Abstract
In a previous study, we showed that electroacupuncture (EA) applied to the SI-6 point on the contralateral forelimb produces long-lasting and powerful analgesia in pain caused by ankle sprain in a rat model. To investigate the underlying mechanism of EA analgesia, the present study tested the effects of various antagonists to known endogenous analgesic systems in this model. Ankle sprain was induced in anesthetized rats by overextending their right ankle with repeated forceful plantar flexion and inversion of the foot. When rats developed pain behaviors (a reduction in weight bearing of the affected hind limb), EA was applied to the SI-6 point on the contralateral forelimb for 30 minutes under halothane anesthesia. EA significantly improved the weight-bearing capacity of the affected hind limb for 2 hours, suggesting an analgesic effect. The alpha-adrenoceptor antagonist phentolamine (2 mg/kg, i.p. or 30 μg, i.t.) completely blocked the EA-induced analgesia, whereas naloxone (1 mg/kg, i.p.) and failed to block the effect. These results suggest that EA-induced analgesia is mediated by alpha-adrenoceptor mechanisms. Further experiments showed that intrathecal administration of yohimbine (10 μg), an α2-adrenergic antagonist, reduced the EA-induced analgesia in a dose-dependent manner, whereas terazosin (10 μg), an α1-adrenergic antagonist, did not produce any effect. These data suggest that the analgesic effect of EA in ankle sprain pain is, at least in part, mediated by spinal α2-adrenoceptor mechanisms.
INTRODUCTION
The endogenous opioid system has received much attention as the key underlying mechanism of acupuncture analgesia (Han and Terenius 1982; He 1987; Han 1993; Mayer 2000), ever since it was first demonstrated that the analgesic effect of acupuncture could be reversed by naloxone, an opioid antagonist (Pomeranz and Chiu 1976; Mayer et al. 1977). However, under certain circumstances, opioid antagonists have failed to reverse acupuncture-induced effects in rabbits (McLennan et al. 1977), in rats (Das et al. 1984; Bossut et al. 1991; Kwon et al. 2001; Koo et al. 2002), and in humans (Chapman et al. 1980; Chapman et al. 1983). Therefore, the mechanism of acupuncture analgesia is still unclear. The contradictory findings on the actions of naloxone on the effects of acupuncture suggest that multiple biological mechanisms are involved and that different conditions may trigger different mechanisms. In fact, besides endogenous opioids, monoaminergic neurotransmitters may play an additional role in acupuncture analgesia (Cheng and Pomeranz 1981; Han and Terenius 1982; Takeshige et al. 1992; Mayer 2000).
Our previous study showed that electroacupuncture (EA) produces a powerful analgesic effect in a rat model of ankle sprain pain. It is unlikely that the analgesic effect on ankle sprain pain was mediated by the endogenous opioid system because, in a previous study, systemic injection of opioid antagonists failed to block EA-induced analgesia (Koo et al., 2002). On the other hand, the monoaminergic system is a well-known pain modulation system (Millan 2002) and monoaminergic antagonists are shown to attenuate EA-induced antinociceptive effects in studies of acute evoked pain (Takeshige et al. 1980; Cheng and Pomeranz 1981; Takeshige et al. 1992). In addition, it has been reported that synthesis and release of serotonin (5-HT) and norepinephrine in the central nervous system are accelerated by manual or electronic acupuncture (Han 1986). Therefore, the present study was designed to test whether or not the monoaminergic system is involved in EA analgesia in a rat model of ankle sprain pain and if so, to identify which types of monoaminergic receptors are involved.
MATERIALS AND METHODS
Experimental animals
Experiments were performed on young adult male Sprague-Dawley rats (200−320 g, Harlan Sprague-Dawley, Indianapolis, IN, USA). Animals were housed in groups of two in plastic cages with soft bedding and were provided free access to food and water under a 12/12 hour reversed light-dark cycle (dark cycle: 8:00 A.M.–8:00 P.M.). All animals were acclimated for 7 days before the experiment began. Animal experiments were carried out in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals, and experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch.
Procedure for ankle sprain
The rats were placed under general anesthesia and the right ankle was sprained by manually overextending the lateral ligaments without breaking them to imitate a lateral ankle sprain in a human being (Cotler 1984). Details of the ankle sprain procedure are described in our earlier study (Koo et al. 2002). In brief, under halothane anesthesia (in the flow of oxygen; 3% for induction and 1.5% for maintenance), the right hind foot of each rat was repeatedly bent in the direction of simultaneous inversion and plantar flexion 60 times during a 1-minute period with gradually increasing force so that the foot could eventually be bent to a position of 90° inversion and 90° plantar flexion from the resting position. The foot was then further inverted repeatedly 60 times during the next 1-minute period so that it eventually reached a 180° inversion (the paw facing completely upward). These two 1-minute procedures were repeated so that the total procedure took 4 minutes. Anesthesia was discontinued, and the rats recovered from anesthesia within 5−10 minutes.
Intrathecal catheter implantation
Intrathecal catheters were implanted in the rats under halothane anesthesia. The back of the rat was shaved and wiped with povidone-iodine (10%). The lamina and articular process of the T12 vertebrae were carefully removed by using a rongeur, and the dura mater was exposed. A pinhole was made in the dura using a pair of microscissors, and a PE-10 catheter (Becton Dickinson, Sparks, MD, USA) was inserted into the spinal subarachnoid space between T12 and T13. The tip of the catheter was aimed at the lumbar enlargement (about 1 cm caudal to the insertion point). The tubing was secured to the muscles at multiple sites and fed subcutaneously to the mid-thoracic level in order to expose the tip to the dorsal midline position. The tip of the tubing was sealed, and the incision was closed. After full recovery from anesthesia, rats were returned to their cages and housed individually. After one week of recovery from catheter implantation, the rats underwent the ankle sprain procedure described above. At the end of each experiment, the area of catheter implantation was re-exposed, and the status of the tubing was examined. An injection of 5 μl of Evans blue dye (0.5%) showed that the tubing permitted free passage of injected material in all cases.
Drug treatment
The effect of drug treatment was examined by comparing the effects of two drugs (either two different compounds or one compound and saline control) in cross-over design by administering two compounds in the same rats in two consecutive days (1 and 2 days post ankle sprain) in a random sequence (see Results for detail procedure).
The effect of two different drugs on EA-induced analgesia was tested by systemic intraperitoneal injection. The drugs tested included naloxone hydrochloride (1 mg/kg; Endo, Chadds Ford, PA, USA) and phentolamine mesylate (2 mg/kg; RBI, Natick, MA, USA). For the intrathecal injections, phentolamine mesylate (30 μg, RBI, Natick, MA, USA), idazoxan hydrochloride (30 μg, Sigma, St Louis, MO, USA), terazosin hydrochloride (10 μg, Abbott Laboratories, North Chicago, IL, USA), or yohimbine hydrochloride (10 μg, Sigma, St Louis, MO, USA) in 10 μl of saline was given immediately after termination of EA stimulation. The control group received 10 μl of saline alone. After each intrathecal injection, the catheter was flushed with 10 μl of saline.
Electroacupuncture (EA)
After placing the rats under gaseous anesthesia (halothane 1.0% in air), EA was applied by electrically stimulating an acupuncture point with a pair of bipolar stimulating electrodes, which were modified acupuncture needles. Two stainless steel acupuncture needles (0.3 mm in diameter and 30 mm in length) were mounted on a holder with 1 mm separation between the tips. The needle set was inserted into a specified acupoint at a depth of 5 mm, and electrical stimulation was applied by a Grass S88 stimulator equipped with a SIU5 isolation unit (Grass Medical Instruments, Quincy, MA, USA). Trains of four pulses (1 ms long square wave pulses, 100 Hz of intra-train frequency), repeated at a rate of 2 Hz, were delivered at an intensity of 10 times the muscle twitch threshold (the muscle twitch threshold was about 200 μA). The current delivered was monitored at all times, and the polarity was reversed every 60 seconds to prevent polarization of the electrodes. The total duration of EA stimulation was 30 minutes. Immediately after the termination of EA, anesthesia was discontinued, and the rats usually resumed full activity within 5−10 minutes. Details of the EA procedure are described elsewhere (Koo et al. 2002).
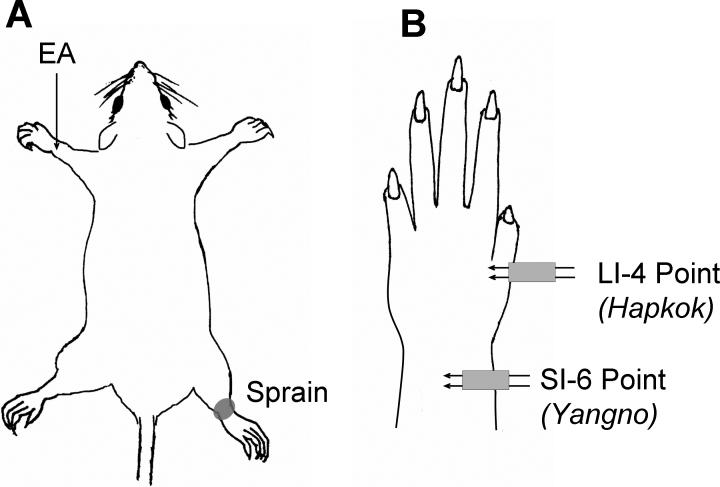
EA was applied to two different sites on the forelimb contralateral to the side of the sprained ankle (Fig. 1). These two sites are equivalent to known human acupoints: the SI-6 (‘Yangno’) and LI-4 (‘Hapkok’) acupoints which are terms designated by the World Health Organization (1993). The SI-6 point is located at the posterior distal end of the forearm between the radius and ulna and the LI-4 point is located in the triangular space between the thumb and the index finger. These two points are about 10 mm apart in the rat. Fig. 1 shows the general layout of the locations in which EA was applied in this study.
Fig. 1.
Schematic drawings showing the locations of sprained ankle and electroacupuncture (EA). (A) The sprain was produced on the right ankle, and EA was applied to the acupoints on the left forelimb. (B) A detailed view of the left forelimb showing the locations of EA application. EA was applied with a stimulation needle set consisting of two acupuncture needles separated by 1 mm, and electrical current was applied between the two needles. The SI-6 point was used as the main point, and the LI-4 point was used as a control (sham stimulation) point.
Behavioral tests
All behavioral tests were performed by experimenters who were blinded to the exact experimental procedure. However, experiments with intrathecal injection were done in a non-blinded fashion.
To estimate the level of pain in the sprained ankle, we measured the weight-bearing force (WBF) of the affected foot. In our previous study, we found that the reduction in weight bearing of the foot after ankle sprain (limping) was likely due to pain. Furthermore, an improvement in weight bearing can be interpreted as a sign of analgesia because systemic injection of morphine restores weight bearing after ankle sprain in a dose-dependent manner (Koo et al. 2002).
Each rat was allowed to walk through a long, narrow plastic chamber (10 cm width, 10 cm high, and 60 cm long). An electronic balance (Acculab, Pocket Pro 250-B, Newton, PA, USA) was placed on the floor at the midway point of the walking path. The rectangular plate of the balance was placed so as to cover half the width of the walking path so that only the limbs on one side would step on the balance. The analog signal of the balance was fed into a digital oscilloscope (Nicolet model 410, Madison, WI, USA). The magnitude of the signal representing the WBF was used for further data analysis (as described in Results).
Data analysis
Data are expressed as mean ± standard error of the mean (SEM). Statistical tests were conducted using two-way repeated-measures analysis of variance (ANOVA) followed by all pair-wise multiple comparison procedures (Tukey test) or by the cross-over repeated ANOVA followed by between group comparison with the least square method (Davis, 2002; Kirk, 1995; Senn, 1993), using the SAS statistical package (SAS, version 9.1). For cross-over designed experiments, carryover effects were assumed to be negligible since the baseline values of the first and second treatment groups were essentially identical and had minimal variability. A p value of less than 0.05 was considered to be statistically significant.
RESULTS
EA-induced analgesia on ankle sprain pain
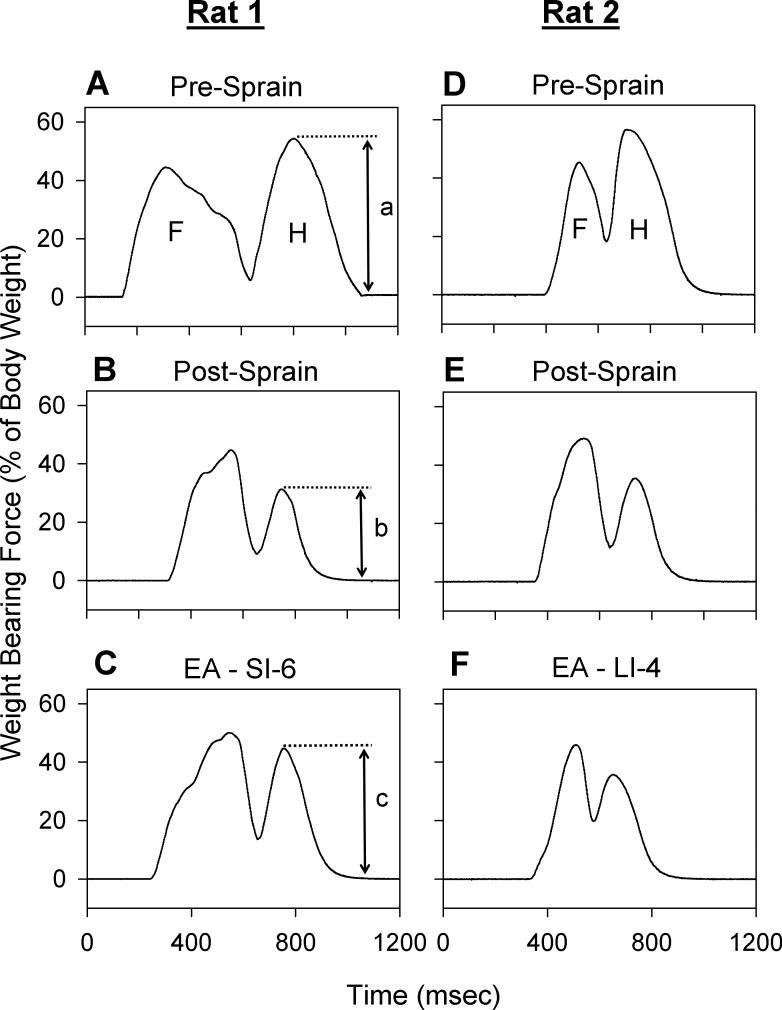
The weight-bearing force (WBF) of the right hind limb was measured before and after ankle sprain, and also after EA application. As shown in Fig. 2, the WBF of the hind limb was approximately 60% of total body weight in normal rats (Fig. 2A, D) and then decreased to approximately 30% one day after ankle sprain (Fig. 2B, E). Our previous study (Koo et al. 2002) documented that the decrease in WBF was due to limping to favor the limb associated with pain in the sprained ankle. One hour after EA application to the SI-6 point of the contralateral forelimb, the WBF of the ankle sprained hind limb recovered to approximately 45% of body weight (Fig. 2C). Such recovery of WBF is interpreted as a sign of analgesic effect.
Fig. 2.
Effects of EA in rats with sprained ankles. Graphs show analog output for the stepping force of the forelimb (F) and hind limb (H) on one side (the right side) of two rats (A, B, C for rat #1 and D, E, F for rat #2) obtained during walking. Weight-bearing force (WBF) is expressed as a percentage of each rat's body weight. In normal conditions (A, D), the hind limb bore about 50−60% of the body weight (height labeled as “a”). One day after ankle sprain (B, E), the WBF of the hind limb with the sprained ankle was reduced to about half (height labeled as “b”). One hour after a 30-minute application of EA to the SI-6 point, WBF significantly recovered in rat #1 (C, height labeled as “c”), whereas the same EA applied to the LI-4 point had no effect in rat #2 (F). For group data treatment, percentage maximum recovery value after EA was calculated as follow using the height values labeled as “a–c” in panels A–C: percentage maximum recovery = ([c – b]/[a – b]) × 100. Therefore, recovery is 100% (full) when the height of c reaches that of a, and is 0% (no recovery) when the height of c remains equal to that of b.
Although EA applied to the SI-6 point produced a partial recovery of WBF, identical EA applied to a neighboring acupoint (LI-4, about 10 mm away) failed to demonstrate any improvement in WBF (Fig. 2F), suggesting that EA effect is site specific.
Most of the experiments in this study were conducted in a two-day span after ankle sprain (24 and 48 hours post-sprain). Because pre-EA baseline values were different between 1 and 2 post-sprain days due to gradual spontaneous recovery, the baseline value of WBF was measured before each experiment, and data were expressed as the percentage of maximum recovery. To show how the percentage of maximum recovery was calculated, the magnitudes of hind limb WBF in different conditions are labeled as a (normal), b (after ankle sprain), and c (after a treatment) in Fig. 2A-2C. Percentage of maximum recovery = ([c – b]/[a – b]) × 100. Therefore, recovery is 100% (full) when the magnitude of c reaches that of a, and it is 0% (no recovery) when the magnitude of c remains the same as that of b.
Fig. 3A shows group data on EA-induced analgesia on ankle sprain pain expressed as percentage of maximum recovery. As shown in Fig. 3A, EA applied to the SI-6 point in 10 rats with a sprained ankle (1 day post-sprain) produced significant recovery of WBF to about 40% of full recovery lasting about 2 hours. On the other hand, identical EA applied to the LI-4 point in another 10 rats with a sprained ankle produced no recovery of WBF (group factor with 2-way repeated-measures ANOVA [time factor repetition]: F = 11.702, p = 0.003).
Fig. 3.
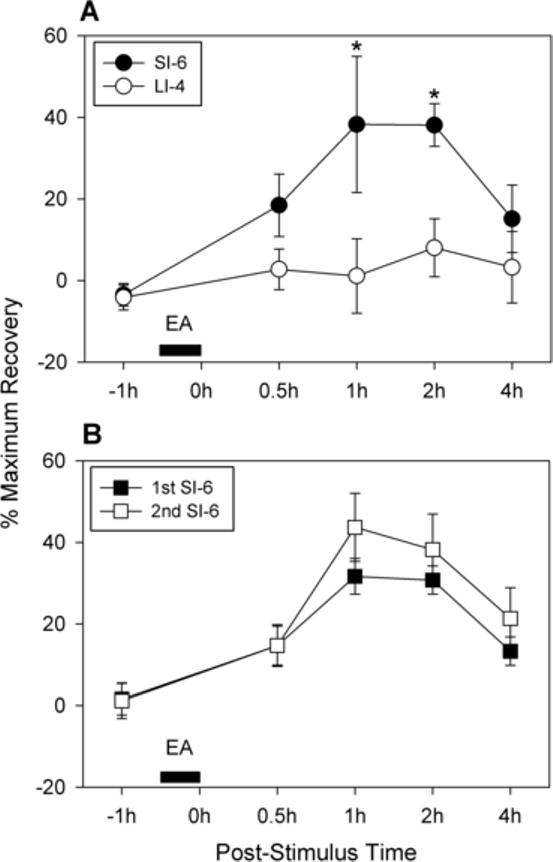
Group data on EA-induced analgesia on ankle sprain pain expressed as percentage maximum recovery. Graphs are plotted for the average values (± SEM) of percentage of maximum recovery value (100% means full recovery of weight-bearing force [WBF] to pre-ankle sprain levels). (A) Comparison of effects of EA applied to the SI-6 and LI-4 points in two groups of rats (10 rats in each group). EA was applied 1 day after ankle sprain. EA to the SI-6 point (for 30 minutes) produced significant recovery of WBF to about 40% of full recovery lasting about 2 hours. On the other hand, identical EA applied to the LI-4 point produced no recovery of WBF. An asterisk (*) indicates a value significantly (p < 0.05) different from the equivalent value with LI-4 stimulation by the two-way repeated measures ANOVA (time factor repetition) followed by Tukey multiple comparisons. (B) Comparison of effects of EA applied to the SI-6 points repeated in two consecutive days (1st and 2nd post-sprain days) on a group of 7 rats. Two EA applications produced a comparable recovery of WBF (group factor with 2-way repeated-measures ANOVA [two-factor repetition]: F = 3.062, p = 0.131).
Because most experiments were conducted within a two-day span after ankle sprain and the data collected in those two days were pooled, it was necessary to test whether EA produced the same effect in those two days. In 7 rats with a sprained ankle, EA applications to the SI-6 point were repeated on the first and second post-sprain days. As shown in Fig. 3B, those two EA applications produced a comparable recovery of WBF (group factor with 2-way repeated-measures ANOVA [two-factor repetition]: F = 3.062, p = 0.131), suggesting that pooling data from two post-sprain days is justified.
Effect of intraperitoneal injection of an adrenergic antagonist on EA-induced analgesia
We first examined the systemic effect of an adrenergic antagonist on EA-induced analgesia. Experiments were conducted using the cross-over design with injections of compounds in a 2-day period after ankle sprain. One day after ankle sprain, eight rats were divided randomly into two groups. In one group of four rats, phentolamine (2 mg/kg) was administered intraperitoneally immediately after the termination of EA, which was applied to the contralateral SI-6 point for 30 minutes. In the other group of four rats, saline vehicle was injected immediately after the termination of EA applied to the same point. The weight-bearing force of the affected hind limb was measured at 0.5, 1, 2, and 4 hours after phentolamine or saline treatment. On the following day (the second post-sprain day), the procedures for these two groups were reversed. Therefore, all eight rats received phentolamine and saline injection in a 2-day period in a random sequence. Data on weight-bearing force were expressed as the percentage of maximum recovery. Fig. 4A shows group data of the eight rats. The data were analyzed using a cross-over repeated ANOVA model and fixed factors, ‘injection sequence’, ‘post-injection time’, and ‘treatment group’, were fitted to the data with the correlated error term. The analysis showed that the treatment factor for the two groups was significantly different (F = 8.18, p = 0.0095), whereas the factor for the injection sequence was not (F = 0.74, p = 0.398). Although the saline group showed a long-lasting recovery in WBF following EA treatment, the phentolamine group failed to show any improvement after EA, indicating that the analgesic effect of EA was blocked by systemic injection of phentolamine, an α-adrenergic antagonist. These data suggest that the adrenergic system is involved in mediating EA-induced analgesia in ankle sprain pain.
Fig. 4.
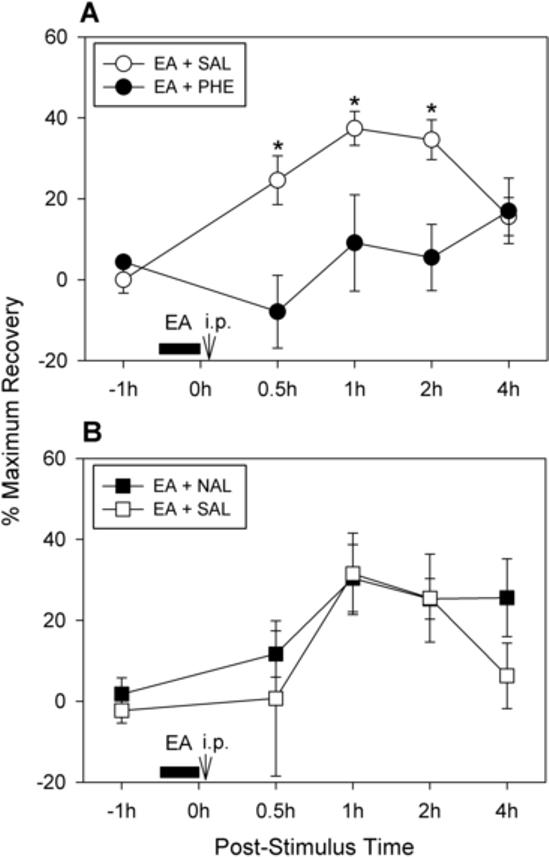
Effects of systemic application of α-adrenergic and opioid receptor antagonists on EA-induced analgesia in rats with sprained ankles. Graphs are plotted for the average values (± SEM) of percentage of maximum recovery value. (A) Effects of an α-adrenergic receptor antagonist on EA-induced analgesia (n = 8 rats). Phentolamine (2 mg/kg in 0.2 ml saline) or the same volume of saline was given intraperitoneally immediately after termination of EA (applied to the SI-6 point on the contralateral forelimb for 30 minutes). The experiment was done with the cross-over design so that all 8 rats received both phentolamine and saline alone within a two-day span (1st and 2nd post-sprain days). There was no significant improvement in weight bearing force (WBF) in the phentolamine-injected group, whereas the saline injection group showed a long-lasting recovery of WBF. An asterisk (*) indicates a value significantly (p < 0.05) different from the equivalent value after phentolamine injection, using cross-over repeated ANOVA with the least square method. (B) Effects of an opioid antagonist on EA-induced analgesia in rats with sprained ankles (n = 7 rats). Either naloxone (1 mg/kg in 0.2 ml of saline) or the same volume of saline was given intraperitoneally immediately after termination of EA (applied to the SI-6 point on the contralateral forelimb for 30 minutes). The experiment was done with the cross-over design so that all 7 rats received both naloxone and saline in the two-day span (1st and 2nd post-sprain days). Both the saline and naloxone group showed a long-lasting recovery of WBF following EA treatment (group factor with cross-over repeated ANOVA: F = 0.62, p = 0.443).
Effect of intraperitoneal injection of an opioid antagonist on EA-induced analgesia
For the next experiment, we examined the possible involvement of the endogenous opioid system in EA-induced analgesia in ankle sprain pain. Experiments were again conducted in cross-over design with injections of compounds during the first two days after ankle sprain. One day after ankle sprain, seven rats were divided randomly into two groups. In one group of four rats, naloxone (1 mg/kg) was given intraperitoneally immediately after the termination of EA, which was applied to the contralateral SI-6 point for 30 minutes. In the other group of three rats, saline vehicle was injected immediately after the termination of EA applied to the same point. The WBF of the affected hind limb was measured at 0.5, 1, 2, and 4 hours after phentolamine or saline treatment. The next day (2nd post-sprain day), the procedures for these two groups were reversed. Therefore, all seven rats received naloxone and saline injection in a 2-day period in a random sequence. Data on weight-bearing force were expressed as the percentage of maximum recovery. Fig. 4B shows group data of the seven rats. The data were analyzed using a cross-over repeated ANOVA model and fixed factors, ‘injection sequence’, ‘post-injection time’, and ‘treatment group’, fitted to the data with the correlated error term. The analysis showed that neither the treatment group factor (F = 0.62, p = 0.443) nor the sequence factor (F = 0.07, p = 0.788) was significantly different. Both the saline and naloxone groups showed a long-lasting recovery of WBF following EA treatment. These data suggest that the endogenous opioid system is not involved in mediating EA-induced analgesia in ankle sprain pain.
Effect of intrathecal injection of an adrenergic antagonist on EA-induced analgesia
Because the adrenergic system seems to be involved in mediating EA-induced analgesia, the possible involvement of the adrenergic system in the spinal cord was examined by repeating the experiment with intrathecal administration of phentolamine. Intrathecal catheters were implanted in seven rats. One week later, the ankles of these rats were sprained. Experiments were again conducted in cross-over design with injections of compounds in a 2-day period after ankle sprain. One day after ankle sprain, seven rats were divided randomly into two groups. In one group of four rats, phentolamine (30 μg in 10 μl of saline followed by a flush with 10 μl of saline) was delivered intrathecally immediately after the termination of EA, which was applied to the SI-6 point for 30 minutes. In the other group of three rats, saline (20 μl) was injected intrathecally immediately after the termination of EA application. Weight-bearing force was measured at 0.5, 1, 2, and 4 hours after drug treatment. The next day, the procedures for these two groups were reversed. Therefore, all seven rats received phentolamine and saline injections over a 2-day period in random order. The pre-EA baseline values were measured before each experiment, and data were expressed as the percentage of maximum recovery as shown in Fig. 5A. The data were analyzed using a cross-over repeated ANOVA model and fixed factors, ‘injection sequence’, ‘post-injection time’, and ‘treatment group’, were fitted to the data with the correlated error term. The analysis showed that the treatment group factor for the two groups was significantly different (F = 20.67, p = 0.0003), whereas the factor for the injection sequence was not (F = 0.08, p = 0.782). These data indicate that intrathecal administration of phentolamine completely blocked the EA-induced analgesic effect, suggesting that EA-induced analgesia in ankle sprain pain is mediated by spinal α-adrenoceptors.
Fig. 5.
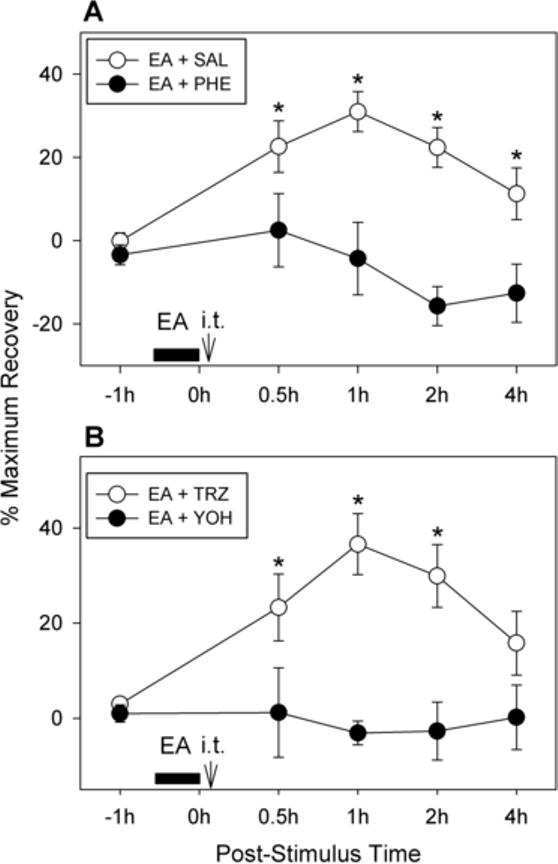
Effects of intrathecal application of α-adrenergic receptor antagonists on EA-induced analgesia in rats with sprained ankles. Graphs are plotted for the average values (± SEM) of percentage of maximum recovery value. A: Effects of intrathecal application of a mixed α-adrenergic receptor antagonist on EA-induced analgesia (n = 7 rats). Phentolamine (30 μg in 10 μl of saline) or the same volume of saline was given intrathecally (through an implanted catheter) immediately after termination of EA (applied to the SI-6 point on the contralateral forelimb for 30 minutes). The experiment was done with the cross-over design so that all 7 rats received both phentolamine and saline alone in a two-day span (1st and 2nd post-sprain days). There was no significant improvement in weight bearing in the phentolamine-injected group, whereas the saline injection group showed a long-lasting recovery of WBF. An asterisk (*) indicates a value significantly (p < 0.05) different from the equivalent value after phentolamine injection, using cross-over repeated ANOVA with the least square method. (B) Effects of intrathecal application of specific α1- and α2-adrenergic receptor antagonists on EA-induced analgesia (n = 8 rats). Yohimbine (an α2-adrenoceptor blocker, 10 μg/10 μl of saline) or terazosin (an α1-adrenoceptor blocker, 10 μg/10 μl of saline) was given intrathecally through an implanted catheter immediately after termination of EA (applied to the SI-6 point on the contralateral forelimb for 30 minutes). The experiment was done with the cross-over design so that all 8 rats received both yohimbine and terazosin in a two-day span (1st and 2nd post-sprain days). There was no significant improvement of weight bearing in the yohimbine-injected group, whereas the terazosin injection group showed a long-lasting recovery of WBF. An asterisk (*) indicates a value significantly (p < 0.05) different from the equivalent value after yohimbine injection, using cross-over repeated ANOVA with the least square method.
Effect of intrathecal injection of α-adrenergic antagonist subtypes on EA-induced analgesia
Because our data indicated that spinal α-adrenoceptors may be involved in mediating EA-induced analgesia, we investigated the subtype of α-adrenoceptors that may be involved by using specific α1- and α2-adrenoceptor antagonists. Intrathecal catheters were implanted in eight rats. One week later, the ankles of these rats were sprained. Experiments were again conducted in cross-over design with injections of compounds in the 2-day period after ankle sprain. One day after ankle sprain, eight rats were divided randomly into two groups of four. In one group, terazosin (an α1-adrenoceptor antagonist; 10 μg/10 μl saline) was injected intrathecally immediately after the termination of EA application at the SI-6 point. In the other group of rats, yohimbine (an α2-adrenoceptor antagonist; 10 μg/10 μl of saline) was injected intrathecally immediately after the termination of EA. The WBF of the affected hind limb was measured before and at 0.5, 1, 2, and 4 hours after the drug treatment. The next day, the procedures for these two groups were reversed. Therefore, all eight rats received both terazosin and yohimbine over a 2-day period in random order. The data were expressed as the percentage of maximum recovery as shown in Fig. 5B. The data were analyzed using a cross-over repeated ANOVA model and fixed factors, ‘injection sequence’, ‘post-injection time’, and ‘treatment group’, were fitted to the data with the correlated error term. The analysis showed that the treatment group factor for the two groups was significantly different (F = 23.31, p < 0.0001), whereas the factor for the injection sequence was not (F = 1.44, p = 0.243). These data indicate that the EA-induced analgesic effect was completely blocked by intrathecal yohimbine treatment, but not by terazosin injection, suggesting that EA-induced analgesia in ankle sprain pain is mediated by spinal α2-adrenoceptors.
The effect of lower doses of intrathecal yohimbine on EA-induced analgesia in ankle sprain pain was also examined in another group of seven rats. Intrathecal injection of 1 μg of yohimbine produced a negligible effect (n = 6), while 5 μg of yohimbine (n = 7) slightly reduced EA-induced analgesia, although the reduction was not statistically significant.
We also tested intrathecal injection of idazoxan, another type of α2-adrenoceptor antagonist, on EA-induced analgesia in ankle sprain pain. In four rats, injection of 30 μg (in 10 μl of saline) almost completely blocked EA-induced analgesia (percentage maximum recovery values at 1 and 2 hours after injection were 6.9 ± 5.0 and −1.6 ± 8.1, respectively).
DISCUSSION
The present study examined the neurotransmitters involved in electroacupuncture-induced analgesia in ankle sprain pain in rats. After ankle sprain, rats decrease weight bearing on the affected hind limb. EA treatment produces temporary reversal of this effect, suggesting that EA has an analgesic effect. This EA-induced analgesia was completely blocked by a systemic injection of the α-adrenergic antagonist phentolamine or by an intrathecal injection of the α2-adrenoceptor antagonist yohimbine, but not by the α1-adrenoceptor antagonist terazosin. The data suggest that EA-induced analgesia is mediated by α2-adrenoceptors in the spinal cord.
The involvement of the endogenous opioid system is a well-established hypothesis for the explanation of acupuncture analgesia. Several previous studies, however, have indicated that opioid antagonists have failed to interfere with acupuncture analgesia under certain circumstances (McLennan et al., 1977; Bossut et al., 1991; Chapman et al., 1980, 1983; Takeshige et al., 1980, 1992; Koo et al., 2002). In fact, a recent study (Harbach et al., 2006) concluded that beta-endorphin is released by stress but not by the acupuncture procedure per se during acupuncture treatment. On the other hand, there is evidence suggesting that non-opioid systems may be involved in EA-induced analgesia. For instance, administration of 5-HT or catecholamine antagonists or depletion of cellular monoamine content blocked the EA-induced analgesic effect, but an opioid antagonist failed to do so (Cheng and Pomeranz, 1981; Takeshige et al., 1980). The antinociceptive effect produced by chemical stimulation of an acupuncture point was also blocked by an α2-adrenoceptor antagonist but not by an opioid antagonist in a visceral pain model (Kwon et al., 2001). These results suggest the possible involvement of the monoaminergic system, noradrenergic and/or serotonergic, in the mediation of acupuncture analgesia. It appears that the analgesic effect induced by acupuncture in various conditions may be mediated by different mechanisms depending on the specific conditions. It is also possible that stimulation of different acupuncture points may trigger different mechanisms. Future systematic studies are warranted to resolve these issues.
The present study tested the possible involvement of the monoaminergic system in EA-induced analgesia in ankle sprain pain because an opioid antagonist, naloxone, was not effective in blocking EA-induced analgesia. This study confirms our previous results of failed attempts to block EA effect by opioid antagonists, both naloxone and naltrexone (a longer-lasting opioid antagonist) (Koo et al., 2002). The descending adrenergic pathway is considered one of the major spinal analgesic systems originating from the brainstem (Yaksh 1985; Proudfit 1988; Millan 2002). Thus, release of spinal norepinephrine (NE) and activation of spinal α2-adrenoceptors represent important components of descending control of nociception. Many studies have shown a role of the adrenergic descending pathway in the modulation of nociception. For example, spinal administration of NE (Reddy et al. 1980; Yaksh 1985; Eisenach et al. 1996; Shinomura et al. 1999) or electrical stimulation of central noradrenergic cells induced powerful antinociception (Stamford 1995; Nuseir and Proudfit 2000). Further studies found that the antinociceptive effect of NE is mediated by α2-adrenoceptors (Howe et al. 1983; Fleetwood-Walker et al. 1985; Yaksh 1985). The activation of α2-adrenoceptors increases potassium conductance in dorsal horn neurons, which produces hyperpolarization and decreases excitability, thereby contributing to analgesia (North and Yoshimura, 1984; Ocana and Baeyens, 1993; Grudt et al., 1995). On the other hand, α2-adrenoceptor agonists reduce the stimulus-evoked release of substance P (Kuraishi et al., 1985; Pang and Vasko, 1986) and glutamate (Kamisaki et al., 1993; Ueda et al., 1995) in the spinal cord, thus suppressing nociceptive transmission. The locus ceruleus in the brainstem is a major cell group of catecholaminergic neurons and is known to project to the spinal cord. Antinociception induced by stimulation of the locus ceruleus is reduced by intrathecal injection of a nonselective adrenergic antagonist, phentolamine, or a selective α2 antagonist, yohimbine, but not by a selective α1 antagonist, prazosin (Jones and Gebhart, 1986). The fact that the analgesic effect induced by EA was reversed by phentolamine or yohimbine, but not by terazosin, clearly agrees with previous studies finding that analgesia is mediated by α2-adrenoceptors. In addition, there have been reports that stimulation of peripheral Aδ and C fibers activates the descending adrenergic system and releases NE in the spinal cord (Tyce and Yaksh, 1981; Men and Matsui, 1994). Thus, we propose that EA activates noradrenergic bulbospinal neurons, resulting in spinal NE release and activation of dorsal horn α2-adrenoceptors. Thus, NE suppresses noxious inputs from the ankle, restoring weight bearing in the affected foot of rats in this model.
The present study used various pharmacological receptor antagonists. Specificity and appropriate dose are important issues in such studies, particularly for antagonists with negative results. We used 1 mg/kg of naloxone, which is a comparable systemic dose reported to block acupuncture analgesia in rodent in a previous study (Pomeranz and Chiu, 1976). In our previous study (Koo et al., 2002), we also tested a much higher dose (10 mg/kg) of naltrexone, which was confirmed to block the effect of 5 mg/kg of morphine. Therefore, we feel that the negative result using the opioid receptor antagonist is valid. There are fewer problems with phentolamine or yohimbine because they were effective at the doses used. Although we primarily used yohimbine as an α2-adrenoceptor blocker, intrathecal injection of idazoxan, another type of α2-adrenoceptor selective antagonist, also blocked EA-induced analgesia. We used terazosin as a selective α1-adrenoceptor antagonist rather than the perhaps more commonly used prazosin to test the reversal of the EA effect in ankle sprain pain. The reason is that terazosin produces a longer-lasting effect (Akduman and Crawford, 2001; Howe et al., 1983), and our previous studies suggest that an effective dose of terazosin is equivalent to that of the α2-adrenoceptor blockers (yohimbine and idazoxan) (Lee et al., 1999; Moon et al., 1999). These results strongly suggest that EA-induced analgesia is mediated by α2-adrenoceptors in the spinal cord.
In summary, we have demonstrated a powerful analgesic effect of EA applied to the contralateral forelimb in rats with ankle sprain. This analgesic effect was reversed by spinal application of α2-adrenoceptor antagonists, but not by a systemic opioid antagonist. These data suggest that EA-induced analgesia in ankle sprain pain is mediated by spinal α2-adrenoceptors. Further study is needed to identify which brain region is activated by EA stimulation in ankle-sprained rats to release norepinephrine in the spinal cord.
ACKNOWLEDGMENTS
This work was supported by NIH Grants AT001474, NS031680, and NS011255. STK was supported, in part, by the Brain Korea 21 project. We would like to express our gratitude to Ms. Denise Broker for her excellent assistance in editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akduman B, Crawford ED. Terazosin, doxazosin, and prazosin: current clinical experience. Urology. 2001;58(6 Suppl 1):49–54. doi: 10.1016/s0090-4295(01)01302-4. [DOI] [PubMed] [Google Scholar]
- Bossut DF, Huang ZS, Sun SL, Mayer DJ. Electroacupuncture in rats: evidence for naloxone and naltrexone potentiation of analgesia. Brain Res. 1991;549:36–46. doi: 10.1016/0006-8993(91)90596-n. [DOI] [PubMed] [Google Scholar]
- Chapman CR, Benedetti C, Colpitts YH, Gerlach R. Naloxone fails to reverse pain thresholds elevated by acupuncture: acupuncture analgesia reconsidered. Pain. 1983;16:13–31. doi: 10.1016/0304-3959(83)90082-9. [DOI] [PubMed] [Google Scholar]
- Chapman CR, Colpitts YM, Benedetti C, Kitaeff R, Gehrig JD. Evoked potential assessment of acupunctural analgesia: attempted reversal with naloxone. Pain. 1980;9:183–197. doi: 10.1016/0304-3959(80)90006-8. [DOI] [PubMed] [Google Scholar]
- Cheng RSS, Pomeranz B. Monoaminergic mechanism of electroacupuncture analgesia. Brain Res. 1981;215:77–92. doi: 10.1016/0006-8993(81)90492-3. [DOI] [PubMed] [Google Scholar]
- Cotler JM. Lateral ligamentous injuries of the ankle. In: Hamilton WC, editor. Traumatic disorders of the ankle. Springer; New York, NY: 1984. pp. 113–123. [Google Scholar]
- Das S, Chatterjee TK, Ganguly A, Ghosh JJ. Role of adrenal steroids on electroacupuncture analgesia and on antagonising potency of naloxone. Pain. 1984;18:135–143. doi: 10.1016/0304-3959(84)90881-9. [DOI] [PubMed] [Google Scholar]
- Davis CS. Statistical methods for the analysis of repeated measurements. Springer-Verlag; New York: 2002. pp. 134–145. [Google Scholar]
- Eisenach JC, De Kock M, Klimscha W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984−1995). Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Mitchell R, Hope PJ, Molony V, Iggo A. An alpha 2 receptor mediates the selective inhibition by noradrenaline of nociceptive responses of identified dorsal horn neurones. Brain Res. 1985;334:243–54. doi: 10.1016/0006-8993(85)90216-1. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Williams JT, Travagli RA. Inhibition by 5-hydroxytryptamine and noradrenaline in substantia gelatinosa of guinea-pig spinal trigeminal nucleus. J Physiol. 1995;485:113–120. doi: 10.1113/jphysiol.1995.sp020716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Terenius L. Neurochemical basis of acupuncture analgesia. Annu Rev Pharmacol Toxicol. 1982;22:193–220. doi: 10.1146/annurev.pa.22.040182.001205. [DOI] [PubMed] [Google Scholar]
- Han JS. Electroacupuncture: an alternative to antidepressants for treating affective diseases? Int J Neurosci. 1986;29:79–92. doi: 10.3109/00207458608985638. [DOI] [PubMed] [Google Scholar]
- Han JS. Acupuncture and stimulation produced analgesia. In: Born GVR, Cuatrecasas P, Herken H, editors. Handbook of experimental pharmacology, 104-Opioids I. Springer; Berlin: 1993. pp. 105–125. [Google Scholar]
- Harbach H, Moll B, Boedeker RH, Vigelius-Rauch U, Otto H, Muehling J, Hempelmann G, Markart P. Minimal immunoreactive plasma beta-endorphin and decrease of cortisol at standard analgesia or different acupuncture techniques. Eur J Anaesthesiol. 2006;12:1–7. doi: 10.1017/S0265021506001906. [DOI] [PubMed] [Google Scholar]
- He LF. Involvement of endogenous opioid peptides in acupuncture analgesia. Pain. 1987;31:99–121. doi: 10.1016/0304-3959(87)90011-X. [DOI] [PubMed] [Google Scholar]
- Howe JR, Wang JY, Yaksh TL. Selective antagonism of the antinociceptive effect of intrathecally applied alpha adrenergic agonists by intrathecal prazosin and intrathecal yohimbine. J Pharmacol Exp Ther. 1983;224:552–558. [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. Characterization of coeruleospinal inhibition of the nociceptive tail-flick reflex in the rat: mediation by spinal alpha 2-adrenoceptors. Brain Res. 1986;364:315–330. doi: 10.1016/0006-8993(86)90844-9. [DOI] [PubMed] [Google Scholar]
- Kamisaki Y, Hamada T, Maeda K, Ishimura M, Itoh T. Presynaptic alpha 2 adrenoceptors inhibit glutamate release from rat spinal cord synaptosomes. J Neurochem. 1993;60:522–526. doi: 10.1111/j.1471-4159.1993.tb03180.x. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the behavioral sciences. 3rd Ed. Brooks/Cole Publishing Co.; Pacific Grove, CA: 1995. pp. 349–355. [Google Scholar]
- Koo ST, Park YI, Lim KS, Chung K, Chung JM. Acupuncture analgesia in a new rat model of ankle sprain pain. Pain. 2002;99:423–431. doi: 10.1016/S0304-3959(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Hirota N, Sato Y, Kaneko S, Satoh M, Takagi H. Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res. 1985;359:177–182. doi: 10.1016/0006-8993(85)91426-x. [DOI] [PubMed] [Google Scholar]
- Kwon YB, Kang MS, Han HJ, Beitz AJ, Lee JH. Visceral antinociception produced by bee venom stimulation of the Zhongwan acupuncture point in mice: role of alpha(2) adrenoceptors. Neurosci. Lett. 2001;308:133–137. doi: 10.1016/s0304-3940(01)01989-9. [DOI] [PubMed] [Google Scholar]
- Lee DH, Liu X, Kim HT, Chung K, Chung JM. Receptor subtype mediating the adrenergic sensitivity of pain behavior and ectopic discharges in neuropathic Lewis rats. J Neurophysiol. 1999;81:2226–2233. doi: 10.1152/jn.1999.81.5.2226. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Price DD, Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 1977;121:368–372. doi: 10.1016/0006-8993(77)90161-5. [DOI] [PubMed] [Google Scholar]
- Mayer DJ. Biological mechanisms of acupuncture. Prog Brain Res. 2000;122:457–477. doi: 10.1016/s0079-6123(08)62157-3. [DOI] [PubMed] [Google Scholar]
- McLennan H, Gilfillan K, Heap Y. Some pharmacological observations on the analgesia induced by acupuncture in rabbits. Pain. 1977;3:229–238. doi: 10.1016/0304-3959(77)90004-5. [DOI] [PubMed] [Google Scholar]
- Men DS, Matsui Y. Activation of descending noradrenergic system by peripheral nerve stimulation. Brain Res Bull. 1994;34:177–182. doi: 10.1016/0361-9230(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Moon DE, Lee DH, Han HC, Xie J, Coggeshall RE, Chung JM. Adrenergic sensitivity of the sensory receptors modulating mechanical allodynia in a rat neuropathic pain model. Pain. 1999;80:589–595. doi: 10.1016/S0304-3959(98)00252-8. [DOI] [PubMed] [Google Scholar]
- North RA, Yoshimura M. The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J Physiol. 1984;349:43–55. doi: 10.1113/jphysiol.1984.sp015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuseir K, Proudfit HK. Bidirectional modulation of nociception by GABA neurons in the dorsolateral pontine tegmentum that tonically inhibit spinally projecting noradrenergic A7 neurons. Neuroscience. 2000;96:773–783. doi: 10.1016/s0306-4522(99)00603-x. [DOI] [PubMed] [Google Scholar]
- Ocana M, Baeyens JM. Differential effects of K+ channel blockers on antinociception induced by alpha 2-adrenoceptor, GABAB and kappa-opioid receptor agonists. Br J Pharmacol. 1993;110:1049–1054. doi: 10.1111/j.1476-5381.1993.tb13919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang IH, Vasko MR. Morphine and norepinephrine but not 5-hydroxytryptamine and gamma-aminobutyric acid inhibit the potassium-stimulated release of substance P from rat spinal cord slices. Brain Res. 1986;376:268–279. doi: 10.1016/0006-8993(86)90189-7. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, Chiu D. Naloxone blockade of acupuncture analgesia: endorphin implicated. Life Sci. 1976;19:1757–1762. doi: 10.1016/0024-3205(76)90084-9. [DOI] [PubMed] [Google Scholar]
- Proudfit HK. Pharmacologic evidence for the modulation of nociception by noradrenergic neurons. Prog Brain Res. 1988;77:357–370. doi: 10.1016/s0079-6123(08)62802-2. [DOI] [PubMed] [Google Scholar]
- Reddy SVR, Maderdrut JL, Yaksh TL. Spinal cord pharmacology of adrenergic agonist-mediated antinociception. J Pharmacol Exp Ther. 1980;213:525–533. [PubMed] [Google Scholar]
- Senn S. Cross-over trials in clinical research. John Wiley & Sons; New York: 1993. pp. 9–16. [Google Scholar]
- Shinomura T, Nakao S, Adachi T, Shingu K. Clonidine inhibits and phorbol acetate activates glutamate release from rat spinal synaptoneurosomes. Anesth Analg. 1999;88:1401–1405. doi: 10.1097/00000539-199906000-00037. [DOI] [PubMed] [Google Scholar]
- Stamford JA. Descending control of pain. Br. J. Anaesth. 1995;75:217–227. doi: 10.1093/bja/75.2.217. [DOI] [PubMed] [Google Scholar]
- Takeshige C, Sato T, Komugi H. Role of peri-aqueductal center gray in acupuncture analgesia. Acupuncture & Electro-Therapeutics Res. 1980;5:323–337. [Google Scholar]
- Takeshige C, Sato T, Mera T, Hisamitsu T, Fang J. Descending pain inhibitory system involved in acupuncture analgesia. Brain Res Bull. 1992;29:617–634. doi: 10.1016/0361-9230(92)90131-g. [DOI] [PubMed] [Google Scholar]
- Tyce GM, Yaksh TL. Monoamine release from cat spinal cord by somatic stimuli: an intrinsic modulatory system. J Physiol. 1981;314:513–529. doi: 10.1113/jphysiol.1981.sp013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Oyama T, Kuraishi Y, Akaike A, Satoh M. Alpha 2-adrenoceptor-mediated inhibition of capsaicin-evoked release of glutamate from rat spinal dorsal horn slices. Neurosci Lett. 1995;188:137–139. doi: 10.1016/0304-3940(95)11397-f. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Standard acupuncture nomenclature. World Health Organization, Regional Office for the Western Pacific; Manila: 1993. [Google Scholar]
- Yaksh TL. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol Biochem Behav. 1985;22:845–858. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]