Abstract
Imbalance of signals that control cell survival and death results in pathologies, including cancer and neurodegeneration. Two pathways that are integral to setting the balance between cell survival and cell death are controlled by lipid-activated protein kinase B (PKB)/Akt and Ca2+. PKB elicits its effects through the phosphorylation and inactivation of proapoptotic factors. Ca2+ stimulates many prodeath pathways, among which is mitochondrial permeability transition. We identified Ca2+ release through inositol 1,4,5-trisphosphate receptor (InsP3R) intracellular channels as a prosurvival target of PKB. We demonstrated that in response to survival signals, PKB interacts with and phosphorylates InsP3Rs, significantly reducing their Ca2+ release activity. Moreover, phosphorylation of InsP3Rs by PKB reduced cellular sensitivity to apoptotic stimuli through a mechanism that involved diminished Ca2+ flux from the endoplasmic reticulum to the mitochondria. In glioblastoma cells that exhibit hyperactive PKB, the same prosurvival effect of PKB on InsP3R was found to be responsible for the insensitivity of these cells to apoptotic stimuli. We propose that PKB-mediated abolition of InsP3-induced Ca2+ release may afford tumor cells a survival advantage.
Keywords: signaling, cell death, cancer
Protein kinase B (PKB) is a central player in regulating many signaling pathways controlling cell metabolism, growth, and survival (1, 2). PKB elicits these effects by phosphorylating and regulating the activity of downstream targets such as glycogen synthase kinase 3β and Bad, or via transcription factors such as Forkhead (1, 3). Because of this critical role of PKB, gain or loss of function is manifest in major disease phenotypes such as cancer and type 2 diabetes (1, 4–6).
Ca2+ released from the endoplasmic reticulum (ER) through inositol 1,4,5-trisphosphate (InsP3) receptors (InsP3Rs) plays a key role in regulating physiological processes (7). However, under pathological conditions, InsP3-induced Ca2+ release (IICR) can be subverted to promote cell death pathways (8–10). The importance of IICR in cell death is underlined by the uncovering of functional interactions with a number of proteins with known proapoptotic and antiapoptotic activity. Notable among these are Bcl-2, Bcl-XL, and cytochrome c (11–14). PKB has also recently been shown to phosphorylate the InsP3R, with consequences for cell survival (15).
We investigated whether cross-talk between the phosphatidylinositol 3-kinase (PI3K)/PKB and InsP3/Ca2+ signaling pathways regulated how cells responded to death-inducing stimuli. We determined that PKB-mediated phosphorylation of InsP3R results in a decrease in the magnitude of IICR and resultant flux of Ca2+ from the ER to mitochondria. Moreover, we show that this decrease in Ca2+ flux caused by PKB-mediated phosphorylation of InsP3Rs contributes to protection from the effects of apoptotic stimuli. This prosurvival action of PKB was also apparent in a glioblastoma cell line (U87) that exhibits increased PKB activity caused by a deletion in the gene encoding the phosphatidylinositol 3,4,5 trisphosphate (PIP3) phosphatase, PTEN. Together, these results contribute to a mechanism by which PKB and IICR interact to regulate cell death and survival.
Results
Ca2+ Release from Intracellular Stores Is Regulated by PKB.
Growth factor status and genetic factors that enhance PKB activity significantly impact on cell fate [supporting information (SI) Fig. 7]. On the other hand, the occurrence of Ca2+ signals during the cell death process, and the protection from apoptosis afforded by buffering intracellular Ca2+, places Ca2+ at a central position in promoting cell death (SI Fig. 7).
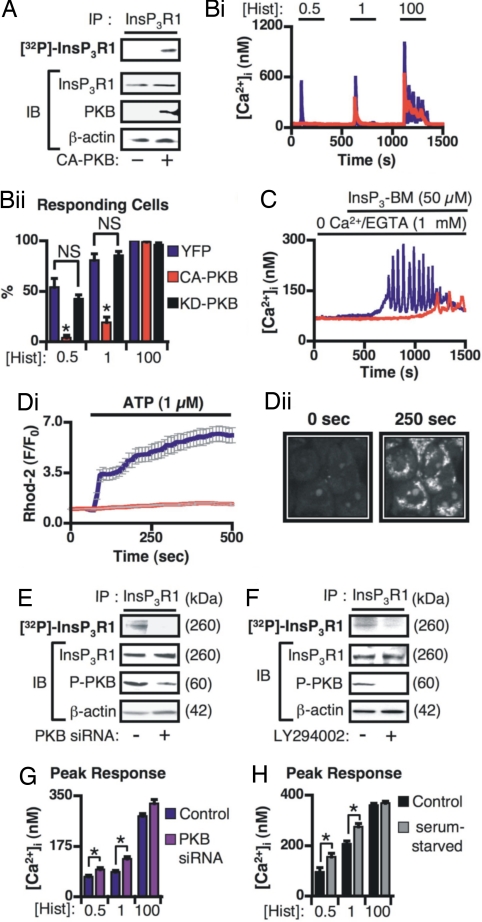
Because of the central roles of both PKB and Ca2+ in regulating cell death, we investigated whether PKB-mediated phosphorylation of InsP3Rs, and suppression of Ca2+ signals, contributed to the prosurvival role of PKB. Inducible overexpression of constitutively active PKB (CA-PKB) promoted the phosphorylation of InsP3Rs (Fig. 1A and SI Fig. 8 for characterization of CA-PKB-expressing cell lines). The example Ca2+ traces and the histogram of the percentage of responding cells in Fig. 1 Bi and Bii, respectively, illustrate the significant inhibition of histamine-induced Ca2+ release by CA-PKB overexpression. The time from agonist addition to peak response (latency) and the percentage of cells exhibiting Ca2+ oscillations after application of 100 μM histamine was also reduced in CA-PKB-expressing cells (SI Fig. 9 Ai and Aii). Kinase dead (KD)-PKB had no effect on agonist-induced Ca2+ signals (Fig. 1B and SI Fig. 9A). Ca2+ release stimulated by cell permeant InsP3 (InsP3-BM) was also reduced by CA-PKB overexpression, indicating that PKB was directly modulating IICR (Fig. 1C and SI Fig. 9B). The PKB-mediated inhibition of agonist-induced Ca2+ release was not caused by a decrease in ER luminal Ca2+ content because the integrated cytosolic Ca2+ transient [area under the curve (AUC)] induced by the irreversible sarco/ER ATPase (SERCA) pump inhibitor thapsigargin was unaffected by CA-PKB expression (29,860 ± 1,118 nM·s vs. 31,220 ± 767.7 nM·s; P > 0.05 in YFP- or CA-PKB-expressing cells, respectively).
Fig. 1.
IICR and mitochondrial Ca2+ uptake is regulated by PKB. (A) CA-PKB expression promotes phosphorylation of InsP3R1. (Upper) An autoradiograph of 32P-labeled InsP3Rs. (Lower) Immunoblots (IB) of proteins present in the lysates used as input for the IPs. InsP3Rs were immunoprecipitated from control cells (−) or cells expressing CA-PKB as shown (+). (Bi and Bii) Histamine-induced Ca2+ signals in HeLa cells expressing CA-PKB (n = 89 cells), YFP (n = 64 cells), or KD-PKB (n = 74 cells). (Bi) Typical responses from individual HeLa cells stimulated with the histamine concentrations (μM) shown. (Bii) Averaged data showing the effect of CA-PKB or KD-PKB expression on the proportion of responding cells. (C) Typical Ca2+ responses recorded in control and CA-PKB-expressing cells stimulated with InsP3 ester (InsP3-BM). (Di) Average background subtracted, mitochondrial Rhod-2 fluorescence changes in control and CA-PKB expressing HeLa cells. (Dii) Confocal images of Rhod-2 fluorescence in HeLa cells stimulated with 1 μM ATP. Images captured at the indicated time points are shown. (E and F) (Upper) Autoradiographs of 32P-labeled InsP3Rs. (Lower) Immunoblots (IB) of the indicated proteins in the lysates used as input for the IPs. (E) InsP3R phosphorylation in HeLa cell transfected with PKB siRNA or control siRNA. (F) Effect of LY294002 onInsP3R phosphorylation in HeLa cells grown under serum-replete normal growth conditions. (G) Impact of PKB siRNA or control siRNA on the amplitude of Ca2+ responses induced by the concentrations of histamine (μM) indicated. (H) Amplitude of Ca2+ responses induced by the concentrations of histamine (μM) shown in control and serum-starved HeLa cells. The data represent mean ± SEM. * indicates P < 0.05. NS indicates not significant.
As Ca2+ released from InsP3Rs is taken up in a privileged manner by mitochondria, mitochondrial Ca2+ levels represent an exquisitely sensitive measure of Ca2+ released from ER-localized InsP3Rs. Using confocal imaging of mitochondrially compartmentalized Rhod-2 (16), a significantly lower level of agonist-induced mitochondrial Ca2+ uptake was observed in CA-PKB-expressing cells compared with controls (Fig. 1 Di and Dii).
Because serum-starvation sensitized cells to apoptotic stimuli (SI Fig. 7), we investigated whether InsP3Rs were phosphorylated under normal serum-replete growth where PKB is tonically active. Reduction of PKB expression by siRNA, or activity, using LY294002 (inhibits PI3K, which lies directly upstream of PKB) significantly decreased 32P-labeling of InsP3Rs to a level that was not detectable above background (Fig. 1 E and F).
The tonic activity of endogenous PKB also regulated agonist-induced Ca2+ release. As would be expected of an InsP3R inhibitor, reduction of endogenous PKB expression by siRNA, or its activity by serum starvation, resulted in an enhancement of agonist-induced Ca2+ release (Fig. 1 G and H). Together, these data suggest that phosphorylation of InsP3R by PKB significantly reduces its sensitivity to InsP3. Moreover, InsP3Rs are phosphorylated by endogenous, tonically active PKB (in serum), CA-PKB, and PKB that had been activated by physiological stimuli.
S2681 in InsP3R1 Regulates IICR.
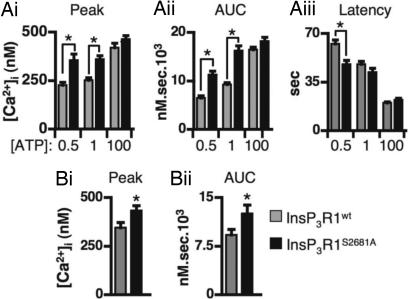
To further isolate the contribution of PKB phosphorylation of InsP3Rs to Ca2+ release, IICR was quantitated in cells overexpressing InsP3Rs in which the phosphorylatable serine in the PKB consensus site was mutated to an unreactive alanine (S2681A in InsP3R1) (15). COS-7 cells expressing InsP3R1S2681A had greater ATP-induced Ca2+ responses than their InsP3R1wt-expressing counterparts (Fig. 2Ai, Aii, and Aiii; the peak, AUC, and latency of the Ca2+ signal are shown). These effects were not caused by differences in expression levels of the wild-type or mutated receptors or differences in their intracellular distribution (SI Figs. 10B and 11 and data not shown). COS-7 cells were used for these experiments because they express low levels of endogenous InsP3R1 and have been successfully used to study InsP3R function (17). Moreover, in these cells, heterologously expressed wild-type InsP3Rs can be overexpressed at a level significantly greater than endogenous receptors, are correctly targeted, and exhibit significantly increased agonist-induced Ca2+ release and decreased latency of the Ca2+ transient (SI Fig. 11).
Fig. 2.
InsP3R1-S2681 regulates agonist-induced Ca2+ release. (A i–iii) Averaged data of ATP-induced Ca2+ signals in COS-7 cells expressing InsP3R1wt (n = 293 cells) or InsP3R1S2681A (n = 192 cells). Peak Ca2+ response (Ai), AUC (Aii), and latency (Aiii) are shown for the ATP concentrations (μM) indicated. (B i and ii) Carbachol-induced Ca2+ responses in M3-expressing DT40 cells transiently transfected with InsP3R1wt (n = 35 cells) or InsP3R1S2681A (n = 44 cells). * indicates that the data are statistically significant (P < 0.05).
Agonist-induced Ca2+ release was also monitored in M3 muscarinic receptor-expressing DT40 InsP3R triple knockout chicken B lymphocyte cell line (DT40 TKO) (18). The peak and AUC of the Ca2+ signal induced by carbachol was significantly greater in cells transiently transfected with InsP3R1S2681A than cells transfected with InsP3R1wt (Fig. 2 Bi and Bii). Together, the data derived from HeLa (Fig. 1), COS-7, and DT40 cells support the conclusion that phosphorylation of InsP3R1-S2681 by PKB was inhibitory to Ca2+ release.
PKB Interacts with InsP3R1.
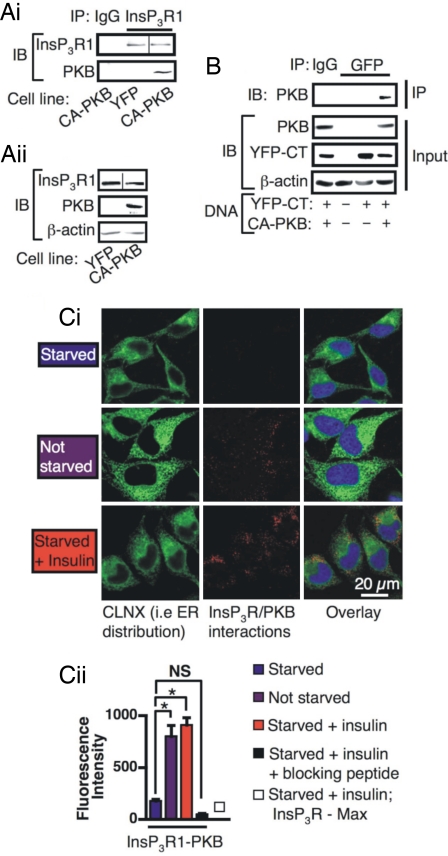
Because protein kinases often reside in a complex with their substrates, whether PKB and InsP3Rs interact was next investigated. Using coimmunoprecipitation (co-IP), an interaction between overexpressed CA-PKB and endogenous full-length InsP3Rs was detected in HeLa cells (Fig. 3 Ai and Aii). KD-PKB did not interact with InsP3R1 (data not shown). PKB also interacted with a YFP-tagged ER-localized NH2-terminally truncated InsP3R1 that encompassed only the amino acids COOH-terminal of transmembrane domains 5 (YFP-CT) (Fig. 3B).
Fig. 3.
PKB interacts with InsP3R1. (A i and ii) Co-IPs of full-length InsP3R1 with PKB. A line is shown in the images where different parts of the same gel have been grouped. (Ai) IPs using anti-InsP3R1 antibody or preimmune IgG were performed on lysates prepared from HeLa cells expressing YFP or CA-PKB. (Aii) Immunoblot showing the proteins present in the lysates used as input for the IP in Ai. (B) Co-IP of PKB with the InsP3R1 COOH terminus. IPs using anti-GFP antibody or preimmune IgG control from lysates prepared from cells transfected with YFP-CT and/or CA-PKB DNA. (Top) An immunoblot (IB) to detect co-IP of PKB. (Middle and Bottom) Immunoblots of the proteins present in the lysates used for IP. (C i and ii) In situ proximity ligation assay in HeLa cells. (Ci) The red pseudocoloration indicates an interaction between endogenous InsP3R1 and PKB. The ER is indicated by the CLNX staining in green. Nuclei are shown by the blue DAPI staining. (Cii) Quantitation of proximity ligation assay (n = 50 cells per condition). The bars represent the mean intensity ± SEM of AlexaFluor 561 fluorescence. * denotes that the data are significantly different (P < 0.05).
Because PKB is activated at the plasma membrane, but the majority of InsP3Rs reside on the ER, we next set out to establish whether activated endogenous PKB could gain access to InsP3Rs on the ER. Using an in situ proximity ligation assay (19), a significant InsP3R-PKB interaction was detected in cells grown under serum-replete normal growth conditions (see images and histogram of mean cellular fluorescence intensity, Fig. 3 Ci and Cii). An insulin-dependent increase in PKB-InsP3R1 interaction was also detected (Fig. 3 Ci and Cii). Controls that either included the InsP3R antibody immunizing peptide in the staining procedure or replacing the PKB probe with a probe for the Max protooncogene were negative (Fig. 3Cii). Colocalization analysis revealed that the sites where PKB and InsP3R1 interacted [rolling circle amplification (RCA) product present] overlapped with the distribution of calnexin, and therefore were localized to the ER (Pearson's correlation coefficient = 0.44 ± 0.01). Using classical colocalization analysis of confocal images of immunostained cells, we found that the distribution of both total and phosphorylated active PKB also overlapped with endogenous InsP3Rs (SI Fig. 12). Together, these data show that active PKB is present not only at the plasma membrane where it is activated, but is also localized in the cytosol, where it interacts with InsP3Rs.
Phosphorylation of InsP3Rs by PKB Inhibits Apoptosis.
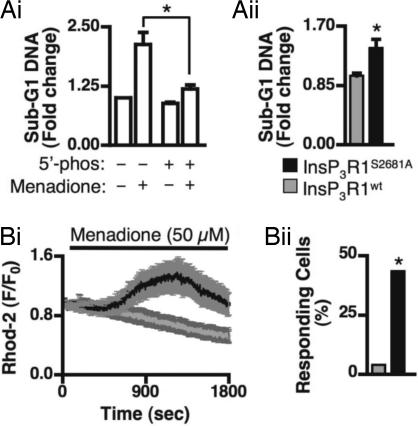
To specifically test the role of PKB-dependent phosphorylation of InsP3Rs onapoptosis, menadione-induced apoptosis was measured in COS-7 cells expressing either InsP3R1wt or InsP3RS2681A. This cell death stimulus was used because its action was sensitive to InsP3-metabolizing 5′-phosphatase expression and therefore is IICR-dependent (Fig. 4Ai). Cells expressing InsP3R1S2681A exhibited significantly higher levels of menadione-induced apoptosis than cells expressing InsP3R1wt (Fig. 4Aii). A similar effect was also observed when staurosporine was used to induce apoptosis (SI Fig. 13).
Fig. 4.
InsP3R1S2681A-expressing cells exhibit increased mitochondrial Ca2+ uptake and apoptosis. (A i and ii) Sub-G1 DNA content of COS-7 transfected with the indicated expression vectors and treated with 50 μM menadione (experiments performed in triplicate on 3 separate days). (Bi) Menadione-induced mitochondrial Ca2+ uptake in COS-7 cells transfected with InsP3R1WT (gray trace) or InsP3RS2681A (black trace). (Bii) Proportion of InsP3R1WT- expressing (n = 23) or InsP3RS2681A-expressing (n = 25) cells that showed a significant mitochondrial response to menadione. * denotes that the data are significantly different (P < 0.05).
We next tested the idea that PKB-mediated regulation of InsP3R activity and Ca2+ flux from the ER to the mitochondria contributed to the mechanism by which PKB elicited its prosurvival effects. Significantly, menadione-induced mitochondrial Ca2+ increases were considerably reduced in cells overexpressing InsP3R1S2681A, but not cells expressing InsP3R1wt (Fig. 4B).
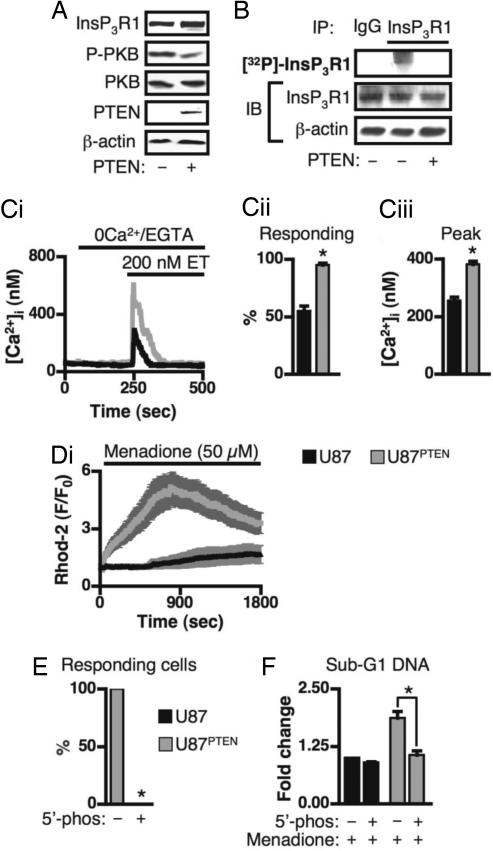
PKB Regulation of IICR and Apoptosis in Glioblastoma Cells.
Many cancers exhibit increased PKB activity as a result of mutation or deletion of the gene encoding the lipid phosphatase PTEN (6). This mechanism underlies the resistance to apoptotic stimuli of the U87 glioblastoma cell line (20). Re-expression of PTEN in these cells (U87PTEN) decreases PKB activity (Fig. 5A) and rescues their sensitivity to an apoptotic stimulus (SI Fig. 14). We investigated whether InsP3R was a target for the prosurvival effect of enhanced PKB activity in these cells. InsP3R1 immunoprecipitated from U87 cells were more highly phosphorylated than similarly treated U87PTEN cells (Fig. 5B). IICR, stimulated by endothelin (ET), was lower in U87 cells than in U87PTEN cells (Fig. 5C). Furthermore, mitochondrial Ca2+ increases after exposure to menadione were significantly lower in the U87 cells than the U87PTEN cells (Fig. 5D). Both menadione–induced mitochondrial Ca2+ uptake and apoptosis in the U87PTEN cells were inhibited by expression of InsP3 5′-phosphatase (Fig. 5 E and F), indicating that these processes depended on IICR. Thus, the U87 cells are less sensitive to apoptosis because phosphorylation of their InsP3Rs reduces Ca2+ release and subsequent transfer of Ca2+ to the mitochondria.
Fig. 5.
InsP3R phosphorylation results in decreased agonist-induced Ca2+ release and menadione-induced mitochondrial Ca2+ uptake in PTEN-deficient, U87 glioblastoma cells. (A) Immunoblots of the indicated proteins in lysates prepared from U87 and U87PTEN cells (indicated by PTEN − and +, respectively). (B) (Top) Autoradiograph of 32P-labeled InsP3Rs immunoprecipitated from U87 and U87PTEN cells. (Middle and Bottom) The proteins present in the lysate used as input for the IP. (C i–iii) ET-induced Ca2+ release in U87 cells (black; n = 190 cells) and U87PTEN cells (gray; n = 183 cells). (Ci) Typical traces of ET-induced Ca2+ release in U87 and U87PTEN cells. (Cii) Proportion of responding cells. (Ciii) Peak Ca2+ response. (Di) Menadione-induced mitochondrial Ca2+ uptake in U87 cells (black trace; n = 25) and U87PTEN cells (gray trace; n = 14). (E) Proportion of YFP-expressing (n = 50) and InsP3 5′-phosphatase-expressing (n = 50) U87PTEN cells that exhibited menadione-induced increases in mitochondrial Ca2+. (F) Menadione-stimulated cell death in U87PTEN cells depended on InsP3Rs. U87 and U87PTEN cells were adenovirally infected with InsP3 5′-phosphatase or YFP, and subsequently stimulated with 50 μM menadione (experiments performed in triplicate on 3 separate days). * indicates that the data are significantly different (P < 0.05).
Discussion
In this study, we have identified and characterized a mechanism by which the prosurvival kinase PKB protects cells from apoptosis-inducing stimuli that engage the InsP3R/Ca2+-dependent cell death pathway. We also provide evidence for a fundamental role of this mechanism in regulating the sensitivity of a cancer cell line to apoptotic stimuli.
The data presented here shows that agonist-induced Ca2+ release is inhibited by PKB-mediated phosphorylation of InsP3Rs. Significantly, agonist-induced Ca2+ release was regulated by endogenous tonically active PKB and CA-PKB that had been overexpressed. Moreover, increased cellular PKB activity significantly impacted the agonist-induced increase in mitochondrial Ca2+, which is a proximal sensor of Ca2+ release through InsP3Rs (21, 22). Because PKB activation had no effect on ER store loading or InsP3R expression levels, but prevented Ca2+ release in response to cell-permeant InsP3, we concluded that PKB was directly regulating InsP3R activity. This conclusion was further supported by the enhanced agonist-induced Ca2+ release observed in cells expressing mutated InsP3Rs that could not be phosphorylated by PKB compared with cells overexpressing wild-type receptors.
The data presented here contrasts with that previously reported by Khan et al. (15), which indicated that mutation of S2681A did not affect IICR. Possible explanations for these discrepancies are that in the study of Khan et al. increases in intracellular Ca2+ were presented as a normalized change in fluorescence but not absolute Ca2+ values. Thus, they could only conclude that there was no effect of InsP3R1 S2681 phosphorylation on the EC50 for Ca2+ release. A further possibility is that because of the low tonic level of PKB activity in DT40 cells (SI Fig. 15 and ref. 23), the wild-type InsP3R would not have been highly phosphorylated, and thus the effect of InsP3R1S2681A would have been small. Unlike Khan et al. (15), we were able to detect a small, but significant, effect of the S2681A mutation on Ca2+ release in DT40 cells, although it was less than that observed in COS-7 cells. A possible explanation for our ability to detect an effect of S2681A mutation upon Ca2+ release is that unlike Khan et al., we used DT40 cells stably expressing muscarinic receptors rather than cells in which muscarinic receptors were transiently transfected.
Our data show that during the life cycle of normally dividing cells InsP3Rs are tonically phosphorylated by PKB. Stimulation of serum-starved cells with insulin or FBS also promoted PKB-dependent phosphorylation of InsP3Rs (SI Fig. 10A). Experiments performed here also corroborated the identification of S2681 as the serine residue phosphorylated by PKB in InsP3R1 by Khan et al. (15) (SI Fig. 10 B and C) and demonstrated that S2681 was the site for phosphorylation by endogenous PKB that had been physiologically activated. We also showed in vitro phosphorylation of this site in recombinant InsP3R1 COOH terminus by recombinant PKB, indicating that PKB was sufficient to catalyze the phosphorylation of InsP3Rs, and that no accessory factors are required. We report that InsP3R3 is also phosphorylated by PKB, both in vitro by recombinant active PKB (SI Fig. 10D) and after insulin stimulation in intact cells (data not shown). Together, our data satisfies the criteria laid out by Manning and Cantley (1) for a protein to constitute a PKB substrate. Interestingly, in the absence of activated PKB, no InsP3R phosphorylation was observed. Because InsP3Rs are a substrate for a number of other protein kinases, including PKA and CaMKII (24, 25), it might be expected that basal activity of these enzymes may also lead to a low level of phosphorylation of InsP3R1. However, in the studies cited above, it appears that unless stimulated appropriately little InsP3R phosphorylation is detected. The remarkable degree of sequence conservation through evolution of the PKB consensus site in InsP3Rs is suggestive of a fundamental role of phosphorylation of this region in InsP3R function. The COOH terminus of InsP3Rs is critically important in the regulation of channel gating and is a hotspot for interactions with other proteins, including huntingtin-associated protein, Bcl-XL, protein phosphatase 1a, and cytochrome c (12, 14, 26–28). Akin to other protein kinases/substrate relationships (29), we also detected an interaction between PKB and the InsP3R. Using an in situ proximity ligation technique, we detected this interaction between endogenous PKB and InsP3Rs in cells either maintained in serum-containing medium or stimulated with insulin. Significantly, this PKB–InsP3R association occurred throughout the ER, and not only at the plasma membrane where PKB is primarily activated. Activated PKB was, however, detected throughout the cell. Although PIP3 has previously been localized to the ER (30), we have shown a clear visualization of endogenous activated PKB interacting with one of its substrates in a location distal from the plasma membrane.
By investigating the effect of mutagenesis of the PKB consensus site in InsP3R1 on cell death and mitochondrial Ca2+ increases stimulated by agents that cause apoptosis in an IICR-dependent manner (14, 31), we also determined that IICR was a bona fide prosurvival target of PKB. The relevance of these findings to disease was shown in the U87 glioblastoma cancer cell line that exhibits increased PKB activity (20). In these cells, we detected increased InsP3R phosphorylation and decreased agonist-induced Ca2+ release when compared with derivatives of these cells in which PTEN was re-expressed. Furthermore, unlike their PTEN-expressing derivatives, the U87 cells were also recalcitrant to menadione-induced apoptosis and did not exhibit any mitochondrial Ca2+ uptake after menadione treatment. From these data we conclude that phosphorylation of InsP3Rs by hyperactive PKB was significantly responsible for the lack of sensitivity of the U87 cells to apoptotic stimuli.
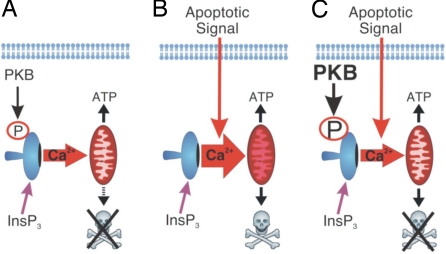
In summary, we provide evidence for a mechanism by which InsP3R-mediated apoptosis is inhibited and by which PKB elicits its prosurvival effects (Fig. 6). This model also predicts that PKB constitutes a link between the cellular environment and growth factor status and can dynamically control IICR to determine cell fate. The significant prosurvival effect of PKB-mediated phosphorylation of InsP3Rs places regulation of IICR high up in the hierarchy of the prosurvival targets of PKB. Indeed, IICR is an upstream signal in the activation of other proapoptotic pathways such as those mediated by Bad and calpains (9). Although our data focus on the role of PKB modulation of IICR in apoptosis, it is likely that this functional interaction also impacts other aspects of Ca2+ signaling. The recent descriptions of role for IICR in controlling autophagy (32) and cellular metabolism (12), which are also targets for the PKB pathway, support this idea. In addition, our data contribute to the developing model that regulation of IICR is a nexus at which multiple signaling pathways converge to determine the physiological output of a given cellular stimulus.
Fig. 6.
Model depicting the functional interaction between PKB and InsP3Rs. (A) Under normal growth conditions with physiological PKB activity InsP3Rs are basally phosphorylated, transfer of Ca2+ to the mitochondria is minimal, and ATP synthesis is promoted. (B) In the absence of growth factors, PKB activity is reduced, and the level of InsP3R phosphorylation is diminished. Thus, upon toxin application Ca2+ flux from the ER to the mitochondria is enhanced, causing permeability transition and cell death. (C) In situations with enhanced PKB activity, such as during cancer or the presence of growth factor stimulation, the level of InsP3R phosphorylation is increased, thereby decreasing the flux of Ca2+ from the ER to the mitochondria in response to an apoptotic stimulus. Agonist-induced Ca2+ release is also suppressed under these conditions.
Materials and Methods
Materials.
Cell culture reagents, NuPage gels, AlexaFluor-conjugated secondary antibodies, Ca2+ indicator dyes, and pluronic were from Invitrogen. Blasticidin, Zeocin, and tetracycline-free FBS were from InvivoGen. Primary antibodies, unless otherwise stated, were from Cell Signaling Technology. All other chemicals, unless stated otherwise, were purchased from Sigma.
Generation of Expression Vectors.
Expression plasmids for myristoylated CA and KD (K179A) forms of PKB have been described (33). The cDNAs encoding CA-PKB and KD-PKB were subcloned into the tetracycline-inducible expression vector pcDNA4-TO (Invitrogen), and pcDNA4-TO-YFP was created by subcloning the YFP cDNA from pEYFP-C1 (Clontech) into pcDNA4-TO. InsP3R1S2681A was generated by QuikChange Mutagenesis (Stratagene) using pcDNA3-mouse InsP3R1 (provided by K. Mikoshiba, University of Tokyo, Tokyo) as template. The COOH-terminal mutant S2681A (CTS/A) was made as above. Constructs were verified by sequencing. YFP-tagged InsP3R1 COOH terminus (YFP-CT; amino acids 2431–2749) has been described (34).
Generation of Stable Cell Lines.
Tet suppressor HeLa cells were from Invitrogen and maintained as prescribed. Stable PKB- and YFP-expressing cell lines were generated after transfection by using GeneJuice (Merck) or JetPEI (Qbiogene) according to the manufacturer's guidelines. Expression was induced with 1 μg/ml doxycycline for 24 h.
Transient Transfection.
HeLa and COS-7 cell lines were from ECACC and maintained as described (35). For knockdown of endogenous PKB, cells were transfected with validated siRNA (Cell Signaling Technology) by using Lipofectamine 2000 (Invitrogen). Scrambled siRNA was used as control. For DNA transfection, Lipofectamine 2000 or JetPei (Qbiogene) was used. For imaging studies, InsP3R transfected cells were identified by cotransfection with pEYFP-C1 (Clontech) at a 1:5 molar ratio. GFP-tagged InsP3 5′-phosphatase was transduced by using adenovirus. Cells were infected at a multiplicity of infection of 100 plaque-forming units 24 h before analysis or treatment. A GFP-expressing adenovirus was used as control. Adenoviral vectors were prepared according to the supplier's instructions (Microbix). M3-muscarinic receptor-expressing DT40 InsP3R triple knockout chicken B-lymphocytes were from David Yule (University of Rochester, Rochester, NY) and cultured as described (18). DT40 cells were transfected with an AMAXA Nucleofector with solution T and program B-023. In brief, 2 × 106 cells were first resuspended in 100 μl of solution T. To the cells in solution T, 10 μg of empty vector or InsP3R1 expression vector was added together with 3 μg of a DsRed expression vector, which was used a transfection marker.
Ca2+ Imaging.
Fura-2 imaging was performed as described (35). Experiments were performed on three coverslips of 20 or more cells per day on 3 separate days. Mitochondrial Ca2+ imaging with Rhod-2 was performed as described (16). Experiments were performed by using a VoxCell Scan confocal imaging system (Visitech Ltd) equipped with a Hamamatsu ORCA-ER camera. The confocal scan head was attached to either an Olympus IX70 inverted microscope configured with a ×40, 1.35 n.a. UAPO oil immersion objective (Figs. 4 and 5) or a Nikon TE2000 equipped with ×40, 1.3 n.a. oil immersion objective (Fig. 1). Image analysis was performed with ImageJ.
Immunoblotting.
Immunoblots were performed essentially as described (35). For detection of PKB, 15–40 μg of cell lysate was separated on 7% or 10% SDS/PAGE or 4–12% precast Bis-Tris NuPAGE gradient gels. Proteins were detected with polyclonal or monoclonal PKB antibodies (both diluted 1:1,000). β-Actin was used as a loading control (mAb 1:10,000; Abcam). Phospho-PKB (S473) (1:1,000) was detected after stripping of total PKB antibody. InsP3R1 was detected by using a polyclonal antibody generated in house against the COOH terminus of the protein as described (36).
32P Labeling of InsP3Rs.
Labeling experiments were performed similar to that described (35). In this study, 300 μg of protein lysate prepared from 2 × 35-mm dishes per condition was used for IP.
In Situ Proximity Ligation Assay.
This assay was performed as described (19). Proximity probes were constructed by conjugating oligonucleotides to a monoclonal PKB antibody and a polyclonal InsP3R1 antibody. A probe for the Max oncogenic transcription factor has been described (19) and was used as a control. Cells were counterstained with an anticalnexin (CLNX) polyclonal antibody (Sigma) and visualized by using an AlexaFluor 488-conjugated secondary antibody. Coverslips were mounted in Vectashield containing DAPI (blue) (Vector Laboratories). Images were captured with a Zeiss LSM 510 META confocal microscope using a plan apochromat ×60, 1.40 n.a. oil immersion objective configured with LSM software version 3.2. Cellular RCA products were quantitated with ImageJ. To this end, mean pixel intensity of region of interest of the image not covered by a cell was subtracted from the mean pixel intensity of a region of interest that was drawn around each cell. The data are presented as mean pixel intensity. The brightness of sample images was increased for presentation. Pearson's colocalization analysis was performed with Volocity software using the region of interest threshold option (37) (version 4.01; Improvision).
Co-IP of InsP3Rs and PKB.
Lysates were prepared as for immunoblotting. Thirty micrograms of each lysate was retained for immunoblot analysis, and IPs were performed on 500–1,000 μg of the remaining protein as described (35). InsP3Rs, YFP-tagged proteins, and PKB were immunoprecipitated by using 2 μl of anti-InsP3R1 or anti-GFP polyclonal antibodies or 2 μl of monoclonal PKB antibody, respectively.
Induction of Apoptosis and FACS Analysis.
When experiments involved transfection or adenoviral infection, growth media were replaced with fresh media or media containing apoptosis-inducing agent after 24 h. Cells were treated for 6 h with 50 μM Menadione. Cells in the media were retained and pooled with remaining adherent cells that were harvested by trypsinization. Cells were collected by centrifugation at 1,200 × g for 5 min and fixed in 70% EtOH/PBS overnight. Cells were then pelleted, incubated for 1 h at 37°C in 600 μl of propidium iodide (PI) buffer (PBS, pH 7.4, 0.4 μg/ml PI, 0.4 μg/ml RNaseA, 0.3% IGEPAL), and analyzed by FACS. Data are presented as fold changes in sub-G1 population compared with the experimental condition indicated.
Data Analysis.
Statistical analysis was calculated by using Student's t test, ANOVA, and posthoc Tukey test or χ2 test. Data are presented as mean ± SEM. Significance was accepted at P < 0.05.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Profs. K. Mikoshiba, C. Erneux (University of Brussels, Brussels, Belgium), C. Taylor (University of Cambridge), and B. Burgering (University Medical Center of Utrecht, Utrecht, The Netherlands) for cDNAs; Stuart Conway (University of St. Andrews, St. Andrews, Scotland) for InP3-BM, J. Hanson, E. Vermassen, G. Morgan, S. Walker, S. Cook, L. Bauwens, M. Taussig, C. Taylor, and Y. Sun for help and discussion; and the Engineering and Physical Sciences Research Council Mass Spectrometry service (Swansea, U.K.). This work was supported in part by Fonds Wetenschappelijk Onderzoek-Vlaanderen Grant 3.0207.99, Program on Interuniversity Poles of Attraction (Belgian Science Policy) Grant P5/05, Concerted Actions of the Katholieke Universiteit Leuven Grant 99/08, The Babraham Institute, The Royal Society, Human Frontier Science Program, and Biotechnology and Biological Sciences Research Council Grant C19767.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711324105/DC1.
References
- 1.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanada M, Feng J, Hemmings BA. Structure, regulation, and function of PKB/AKT: A major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 4.Thompson JE, Thompson CB. Putting the rap on Akt. J Clin Oncol. 2004;22:4217–4226. doi: 10.1200/JCO.2004.01.103. [DOI] [PubMed] [Google Scholar]
- 5.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 6.Leslie NR, Downes CP. PTEN function: How normal cells control it and tumor cells lose it. Biochem J. 2004;382:1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 8.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP3-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 10.Hanson CJ, Bootman MD, Roderick HL. Cell signaling: IP3 receptors channel calcium into cell death. Curr Biol. 2004;14:R933–R935. doi: 10.1016/j.cub.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Choe CU, Ehrlich BE. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: Sometimes good and sometimes bad teamwork. Sci STKE. 2006 doi: 10.1126/stke.3632006re15. [DOI] [PubMed] [Google Scholar]
- 12.White C, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R, et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boehning D, et al. Cytochrome c binds to inositol (4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 15.Khan MT, et al. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2006;281:3731–3737. doi: 10.1074/jbc.M509262200. [DOI] [PubMed] [Google Scholar]
- 16.Collins TJ, Lipp P, Berridge MJ, Bootman MD. Mitochondrial Ca2+ uptake depends on the spatial and temporal profile of cytosolic Ca2+ signals. J Biol Chem. 2001;276:26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- 17.Boehning D, Joseph SK. Functional properties of recombinant type I, type III inositol 1, 4,5-trisphosphate receptor isoforms expressed in COS-7 cells. J Biol Chem. 2000;275:21492–21499. doi: 10.1074/jbc.M001724200. [DOI] [PubMed] [Google Scholar]
- 18.Wagner LE, 2nd, Betzenhauser MJ, Yule DI. ATP binding to a unique site in the type-1 S2- inositol 1,4,5-trisphosphate receptor defines susceptibility to phosphorylation by protein kinase A. J Biol Chem. 2006;281:17410–17419. doi: 10.1074/jbc.M601340200. [DOI] [PubMed] [Google Scholar]
- 19.Söderberg O, et al. Direct observation in situ of individual endogenous protein complexes by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 20.Kotelevets L, et al. The lipid phosphatase activity of PTEN is critical for stabilizing intercellular junctions and reverting invasiveness. J Cell Biol. 2001;155:1129–1135. doi: 10.1083/jcb.200105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: The ER/mitochondria Ca2+ liaison. Sci STKE. 2004 doi: 10.1126/stke.2152004re1. [DOI] [PubMed] [Google Scholar]
- 22.Szabadkai G, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pogue SL, Kurosaki T, Bolen J, Herbst R. B cell antigen receptor-induced activation of Akt promotes B cell survival and is dependent on Syk kinase. J Immunol. 2000;165:1300–1306. doi: 10.4049/jimmunol.165.3.1300. [DOI] [PubMed] [Google Scholar]
- 24.Yule DI, Straub SV, Bruce JI. Modulation of Ca2+ oscillations by phosphorylation of Ins(1,4,5)P3 receptors. Biochem Soc Trans. 2003;31:954–957. doi: 10.1042/bst0310954. [DOI] [PubMed] [Google Scholar]
- 25.Bare DJ, et al. Cardiac type inositol 1,4,5-triphosphate receptor: Interaction and modulation by calcium calmodulin-dependent protein kinase II. J Biol Chem. 2005;280:15915–15920. doi: 10.1074/jbc.M414212200. [DOI] [PubMed] [Google Scholar]
- 26.Tang TS, et al. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schug ZT, Joseph SK. The role of the S4–S5 linker and C-terminal tail in inositol 1,4,5-trisphosphate receptor function. J Biol Chem. 2006;281:24431–24440. doi: 10.1074/jbc.M604190200. [DOI] [PubMed] [Google Scholar]
- 28.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 29.Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 30.Lindsay Y, et al. Localization of agonist-sensitive PtdIns(3,4,5)P3 reveals a nuclear pool that is insensitive to PTEN expression. J Cell Sci. 2006;119:5160–5168. doi: 10.1242/jcs.000133. [DOI] [PubMed] [Google Scholar]
- 31.Gerasimenko JV, et al. Menadione-induced apoptosis: Roles of cytosolic Ca2+ elevations and the mitochondrial permeability transition pore. J Cell Sci. 2002;115:485–497. doi: 10.1242/jcs.115.3.485. [DOI] [PubMed] [Google Scholar]
- 32.Criollo A, et al. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14:1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- 33.Dufner A, et al. Protein kinase B localization and activation differentially affect S6 kinase 1 activity and eukaryotic translation initiation factor 4E-binding protein 1 phosphorylation. Mol Cell Biol. 1999;19:4525–4534. doi: 10.1128/mcb.19.6.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker AK, Gergely FV, Taylor CW. Targeting of inositol 1,4,5-trisphosphate receptors to the endoplasmic reticulum by multiple signals within their transmembrane domains. J Biol Chem. 2004;279:23797–23805. doi: 10.1074/jbc.M402098200. [DOI] [PubMed] [Google Scholar]
- 35.Kasri NN, et al. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J. 2004;23:312–321. doi: 10.1038/sj.emboj.7600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wojcikiewicz RJ. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 37.Manders EM, et al. Dynamics of three-dimensional replication patterns during the S-phase, analyzed by double labeling of DNA, confocal microscopy. J Cell Sci. 1992;103:857–862. doi: 10.1242/jcs.103.3.857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.