Abstract
The oculomotor periphery was formerly regarded as a simple mechanism executing complex behaviors explicitly specified by innervation. It is now recognized that several fundamental aspects of ocular motility are properties of the extraocular muscles (EOMs) and their associated connective tissue pulleys. The Active Pulley Hypothesis proposes that rectus and inferior oblique EOMs have connective tissue soft pulleys that are actively controlled by the direction action of the EOMs’ orbital layers. Functional imaging and histology have suggested that the rectus pulley array constitutes an inner mechanism, similar to a gimbal, that is rotated torsionally around the orbital axis by an outer mechanism driven by the oblique EOMs. This arrangement may mechanically account for several commutative aspects of ocular motor control, including Listing’s law, yet permits implementation of noncommutative motility as during the vestibulo-ocular reflex. Recent human behavioral studies, as well neurophysiology in monkeys, are consistent with mechanical rather than central neural implementation of Listing’s law. Pathology of the pulley system is associated with predictable patterns of strabismus that are surgically treatable when the pathologic anatomy is characterized by imaging. This mechanical determination may imply limited possibilities for neural adaptation to some ocular motor pathologies, but indicates greater potential for surgical treatments.
Ophthalmologists routinely interpret the ocular motility examination to assess the status of cranial nerves and central ocular motor processing. While normal motility is easily interpreted, the interpretation of abnormal motility can be quite complex, since it is founded on an understanding of the anatomy of the extraocular muscles (EOMs), the orbital connective tissues, and general principles of motor innervation that ordinarily coordinate binocular movements. Our understanding of even fundamental gross EOM anatomy has been clarified by imaging methods developed in the late 20th century. These insights have in turn motivated investigations that have altered fundamental understanding of ocular motility.
Classical Anatomy
There are six striated oculorotary EOMs, configured as antagonist pairs [1,2]. The medial (MR) and lateral rectus (LR) EOMs rotate the eye horizontally, with the MR accomplishing adduction and the LR accomplishing abduction. The superior (SR) and inferior rectus (IR) EOMs form a vertical antagonist pair, with the SR supraducting and the IR infraducting the globe. However, the vertical rectus EOMs have additional actions not strictly antagonistic. The superior (SO) and inferior oblique (IO) EOMs form an antagonist pair implementing torsion around the line of sight. The SO intorts, while the IO extorts. The oblique EOMs have additional actions that are not strictly antagonistic.
EOM Layers
The oculorotary EOMs, but not the lid-elevating levator palpebrae superioris muscle, are bilaminar [3]. The global layer (GL), containing in humans a maximum of approximately 10,000–15,000 fibers in the mid-length of the EOM, is located adjacent to the globe in rectus EOMs and in the central core of the oblique EOMs [4, 5]. In rectus EOMs and the SO, the GL anteriorly becomes continuous with the terminal tendon that inserts on the sclera [6]. Each rectus orbital layer (OL) contains 40–60% of all the EOM’s fibers. The OL terminates well posterior to the sclera, and at least some of its fibers insert on connective tissue pulleys [6, 7]. The OL is located on the orbital surface of rectus EOMs, sometimes C shaped, and constitutes the concentric peripheral layer of the oblique EOMs.
Gross Structure of EOMs
Rectus EOMs originate in the orbital apex from the fibrous annulus of Zinn. The SO muscle originates from the periorbita of the superonasal orbital wall. The rectus EOMs course anteriorly through loose lobules of fat and connective tissues that form sheathes as the EOMs penetrate posterior Tenon’s fascia. Despite common clinical terminology to the contrary, there exists no ‘muscle cone’ of connective tissue forming bridges among the adjacent rectus EOM bellies in the mid to deep orbit. The SO muscle remains tethered to the periorbita via connective tissues as it courses anteriorly, thins to become continuous with its long, thin tendon. The concentric OL of the SO terminates posterior on a peripherally located sheath [5]. Both the SO sheath and tendon pass through the trochlea, a cartilaginous rigid pulley attached to the superonasal orbital wall. After reflection in the trochlea, the SO tendon passes inferior to the SR, thins, and flattens as it spreads out to its broad scleral insertion posterolaterally on the globe [5]. The IO muscle originates much more anteriorly from the periorbita of the inferonasal orbital rim adjacent to the anterior lacrimal crest, continuing laterally to enter its connective tissue pulley inferior to the IR where the IO penetrates Tenon’s fascia [8].
Notwithstanding the implications of many textbooks, a recent fundamental insight is that rectus EOMs do not follow straight-line paths from their origins to their scleral insertions. In eccentric gaze, rectus EOM paths are inflected sharply at discrete points in the anterior orbit. Even in the 19th century, it was supposed that inflections in EOM paths might be due to orbital connective tissues acting as pulleys, although the archaic concept of early anatomists was largely concerned with bowing of rectus EOM paths away from the orbital center rather than prevention of muscle sideslip relative to the orbital wall [9, 10]. The modern concept of pulleys was first conceived by Joel M. Miller [11], and any eponym applied to rectus pulleys should be his. The ‘pulleys of Miller’ change the anterior paths of rectus EOMs, and thus their pulling directions, in an orderly way during duction. This is shown in the axial magnetic resonance images (MRI) in figure 1, illustrating that the anterior path of the IR muscle changes by half the change in the angle of duction. MRI has shown the same behavior for all the rectus EOMs. The anterior path angle of a rectus EOM changes by half the amount of duction [12].
Fig. 1.
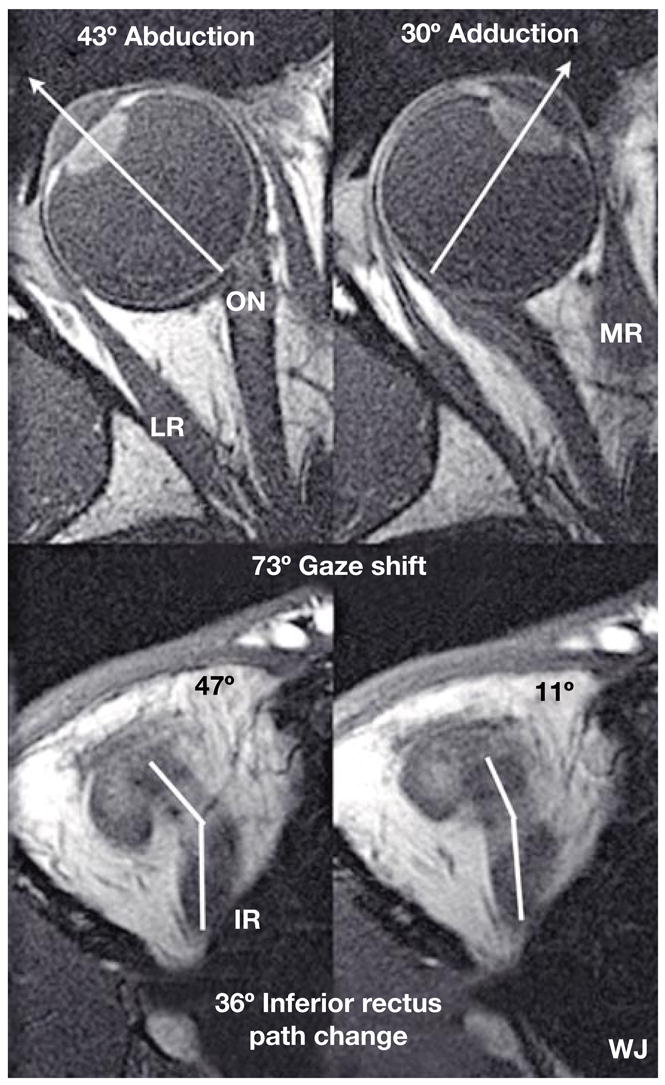
Axial MRI images of a right orbit taken at the level of the lens, fovea, and optic nerve (top row), and simultaneously in an inferior plane along the IR muscle path (bottom row), in abduction (left) and adduction (right). Note the bisegmental IR path, with an inflection corresponding to the IR pulley. For this 73° horizontal gaze shift, there was a corresponding 36° shift in IR muscle path anterior to the inflection at its pulley. By permission from Demer [19].
Structure of Pulleys
Rectus EOM inflection points constitute the functional pulleys of Miller. Anterior to these, rectus EOM paths follow the scleral insertions in eccentric gazes. The pulleys thus act mechanically as rectus EOM origins. The EOM segment between the scleral insertion and pulley defines the direction of force applied to the globe. Pulleys consist of discrete rings of dense collagen encircling the EOM, transitioning gradually into less substantial but broader collagenous sleeves. Anteriorly, these sleeves thin to form slings convex to the orbital wall, and more posteriorly the sleeves thin to form slings convex toward the orbital center. The anterior pulley slings have also been called the ‘intermuscular septum’, a time-honored but not functionally specific term that may be eventually supplanted by more specific terminology. Electron microscopy demonstrates the fibrils of collagen in the pulleys to have an interlaced configuration suited to high internal rigidity [13]. There are bands of smooth muscle (SM) in the pulley suspensions [14, 15], and particularly in a distribution called the inframedial peribulbar SM between the MR and IR pulleys [16]. The overall structure of the orbital connective tissues in schematized in figure 2.
Fig. 2.
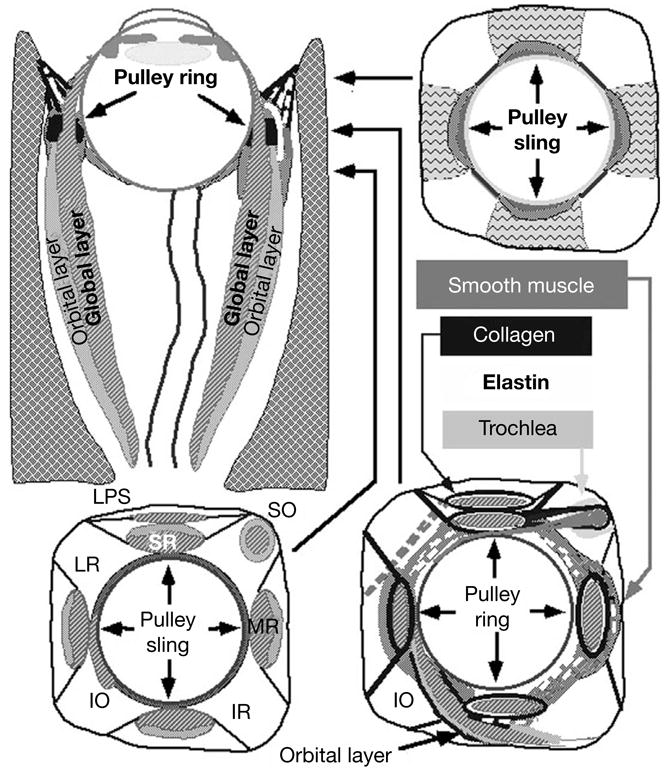
Diagram of the orbita. Coronal views are depicted at levels indicated on axial view. Functional pulleys are at level depicted at lower right. LG = Lacrimal gland; LPS = levator palpebrae superioris muscle; SOT = superior oblique tendon. By permission from Demer [31].
The IR pulley is coupled to the IO pulley in a bond forming part of Lockwood’s ligament, the connective tissue ‘hammock’ across the inferior orbit [8, 16]. The IR and IO pulleys are composed of a common collagenous sheath stiffened by dense elastin. The OL of the IR inserts on its pulley and does not continue anteriorly. The OL of the IO muscle inserts partly on the conjoined IO-IR pulleys, partly on the IO sheath temporally and partly on the inferior aspect of the LR pulley. Elastin and SM occur in the Lockwood’s ligament region of posterior Tenon’s fascia supporting the IR-IO pulley. The inframedial peribulbar SM originates on the nasal aspect of this conjoint pulley, and is positioned upon contraction to displace the pulley nasally. The SM retractors of the lower eyelid (‘Muller’s inferior tarsal muscle’) and connective tissues extending to the inferior tarsal plate are also coupled to the conjoint IR-IO pulley, coordinating lower eyelid position with vertical eye position during vertical gaze shift. The SM of the pulley system has autonomic innervation, including three likely pathways: (1) sympathetic with a norepinephrine projection from the superior cervical ganglion; (2) cholinergic parasympathetic, probably from the ciliary ganglion, and (3) nitroxidergic, probably from the pterygopalatine ganglion [15].
Although the rigid SO pulley – the trochlea – has been known since antiquity [17, 18], its immobility is exceptional, and also unique that the SO’s OL inserts via the SO sheath on the SR pulley’s medial aspect [5]. Net SO pulling direction probably changes half as much as duction despite an immobile pulley, because of the uniquely thin, broad SO tendon wrapping over the globe [19].
Most of these anatomical relationships are evident in gross dissections and surgical exposures. After surgical transposition of a rectus tendon (for the treatment of, e.g., strabismus due to LR palsy), the path of the transposed EOM continues to be obliquely toward the original pulley location. The effect of rectus EOM transposition can be improved by suture fixation from a posterior point on the transposed EOM belly to the sclera adjacent to the palsied EOM [20], a maneuver shown by MRI to displace the pulley further in the transposed direction [21].
Functional Anatomy of Pulleys
The insertion of each rectus EOM’s OL on its pulley appears to be the main driving force translating (linearly moving) that pulley posteriorly during EOM contraction. There is consensus that, in both humans and monkeys, fibers on the orbital surface of each rectus EOM insert into the dense encircling tissue [4, 6] in a distributed manner over an anteroposterior region in which successive bundles of fibers extend up to 1 mm into the surrounding connective tissue1 [7]. Imaging by MRI suggests that these enveloping tissues move in coordination with the insertion and underlying sclera, although histological examinations show the absence of direct connections between these tissues. The connective tissue sleeves themselves have a substantial anteroposterior extent along which connective tissue thickness varies [14], and it has not been possible to histologically identify the precise sites causing EOM path inflections. Consequently, actual pulley locations have been determined from functional imaging by MRI in vivo, rather than by histological examination of dead tissues not subjected to physiological striated and smooth EOM forces.
Since the EOMs must pass through their pulleys and pulleys encircle the EOMs, pulley locations may be inferred from EOM paths even if pulley connective tissues cannot be imaged directly. Quantitative determinations of pulley locations and shifts during ocular rotation have been obtained from coronal MRIs in secondary and tertiary gazes associated with EOM path inflection at the pulleys. Imaging in tertiary (combined horizontal and vertical) gaze positions is particularly informative, since such images show changes in the anteroposterior position of the EOM path inflections [12]. These data have confirmed that all four rectus pulleys move anteroposteriorly in coordination with their scleral insertions, by the same anteroposterior amounts. Being partially coupled to the mobile IR pulley, the IO pulley shifts anteriorly in supraduction, and posteriorly in infraduction. Quantitative MRI shows that the IO pulley moves anteroposteriorly by half as much as the IR insertion [8]. To date, the MRI studies in living subjects have been consistent with histological examinations of the same regions in cadavers that were also examined by MRI prior to embedding and sectioning [16].
Although MRI indicates that rectus and IO pulleys are mobile along the axes of their respective EOMs, pulleys are located stably and stereotypically in the planes transverse to the EOM axes. The 95% confidence intervals for the horizontal and vertical coordinates of normal rectus pulleys range over less than ± 0.6mm [22]. Precise placement of rectus pulleys is important since the pulleys act as the EOM’s functional mechanical origins. Pulley stability in the coronal plane implies a high degree of stiffness of the suspensory tissues of the pulleys. The Active Pulley Hypothesis (APH) supposes that the anteroposterior mobility of the pulleys is accomplished by application of substantial force by the OL of each EOM (fig. 2). Aging causes cause inferior sagging of horizontal rectus pulley positions, which shift downward by 1–2 mm from young adulthood to the seventh decade [23]. Vertical rectus pulley positions change little with aging [23].
The globe itself makes small translations – linear shifts – during ocular duction, as determined by high-resolution MRI in normal humans [23]. For example, the globe translates 0.8 mm inferiorly from 22° downward gaze to 22° upward gaze, and it also translates slightly nasally in both abduction and adduction. While small, these translations affect EOM force directions since the globe center is only 8 mm anterior to the plane of the rectus pulleys.
Pulleys prevent EOM sideslip during globe rotations, but physiologic transverse shifts of rectus pulleys can also occur. Gaze-related changes in rectus pulley positions have been determined by tracing EOM paths with coronal MRI using a coordinate system relative to the center of the orbit [24]. The MR pulley translates 0.6mm superiorly from 22° infraduction to 22° supraduction. The LR pulley translates 1.5mm inferiorly from infraduction to supraduction. The IR pulley shifts 1.1 mm medially in supraduction, but moves 1.3 mm temporally in infraduction. The SR pulley is relatively stable in the mediolateral direction, but moves inferiorly in supraduction, and superiorly in infraduction. Gaze-related shifts in rectus pulley positions are uniform among normal people.
Kinematics of Pulleys
Joel M. Miller first suggested that orbitally fixed pulleys would make the eye’s rotational axis dependent on eye position [11]. Miller’s crucial insight has proved fundamental to ocular kinematics, the rotational properties of the eye. Sequential rotations are not mathematically commutative, so that final eye orientation depends on the order of rotations [25]. Each combination of horizontal and vertical orientations could be associated with infinitely many torsional positions [26], but the eye is constrained (when the head is upright and immobile) by Listing’s law (LL): ocular torsion in any gaze direction is that which it would have it if it had reached that gaze direction by a single rotation from primary eye position about an axis lying in Listing’s plane (LP) [27]. LL is satisfied if the ocular rotational velocity axis shifts by half of the shift in ocular duction [28]. For example, if the eye supraducts 20°, then the vertical velocity axis about which it rotates for subsequent horizontal movement should tip back by 10°. This is called the ‘half-angle rule’, or the velocity domain formulation of LL. Conformity to the half angle rule makes the sequence of ocular rotations appear commutative to the brain [29]. Commutativity is the critical feature of the pulley system.
The APH explains how rectus pulley position can implement the half angle kinematics required by LL [2, 6, 12, 19]. The EOMs rotate the globe about axes perpendicular to the tendon paths near the insertion. In figure 3a, b, it is seen from simple small angle trigonometry that a horizontal rectus EOM’s pulling direction tilts posteriorly by half the angle of supraduction if the pulley is located as far posterior to globe center as the insertion is anterior to globe center. If all rectus EOMs and their pulleys are arranged similarly, this configuration mechanically enforces LL since all the rectus forces rotating the globe observe half angle kinematics.
Fig. 3.
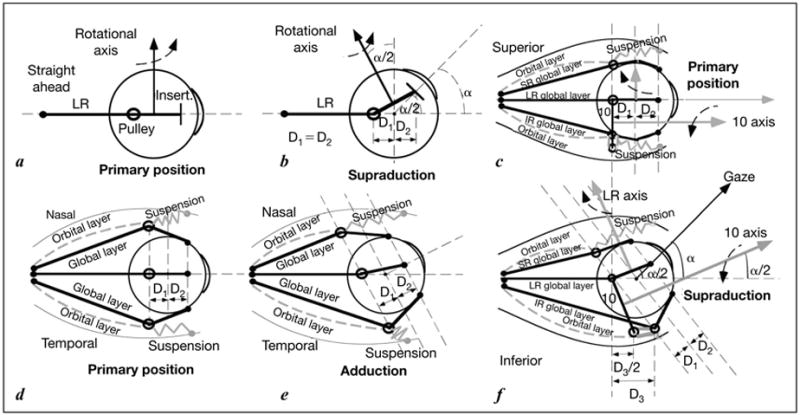
Diagram of EOM and pulley behavior for half angle kinematics conforming to LL. a Lateral view. Rotational velocity axis of the EOM is perpendicular to the segment from pulley to scleral insertion. The velocity axis for the LR is vertical in primary position. b Lateral view. In supraduction to angle alpha, the LR velocity axis tilts posteriorly by angle alpha/2 if distance D1 from pulley to globe center is equal to distance D2 from globe center to insertion. c Lateral view. In primary position, terminal segment of the IO muscle lies in the plane containing the LR and IR pulleys into which the IO’s orbital layer inserts. The IO velocity axis parallels primary gaze. d Superior view of rectus EOMs and pulleys in primary position, corresponding to a. e Superior view. In order for adduction to maintain D1 = D2 in an oculocentric reference, the MR pulley must shift posteriorly in the orbit, and the LR pulley anteriorly. This is proposed to be implemented by the orbital layers of these EOMs, working against elastic pulley suspensions. f Lateral view similar to c. In supraduction to angle alpha, the IR pulley shifts anteriorly by distance D3, as required by the relationship shown in e. The IO pulley shifts anteriorly by D3/2, shifting the IO velocity axis superiorly by alpha/2. By permission from Demer [19].
If only primary and secondary gaze positions were required, rectus pulleys could be rigidly fixed to the orbit. However, it has been proven mathematically that perfect agonist-antagonist EOM alignment is possible only if pulley locations move in the orbit [30]. Tertiary gazes such as adducted supraduction require the rectus pulleys actively to shift anteroposteriorly in the orbit along the EOM’s length, maintaining a fixed oculocentric relationship (fig. 3d, e). The APH proposes that pulley shifts are generated by the contraction of the OLs acting against the elasticity of the pulley suspensions [1, 6, 12, 31]. This behavior could not be due to attachment of rectus pulleys to the sclera. Not only does serial section histology show no such attachment, but also the sclera moves freely relative to pulleys transverse to the EOM axes. Anteroposterior rectus pulley movements persist even after enucleation [32], when the MR path inflection at its pulley continues to shift anteroposteriorly with horizontal versions, but the angle of inflection sharpens to as much as 90° at the pulley [32].
Despite coordinated movements, however, it is supposed that ocular rotation by the OL and pulley translation by the GL require different EOM actions and neural commands. The mechanical load on the GL is predominantly the viscosity of the relaxing antagonist EOM, proportional to rotational speed [33]. The load on the OL, however, is due to the pulley suspension elasticity, which is independent of rotational speed, but proportional to the angle of eccentric gaze. Laminar electromyography in humans shows high, phasic activity in the GL during saccades, with only a small maintained change in activity in eccentric gaze [33]. In the OL, electromyography shows sustained, high activity in eccentric gaze, but no phasic activity during saccades. In cat, the most powerful and fatigue-resistant LR motor units, comprising 27% of all units, innervate both the OL and GL [34]. These ‘bilayer’ motor units would command similar tonic contraction in the two layers, an arrangement convenient to maintain pulley position relative to the EOM insertion. Other motor units project selectively to either the OL or GL [34], as might be appropriate for control of differing viscous loads.
While the rectus EOMs by themselves seem capable of implementing LL [35], some important eye movements do not conform to LL. Violations of LL occur during the vestibulo-ocular reflex (VOR) [36, 37] and during convergence [38, 39]. These violations may be due to the action of the oblique EOMs. The IO muscle’s functional anatomy also appears suited to half angle kinematics. The IO pulley shifts anteroposteriorly by half of vertical ocular duction [8], shifting the IO’s rotational axis by half of vertical duction (fig. 3d, f) [8]. The broad, thin SO insertion on the sclera resists sideslip by virtue of its shape. The SO approximates half angle kinematics because the distance from trochlea to globe center is approximately equal to the distance from globe center to insertion, the SO rotational axis shifts by half the horizontal duction [19].
Optimal stereopsis requires torsional cyclovergence to align corresponding retinal meridia [40]. In central gaze, excyclotorsion occurs in convergence that violates LL [41]. During asymmetrical convergence to a target aligned to one eye, this extorsion occurs in both eyes, interpretable as temporal tilting of LP for each eye [38]. A form of Herring’s law of equal innervation probably exists for the vergence system, such that both eyes receive symmetric version commands for remote targets, and mirror symmetric vergence commands for near targets [42].
MRI during convergence to a target aligned to one eye has been performed using mirrors and has allowed the effect of convergence to be distinguished from that of adduction [43]. In the aligned orbit, there was a 0.3–0.4mm extorsional shift of most rectus pulleys corresponding to about 1.9° [43], similar to globe extorsion [44]. It appears that during convergence, the rectus pulley array rotates about the long axis of the orbit in coordination with ocular torsion, changing the torsional pulling directions of all rectus EOMs but maintaining half angle dependence on horizontal and vertical duction. This would cause a parallel, torsional offset in LP.
While it is possible that globe torsion might passively rotate the rectus pulley array, the high stiffness of the rectus pulley suspensions necessary to stabilize them against sideslip would severely limit such passive torsional shifts, always to less than ocular torsion [43]. An active mechanism has been suggested for the torsional pulley shifts in convergence that equal ocular torsion. The OL of the IO muscle inserts on the IR pulley and, at least in younger specimens, also on the LR pulley [8]. Contraction of the IO OL would directly extorsionally shift the LR and IR pulleys. Contractile IO thickening has been directly demonstrated by MRI during convergence [43]. Inferior LR pulley shift could be coupled to lateral SR pulley shift via the dense connective tissue band between them [45]. The OL of the SO muscle inserts on the SO sheath posterior to the trochlea, with both tendon and sheath reflected at that rigid pulley [5]. Anterior to the trochlea, the SO sheath inserts on the SR pulley’s nasal border. Relaxation of the SO OL during convergence is consistent with single unit recordings in the monkey trochlear nucleus [46], and could contribute to extorsion of the pulley array. The inframedial peribulbar SM might also contribute to rectus pulley extorsion in convergence [16].
Controversy Concerning Pulleys
Because of their distributed nature, some doubt the existence of EOM pulleys of Miller, with the alternative suppositions being that the penetrations of the rectus EOMs through Tenon’s fascia are unimportant, or that the connective tissues serve only to limit the range of ductions [47]. Histological evidence has previously been presented suggesting the presence of EOM pulleys in rodents [48]. Ruskell et al. [7] have proposed that OL insertion into connective tissue sleeves may be a general feature of all mammals. They studied isolated human and monkey rectus EOMs near their pulleys, reporting tendons leaving the orbital surface of the EOMs to insert in sleeves or other surrounding connective tissues. Ruskell et al. [7] considered their results to confirm and extend the observation that the OL fibers separate from the GL fibers and insert in the sheath, and that OL fibers are unlikely to contribute much to duction. Histological study in rat, including 3-D reconstruction, suggested insertion of the OL of the IR on a pulley [49], consistent with the APH.
Dimitrova et al. [50] electrically stimulated eye movements from central to secondary gazes in anesthetized cats and monkeys before and after removal of the LR pulley. Although this surgery predictably increased the amplitude and velocity of horizontal eye movements, there was no significant effect on vertical eye movements [50]. Dimitrova et al. [50] interpreted the increase in eye movement size to transmission of OL force to the tendon, although they also noted that reduction in elastic load associated with pulley removal would also increase eye movement. Their experiment was not a test of the APH’s implications for LL, which would have required investigation of tertiary gazes.
LL Is Mechanical
Long regarded as an organizing principle of ocular motility, LL reduces ocular rotational freedom from three (horizontal, vertical, and torsional) to only two degrees (horizontal and vertical) during visually guided eye movements with the head upright and stationary [28]. The classic formulation of LL states that, with the head upright and immobile, any eye position can be reached from primary position by rotation about one axis lying in LP. Conformity with LL can be demonstrated by expressing ocular rotational axes as ‘quaternions’ that, when plot into LP [25].
Unlike 1-D velocity that is the time derivative of position, 3-D eye velocity is a mathematical function both of eye position and its derivative. The time derivative of each component of 3-D eye position is called coordinate velocity, but this differs from 3-D velocity in a way critical to neural control of saccades [29, 51–53]. Tweed et al. [54] have pointed out that the ocular position axis will be constrained to a plane if, in the velocity domain, the ocular velocity axis changes by half the amount of duction. This can be expressed as a tilt angle ratio of one half. Since in most situations the eye begins in LP, a tilt angle ratio of one half constrains the eye to remain in LP, and so satisfies LL. However, if eye position were somehow to begin outside LP at the onset of an eye movement that subsequently conforms to the velocity domain formulation of LL, eye position would remain in a plane parallel to but displaced from LP.
Violation of LL during the VOR occurs since the VOR compensates for head rotation about any arbitrary axis [37, 55, 56]. However, the VOR does not violate LL ideally, but has a non-half angle dependency of rotational velocity axis on eye position. The ideal tilt angle ratio for the VOR would be zero. However, ocular torsion during the VOR does depend on eye position in the orbit; the VOR axis shifts by about one quarter of duction relative to the head, and thus a tilt angle ratio near 0.25 [37, 55, 56]. During well-controlled, whole-body transient yaw rotation at high acceleration, the VOR exhibits quarter angle behavior beginning at a time indistinguishable for the earliest VOR response [57, 58]. Such kinematics would be consistent with neural drive to a mechanical implementation of quarter angle VOR kinematics as part of the minimum latency reflex, and a different mechanical specification of saccadic half angle behavior.
Neural and mechanical roles in determination of ocular kinematics have been controversial. Before modern descriptions of the orbita, it seemed obvious that LL was implemented neurally in premotor circuits as an intrinsic feature of central ocular motor control [59–63]. The APH then proposed to account for LL mechanically, but physiologic violations of LL continued to suggest a role for central neural [64]. A neural role in LL appeared tenable given the observation of ocular extorsion and temporal tilting of LP during convergence [39, 65] associated with torsional repositioning of the rectus EOM pulley array [43] and alteration in discharge of trochlear motoneurons [46].
Crane et al. [66] studied the transition between the angular VOR’s quarter angle strategy and saccades’ half-angle behavior. These investigators used the yaw angular VOR to drive ocular torsion out of LP, and then used a visual target to evoke a vertical saccade. This is an unusual situation in which the velocity and position domain formulations of LL are no longer equivalent. To return the saccade’s position domain rotational axis to LP would require that the saccade’s velocity axis violate the half angle rule in the process of canceling the initial non-LP torsion. If instead the saccade’s velocity axis conformed to the half angle rule, the saccade would begin and end with the non-LP torsion induced by the VOR. Crane et al. [66] showed that saccades observed half angle kinematics in the velocity domain, and maintained any non-LL initial torsion. This result suggests that the half angle velocity relationship is the fundamental principle underlying LL, as would be expected from coordinated APH behavior of the rectus pulleys. However, torsion returning the eye to LP has been observed during both horizontal and vertical saccades after torsional optokinetic nystagmus had driven the eye out of LP [67], a difference perhaps related to the entrainment of quick phases during nystagmus, and seemingly impossible to implement with a purely mechanical system [67]. Reconciliation of these findings would require differences in neural control of visual saccades vs. vestibular quick phases, a possibility [66] given the known ability of the vestibular system to drive saccades [68].
The functional anatomy of human EOMs has been examined by MRI during ocular counterrolling (OCR), a static torsional VOR mediated by the otoliths [69]. The coronal plane positions of the rectus EOMs shifted torsionally in the same direction as OCR. While OCR was not measured, the torsion of the rectus pulley array was roughly half of OCR reported by other eye movement studies. The torsional shift of the rectus pulley array half of OCR would change rectus EOM pulling directions by one quarter of OCR (fig. 4), ideal for quarter angle VOR kinematics. During OCR, oblique EOMs exhibited changes in cross section consistent with their possible roles in torsional positioning of rectus pulleys [69]. This finding, considered in the context of saccade kinematics during the VOR [66], suggests that the array of the four rectus pulleys constitute a kind of ‘inner gimbal’ that conforms to Listing’s half angle kinematics for visually guided movements such as fixations and saccades, but which is rotated by the oblique EOMs to implement eye movements such as the slow and quick phases of the VOR.
Fig. 4.
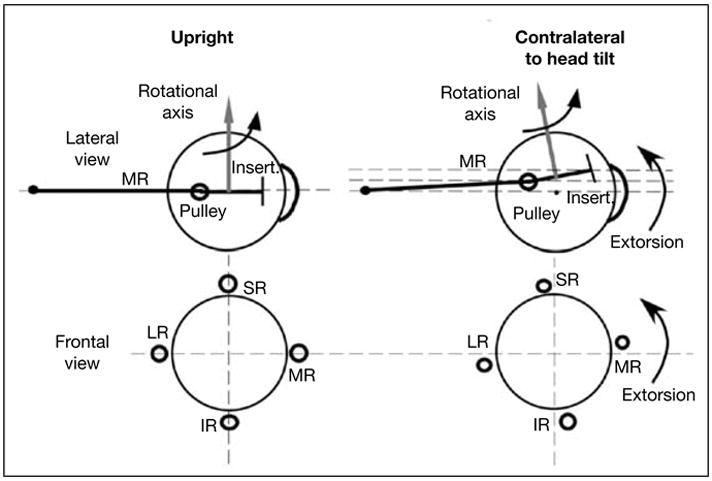
Diagram of effects of head tilt on rectus pulleys in lateral (top row) and frontal (bottom row) views. With head upright, the IR, LR, MR, and SR pulleys are arrayed in frontal view in a cruciate pattern. The MR passes through its pulley, represented as a ring, to its scleral insertion. The rotational velocity axis imparted by the MR is perpendicular to the segment from pulley to insertion. The pulley array extorts during contralateral head tilt. Since during head tilt the MR pulley shifts superiorly by the half the distance the insertion shifts, the MR’s velocity axis changes by one fourth the ocular torsion. By permission from Demer and Clark [69].
Older recordings of trochlear motoneuron discharge suggest that ocular extorsion during convergence is neurally commanded [46]. If the ocular torsion specified by LL were similarly neurally commanded, torsional commands should be reflected in discharge patterns of neurons innervating the oblique and vertical rectus EOMs. Ghasia and Angelaki [70] recorded activities of motoneurons and nerve fibers innervating the vertical rectus and oblique EOMs in monkeys during smooth pursuit conforming to LL. There were no neural commands for LL torsion in motor units innervating the cyclovertical EOMs [51]. This evidence for a mechanical basis of LL was also supported by the experiment of Klier et al. [71] in which electrical stimulation was delivered to the abducens nerve (CN6) of alert monkeys to evoke saccade-like movements. Klier et al. [71] demonstrated that the evoked saccades had half angle kinematics conforming to LL. The decisive conclusion from these two experiments is that LL has a mechanical basis, and is not specified by the instantaneous neural commands. These two results were predicted by the APH [6], while the neural theory of LL predicted opposite results in both cases [62]. However, the neurons driving the cyclovertical EOMs not only did not command half angle LL torsion, but also did not command quarter angle kinematics for the VOR [70]. This suggests that quarter angle VOR kinematics are also mechanical, rather than neural. An early suggestion had been made than quarter angle behavior could be implemented mechanically by retraction of rectus pulleys [6], but subsequent recognition that this idea would be unrealistic [61] led to abandonment of the concept of pulley retraction [2, 43]. Furthermore, uncoordinated antero-posterior shift in pulley location would be inconsistent with the recent experiments of Crane et al. [66] demonstrating transition between quarter angle VOR, and half angle saccade behavior without measurable latency. The foregoing results seemingly require that quarter angle VOR behavior arise from mechanical phenomena not previously considered.
Implications for Neural Control
Some tentative conclusions can now be reached concerning neural control of eye movements generally, and some older data probably should be reinterpreted. Central neural signals correlated with all types of eye movements would be expected to reflect effects of torsional reconfiguration of rectus pulleys during the VOR. Recordings from burst neurons in monkeys appear compatible with the torsional shift of rectus pulleys transverse to the EOM axes in the direction of OCR induced by head tilt [72]. In monkeys, the displacement plane for 3-D eye positions during pursuit and saccades shifts opposite to changes in head orientation relative to gravity [73], and such shifts may be dynamic during semicircular canal stimulation [74, 75]. Hess and Angelaki have suggested that shift in LP is mediated by the otolith input to the 3-D neural integrator [73], but the finding may be reconciled with the observation that lesion of the integrator in the rostral interstitial nucleus of the medial longitudinal fasciculus also abolishes the torsional shift in LP associated with OCR [76] if the torsional shift of pulleys is mediated by the 3-D neural integrator. If so, it would be predicted that integrator lesion would abolish counterroll of the pulleys during vestibular stimulation, by blocking polysynaptic vestibular input to the oblique EOMs whose tonic activity presumably maintains torsional pulley array orientation.
In monkeys, the preferred directions of saccadic neurons in the superior colliculus shift in the opposite direction, and by slightly more than half the amount, of head tilt [77]. Based on simultaneous measurements of OCR and preferred directions of superior collicular neurons, Frens et al. [77] concluded that the changes in EOM pulling directions are probably about two thirds of ocular torsion.
Regardless of the ocular motor subsystem involved, torsional rectus pulley shifts during the VOR would preserve the advantage of apparent commutativity of the peripheral ocular motor apparatus for concurrent saccades and pursuit. This commutativity would be valuable even though higher-level sensorimotor transformations must account for 3-D geometrical effects of eye and head orientation [64, 77–79], and is incorporated in some modern models of ocular motor control [29, 52, 76, 78, 80]. Neural processing for the VOR must be generated in 3-D, based on transduction of head motion in three degrees of freedom, and on 3-D eye orientation in the head.
Some low level visuomotor processing may be simpler than previously believed. In saccade programming, retinal error could be mapped onto corresponding zero-torsion motor error commands within LP as modeled by the ‘displacement-feedback’ model of Crawford and Guitton [81]. This model, with a downstream mechanism for half angle behavior, can simulate the visuomotor transformations necessary for accurate and kinematically correct saccades within a reasonable oculomotor range, but had been rejected by Crawford and Guitton who supposed that saccades from non-LL torsional starting positions return to LP [81]. Recent demonstration by Crane et al. [66] that such saccades maintain their initial non-LL torsion while nevertheless conforming to half angle kinematics suggests that the ‘displacement-feedback’ model, lacking in a neural representation of LL, is plausible for control of visual saccades. In the context of realistic mechanical properties of EOM pulleys, sensorimotor integration of saccades does not require explicit neural computation of ocular torsion. This simplification solves some complexity, but merely moves other kinematic problems to a higher level. When head movements are involved, neural consideration of torsion is geometrically unavoidable for accurate localization of visual targets [78, 81].
Several aspects of ocular kinematics are thus implemented by an intricate mechanical arrangement, rather than by complex neural commands to a simpler mechanical arrangement. This insight alters the interpretation of common situations, and offers hope of mechanical (i.e., surgical) solutions to clinical disorders that might earlier have been believed to have neural origins. If the APH is correct, oblique EOM function would not be critical for LL [35], although oblique tone might set initial LP orientation. This is supported by the finding in chronic SO paralysis that LL is observed, albeit with temporal tilting of LP [82, 83], and that this temporally tilted LP is not changes by vergence as is normally the case [84]. The orientation of LP varies considerably both among individuals, and between eyes of the same individuals, making it unlikely that absolute LP orientation is very important to either vision of ocular motor control [58]. Although oblique EOMs do not actively participate in generation of LL, elastic tensions arising from stretching and relaxation of oblique EOMs would create torques violating LL unless their innervations were adjusted to compensate [51]. Consequently, recordings of small changes in oblique EOM innervations during pursuit movements conforming to LL [70, 85] do not negate a pulley contribution, nor do dynamic violations of LL during saccades in SO palsy [82].
Implications for Strabismus
Thinking about cyclovertical strabismus has been dominated by the historical concept of EOM weakness, typified by the terms ‘paresis’ and ‘paralysis’ [86]. This is likely because cranial nerves innervating EOMs are susceptible to damage by trauma and compression, and because when the concept of EOM weakness became dominant, neuroscience was too primitive to offer alternative explanations [86]. Deeply engrained clinical concepts require modification.
Prototypic for cyclovertical strabismus is SO palsy. Theoretical, experimental, and much clinical evidence supports the idea that acute, unilateral SO palsy produces a small ipsilateral hypertropia that increases with contralateral gaze, and with head tilt to the ipsilateral shoulder [87, 88]. The basis of this ‘3-step test’ is traditionally believed related to OCR, so that the eye ipsilateral to head tilt is normally intorted by the SO and SR EOMs whose vertical actions cancel [89]. However, ipsilateral to a palsied SO, unopposed SR elevating action is supposed to create hypertropia. The 3-step test has been the cornerstone of diagnosis and classification of cyclovertical strabismus for generations of clinicians [90,91]. When the 3-step test is positive, strabismologists infer SO weakness and attribute the large amount of interindividual alignment variability to secondary changes [83] such as ‘IO overaction’ and ‘SR contracture’. The 3-step test’s mechanism is generally misunderstood. Kushner [92] has pointed out that were traditional teaching true, then IO weakening, the most common surgery for SO palsy, should increase the head tilt-dependent change in hypertropia; the opposite is observed. Among numerous inconsistencies with common clinical observations [92], bilateral SO palsy should cause greater head tilt-dependent change in hypertropia than unilateral SO palsy; however, the opposite is found [93]. Modeling and simulation of putative effects of head tilt in SO palsy suggest that SO weakness alone cannot account for typical 3-step test findings [94, 95].
High-resolution MRI has quantified normal changes in SO cross-section with vertical gaze, and SO atrophy and loss of gaze-related contractility typical of SO palsy [96–99]. Neurosurgical SO denervation rapidly produces neurogenic atrophy and ablates contractile thickening normally observed in infraduction. A striking and consistent MRI finding has been the nonspecificity of the 3-step test for structural abnormalities of the SO belly, tendon, and trochlea, found in only in ~50% of patients [100]. Even in patients selected because MRI demonstrated profound SO atrophy, there was no correlation between clinical motility and IO size or contractility [99]. A possible explanation for some of this discrepancy might be putative SO tendon laxity, assessed intraoperatively by a qualitative and perhaps unreliable judgment made during application of traction with forceps [101–103]. Multiple conditions can simulate the ‘SO palsy’ pattern of incomitant hypertropia [104]. Vestibular lesions cause head-tilt dependent hypertropia, also known as skew deviation [105] that can mimic SO palsy by the 3-step test [106]. Pulley heterotopy can simulate SO palsy [107], and is probably not its result, since SO atrophy is not associated with significant alterations in pulley position in central gaze [108].
Craniosynostosis is a congenital disorder in which skull shape is distorted by premature fusion of the sutures among cranial bones. While various eponyms have been attached based on variable expressivity (e.g. Crouzon, Pfeiffer), these are largely due to known gene mutations affecting bone formation [109]. Strabismus is prevalent in craniosynostosis, particularly large V and A patterns [110, 111], yet responds poorly to oblique EOM surgery [112]. Rectus EOM paths may be markedly abnormal in craniosynostosis [107, 113], imparting abnormal pulling directions. It has been proposed that because the EOM pulley array is anchored to the bony orbit at discrete points [45], bony abnormality alters EOM pulling directions by malpositioning pulleys. Typically, the heterotopic array of rectus pulleys is extorted or intorted, not necessarily symmetrically. Computer simulations suggest rectus pulley malpositioning as in craniosynostosis can produce incomitant strabismus [114, 115]. Extorsion of the pulley array is associated with V patterns, and intorsion associated with A patterns [116].
Surgical Treatment of Pulley Pathology
Surgery for pulley disorders has recently emerged for treatment of three types of pathologies [19].
Pulley Heterotopy
Milder pulley heterotopy not apparently associated with craniosynostosis may involve the stable malpositioning of one or several rectus pulleys [114, 115]. Initial efforts to treat heterotopy involved transpositions of the scleral insertions of EOMs whose pulleys were heterotopic [19], later augmented by fixations of EOM bellies to the underlying sclera ~8mm posteriorly [113]. While MRI has demonstrated that this does shift the involved rectus pulley in the desired direction [21, 117], because the pulley does not shift as far as the insertion, the operation introduces undesirable ocular torsion opposite the direction of transposition. Since normal pulleys are not fixed to sclera, posterior fixation also compromises normal pulley kinematics and introduces abnormal globe translation during duction [117]. Newer approaches to pulley heterotopy involve surgery on connective tissues suspending the pulleys. A technically convenient approach to treatment of inferior displacement of the LR pulley is to shorten and stiffen the ligament coupling the LR and SR pulleys. Extreme pulley heterotopy is associated with esotropia and hypotropia in axial high myopia [118, 119]. In this condition, historically misnamed the ‘heavy eye syndrome,’ the LR pulley shifts inferiorly to approach the IR, and the globe correspondingly shifts superotemporally out of the rectus pulley array. It has been recently reported that surgical anastomosis of the lateral margin of the IR belly with the superior margin of the LR belly is highly effective in correcting esotropia associated with the ‘heavy eye syndrome,’ since the procedure normalizes EOM paths relative to the globe in a manner impossible for more conventional strabismus surgery [120].
Pulley Instability
Normal pulleys shift only slightly in the coronal plane even during large ductions [24]. Large gaze-related shifts or one or more pulleys are associated with incomitant strabismus [19, 121]. Pulley instability has also been termed ‘gaze-related pulley shift’ [122]. Inferior LR pulley shift in adduction produces restrictive hypotropia closely resembling Brown syndrome caused by hindrance of SO travel in the trochlea [123], or ‘X’ pattern exotropia characterized by greater deviation in both up and down than in central gaze [121]. Early efforts to treat pulley instability consisted of posterior fixation of the involved EOM to the underlying sclera, and were intended to prevent posterior sideslip of the EOM belly. More recent physiologically driven approaches involve pulley suspensions directly, tightening lax connective tissue bands that presumably permitted the pulley shift.
Pulley Hindrance
The third recognized pathology is pulley hindrance, in which normal posterior shift with EOM contraction is mechanically impeded [124], often inducing abnormal globe translation. Intentionally created hindrance can be therapeutic, as long known for posterior fixation (also known as ‘retroequatorial myopexy’ and ‘fadenoperation’) of an EOM to the underlying sclera. An operation intended to reduce an EOM’s effect in its field of action, posterior fixation was originally supposed to work by reduction in the EOM’s arc of contact, reducing its rotational lever arm [125]. Imaging by MRI demonstrates this mechanism incorrect, but several lines of evidence indicate that posterior fixation actually works by hindering posterior shift of the contracting EOM’s pulley, mechanically restricting EOM action [126]. A technically simpler and safer modification of posterior fixation has recently been introduced by us in which the MR pulley suspension is placed under tension and the MR pulley sutured to the EOM belly; this operation is at least as effective as posterior fixation with scleral suturing in treatment of accommodative esotropia with excessive accommodative convergence [127].
Central to the initial recognition of pulleys was the stability of rectus EOM paths after large surgical transpositions of the scleral insertions. Only slight shifts of pulleys are observed by MRI after transposition [21, 117]. Posterior suture fixation of the transposed EOMs as described by Foster [20] shifts the pulley farther into the direction of the transposed insertion. This changes the pulling direction to mimic more closely that of the paralyzed EOM, increasing the effectiveness of transposition [117].
Conclusion
The fundamental anatomy of the ocular motor effector apparatus fundamentally differs from traditional teaching. The following encapsulates this author’s broad concept of the orbita, simplified here for heuristic purposes. Rather than consisting of mechanically simple EOMs rotating the eye under explicit neural control of every kinematic nuance, the ocular motor system consists of a rather intricate mechanical arrangement comprised of a trampoline-like suspension supported by the rectus EOMs and their associated connective tissues, which in turn is circumferentially controlled by the obliques. Rectus EOMs and their pulleys constitute the inner suspension that implements kinematics in 2-D corresponding largely to the 2-D organization of the retina and subcortical visual system, and so mechanically implements LL without additional neural specification. The inner suspension has effectively commutative properties. Analogous to a gimbal arrangement (but importantly different from a gimbal in some respects), the outer suspension moves the inner under the drive from the oblique EOMs to generate torsion not conforming to LL, and noncommutatively influences the inner suspension. The degree to which neural adaptations can compensate for ocular kinematics that normally are mechanically determined is a crucial question, since the answer will inform us about the clinical significance of many disorders of ocular motility, and the degree to which they may be amenable to surgical treatment.
Acknowledgments
This work was supported by US Public Health Service grants EY08313, EY00331, and DC005224. J. Denier received an award from Research to Prevent Blindness and is Leonard Apt Professor of Ophthalmology.
Footnotes
While they may properly be said to have dual insertions, the OL and GL insertions are not widely displaced. The OLs and GLs of EOMs do not bifurcate widely before inserting as might have been misunderstood from the diagrammatic implications of some authors who intended to emphasize the differing neural control and possible proprioception of the two layers [22]. The concept of dual insertions does not necessarily imply that every fiber in each layer terminates in that layer’s insertion, since fibers may terminate on one another short of the insertion in myomyous junctions [23].
References
- 1.Demer JL. Extraocular muscles, chapter 1. In: Jaeger EA, Tasman PR, editors. Duane’s Clinical Ophthalmology. Vol. 1. Philadelphia: Lippincott; 2000. pp. 1–23. [Google Scholar]
- 2.Demer JL. Anatomy of strabismus. In: Taylor D, Hoyt C, editors. Pediatric Ophthalmology and Strabismus. 3. London: Elsevier; 2005. pp. 849–861. [Google Scholar]
- 3.Porter JD, Baker RS, Ragusa RJ, Brueckner JK. Extraocular muscles: basic and clinical aspects of structure and function. Surv Ophthalmol. 1995;39:451–484. doi: 10.1016/s0039-6257(05)80055-4. [DOI] [PubMed] [Google Scholar]
- 4.Oh SY, Poukens V Demer JL. Quantitative analysis of rectus extraocular muscle layers in monkey and humans. Invest Ophthalmol Vis Sci. 2001;42:10–16. [PubMed] [Google Scholar]
- 5.Kono R, Poukens V, Demer JL. Superior oblique muscle layers in monkeys and humans. Invest Ophthalmol Vis Sci. 2005;46:2790–2799. doi: 10.1167/iovs.04-1147. [DOI] [PubMed] [Google Scholar]
- 6.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–1290. [PubMed] [Google Scholar]
- 7.Ruskell GL, Kjellevold Haugen IB, Bruenech JR, van der Werf F. Double insertions of extraocular rectus muscles in humans and the pulley theory. J Anat. 2005;206:295–306. doi: 10.1111/j.1469-7580.2005.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demer JL, Oh SY, Clark RA, Poukens V. Evidence for a pulley of the inferior oblique muscle. Invest Ophthalmol Vis Sci. 2003;44:3856–3865. doi: 10.1167/iovs.03-0160. [DOI] [PubMed] [Google Scholar]
- 9.Sappey PC. Traite D’Anatomie Descriptive Avec Figures Intercalees Dans Le Texte. Paris: Delahaye et Lecrosnier; 1888. [Google Scholar]
- 10.Sappey PC. The motor muscles of the eyeball [translation from the French] Strabismus. 2001;9:243–253. doi: 10.1076/stra.9.4.243.696. [DOI] [PubMed] [Google Scholar]
- 11.Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–240. doi: 10.1016/0042-6989(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 12.Kono R, Clark RA, Demer JL. Active pulleys: magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci. 2002;43:2179–2188. [PubMed] [Google Scholar]
- 13.Porter JD, Poukens V, Baker RS, Demer JL. Cytoarchitectural organization of the medial rectus muscle pulley in man. Invest Ophthalmol Vis Sci. 1995;36:S960. [Google Scholar]
- 14.Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–1136. [PubMed] [Google Scholar]
- 15.Demer JL, Poukens V, Miller JM, Micevych P. Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest Ophthalmol Vis Sci. 1997;38:1774–1785. [PubMed] [Google Scholar]
- 16.Miller JM, Demer JL, Poukens V, Pavlowski DS, Nguyen HN, Rossi EA. Extraocular connective tissue architecture. J Vis. 2003;3:240–251. doi: 10.1167/3.3.5. [DOI] [PubMed] [Google Scholar]
- 17.Fink WH. Surgery of the Vertical Muscles of the Eye. Springfield (IL): Thomas; 1962. pp. 37–121. [Google Scholar]
- 18.Helveston EM, Merriam WW, Ellis FD, Shellhamer RH, Gosling CG. The trochlea: a study of the anatomy and physiology. Ophthalmology. 1992;80:124–133. doi: 10.1016/s0161-6420(82)34835-6. [DOI] [PubMed] [Google Scholar]
- 19.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus. The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–738. doi: 10.1167/iovs.03-0464. [DOI] [PubMed] [Google Scholar]
- 20.Foster RS. Vertical muscle transposition augmented with lateral fixation. J AAPOS. 1997;1:20–30. doi: 10.1016/s1091-8531(97)90019-7. [DOI] [PubMed] [Google Scholar]
- 21.Clark RA, Rosenbaum AL, Demer JL. Magnetic resonance imaging after surgical transposition defines the anteroposterior location of the rectus muscle pulleys. J AAPOS. 1999;3:9–14. doi: 10.1016/s1091-8531(99)70088-1. [DOI] [PubMed] [Google Scholar]
- 22.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797. [PubMed] [Google Scholar]
- 23.Clark RA, Demer JL. Effect of aging on human rectus extraocular muscle paths demonstrated by magnetic resonance imaging. Am J Ophthalmol. 2002;134:872–878. doi: 10.1016/s0002-9394(02)01695-1. [DOI] [PubMed] [Google Scholar]
- 24.Clark RA, Miller JM, Demer JL. Location and stability of rectus muscle pulleys inferred from muscle paths. Invest Ophthalmol Vis Sci. 1997;38:227–240. [PubMed] [Google Scholar]
- 25.Haslwanter T. Mathematics of three-dimensional eye rotations. Vision Res. 1995;35:1727–1739. doi: 10.1016/0042-6989(94)00257-m. [DOI] [PubMed] [Google Scholar]
- 26.van den Berg AV. Kinematics of eye movement control. Proc R Soc Lond. 1995;260:191–197. doi: 10.1098/rspb.1995.0079. [DOI] [PubMed] [Google Scholar]
- 27.Ruete CGT. Ocular physiology. Strabismus. 1999;7:43–60. doi: 10.1076/stra.7.1.43.654. [DOI] [PubMed] [Google Scholar]
- 28.Tweed D, Vilis T. Geometric relations of eye position and velocity vectors during saccades. Vision Res. 1990;30:111–127. doi: 10.1016/0042-6989(90)90131-4. [DOI] [PubMed] [Google Scholar]
- 29.Quaia C, Optican LM. Commutative saccadic generator is sufficient to control a 3-D ocular plant with pulleys. J Neurophysiol. 1998;79:3197–3215. doi: 10.1152/jn.1998.79.6.3197. [DOI] [PubMed] [Google Scholar]
- 30.Koene AR, Erklens CJ. Properties of 3D rotations and their relation to eye movement control. Biol Cybern. 2004;90:410–417. doi: 10.1007/s00422-004-0477-3. [DOI] [PubMed] [Google Scholar]
- 31.Demer JL. The orbital pulley system: a revolution in concepts of orbital anatomy. Ann N Y Acad Sci. 2002;956:17–32. doi: 10.1111/j.1749-6632.2002.tb02805.x. [DOI] [PubMed] [Google Scholar]
- 32.Detorakis ET, Engstrom RE, Straatsma BR, Demer JL. Functional anatomy of the anophthalmic socket: insights from magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2003;44:4307–4313. doi: 10.1167/iovs.03-0171. [DOI] [PubMed] [Google Scholar]
- 33.Collins CC. The human oculomotor control system. In: Lennerstrand G, Bach-y-Rita P, editors. Basic Mechanisms of Ocular Motility and Their Clinical Implications. New York: Pergamon; 1975. pp. 145–180. [Google Scholar]
- 34.Shall MS, Goldberg SJ. Lateral rectus EMG and contractile responses elicited by cat abducens motoneurons. Muscle Nerve. 1995;18:948–955. doi: 10.1002/mus.880180905. [DOI] [PubMed] [Google Scholar]
- 35.Porrill J, Warren PA, Dean P. A simple control laws generates Listing’s positions in a detailed model of the extraocular muscle system. Vision Res. 2000;40:3743–3758. doi: 10.1016/s0042-6989(00)00211-x. [DOI] [PubMed] [Google Scholar]
- 36.Smith MA, Crawford JD. Neural control of rotational kinematics within realistic vestibuloocular coordinate systems. J Neurophysiol. 1998;80:2295–2315. doi: 10.1152/jn.1998.80.5.2295. [DOI] [PubMed] [Google Scholar]
- 37.Crane BT, Tian J, Demer JL. Human angular vestibulo-ocular reflex initiation: relationship to Listing’s law. Ann NY Acad Sci. 2005;1039:1–10. doi: 10.1196/annals.1325.004. [DOI] [PubMed] [Google Scholar]
- 38.Steffen H, Walker MF, Zee DS. Rotation of Listing’s plane with convergence: Independence from eye position. Invest Ophthalmol Vis Sci. 2000;41:715–721. [PubMed] [Google Scholar]
- 39.Kapoula Z, Bernotas M, Haslwanter T. Listing’s plane rotation with convergence: role of disparity, accommodation, and depth perception. Exp Brain Res. 1999;126:175–186. doi: 10.1007/s002210050727. [DOI] [PubMed] [Google Scholar]
- 40.Schreiber K, Crawford JD, Fetter M, Tweed D. The motor side of depth vision. Nature. 2001;410:819–822. doi: 10.1038/35071081. [DOI] [PubMed] [Google Scholar]
- 41.Bruno P, van den Berg AV. Relative orientation of primary positions of the two eyes. Vision Res. 1997;37:935–947. doi: 10.1016/s0042-6989(96)00219-2. [DOI] [PubMed] [Google Scholar]
- 42.van Rijn LJ, van den Berg AV. Binocular eye orientation during fixations: Listing’s law extended to include eye vergence. Vision Res. 1993;33:691–708. doi: 10.1016/0042-6989(93)90189-4. [DOI] [PubMed] [Google Scholar]
- 43.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–2085. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 44.Allen MJ, Carter JH. The torsional component of the near reflex. Am J Optom Arch Am Acad Optom. 1967;44:343–349. [PubMed] [Google Scholar]
- 45.Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002;43:2923–2932. [PubMed] [Google Scholar]
- 46.Mays LE, Zhang Y, Thorstad MH, Gamlin PD. Trochlear unit activity during ocular convergence. J Neurophysiol. 1991;65:1484–1491. doi: 10.1152/jn.1991.65.6.1484. [DOI] [PubMed] [Google Scholar]
- 47.van den Bedem SPW, Schutte S, van der Helm FCT, Simonsz HJ. Mechanical properties and functional importance of pulley bands or ‘faisseaux tendineux’. Vis Res. 2005;45:2710–2714. doi: 10.1016/j.visres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 48.Khanna S, Porter JD. Evidence for rectus extraocular muscle pulleys in rodents. Invest Ophthalmol Vis Sci. 2001;42:1986–1992. [PubMed] [Google Scholar]
- 49.Felder E, Bogdanovich S, Rubinstein NA, Khana TS. Structural details of rat extraocular muscles andd three-dimensional reconstruction of the rat inferior rectus muscle and muscle-pulley interface. Vision Res. 2005;45:1945–1955. doi: 10.1016/j.visres.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 50.Dimitrova DM, Shall MS, Goldberg SJ. Stimulation-evoked eye movements with and without the lateral rectus muscle pulley. J Neurophysiol. 2003;90:3809–3815. doi: 10.1152/jn.00622.2003. [DOI] [PubMed] [Google Scholar]
- 51.Quaia C, Optican LM. Dynamic eye plant models and the control of eye movements. Strabismus. 2003;11:17–31. doi: 10.1076/stra.11.1.17.14088. [DOI] [PubMed] [Google Scholar]
- 52.Raphan T. Modeling control of eye orientation in three dimensions. I. Role of muscle pulleys in determining saccadic trajectory. J Neurophysiol. 1998;79:2653–2667. doi: 10.1152/jn.1998.79.5.2653. [DOI] [PubMed] [Google Scholar]
- 53.Raphan T. Modeling control of eye orientation in three dimensions. In: Fetter M, Haslwanter T, Misslisch H, Tweed D, editors. Three-dimensional Kinematics of Eye, Head, and Limb Movements. Amsterdam: Harwood; 1997. pp. 359–374. [Google Scholar]
- 54.Tweed D, Cadera W, Vilis T. Computing three-dimensional eye position quaternions and eye velocity from search coil signals. Vision Res. 1990;30:97–110. doi: 10.1016/0042-6989(90)90130-d. [DOI] [PubMed] [Google Scholar]
- 55.Misslisch H, Tweed D, Fetter M, Sievering D, Koenig E. Rotational kinematics of the human vestibuloocular reflex. III. Listing’s law. J Neurophysiol. 1994;72:2490–2502. doi: 10.1152/jn.1994.72.5.2490. [DOI] [PubMed] [Google Scholar]
- 56.Thurtell MJ, Black RA, Halmagyi GM, Curthoys IA, Aw ST. Vertical eye position-dependence of the human vestibuloocular reflex during passive and active yaw head rotations. J Neurophysiol. 1999;81:2415–2428. doi: 10.1152/jn.1999.81.5.2415. [DOI] [PubMed] [Google Scholar]
- 57.Crane BT, Tian JR, Demer JL. Temporal dynamics of ocular position dependence of the initial human vestibulo-ocular reflex. Invest Ophthalmol Vis Sci. 2005 doi: 10.1167/iovs.05-0172. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crane BT, Tian JR, Demer JL. Human angular vestibulo-ocular reflex initiation: relationship to Listing’s law. Ann N Y Acad Sci. 2005;1039:26–35. doi: 10.1196/annals.1325.004. [DOI] [PubMed] [Google Scholar]
- 59.Crawford JD, Vilis T. Symmetry of oculomotor burst neuron coordinates about Listing’s plane. J Neurophysiol. 1992;68:432–448. doi: 10.1152/jn.1992.68.2.432. [DOI] [PubMed] [Google Scholar]
- 60.Tweed D. Visual-motor optimization in binocular control. Vision Res. 1997;37:1939–1951. doi: 10.1016/s0042-6989(97)00002-3. [DOI] [PubMed] [Google Scholar]
- 61.Misslisch H, Tweed D. Neural and mechanical factors in eye control. J Neurophysiol. 2001;86:1877–1883. doi: 10.1152/jn.2001.86.4.1877. [DOI] [PubMed] [Google Scholar]
- 62.Angelaki DE, Hess BJ. Control of eye orientation: where does the brain’s role end and the muscle’s begin? Eur J Neurosci. 2004;19:1–10. doi: 10.1111/j.1460-9568.2004.03068.x. [DOI] [PubMed] [Google Scholar]
- 63.Angelaki DE. Three-dimensional ocular kinematics during eccentric rotations: evidence for functional rather than mechanical constraints. J Neurophysiol. 2003;89:2685–2696. doi: 10.1152/jn.01137.2002. [DOI] [PubMed] [Google Scholar]
- 64.Klier EM, Crawford JD. Human oculomotor system acounts for 3-D eye orientation in the visual-motor transformation for saccades. J Neurophysiol. 1998;80:2274–2294. doi: 10.1152/jn.1998.80.5.2274. [DOI] [PubMed] [Google Scholar]
- 65.Mok D, Ro A, Cadera W, Crawford JD, Vilis T. Rotation of Listing’s plane during vergence. Vision Res. 1992;32:2055–2064. doi: 10.1016/0042-6989(92)90067-s. [DOI] [PubMed] [Google Scholar]
- 66.Crane BT, Tian J, Demer JL. Kinematics of vertical saccades during the yaw vestibulo-ocular reflex in humans. Invest Ophthalmol Vis Sci. 2005;46:2800–2809. doi: 10.1167/iovs.05-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee C, Zee DS, Straumann D. Saccades from torsional offset positions back to Listing’s plane. J Neurophysiol. 2000;83:3141–3253. doi: 10.1152/jn.2000.83.6.3241. [DOI] [PubMed] [Google Scholar]
- 68.Tian JR, Crane BT, Demer JL. Vestibular catch-up saccades in labyrinthine deficiency. Exp Brain Res. 2000;131:448–457. doi: 10.1007/s002219900320. [DOI] [PubMed] [Google Scholar]
- 69.Demer JL, Clark RA. Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol. 2005;94:3292–3302. doi: 10.1152/jn.01157.2004. [DOI] [PubMed] [Google Scholar]
- 70.Ghasia FF, Angelaki DE. Do motoneurons encode the noncommutativity of ocular rotations? Neuron. 2005;47:281–293. doi: 10.1016/j.neuron.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 71.Klier EM, Meng H, Angelaki DE. Abducens nerve/nucleus stimulation produces kinematically correct three-dimensional eye movements. Soc Neurosci Abstr. 2005 abstract #475.4. [Google Scholar]
- 72.Scherberger H, Cabungcal J-H, Hepp K, Suzuki Y, Straumann D, Henn V. Ocular counterroll modulates the preferred direction of saccade-related pontine burst neurons in the monkey. J Neurophysiol. 2001;86:935–949. doi: 10.1152/jn.2001.86.2.935. [DOI] [PubMed] [Google Scholar]
- 73.Hess BJM, Angelaki DE. Gravity modulates Listing’s plane orientation during both pursuit and saccades. J Neurophysiol. 2003;90:1340–1345. doi: 10.1152/jn.00167.2003. [DOI] [PubMed] [Google Scholar]
- 74.Hess BJM, Angelaki DE. Kinematic principles of primate rotational vestibulo-ocular reflex II. Gravity-dependent modulation of primary eye position. J Neurophysiol. 1997;78:2203–2216. doi: 10.1152/jn.1997.78.4.2203. [DOI] [PubMed] [Google Scholar]
- 75.Hess BJM, Angelaki DE. Kinematic principles of primate rotational vestibulo-ocular reflex. I. Spatial organization of fast phase velocity axes. J Neurophysiol. 1997;78:2193–2202. doi: 10.1152/jn.1997.78.4.2193. [DOI] [PubMed] [Google Scholar]
- 76.Crawford JD, Tweed DB, Vilis T. Static ocular counterroll is implemented through the 3-D neural integrator. J Neurophysiol. 2003;90:2777–2784. doi: 10.1152/jn.00231.2003. [DOI] [PubMed] [Google Scholar]
- 77.Frens MA, Suzuki Y, Scherberger H, Hepp K, Henn V. The collicular code of saccade direction depends on the roll orientation of the head relative to gravity. Exp Brain Res. 1998;120:283–290. doi: 10.1007/s002210050402. [DOI] [PubMed] [Google Scholar]
- 78.Crawford JD, Martinez-Trujillo JC, Kleier EM. Neural control of three-dimensional eye and head movements. Cur Opin Neurosci. 2003;13:655–662. doi: 10.1016/j.conb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 79.Van Opstal AJ, Hepp K, Hess BJ, Straumann D, Henn V. Two- rather than three-dimensional representation of saccades in monkey superior colliculus. Science. 1991;252:1313–1315. doi: 10.1126/science.1925545. [DOI] [PubMed] [Google Scholar]
- 80.Glasauer S, Dieterich M, Brandt T. Central positional nystagmus simulated by a mathematical ocular motor model of otolith-dependent modification of Listing’s plane. J Neurophysiol. 2001;86:1456–1554. doi: 10.1152/jn.2001.86.4.1546. [DOI] [PubMed] [Google Scholar]
- 81.Crawford JD, Guitton D. Visual-motor transformations required for accurate and kinematically correct saccades. J Neurophysiol. 1997;78:1447–1467. doi: 10.1152/jn.1997.78.3.1447. [DOI] [PubMed] [Google Scholar]
- 82.Wong AMF, Sharpe JA, Tweed D. Adaptive neural mechanism for Listing’s law revealed in patients with fourth nerve palsy. Invest Ophthalmol Vis Sci. 2002;43:1796–1803. [PubMed] [Google Scholar]
- 83.Straumann D, Steffen H, Landau K, et al. Listing’s law in acquired and congenital trochlear nerve palsy. Invest Ophthalmol Vis Sci. 2003;44:4282–4292. doi: 10.1167/iovs.02-1181. [DOI] [PubMed] [Google Scholar]
- 84.Migliaccio AA, Cremer PD, Sw ST, Halmagyi GM. Vergence-mediated changes in Listing’s plane do not occur in an eye with superior oblique palsy. Invest Ophthalmol Vis Sci. 2004;45:3043–3047. doi: 10.1167/iovs.04-0014. [DOI] [PubMed] [Google Scholar]
- 85.Angelaki DE, Dickman DJ. Premotor neurons encode torsional eye velocity during smooth-pursuit eye movements. J Neurosci. 2003;23:2971–2979. doi: 10.1523/JNEUROSCI.23-07-02971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Demer JL. Clarity of words and thoughts about strabismus. Am J Ophthalmol. 2001;132:757–759. doi: 10.1016/s0002-9394(01)01099-6. [DOI] [PubMed] [Google Scholar]
- 87.Bielschowsky A. Lectures on motor anomalies. XI. Etiology, prognosis, and treatment of ocular paralyses. Am J Ophthalmol. 1939;22:723–734. [Google Scholar]
- 88.von Noorden GK, Murray E, Wong SY. Superior oblique paralysis. A review of 270 cases. Arch Ophthalmol. 1986;104:1771–1776. doi: 10.1001/archopht.1986.01050240045037. [DOI] [PubMed] [Google Scholar]
- 89.Adler FE. Physiologic factors in differential diagnosis of paralysis of superior rectus and superior oblique muscles. Arch Ophthalmol. 1946;36:661–673. doi: 10.1001/archopht.1946.00890210672001. [DOI] [PubMed] [Google Scholar]
- 90.Scott WE, Kraft SP. Classification and treatment of superior oblique palsies: II. Bilateral superior oblique palsies. In: Caldwell D, editor. Pediaric Ophthalmology and Strabismus: Transactions of the New Orleans Academy of Ophthalmology. New York: Raven Press; 1986. pp. 265–291. [PubMed] [Google Scholar]
- 91.Scott WE, Parks MM. Differential diagnosis of vertical muscle palsies. In: von Noorden GK, editor. Symposium on Strabismus: Transactions of the New Orleans Academy of Ophthalmology. St Louis: Mosby; 1978. pp. 118–134. [Google Scholar]
- 92.Kushner BJ. Ocular torsion: rotations around the ‘WHY’ axis. J AAPOS. 2004;8:1–12. doi: 10.1016/j.jaapos.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 93.Kushner BJ. The diagnosis and treatment of bilateral masked superior oblique palsy. Am J Ophthalmol. 1988;105:186–194. doi: 10.1016/0002-9394(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 94.Robinson DA. Bielschowsky head-tilt test - II. Quantitative mechanics of the Bielschowsky head-tilt test. Vision Res. 1985;25:1983–1988. doi: 10.1016/0042-6989(85)90023-9. [DOI] [PubMed] [Google Scholar]
- 95.Simonsz HJ, Crone RA, van der Meer J, Merckel-Timmer CF, van Mourik-Noordenbos AM. Bielschowsky head-tilt test I – ocular counterrolling and Bielschowsky head-tilt test in 23 cases of superior oblique palsy. Vision Res. 1985;25:1977–1982. doi: 10.1016/0042-6989(85)90022-7. [DOI] [PubMed] [Google Scholar]
- 96.Demer JL, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci. 1995;36:906–913. [PubMed] [Google Scholar]
- 97.Chan TK, Demer JL. Clinical features of congenital absence of the superior oblique muscle as demonstrated by orbital imaging. JAAPOS. 1999;3:143–150. doi: 10.1016/s1091-8531(99)70059-5. [DOI] [PubMed] [Google Scholar]
- 98.Velez FG, Clark RA, Demer JL. Facial asymmetry in superior oblique palsy and pulley heterotopy. JAAPOS. 2000;4:233–239. doi: 10.1067/mpa.2000.105277. [DOI] [PubMed] [Google Scholar]
- 99.Kono R, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior oblique muscle in superior oblique palsy. Ophthalmology. 2003;110:1219–1229. doi: 10.1016/S0161-6420(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 100.Demer JL, Miller MJ, Koo EY, Rosenbaum AL, Bateman JB. True versus masquerading superior oblique palsies: muscle mechanisms revealed by magnetic resonance imaging. In: Lennerstrand G, editor. Update on Strabismus and Pediatric Ophthalmology. Boca Raton (FL): CRC Press; 1995. pp. 303–306. [Google Scholar]
- 101.Plager DA. Traction testing in superior oblique palsy. J Pediatr Ophthalmol Strabismus. 1990;27:136–140. doi: 10.3928/0191-3913-19900501-08. [DOI] [PubMed] [Google Scholar]
- 102.Plager DA. Tendon laxity in superior oblique palsy. Ophthalmology. 1992;99:1032–1038. doi: 10.1016/s0161-6420(92)31854-8. [DOI] [PubMed] [Google Scholar]
- 103.Plager DA, Helveston EM, Fahad B. Superior oblique muscle atrophy/hypodevelopment in superior oblque palsy. Abstr of 22nd Annual AAPOS Mtg. 1996:papers 10. [Google Scholar]
- 104.Kushner BJ. Errors in the three-step test in the diagnosis of vertical strabismus. Ophthalmology. 1987;96:127–132. doi: 10.1016/s0161-6420(89)32933-2. [DOI] [PubMed] [Google Scholar]
- 105.Brodsky ME. Three dimensions of skew deviation. Br J Ophthalmol. 2003;87:1440–1441. doi: 10.1136/bjo.87.12.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Donahue SP, Lavin PJ, Hamed LM. Tonic ocular tilt reaction simulating a superior oblique palsy: diagnostic confusion with the 3-step test. Arch Ophthalmol. 1999;117:347–352. doi: 10.1001/archopht.117.3.347. [DOI] [PubMed] [Google Scholar]
- 107.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? JAAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 108.Clark RA, Miller JM, Demer JL. Displacement of the medial rectus pulley in superior oblique palsy. Invest Ophthalmol Vis Sci. 1998;39:207–212. [PubMed] [Google Scholar]
- 109.Lajeunie E, Catala M, Renier D. Craniosynostosis: from a clinical description to an understanding of bone formation of the skull. Childs Nerv Syst. 1999;15:276–280. doi: 10.1007/s003810050457. [DOI] [PubMed] [Google Scholar]
- 110.Limon de Brown E, Monasterio FO, Feldman MS. Strabismus in plagiocephaly. J Pediatr Ophthalmol Strabismus. 1998;25:180–190. doi: 10.3928/0191-3913-19880701-08. [DOI] [PubMed] [Google Scholar]
- 111.Khan SH, Nischal KK, Dean F, Hayward RD, Walker J. Visual outcomes and amblyogenic risk factors in craniosynostotic syndromes: a review of 141 cases. Br J Ophthalmol. 2003;87:999–1003. doi: 10.1136/bjo.87.8.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Coats DK, Paysse EA, Stager DR. Surgical management of V-pattern strabismus and oblique dysfunction in craniofacial dysostosis. J AAPOS. 2000;4:338–342. doi: 10.1067/mpa.2000.110337. [DOI] [PubMed] [Google Scholar]
- 113.Velez FG, Thacker N, Britt MT, Rosenbaum AL. Cause of V pattern strabismus in craniosynostosis: a case report. Br J Ophthalmol. 2004;88:1598–1599. doi: 10.1136/bjo.2004.048413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clark RA, Demer JL, Miller JM, Rosenbaum AL. Heterotopic rectus extraocular muscle pulleys simulate oblique muscle dysfunction. Abstracts of the American Association for Pediatric Ophthalmology and Strabismus. 1997:39. [Google Scholar]
- 115.Demer JL, Clark RA, Miller JM. Heterotopy of extraocular muscle pulleys causes incomitant strabismus. In: Lennerstrand G, editor. Advances in Strabismology. Buren (Netherlands): Aeolus Press; 1999. pp. 91–94. [Google Scholar]
- 116.Demer JL. A 12 year, prospective study of extraocular muscle imaging in complex strabismus. JAAPOS. 2003;6:337–347. doi: 10.1067/mpa.2002.129040. [DOI] [PubMed] [Google Scholar]
- 117.Clark RA, Demer JL. Rectus extraocular muscle pulley displacement after surgical transposition and posterior fixation for treatment of paralytic strabismus. Am J Ophthalmol. 2002;133:119–128. doi: 10.1016/s0002-9394(01)01264-8. [DOI] [PubMed] [Google Scholar]
- 118.Krzizok TH, Schroeder BU. Measurement of recti eye muscle paths by magnetic resonance imaging in highly myopic and normal subjects. Invest Ophthalmol Vis Sci. 1999;40:2554–2560. [PubMed] [Google Scholar]
- 119.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management: Principles and Techniques. Philadelphia: WB Saunders; 1999. pp. 84–98. [Google Scholar]
- 120.Wong IBY, Leo SW, Khoo BK. Surgical correction of myopia strabismus fixus. Abstracts of 31th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus; 2005. p. 50. [DOI] [PubMed] [Google Scholar]
- 121.Oh SY, Clark RA, Velez F, Rosenbaum AL, Demer JL. Incomitant strabismus associated with instability of rectus pulleys. Invest Ophthalmol Vis Sci. 2002;43:2169–2178. [PubMed] [Google Scholar]
- 122.Demer JL, Kono R, Wright W, Oh SY, Clark RA. Gaze-related orbital pulley shift: A novel cause of incomitant strabismus. In: de Faber JT, editor. Progress in Strabismology. Lisse: Swets and Zeitlinger; 2002. pp. 207–210. [Google Scholar]
- 123.Bhola R, Rosenbaum AL, Ortube MC, Demer JL. High resolution magnetic resonance imaging demonstrates varied anatomic abnormalities in Brown’s syndrome. J AAPOS. 2004 doi: 10.1016/j.jaapos.2005.07.001. in revision. [DOI] [PubMed] [Google Scholar]
- 124.Piruzian A, Goldberg RA, Demer JL. Inferior rectus pulley hindrance: orbital imaging mechanism of restrictive hypertropia following lower lid surgery. J AAPOS. 2004;8:338–344. doi: 10.1016/j.jaapos.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 125.Scott AB. The faden operation: mechanical effects. Am Orthoptic J. 1977;27:44–47. [PubMed] [Google Scholar]
- 126.Clark RA, Isenberg SJ, Rosenbaum SJ, Demer JL. Posterior fixation sutures: a revised mechanical explanation for the fadenoperation based on rectus extraocular muscle pulleys. Am J Ophthalmol. 1999;128:702–714. doi: 10.1016/s0002-9394(99)00356-6. [DOI] [PubMed] [Google Scholar]
- 127.Clark RA, Ariyasu R, Demer JL. Medial rectus pulley posterior fixation is as effective as scleral posterior fixation for acquired esotropia with a high AC/A ratio. Am J Ophthalmol. 2004;137:1026–1033. doi: 10.1016/j.ajo.2004.01.012. [DOI] [PubMed] [Google Scholar]


