Abstract
Cell-cycle exit and differentiation of suprabasal epidermal keratinocytes require nuclear IκB kinase α (IKKα), but not its protein kinase activity. IKKα also is a suppressor of squamous cell carcinoma (SCC), but its mode of action remains elusive. Postulating that IKKα may serve as a transcriptional regulator in keratinocytes, we searched for cell-cycle-related genes that could illuminate this function. IKKα was found to control several Myc antagonists, including Mad1, Mad2, and Ovol1, through the association with TGFβ-regulated Smad2/3 transcription factors and is required for Smad3 recruitment to at least one of these targets. Surprisingly, Smad2/3-dependent Mad1 induction and keratinocyte differentiation are independent of Smad4, the almost universal coregulator of canonical TGFβ signaling. IKKα also is needed for nuclear accumulation of activated Smad2/3 in the epidermis, and Smad2/3 are required for epidermal differentiation. We suggest that a TGFβ–Smad2/3–IKKα axis is a critical Smad4-independent regulator of keratinocyte proliferation and differentiation.
Keywords: epidermis, cornification, terminal differentiation
A critical mediator of NF-κB activation (1), IκB kinase (IKK) consists of two catalytic subunits, IKKα and IKKβ (2–5), and a regulatory subunit, IKKγ/NEMO (6, 7). Despite structural similarity, IKKα and IKKβ have nonredundant functions, with IKKβ being the predominant IKK (1, 8) and IKKα being a critical regulator of keratinocyte differentiation (9, 10). Without IKKα, epidermal keratinocytes exhibit enhanced proliferation and failure to differentiate. Consequently, Ikkα−/− mice are born enshrouded in a taut and thickened, nonstratified, epidermal sheet devoid of barrier function.
The mammalian epidermis is a stratified squamous epithelium in which basal keratinocytes undergo asymmetric cell divisions, giving rise to nonproliferative progeny that embark on a differentiation program as they delaminate and move upward through the spinous and granular layers before generating the cornified layer, which provides the crucial barrier function (11, 12). Without IKKα, this process is blocked, and basal keratinocytes fail to exit the cell cycle (9, 10, 13). Isolated Ikkα−/− keratinocytes proliferate uncontrollably and do not respond to differentiation-inducing signals such as high Ca2+ (9, 13). The reexpression of IKKα in Ikkα−/− keratinocytes induces growth arrest and allows terminal differentiation, but this function depends neither on IKKα's protein kinase activity nor on NF-κB. Instead, it requires nuclear accumulation of IKKα (14).
Recently, IKKα was identified as a tumor suppressor in squamous cell carcinoma (SCC), a type of cancer derived from squamous epithelia of the skin, oral and nasal cavities, esophagus, and other sites (15). Decreased nuclear IKKα expression was found in about one third of oral SCCs, mainly those that exhibit poorly differentiated phenotype and poor prognosis (16). These results strongly suggest that loss of nuclear IKKα contributes to malignant conversion of keratinocytes to less differentiated and hyperproliferative SCC. The mechanism by which IKKα controls the keratinocyte cell cycle is not known, but, as suggested from the above discussion, IKKα function is likely to be exerted in the nucleus.
Here, we describe IKKα target genes involved in negative regulation of keratinocyte proliferation. We also provide evidence that IKKα activates these genes through interaction with TGFβ-regulated Smad transcription factors. Although the prevailing mode of TGFβ action entails the interaction of receptor substrate Smads (R-Smads) with the co-Smad, Smad4 (17, 18), keratinocyte and epidermal differentiation, which depend on Smad2/3 and IKKα, are Smad4-independent. We suggest that IKKα serves as a nuclear cofactor for Smad2/3 in the epidermis.
Results
Identification of IKKα-Dependent Cell-Cycle Regulators.
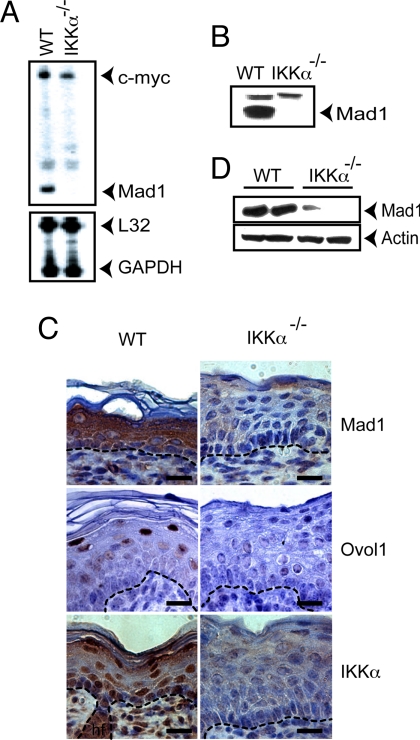
One of the earliest responses to IKKα reexpression in Ikkα−/− keratinocytes is growth arrest (13). Therefore, we examined the IKKα dependence of known cell-cycle regulators. Among the analyzed genes, the one showing the greatest IKKα dependence was Max dimerizer 1 (Mad1) [Fig. 1 A and B and supporting information (SI) Fig. 7]. Mad1 is a transcriptional regulator whose immediate relatives are Mad2, Mad3, and Mad4 proteins that heterodimerize with Max to form Mad/Max dimers that antagonize Myc/Max dimers (19, 20). Mad/Max dimers were linked to the inhibition of cell proliferation and the induction of differentiation in a variety of cell types, including keratinocytes (21), in which c-Myc stimulates proliferation and inhibits differentiation (22). The expression of Ovol1, Mad2, and, to a lesser extent, Mad3 and Mad4 also was reduced in the absence of IKKα (SI Fig. 7A). Interestingly, Ovol1 acts as a c-Myc repressor in keratinocytes (23). Because of the magnitude of its IKKα dependence, we chose Mad1 as a model gene. Mad1 expression was strikingly reduced in the Ikkα−/− epidermis, which also was deficient in the expression of Ovol1 (Fig. 1 C and D).
Fig. 1.
Keratinocyte Mad1 expression is IKKα-dependent. (A) RNA from WT and Ikkα−/− keratinocytes was analyzed for the expression of the indicated genes by RNase protection. (B) Whole-cell lysates of WT and Ikkα−/− keratinocytes were analyzed for Mad1 expression by immunoblotting. (C) Cross-sections of skin from WT and Ikkα−/− E18 fetuses were stained with H&E and antibodies to Mad1, Ovol1, and IKKα. (Scale bars: 25 μm.) (D) Protein lysates of E18 WT and Ikkα−/− skin were analyzed for Mad1 expression by immunoblotting.
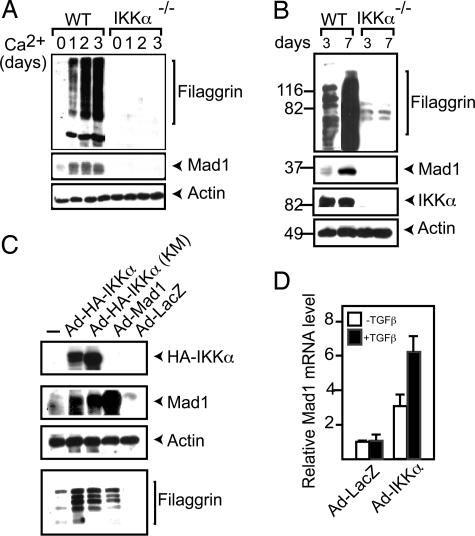
During differentiation triggered by Ca2+ (Fig. 2A) or confluency (Fig. 2B), Mad1 was induced in WT, but not in Ikkα−/−, keratinocytes. The introduction of IKKα, even in a catalytically inactive form, into Ikkα−/− keratinocytes restored Mad1 expression, along with the differentiation marker filaggrin (Fig. 2C). Ectopic Mad1 expression resulted in a modest increase in filaggrin expression, an effect that may be due to the inhibition of Ikkα−/− keratinocyte proliferation (data not shown).
Fig. 2.
Mad1 is induced during keratinocyte differentiation and by TGFβ in an IKKα-dependent manner. (A) WT keratinocytes were incubated with 0.2 mM Ca2+. Cell lysates were prepared when indicated, and filaggrin and Mad1 expression were analyzed by immunoblotting. (B) WT and Ikkα−/− keratinocytes were examined after 3 or 7 days of culture for the expression of Mad1 and filaggrin by immunoblotting. (C) Ikkα−/− keratinocytes were infected with adenoviruses encoding the indicated proteins. After 5 days, cells were lysed, and protein expression was analyzed by immunoblotting. (D) RNA was extracted from IKKα- or LacZ-adenovirus-infected Ikkα−/− keratinocytes incubated with or without TGFβ1 for 2 h. Mad1 mRNA was analyzed by real-time PCR.
IKKα Is Needed for TGFβ-Regulated Gene Expression and Inhibition of Proliferation in Keratinocytes.
In keratinocytes, Mad1 is induced by TGFβ (21), whose growth-inhibitory activity in these cells is partially dependent on Ovol1 (23). Interestingly, Ikkα−/− keratinocytes failed to arrest (SI Fig. 8) and up-regulate Mad1 mRNA (Fig. 2D) also in response to TGFβ1. The reexpression of IKKα restored TGFβ responsiveness (Fig. 2D). The induction of p21WAF1 showed partial IKKα dependence, but Smad7, encoding a TGFβ-inducible inhibitory i-Smad protein (24), was fully inducible in the absence of IKKα (SI Fig. 9). Thus, IKKα is required for many, but not all, TGFβ-elicited transcriptional responses in keratinocytes.
IKKα Interacts with TGFβ-Regulated R-Smads and Translocates to the Nucleus in Response to TGFβ.
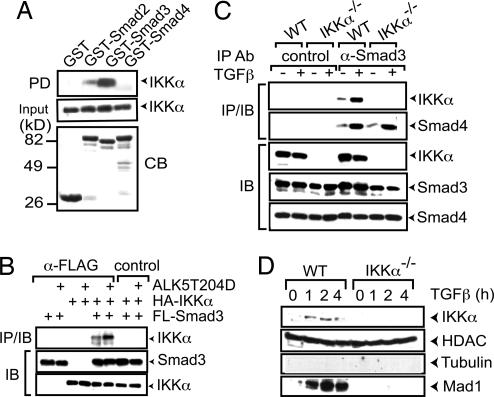
TGFβ family members signal via specific serine/threonine kinase receptors whose activation induces the association of receptor-regulated (R) Smad transcription factors with co-Smad and nuclear accumulation of the resulting complex (25). We examined whether IKKα interacts with R-Smads and co-Smads. GST-pulldown experiments using GST-Smad2–4-fusion proteins and lysates of HaCaT cells containing HA-tagged IKKα showed strong and weak binding of IKKα to Smad3 and Smad2, respectively, but no interaction with Smad4 (Fig. 3A). Transient transfection experiments revealed an association of IKKα with Smad3 that was potentiated by a constitutively activated form of TGFβ type 1 receptor, ALK5(T204D) (Fig. 3B). As predicted by the pulldown experiments, Smad4 did not interact with IKKα unless Smad3 was present (data not shown). An efficient and specific coprecipitation of endogenous IKKα with Smad3 was seen in primary mouse keratinocytes, especially after TGFβ treatment (Fig. 3C).
Fig. 3.
TGFβ stimulates complex formation between IKKα and Smad3 and induces IKKα nuclear translocation. (A) GST Smad2, −3, or −4-fusion proteins on GSH agarose beads were incubated with extracts of HaCaT cells expressing HA-IKKα. After extensive washing, bound proteins were eluted, gel was separated, and the presence of HA-IKKα was examined by immunoblotting. PD, pulldown; CB, Coomassie blue-stained gel. (B) HaCaT cells were transfected with various combinations of HA-IKKα, Flag-Smad3, and ALK5(T204D) expression vectors. After 2 days, whole-cell lysates were immunoprecipitated (IP) with Flag antibodies, gel separated, and immunoblotted (IB) with either Flag or HA antibodies. (C) WT and Ikkα−/− keratinocytes were prepared and treated with or without TGFβ1 for 1 h. Cell lysates were immunoprecipitated (IP) with Smad3 or isotype-matched control antibody, followed by immunoblotting (IB) with the indicated antibodies. (D) WT and Ikkα−/− keratinocytes were incubated with TGFβ1. Nuclear extracts were prepared at the indicated times and analyzed by immunoblotting.
Nuclear accumulation of IKKα is critical for the induction of keratinocyte differentiation (14). TGFβ signaling caused nuclear accumulation of IKKα in primary mouse keratinocytes, whose kinetics paralleled the onset of Mad1 induction (Fig. 3D and SI Fig. 10).
IKKα Is Required for Smad3 Recruitment to Mad1 Chromatin.
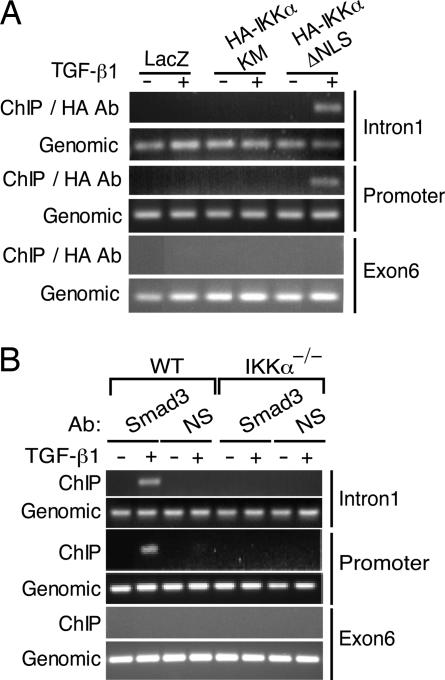
We examined the transcriptional regulatory region of the Mad1 gene and identified potential Smad-binding elements (SBEs) (18) in its 5′-flanking region at positions −534 and −21 and within intron 1 at positions +390 and +394. Mutational study revealed that both of these SBEs are required for IKKα and Smad3 responsiveness (data not shown). ChIP experiments using Ikkα−/− keratinocytes reconstituted with various HA-tagged IKKα constructs revealed TGFβ-induced IKKα binding to the SBE-containing regions of the Mad1 promoter and intron 1, but not to the SBE-devoid exon 6 (Fig. 4A and SI Fig. 11). Chromatin recruitment of IKKα required its NLS, but not its kinase activity (Fig. 4A). Smad3 was recruited to the same regions of the Mad1 gene after TGFβ treatment of WT keratinocytes (Fig. 4B). No Smad3 recruitment to the Mad1 chromatin could be detected in Ikkα−/− keratinocytes. It indicates that IKKα and Smad3 are likely to be present within the same complex.
Fig. 4.
IKKα is required for Smad3 recruitment to the Mad1 regulatory region. (A) Primary keratinocytes were transduced with LacZ, HA-IKKα(KM), or HA-IKKα(ΔNLS) adenoviruses. After 2 days, cells were treated or not with TGFβ1 for 1 h. After fixation, nuclear extracts were prepared, sonicated, and immunoprecipitated with HA antibodies. The presence of 5′ SBE-containing region (promoter), intron 1, and exon 6 sequences was analyzed by PCR. (B) Primary WT and Ikkα−/− keratinocytes were treated with or without TGFβ1 as indicated. After fixation, nuclear extracts were prepared, sonicated, and immunoprecipitated with Smad3 or RelA antibodies. The presence of the 5′ SBE-containing region (promoter) and exon 6 was analyzed by PCR.
TGFβ Signaling Controls Mad1 Expression, IKKα Nuclear Localization, and Keratinocyte Proliferation and Differentiation.
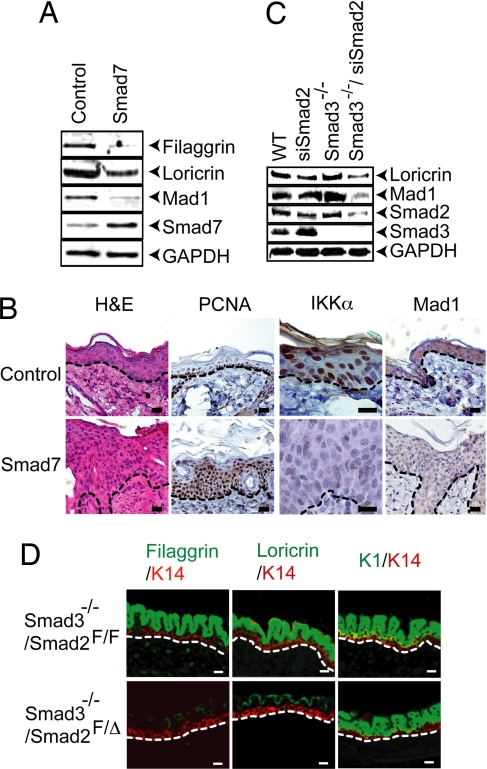
To further investigate the role of Smad transcription factors in the regulation of Mad1 expression, we prepared primary keratinocytes from Smad7 gene-switch-transgenic mice (26). The expression of this i-Smad in keratinocytes cultured in high Ca2+ inhibited Mad1, filaggrin, and loricrin expression (Fig. 5A). The expression of Smad7 between embryonic day 14.5 (E14.5) and birth interfered with proper epidermal differentiation, as indicated by aberrant and reduced filaggrin and loricrin expression (SI Fig. 12). The expression of Smad7 in adult mice prevented nuclear accumulation of IKKα, inhibited Mad1 expression, and led to marked epidermal hyperplasia (Fig. 5B).
Fig. 5.
Abrogation of endogenous Smad2/3 signaling inhibits epidermal differentiation, IKKα nuclear accumulation, and Mad1 expression. (A) Primary keratinocytes were isolated from control and bigenic Smad7 (K5.GLp65/tata.Smad7) (26) mice and cultured to confluency in high-calcium medium with RU486. After 48 h, cells were analyzed by immunoblotting. (B) Paraffin-embedded skin sections were prepared from adult control and Smad7 bigenic mice and were treated with RU486 for 12 days before analysis. Sections were stained with H&E and antibodies to PCNA, IKKα, and Mad1. (C) Primary keratinocytes from WT and Smad3−/− mice were cultured in high-Ca2+ medium for 48 h in the presence or absence of 100 nM Smad2-specific siRNA and analyzed by immunoblotting. (D) Immunofluorescence analysis of skin sections from E15.5 Smad3−/−/Smad2F/F and Smad3−/−/Smad2F/Δ embryos. Dashed lines highlight the border between the epidermis and the dermis. (Scale bars: B and D, 25 μm.)
Next we examined the role of Smad3 and Smad2 in epidermal differentiation. Although it is known that the disruption of either Smad3 or Smad2 alone does not interfere with keratinocyte differentiation (ref. 27 and data not shown), this may be due to redundancy between these two R-Smads. To assess this theory, we used siRNA-mediated knockdown of Smad2 in Smad3−/− keratinocytes and found reduced Ca2+-induced loricrin and Mad1 expression (Fig. 5C). In addition, we crossed Smad2-floxed mice (28) and CrePR1 mice (29) to the Smad3−/− background to allow for keratinocyte-specific Smad2 deletion. Although we did not recover any mouse embryos older than E14.5 lacking both Smad3 and Smad2 in keratinocytes, Smad3−/−/Smad2F/Δ embryos that were alive at E15.5 exhibited dramatic reduction in terminal epidermal differentiation, whose specific markers are filaggrin and loricrin, relative to Smad3−/−/Smad2F/F counterparts (Fig. 5D). Consistent with the Smad2 siRNA-silencing experiment, this result suggests that the reduction of ≈50% of Smad2 protein is sufficient to overcome the compensation for Smad3 loss and inhibit terminal epidermal differentiation.
IKKα-Dependent Smad2/3 Activation and Keratinocyte Differentiation Are Smad4-Independent.
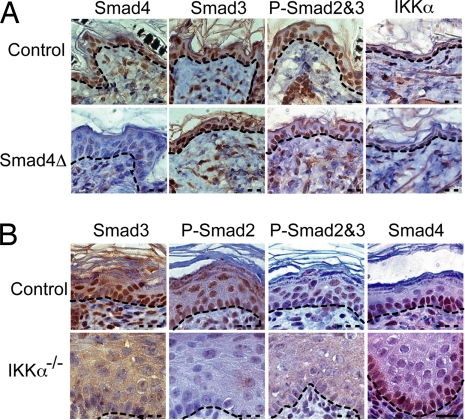
With rare exceptions, as recently demonstrated for TIF1γ-mediated erythroid differentiation (30), most R-Smad-dependent TGFβ responses require Smad4 (17). However, mice lacking Smad4 in epidermal keratinocytes do not show obvious differentiation defects (31, 32). This result prompted us to examine whether Smad4 is required for IKKα-dependent TGFβ-induced gene expression. In adult WT mouse skin, Smad4 was mainly present in basal keratinocytes, whereas nuclear phospho-Smad2/3 and IKKα were similarly distributed in nuclei of both basal and suprabasal keratinocytes (Fig. 6A). In the Smad4-deficient epidermis, IKKα and phospho-Smad2/3 remained nuclear, and their distribution appeared identical to that in WT epidermis. However, in the Ikkα−/− epidermis, IKKα was no longer present, and nuclear staining with phospho-Smad2/3 antibodies was severely diminished, but Smad4 localization was not affected (Fig. 6B).
Fig. 6.
IKKα, but not Smad4, is required for the nuclear accumulation of activated Smad2/3 and keratinocyte differentiation. (A) Paraffin-embedded skin sections were prepared from P14 control and Smad4F/F;K5-CrePR1 (Smad4Δ) mice and were stained with antibodies to Smad4, Smad3, phospho-Smad2,3 (P-Smad2&3), and IKKα. (B) Paraffin-embedded skin sections of control (WT) and Ikkα−/− E18 embryos were stained with antibodies to Smad3, phospho-Smad2 (P-Smad2), P-Smad2&3, and Smad4. Dashed lines highlight the border between the epidermis and the dermis. (Scale bar: in B for A and B, 25 μm.)
Discussion
Analysis of Ikkα-null mice revealed that IKKα is a critical regulator of keratinocyte proliferation and differentiation (9, 13), acting in the nucleus within a kinase-independent manner (14). This view is supported by the finding that keratinocyte-restricted expression of IKKα, which, even as a “kinase-dead” form, rescues epidermal morphogenesis in Ikkα−/− mice (14). However, the transplantation of Ikkα−/− skin onto WT recipients also rescues differentiation (13), and a keratinocyte-specific ablation of Ikkα results in only a partial differentiation defect, preventing formation of the epidermal barrier (33). These results suggest that, in addition to acting autonomously in isolated keratinocytes, IKKα also may act in dermal fibroblasts to produce factors that allow IKKα-deficient keratinocytes to partially differentiate when in contact with a normal dermis. Nonetheless, studies of SCC, cancers derived from squamous epithelial keratinocytes, underscore the importance of nuclear IKKα in negative control of keratinocyte proliferation and demonstrate that its loss results in an aggressive phenotype with poor prognosis (15, 16). To understand how IKKα controls keratinocyte proliferation, we identified cell-cycle genes whose expression is IKKα-dependent. Intriguingly, several of these genes encode negative regulators of Myc action or expression, Mad1, Mad2, and Ovol1 that are induced by TGFβ. These results are consistent with the finding that Myc is a positive regulator of keratinocyte proliferation and an inhibitor of terminal differentiation (22). Importantly, the expression of these genes in TGFβ-treated or differentiating keratinocytes depends on IKKα, but not on Smad4, the canonical coregulator of TGFβ-induced transcription (17, 18).
Investigation of the mechanism by which IKKα controls Mad1 transcription revealed that IKKα interacts with Smad3-containing transcription-activating complexes in a TGFβ-dependent manner and is needed for their recruitment to the Mad1 control region. IKKα is recruited to the same region of the Mad1 control region as Smad3, and our unpublished results reveal that IKKα also interacts with the control region of the Ovol1 gene (data not shown). Most likely, IKKα is recruited to chromatin via its interaction with Smad2/3 because IKKα lacks a recognizable DNA-binding domain. Although IKKα was proposed to act as a histone H3 kinase (34, 35), its function in TGFβ signaling does not depend on its protein kinase activity, which is not TGFβ-responsive (SI Fig. 11). The effect of IKKα on Mad1 expression and keratinocyte differentiation is unique to this particular IKK subunit, and the deletion of IKKβ has no effect on either parameter.
Although the physiological cues that control cell-cycle exit in keratinocytes, a prerequisite for differentiation (11, 12), are ill defined, TGFβ production is elevated during the differentiation of human oral keratinocytes, which can be induced by exogenous TGFβ (36). Furthermore, TGFβ inhibits keratinocyte proliferation and that is partially dependent on the IKKα target Ovol1 (23). We suggest that the antiproliferative effect of TGFβ is exerted via several IKKα-dependent genes, including Mad1, Mad2, and Ovol1. This explains why disruption of any single Mad gene does not perturb keratinocyte differentiation (37–39) and disruption of Ovol1 leads to keratinocyte hyperproliferation, but its effect on differentiation is relatively modest (23).
Until now, however, the role of TGFβ in epidermal differentiation has been obscured by the previous observations that the absence of Smad4, the ubiquitous coregulator of R-Smad-dependent transcription (17, 18), does not interfere with keratinocyte differentiation (31, 32). Until recently (30), Smad4 was thought to be obligatory for all R-Smad-mediated transcriptional responses, therefore the presence of normal epidermis in the absence of Smad4 suggested that TGFβ family members do not have a critical role in keratinocyte differentiation. However, we found that IKKα and activated Smad2/3 accumulate in the nuclei of differentiating keratinocytes independently of Smad4. In addition, IKKα is required for the nuclear accumulation of activated Smad2/3 in epidermis. These results indicate that IKKα is required in mouse keratinocytes for both TGFβ-induced growth arrest and differentiation. In conclusion, IKKα is a critical component of the TGFβ-signaling pathway in keratinocytes, where Smad4 plays a very minor role, if any, in the activation of the differentiation program.
By controlling keratinocyte proliferation, TGFβ and its relatives also may influence keratinocyte differentiation. The ectopic expression of Smad7 in epidermis, at a level sufficient to block Smad2/3 activation (26), prevents nuclear IKKα and Smad2/3 accumulation in epidermal keratinocytes and is associated with abnormal epidermal differentiation. Likewise, silencing of Smad2 in Smad3−/− keratinocytes reduced Mad1 expression and epidermal differentiation, and in vivo reduction of the Smad2 gene dosage in Smad3−/− epidermis resulted in a significant reduction of the expression of epidermal terminal differentiation markers. Therefore, it would be interesting to investigate the exact roles of individual TGFβ family members, which include activins, nodal, and BMPs in keratinocyte differentiation. In addition, the TGFβ–Smad2/3–IKKα axis may not control all aspects of epidermal differentiation, and the expression of barrier-related genes may depend on an interaction between IKKα and the retinoic acid receptor (33).
Experimental Procedures
In Vivo Mouse Models.
Gene-switch-Smad7 mice were generated as described previously (26). Smad7 expression was induced in vivo by topical application of 20 μg per mouse RU486 as described (26). To generate mice with reduced Smad2 gene dosage in the Smad3−/− background, Smad3−/− mice were bred with K14.CrePR1/Smad2F/Smad3+/− mice. Daily RU486 (100 μg per mouse) was injected into pregnant mice to induce Cre-mediated Smad2 deletion in embryonic epidermis, starting at day 12 of gestation. Then Progesterone (0.5 mg per mouse) was coinjected to protect pregnancy as described (29). Epidermal Smad4 KO mice (Smad4Δ) were generated by cross-breeding Smad4-floxed mice (no. 119) (31) with K5.CrePR1 mice, and Smad4 gene deletion in keratinocytes was achieved by topical application of RU486 (20 μg per mouse daily for 5 days, beginning on postnatal day 1 on bigenic mouse skin.
Cell Culture and Transfection.
Primary mouse keratinocytes from WT, Smad3−/−, and Ikkα−/− were cultured as described (13). For Smad7 overexpression in keratinocytes, primary keratinocytes obtained from K5.GLp65/tata.Smad7 were treated with 10−7 M RU486. Keratinocytes differentiation was induced by using 0.35 mM Ca2+ containing medium. HaCaT and HeLa cells were maintained in DMEM supplemented with 10% FBS, penicillin, and streptomycin. HaCaT and HeLa cells were transfected by using Lipofectamine Plus (Invitrogen). Primary keratinocytes were infected with adenoviral vectors as described below For TGFβ treatment, we normally used 100 pM TGFβ1 (Sigma).
Adenoviral Constructs and Infections.
Adenoviruses encoding WT and mutant IKKα were described (13). Adenovirus-expressing Mad1 was constructed by using BD-Adeno-X Expression System1 (BD Bioscience/Clontech) according to the manufacturer's instructions. Adenoviruses encoding Smad3, Smad4, or Smad7 were kindly provided by A. Moustakas (Ludwig Institute for Cancer Research, Uppsala, Sweden). Keratinocytes were infected at a moi of 50. Virus-containing medium was replaced with fresh medium after 1 day.
RNA and Real-Time PCR Analyses.
Total RNA was prepared by using an RNeasy Mini Kit (Qiagen), and cDNA was synthesized with a SuperScript II reverse transcription system (Invitrogen). Real-time quantitative PCR was performed by using cyclophilin mRNA for normalization (14). RNase protection assays were performed as described (40).
GST Pulldown and Coimmunoprecipitations.
GST Smad2–4 were expressed in Escherichia coli and purified on GSH agarose beads. HaCaT cells expressing HA-IKKα were lysed in buffer [10 mM Tris·HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 1 mM DTT, and 1% Nonidet P-40 with proteinase and phosphatase inhibitors]. Then ≈20 μg of purified recombinant GST-fusion protein was mixed with cell lysates at 4°C overnight with rotation, followed by five washes in lysis buffer. The washed beads were boiled in SDS/PAGE sample buffer and, after gel separation, were analyzed by immunoblotting with anti-HA antibody (Santa Cruz Biotechnology).
HaCaT cells transfected with Smad and IKKα expression vectors were lysed. After centrifugation, cleared lysates were incubated with anti-HA or anti-FLAG antibodies (Sigma) on ice. Protein G beads were added at 4°C for 2 h on a rotating platform. After three washes in lysis buffer, immune complexes were isolated, separated by SDS/PAGE, and analyzed by immunoblotting.
ChIP.
ChIP assays were performed as described (41).
Histological, Immunohistochemical, and Immunofluorescence Analysis.
Skin from E18 embryos were fixed in 10% neutral-buffered formalin. Then 5-μm paraffin-embedded skin sections were stained with H&E and specific antibodies. For immunohistochemistry, skin sections were kept at 60°C for 1 h and then incubated either overnight at 80°C in citrate buffer (pH 6) or at 94°C for 40 min in Dako's antigen retrieval solution S2368. A specific signal was detected by using the appropriate Dako EnVision System, HPR (DAB) kit. Immunofluoresence studies were performed as described (42).
Supplementary Material
ACKNOWLEDGMENTS.
We thank E. Bottinger (Mount Sinai School of Medicine, New York) for providing Smad2-floxed mice, A. Moustakas for Smad-expressing adenoviruses, S. Ishii (RIKEN Tsukuba Institute, Tsukuba, Japan) for Smad expression vectors, and X. Dai (University of California, Irvine) for the Ovol1 antibody. This work was supported by Association pour la Recherche sur le Cancer and Human Frontier Science Program Fellowships (to P.D.), a Japanese Society for the Promotion of Science Fellowship (to Y.S.), National Institutes of Health grants (to X.-J.W. and M.K.), and an American Cancer Society Research Professorship (to M.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712044105/DC1.
References
- 1.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 2.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 3.Mercurio F, et al. IKK-1 and IKK-2: Cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 4.Regnier CH, et al. Identification and characterization of an IkappaB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 5.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 6.Rothwarf DM, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 7.Yamaoka S, et al. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y, et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, et al. Limb and skin abnormalities in mice lacking IKKalpha. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs E, Byrne C. The epidermis: Rising to the surface. Curr Opin Genet Dev. 1994;4:725–736. doi: 10.1016/0959-437x(94)90140-x. [DOI] [PubMed] [Google Scholar]
- 12.Koster MI, Roop DR. Asymmetric cell division in skin development: A new look at an old observation. Dev Cell. 2005;9:444–446. doi: 10.1016/j.devcel.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, et al. IKKalpha controls formation of the epidermis independently of NF-kappaB. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- 14.Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- 15.Liu B, et al. A critical role for I kappaB kinase alpha in the development of human and mouse squamous cell carcinomas. Proc Natl Acad Sci USA. 2006;103:17202–17207. doi: 10.1073/pnas.0604481103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda G, Chiba T, Kawashiri S, Satoh T, Imai K. Epigenetic inactivation of IkappaB Kinase-alpha in oral carcinomas and tumor progression. Clin Cancer Res. 2007;13:5041–5047. doi: 10.1158/1078-0432.CCR-07-0463. [DOI] [PubMed] [Google Scholar]
- 17.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 18.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 19.Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–699. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- 20.Zervos AS, Gyuris J, Brent R. Mxi1, a protein that specifically interacts with Max to bind Myc-Max recognition sites. Cell. 1993;72:223–232. doi: 10.1016/0092-8674(93)90662-a. [DOI] [PubMed] [Google Scholar]
- 21.Werner S, Beer HD, Mauch C, Luscher B. The Mad1 transcription factor is a novel target of activin and TGF-beta action in keratinocytes: Possible role of Mad1 in wound repair and psoriasis. Oncogene. 2001;20:7494–7504. doi: 10.1038/sj.onc.1204937. [DOI] [PubMed] [Google Scholar]
- 22.Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28:165–168. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- 23.Nair M, et al. Ovol1 regulates the growth arrest of embryonic epidermal progenitor cells and represses c-myc transcription. J Cell Biol. 2006;173:253–264. doi: 10.1083/jcb.200508196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi H, et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 26.Han G, et al. Smad7-induced beta-catenin degradation alters epidermal appendage development. Dev Cell. 2006;11:301–312. doi: 10.1016/j.devcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Datto MB, et al. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ju W, et al. Deletion of Smad2 in mouse liver reveals novel functions in hepatocyte growth and differentiation. Mol Cell Biol. 2006;26:654–667. doi: 10.1128/MCB.26.2.654-667.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Z, Wang D, Wang XJ, Roop DR. In utero activation of K5CrePR1 induces gene deletion. Genesis. 2002;32:191–192. doi: 10.1002/gene.10064. [DOI] [PubMed] [Google Scholar]
- 30.He W, et al. Hematopoiesis controlled by distinct TIF1gamma and Smad4 branches of the TGFbeta pathway. Cell. 2006;125:929–941. doi: 10.1016/j.cell.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 31.Qiao W, et al. Hair follicle defects and squamous cell carcinoma formation in Smad4 conditional knockout mouse skin. Oncogene. 2006;25:207–217. doi: 10.1038/sj.onc.1209029. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, et al. Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res. 2005;65:8671–8678. doi: 10.1158/0008-5472.CAN-05-0800. [DOI] [PubMed] [Google Scholar]
- 33.Gareus R, et al. Normal epidermal differentiation but impaired skin-barrier formation upon keratinocyte-restricted IKK1 ablation. Nat Cell Biol. 2007;9:461–469. doi: 10.1038/ncb1560. [DOI] [PubMed] [Google Scholar]
- 34.Westerheide SD, Mayo MW, Anest V, Hanson JL, Baldwin AS., Jr The putative oncoprotein Bcl-3 induces cyclin D1 to stimulate G (1) transition. Mol Cell Biol. 2001;21:8428–8436. doi: 10.1128/MCB.21.24.8428-8436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 36.Min BM, Woo KM, Lee G, Park NH. Terminal differentiation of normal human oral keratinocytes is associated with enhanced cellular TGF-beta and phospholipase C-gamma 1 levels and apoptotic cell death. Exp Cell Res. 1999;249:377–385. doi: 10.1006/excr.1999.4489. [DOI] [PubMed] [Google Scholar]
- 37.Dontu G, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Queva C, McArthur GA, Iritani BM, Eisenman RN. Targeted deletion of the S-phase-specific Myc antagonist Mad3 sensitizes neuronal and lymphoid cells to radiation-induced apoptosis. Mol Cell Biol. 2001;21:703–712. doi: 10.1128/MCB.21.3.703-712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreiber-Agus N, et al. Role of Mxi1 in ageing organ systems and the regulation of normal and neoplastic growth. Nature. 1998;393:483–487. doi: 10.1038/31008. [DOI] [PubMed] [Google Scholar]
- 40.Makris C, et al. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell. 2000;5:969–979. doi: 10.1016/s1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- 41.Park JM, et al. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis–CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Wang XJ, Greenhalgh DA, Lu XR, Bickenbach JR, Roop DR. TGF alpha and v-fos cooperation in transgenic mouse epidermis induces aberrant keratinocyte differentiation and stable, autonomous papillomas. Oncogene. 1995;10:279–289. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.