert an effect. When administered as part of a gel, the PROLI/NO nanofiber gel was shown to release NO for 4 days, and this prolonged NO release may partially increase the effect seen. Additionally, the PROLI/NO nanofiber gel inhibited inflammation, as evidenced by less monocyte and leukocyte infiltration, whereas the DPTA/NO nanofiber gel did not. Inhibition of leukocyte infiltration would impact the arterial injury cascade by limiting growth factor and cytokine secretion and subsequent VSMC proliferation and migration. Thus, these properties of the PROLI/NO nanofiber gel may contribute to its heightened efficacy.
Another fascinating aspect of this research is the effectiveness of the NO donors with use of the nanofiber delivery vehicle. Prior work in our laboratory examined the effect of 20 mg of PROLI/NO powder applied to the adventitial surface of the rat carotid artery following balloon injury, and demonstrated 86% inhibition of neointimal hyperplasia at 14 days, as measured by the I/M area ratio (unpublished data). In this study, half as much PROLI/NO (10 mg) resulted in a similar degree of inhibition (77%), and this may be because the gel application is more controlled, and it allows for more even distribution of the drug on the entire external surface of the artery. Additionally, the peptide amphiphile is known to specifically bind heparin, an inherent property which may increase the local heparin concentration at the site of injury and potentiate the anti-proliferative actions of NO. There is precedence for combining diazeniumdiolate NO donors with heparin: Saavedra et al synthesized a heparin-MOM-PIPERAZI/NO conjugate, which released NO on hydrolysis and inhibited adenosine 5’-diphosphate-induced platelet aggregation, but also retained significant heparin-like anticoagulant properties in vitro.50 The two complementary anti-thrombotic mechanisms are likely occurring with application of our treatment gels as well. Regardless, we found that use of this nanofiber gel delivery vehicle provided advantages over the PROLI/NO powder alone, and will enable transition of this therapy to the clinical arena.
While our NO-releasing nanofiber gel is an exciting therapeutic candidate, one potential limiting factor is the potentiated anti-thrombotic actions of heparin and NO. Since both of these products inhibit platelet adherence and aggregation, their combination may lead to increased bleeding if proper hemostasis is not achieved. In 5 animals treated with the DPTA/NO nanofiber gel and 3 animals treated with PROLI/NO nanofiber gel, a superficial hematoma was observed at sacrifice (14 days) versus none in the injury alone group. To overcome this issue, heparin could be substituted with another sulfated glycosaminoglycan without antithrombin-binding properties that would also induce gel formation. One such potential substitute is heparan sulfate, however it is much more costly than heparin. Another limitation is that this study only compares the outcome of one delivery vehicle, namely the self-assembled nanofiber gel. A future study could analyze the efficacy of this gel delivery vehicle against other gel delivery vehicles using the same NO donor. This form of analysis would provide data with respect to the gel delivery vehicle alone. Finally, we do not know if any of the hydrolyzed breakdown products or if the peptide amphiphile or byproducts have effects on the vasculature or specifically, neointimal hyperplasia. Some of these molecular byproducts include the amino acids alanine, glycine, lysine, leucine, and arginine; salts from sodium hydroxide, hydrochloric acid, and possibly trifluoroacetic acid also may be present. The nanofiber gel alone serves as a control, and no statistically significant reduction in neointimal hyperplasia was observed with its application, suggesting that the peptide amphiphile products do not affect results. However, this is something that may need to be examined more specifically in the future.
In conclusion, by combining NO and nanotechnology, we have created a novel approach which successfully inhibited neointimal hyperplasia in the rat carotid artery injury model. While both NO-eluting nanofiber gels inhibited neointimal hyperplasia, the PROLI/NO nanofiber gel was clearly more effective. Before this therapy can be utilized in the clinical arena, it must be evaluated in a large animal model, and long-term studies will be conducted for toxicity as well as efficacy. Overall, this therapy has promising clinical potential as a novel NO-based therapy for open vascular and cardiovascular surgical procedures for the prevention of restenosis and the associated patient morbidity.
Figure 6.
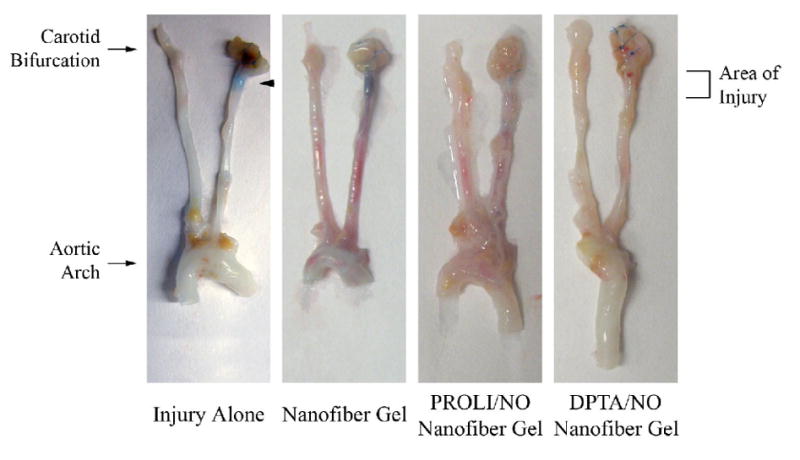
Re-endothelialization of injured rat carotid arteries. Groups include injury alone, and injury with nanofiber, PROLI/NO nanofiber or DPTA/NO nanofiber gels (n=3 per group). Arteries were procured at 7 days post-injury. Arterial areas lacking endothelium stain blue (arrow).
Acknowledgments
This work was supported in part by funding from the National Institutes of Health (NIH, 1K08HL084203 and 5 R01 EB003806-02), the Department of Veterans Affairs, VA Merit Review Grant, the U.S. Army TATRC (W81XWH-05-1-0381), and by the generosity of Mrs. Hilda Rosenbloom. In addition, part of this research was supported with federal funds from the National Cancer Institute, NIH, under Contract N01-CO-12400 with SAIC-Frederick, Inc. and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. Finally, the authors would like to express their thanks to the Northwestern University Institute for BioNanotechnology in Medicine and to Lynnette Dangerfield for her administrative support.
Footnotes
Presented at the Society for Vascular Surgery Meeting, June 2007 in Baltimore, MD Dr Kapadia is the recipient of the 2007 Lifeline Resident Research Prize
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics - 2007 update - A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 Feb 6;115(5):E69–E171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–95. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 3.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–8. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 4.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–7. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubey RK, Jackson EK, Lüscher TF. Nitric oxide inhibits angiotensin II-induced migration of rat aortic smooth muscle cell. Role of cyclic-nucleotides and angiotensin1 receptors. J Clin Invest. 1995 Jul;96(1):141–9. doi: 10.1172/JCI118014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzeng E, Kim YM, Pitt BR, Lizonova A, Kovesdi I, Billiar TR. Adenoviral transfer of the inducible nitric oxide synthase gene blocks endothelial cell apoptosis. Surgery. 1997 Aug;122(2):255–63. doi: 10.1016/s0039-6060(97)90016-7. [DOI] [PubMed] [Google Scholar]
- 8.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994 Nov;94(5):2036–44. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JS, Adrie C, Jacob HJ, Roberts JDJ, Zapol WM, Bloch KD. Chronic inhalation of nitric oxide inhibits neointimal formation after balloon-induced arterial injury. Circ Res. 1996 Feb;78(2):337–42. doi: 10.1161/01.res.78.2.337. [DOI] [PubMed] [Google Scholar]
- 11.Davies MG, Kim JH, Dalen H, Makhoul RG, Svendsen E, Hagen PO. Reduction of experimental vein graft intimal hyperplasia and preservation of nitric oxide-mediated relaxation by the nitric oxide precursor L-arginine. Surgery. 1994 Sep;116(3):557–68. [PubMed] [Google Scholar]
- 12.McNamara DB, Bedi B, Aurora H, Tena L, Ignarro LJ, Kadowitz PJ, et al. L-arginine inhibits balloon catheter-induced intimal hyperplasia. Biochem Biophys Res Commun. 1993 May 28;193(1):291–6. doi: 10.1006/bbrc.1993.1622. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Mattar SG, Lumsden AB. Oral administration of L-arginine reduces intimal hyperplasia in balloon-injured rat carotid arteries. J Surg Res. 1999 Mar;82(1):17–23. doi: 10.1006/jsre.1998.5476. [DOI] [PubMed] [Google Scholar]
- 14.Groves PH, Banning AP, Penny WJ, Newby AC, Cheadle HA, Lewis MJ. The effects of exogenous nitric oxide on smooth muscle cell proliferation following porcine carotid angioplasty. Cardiovasc Res. 1995 Jul;30(1):87–96. [PubMed] [Google Scholar]
- 15.von der Leyen HE, Mann MJ, Dzau VJ. Gene inhibition and gene augmentation for the treatment of vascular proliferative disorders. Seminars in Interventional Cardiology. 1996 Sep;1(3):209–14. [PubMed] [Google Scholar]
- 16.Chen L, Daum G, Forough R, Clowes M, Walter U, Clowes AW. Overexpression of human endothelial nitric oxide synthase in rat vascular smooth muscle cells and in balloon-injured carotid artery. Circ Res. 1998 May 4;82(8):862–70. doi: 10.1161/01.res.82.8.862. [DOI] [PubMed] [Google Scholar]
- 17.Janssens S, Flaherty D, Nong Z, Varenne O, Van Pelt N, Haustermans C, et al. Human endothelial nitric oxide synthase gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation after balloon injury in rats. Circulation. 1998 Apr 7;97(13):1274–81. doi: 10.1161/01.cir.97.13.1274. [DOI] [PubMed] [Google Scholar]
- 18.Varenne O, Pislaru S, Gillijns H, Van Pelt N, Gerard RD, Zoldhelyi P, et al. Local adenovirus-mediated transfer of human endothelial nitric oxide synthase reduces luminal narrowing after coronary angioplasty in pigs. Circulation. 1998 Sep 1;98(9):919–26. doi: 10.1161/01.cir.98.9.919. [DOI] [PubMed] [Google Scholar]
- 19.Kibbe MR, Tzeng E, Gleixner SL, Watkins SC, Kovesdi I, Lizonova A, et al. Adenovirus-mediated gene transfer of human inducible nitric oxide synthase in porcine vein grafts inhibits intimal hyperplasia. J Vasc Surg. 2001 Jul;34(1):156–65. doi: 10.1067/mva.2001.113983. [DOI] [PubMed] [Google Scholar]
- 20.Shears LL, Kibbe MR, Murdock AD, Billiar TR, Lizonova A, Kovesdi I, et al. Efficient inhibition of intimal hyperplasia by adenovirus-mediated inducible nitric oxide synthase gene transfer to rats and pigs in vivo. J Am Coll Surg. 1998 Sep;187(3):295–306. doi: 10.1016/s1072-7515(98)00163-x. [DOI] [PubMed] [Google Scholar]
- 21.Wang K, Kessler PD, Forudi F, Zhou Z, Zhou X, Tarakji K, et al. Local adenoviral-mediated inducible nitric oxide synthase (iNOS) gene transfer inhibits neointimal formation in the porcine coronary stented model. Am J Cardiol. 2001 Sep 11;88(5A):51G–2G. doi: 10.1016/s1525-0016(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 22.Marks DS, Vita JA, Folts JD, Keaney JFJ, Welch GN, Loscalzo J. Inhibition of neointimal proliferation in rabbits after vascular injury by a single treatment with a protein adduct of nitric oxide. J Clin Invest. 1995 Dec;96(6):2630–8. doi: 10.1172/JCI118328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarzacher SP, Lim TT, Wang BY, Kernoff RS, Niebauer J, Cooke JP, et al. Local intramural delivery of L-arginine enhances nitric oxide generation and inhibits lesion formation after balloon angioplasty. Circulation. 1997 Apr 1;95(7):1863–9. doi: 10.1161/01.cir.95.7.1863. [DOI] [PubMed] [Google Scholar]
- 24.Chaux A, Ruan XM, Fishbein MC, Ouyang Y, Kaul S, Pass JA, et al. Perivascular delivery of a nitric oxide donor inhibits neointimal hyperplasia in vein grafts implanted in the arterial circulation. J Thorac Cardiovasc Surg. 1998 Mar;115(3):604–12. doi: 10.1016/S0022-5223(98)70325-3. [DOI] [PubMed] [Google Scholar]
- 25.Fulton GJ, Davies MG, Barber L, Gray JL, Svendsen E, Hagen PO. Local effects of nitric oxide supplementation and suppression in the development of intimal hyperplasia in experimental vein grafts. Eur J Vasc Endovasc Surg. 1998 Apr;15(4):279–89. doi: 10.1016/s1078-5884(98)80030-0. [DOI] [PubMed] [Google Scholar]
- 26.Kaul S, Cercek B, Rengstrom J, Xu XP, Molloy MD, Dimayuga P, et al. Polymeric-based perivascular delivery of a nitric oxide donor inhibits intimal thickening after balloon denudation arterial injury: role of nuclear factor-kappaB. J Am Coll Cardiol. 2000 Feb;35(2):493–501. doi: 10.1016/s0735-1097(99)00543-4. [DOI] [PubMed] [Google Scholar]
- 27.Harnek J, Zoucas E, Sjuve R, Arner A, Ekblad E, Schou H, et al. Local infusion of the nitric oxide donor SIN-1 after angioplasty - Effects on intimal hyperplasia in porcine coronary arteries. Acta Radiologica. 2003 Jul;44(4):395–402. doi: 10.1080/j.1600-0455.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoon J, Wu CJ, Homme J, Tuch RJ, Wolff RG, Topol EJ, et al. Local delivery of nitric oxide from an eluting stent to inhibit neointimal thickening in a porcine coronary injury model. Yonsei Medi J. 2002 Apr;43(2):242–51. doi: 10.3349/ymj.2002.43.2.242. [DOI] [PubMed] [Google Scholar]
- 29.Hou DM, Narciso H, Kamdar K, Zhang P, Barclay B, March KL. Stent-based nitric oxide delivery reducing neointimal proliferation in a porcine carotid overstretch injury model. Cardiovasc Interventional Radiology. 2005 Jan;28(1):60–5. doi: 10.1007/s00270-004-0206-2. [DOI] [PubMed] [Google Scholar]
- 30.Kown MH, Yamaguchi A, Jahncke CL, Miniati D, Murata S, Grunenfelder J, et al. L-arginine polymers inhibit the development of vein graft neointimal hyperplasia. J Thorac Cardiovasc Surg. 2001 May;121(5):971–80. doi: 10.1067/mtc.2001.112532. [DOI] [PubMed] [Google Scholar]
- 31.Keefer LK. Progress toward clinical application of the nitric oxide-releasing diazeniumdiolates. Annu Rev Pharmacol Toxicol. 2003;43:585–607. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- 32.Rajangam K, Behanna HA, Hui MJ, Han XQ, Hulvat JF, Lomasney JW, et al. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 2006 Sep 13;6(9):2086–90. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 33.Behanna HA, Rajangam K, Stupp SI. Modulation of fluorescence through coassembly of molecules in organic nanostructures. J Am Chem Soc. 2007 Jan 17;129(2):321–7. doi: 10.1021/ja062415b. [DOI] [PubMed] [Google Scholar]
- 34.Hrabie JA, Keefer LK. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem Rev. 2002 Apr;102(4):1135–54. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 35.Saavedra JE, Southan GJ, Davies KM, Lundell A, Markou C, Hanson SR, et al. Localizing antithrombotic and vasodilatory activity with a novel, ultrafast nitric oxide donor. J Med Chem. 1996 Oct 25;39(22):4361–5. doi: 10.1021/jm960616s. [DOI] [PubMed] [Google Scholar]
- 36.Hrabie JA, Klose JR, Wink DA, Keefer LK. New nitric oxide-releasing zwitterions derived from polyamines. J Org Chem. 1993 Mar 12;58(6):1472–6. [Google Scholar]
- 37.Kibbe MR, Li J, Nie S, Watkins SC, Lizonova A, Kovesdi I, et al. Inducible nitric oxide synthase (iNOS) expression upregulates p21 and inhibits vascular smooth muscle cell proliferation through p42/44 mitogen-activated protein kinase activation and independent of p53 and cyclic guanosine monophosphate. J Vasc Surg. 2000 Jun;31(6):1214–28. doi: 10.1067/mva.2000.105006. In Process Citation. [DOI] [PubMed] [Google Scholar]
- 38.Yu SM, Hung LM, Lin CC. cGMP-elevating agents suppress proliferation of vascular smooth muscle cells by inhibiting the activation of epidermal growth factor signaling pathway. Circulation. 1997 Mar 4;95(5):1269–77. doi: 10.1161/01.cir.95.5.1269. see comments. [DOI] [PubMed] [Google Scholar]
- 39.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983 Aug;49(2):208–15. [PubMed] [Google Scholar]
- 40.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–33. [PubMed] [Google Scholar]
- 41.Fingerle J, Johnson R, Clowes AW, Majesky MW, Reidy MA. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–6. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg. 1994 Sep;81(9):1254–69. doi: 10.1002/bjs.1800810904. [DOI] [PubMed] [Google Scholar]
- 43.Moller M, Botti H, Batthyany C, Rubbo H, Radi R, Denicola A. Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J Biol Chem. 2005 Mar 11;280(10):8850–4. doi: 10.1074/jbc.M413699200. [DOI] [PubMed] [Google Scholar]
- 44.Masters KS, Lipke EA, Rice EE, Liel MS, Myler HA, Zygourakis C, et al. Nitric oxide-generating hydrogels inhibit neointima formation. J Biomater Sci Polym Ed. 2005;16(5):659–72. doi: 10.1163/1568562053783722. [DOI] [PubMed] [Google Scholar]
- 45.Lipke EA, West JL. Localized delivery of nitric oxide from hydrogels inhibits neointima formation in a rat carotid balloon injury model. Acta Biomater. 2005 Nov;1(6):597–606. doi: 10.1016/j.actbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004 Feb 27;303(5662):1352–5. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 47.Beniash E, Hartgerink JD, Storrie H, Stendahl JC, Stupp SI. Self-assembling peptide amphiphile nanofiber matrices for cell entrapment. Acta Biomaterialia. 2005 Jul;1(4):387–97. doi: 10.1016/j.actbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Harrington DA, Cheng EY, Guler MO, Lee LK, Donovan JL, Claussen RC, et al. Branched peptide-amphiphiles as self-assembling coatings for tissue engineering scaffolds. J Biomed Mater Res Part A. 2006;78a(1):157–67. doi: 10.1002/jbm.a.30718. [DOI] [PubMed] [Google Scholar]
- 49.Guler MO, Hsu L, Soukasene S, Harrington DA, Hulvat JF, Stupp SI. Presentation of RGDS epitopes on self-assembled nanofibers of branched peptide amphiphiles. Biomacromolecules. 2006 Jun;7(6):1855–63. doi: 10.1021/bm060161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saavedra JE, Mooradian DL, Mowery KA, Schoenfisch MH, Citro ML, Davies KM, et al. Conversion of a polysaccharide to nitric oxide-releasing form. Dual mechanism anticoagulant activity of diazeniumdiolated heparin. Bioorg Med Chem Lett. 2000 Apr 17;10(8):751–3. doi: 10.1016/s0960-894x(00)00086-x. [DOI] [PubMed] [Google Scholar]


