Abstract
Modest dietary restriction (DR) prolongs life in a wide range of organisms, spanning single-celled yeast to mammals. Here, we report the use of recent techniques in nutrition research to quantify the detailed relationship between diet, nutrient intake, lifespan, and reproduction in Drosophila melanogaster. Caloric restriction (CR) was not responsible for extending lifespan in our experimental flies. Response surfaces for lifespan and fecundity were maximized at different protein–carbohydrate intakes, with longevity highest at a protein-to-carbohydrate ratio of 1:16 and egg-laying rate maximized at 1:2. Lifetime egg production, the measure closest to fitness, was maximized at an intermediate P:C ratio of 1:4. Flies offered a choice of complementary foods regulated intake to maximize lifetime egg production. The results indicate a role for both direct costs of reproduction and other deleterious consequences of ingesting high levels of protein. We unite a body of apparently conflicting work within a common framework and provide a platform for studying aging in all organisms.
Keywords: diet restriction, geometric framework, longevity, nutrition, aging
Animals that eat less live longer—up to a point. The view that dietary restriction without malnutrition prolongs life has become a central tenet in gerontology (1–3). Yeasts, fruit flies, nematode worms, and mice have become model systems for studying dietary restriction and aging, with some striking commonalities evident at the molecular and cellular levels (1–6). There is growing interest in the relationship between dietary restriction and quality and length of life in humans, although experimental data are lacking and difficult to obtain (7).
It is widely held that the life-extending effects of dietary restriction (DR) are due to caloric restriction (CR) (1, 8, 9), but recently this view has been challenged by experiments suggesting that specific nutrients (proteins and certain amino acids) rather than energy are responsible (10–12). Claims and counterclaims in the debate over the roles of energy and specific nutrients in aging (8, 11–14) have been hampered by one or more of the following problems (15). First, there is a lack of a suitable concept for baseline energy and nutrient intake, that is, dietary restriction relative to what? Thus, it may be that diet-restricted animals live longer either because eating less extends life, or because the normal nutritional regime in the laboratory is harmfully nutrient-rich in relation to requirements (15–17). Second, there have been too few dietary treatments used within an experiment to allow the effects of nutrients and energy to be partitioned. Hence, data from studies on rodents and Drosophila, which are claimed to prove the primacy of calories in influencing longevity, are open to alternative explanations (15). Finally, in the notable case of Drosophila, no study to date has measured how much flies actually eat throughout their lives. Rather, dietary restriction has been assumed to have occurred after dilution of the diet, without taking account of compensatory feeding or changes in the ratio of nutrients in the diet (14, 15, 18). In this article, we have used recent advances in nutritional research, a state-space platform known as the Geometric Framework (19, 20), to show how these issues can be resolved.
Results
We individually confined 1,008 mated female Canton-S Drosophila to one of 28 diets varying in both the ratio of yeast to sucrose and in the total concentration of yeast and sugar combined. The aim was to generate a detailed map of protein-carbohydrate intake space, on which response surfaces for variables related to aging and fecundity could be regressed. Liquid diets were provided in 5-μl glass capillaries (19, 21) and food intake and egg production were measured over 3-day intervals until death (see Materials and Methods). Parametric response surfaces for the three key life-history variables, individual lifespan (LS), lifetime egg production (LEP), and rate of egg production (REP = LEP/LS) were fitted over the protein-carbohydrate intake array, and then visualized by using nonparametric thin-plate splines (22). This is a powerful statistical procedure, given that surface-fitting regressions are based on ≈1,000 independent points (individually housed flies), representing 28 diet treatments.
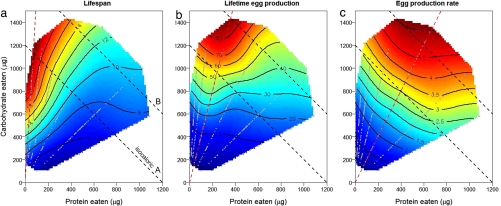
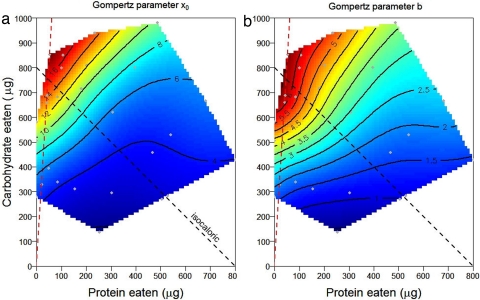
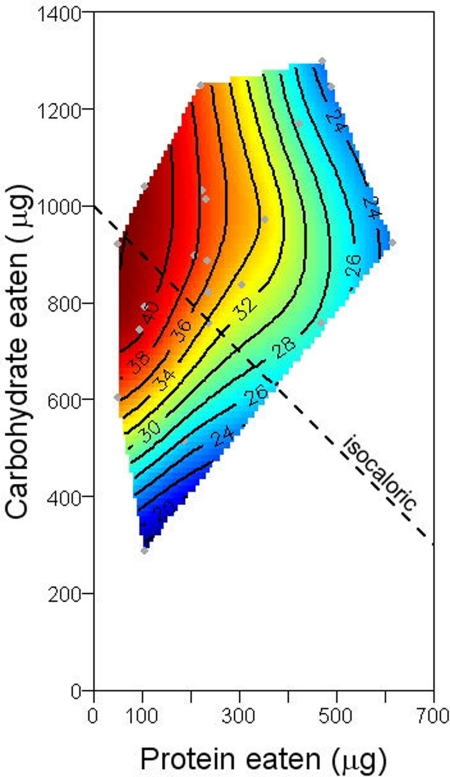
The shapes of the response surfaces differed markedly according to the performance variable (Fig. 1). Flies lived longest on a diet comprising a 1:16 ratio of protein to carbohydrate and survived progressively less long as this ratio rose (Fig. 1a). To distinguish the contributions of differences in the latency to commence dying and the age-specific rate of mortality, three-parameter Gompertz equations were fitted to cumulative mortality data within each of the 28 treatments, based on the lifespans of the 36 individually housed replicate flies [see Materials and Methods and supporting information (SI) Fig. 6]. Coefficient x0 indicates the time to the onset of rapid mortality (the point of inflection on the curve), whereas coefficient b is related to the steepness of the mortality curve thereafter, with high values indicating lower age-dependent increases in mortality rate. Surfaces for the 28 values of x0 and b were fitted over the associated mean protein-carbohydrate intake array (Fig. 2) and show that extended lifespan on low P:C diets was associated with both a later onset of mortality and a slower increase in mortality thereafter.
Fig. 1.
Effects of protein (P) and carbohydrate (C) intake on lifespan (a), lifetime egg production (b), and egg production rate (c), recorded for individual flies confined to 1 of 28 diets varying in both the ratio and the total amount of P and C. Gray dots are actual intakes over the first 6 days of individual flies. Brown dashed lines represent the nutritional ratio at which each fitness component was maximized. Black dashed lines show isocaloric intakes.
Fig. 2.
Effects of protein and carbohydrate intake on coefficients of the three-parameter Gompertz equation [y = a exp(−exp((x0 − x)/b))], fitted to lifespan data recorded from individual flies for each of the 28 diets. (a) x0, the inflection point representing the age of onset of increased mortality; (b) the rate of age-dependent increase in mortality (large numbers represent slow aging). Gray dots are mean intakes over 6 days for each diet. Brown dashed lines represent the nutritional rail on which each parameter was maximized. Black dashes are isocaloric lines.
The surface for lifetime egg production differed significantly from the surface for lifespan (partial F5, 1496 = 32.0, P < 0.0005; see SI Table 1 for estimated surface gradients), reaching a peak at a higher P:C (1:4) (Fig. 1b). Isoclines for LEP fell away from the peak at both lower and higher intakes of protein, and with a decline in carbohydrate intake (see SI Fig. 7 for plots of cumulative egg production across the 28 treatments). The rate of egg production was greatest at an even higher protein intake, ascending to a peak at P:C 1:2 (surface comparisons: LEP vs. REP, partial F5, 1496 = 10.3, P < 0.0005; LS vs. REP, partial F5, 1496 = 72.5, P < 0.0005).
Ingesting a target amount of protein in relation to carbohydrate enables egg production to be maximized, at a cost to longevity, but further protein intake reduces both egg production and longevity, even when total energy intake is not limiting. Egg laying rate is positively associated with longevity across the whole data set (regression, β = 0.783, F1,972 = 35.8, P < 0.0005), and when the effects of protein and carbohydrate are controlled for by fitting full diet response surface (P, C, P2, C2, P × C) plus egg production rate, this significant positive association remains (βegg production rate = 0.529, F1,755 = 13.6, P < 0.0005) .
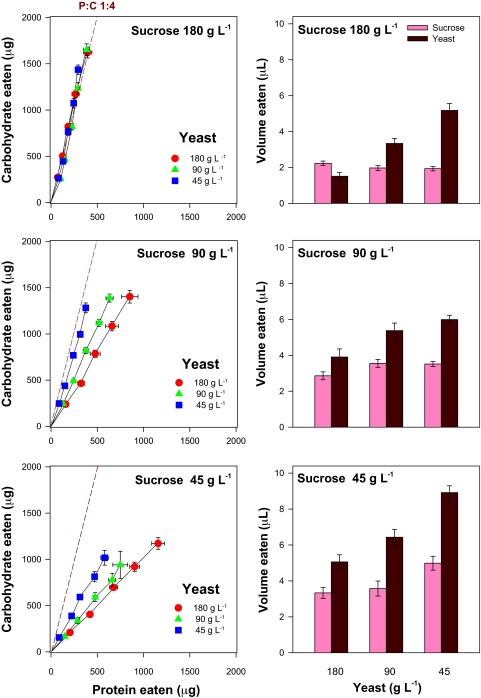
In a separate experiment, 225 flies were offered a choice of capillaries, one containing a yeast solution (45, 90, or 180 g·liter−1) and the other a sucrose solution (45, 90, or 180 g·liter−1). The aim was to establish to what extent—and to which macronutrient ratio—flies regulated their intake of protein and carbohydrate by adjusting the volume they ingested to account for differences in the concentration of nutrients in the capillaries. There was clear evidence that flies regulated intake of both macronutrients (Fig. 3). In four of the nine treatments, flies converged on a closely similar trajectory of protein-carbohydrate intake, requiring substantial differences in volumes of food to be imbibed between these treatments (Fig. 3). This “intake target” (15, 19) trajectory was P:C 1:4; the same as that which led to maximal lifetime egg production in the no-choice experiment (Fig. 1).
Fig. 3.
Mean ± SEM for macronutrient intake and volume consumed by flies provided with a 5-μl glass capillary containing one of three concentrations of yeast (Y) solution and another capillary with one of three sucrose (S) concentrations. (Left) Cumulative protein–carbohydrate intake trajectories at 3-day intervals over 15 days. (Right) This column plots the volume of the solutions imbibed over the first 6 days. In four treatments (S:Y 180:180, 180:90, 180:45, and 90:45), flies converged on a P:C 1:4 trajectory, which maximized lifetime egg production in no-choice experiments (Fig. 1). In the other five choice treatments, the P:C trajectory was more protein-biased because of flies compensating for limiting carbohydrate by feeding from the yeast solution, rather than by increasing intake of dilute sucrose, with associated longevity costs (Fig. 4).
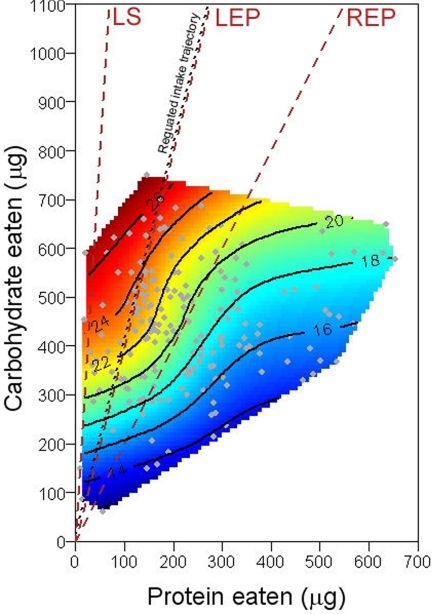
When sugar was present at 180 g·liter−1, flies were able to maintain protein intake across the full fourfold range of yeast dilutions (45, 90, and 180 g·liter−1). They were also able to maintain protein and carbohydrate intake when sugar was present at 90 g·liter−1 and yeast at 45 g·liter−1. In the remaining five choice treatments, however, compensatory feeding, although evident, was not complete. A clear pattern was apparent in these treatments: when the sugar solution was diluted, limiting carbohydrate was gained partly by consuming more of the sugar solution, but also by eating more yeast, which contains 24% carbohydrate. The latter came at the price, however, of ingesting excess protein (and correlated micronutrients) relative to the target ratio. Consequently, when a response surface was constructed for lifespan over the choice intake array, longevity was greatest for flies that converged at the intake target and fell with distance from the target (Fig. 4), as predicted from the no-choice surface in Fig. 1.
Fig. 4.
Longevity landscape fitted to 6-day macronutrient intake by individual flies (gray dots) across the nine treatments in the choice experiment. Dashed brown lines show P:C ratios that maximized lifespan (LS), lifetime egg production (LEP), and rate of egg production (REP) in no-choice experiments (Fig. 1).
We next reconciled our data against a compilation of previously published results on Drosophila, in which substantially smaller numbers of diet compositions were used and intake was not measured. Fig. 5 plots data from five classic studies (11, 13, 18, 23, 24), between them spanning 22 diet compositions. We estimated the protein and carbohydrate intake of flies fed these diets by interpolating within the intake array in Fig. 1 (SI Table 2). Next, we constructed a surface by using reported data for median lifespan. Despite procedural and fly strain differences between the studies, the resulting surface is strikingly similar in shape to that in Fig. 1a. The overall elevation is higher (i.e., overall, our flies lived less long), but this is likely the result of differences in protocol (e.g., we housed flies singly rather than in groups), and the greater difficulty for an aged fly of accessing food in capillaries as compared with a food block.
Fig. 5.
Response surface for median lifespan mapped onto an estimated intake array derived from 22 diets from five published studies in which diet composition was manipulated but in which intake was not measured. Note the strong similarity in shape of the landscape to ours in Fig. 1.
To demonstrate that the responses described in Fig. 1 were directly due to diet composition, not to incidental effects arising from the capillary feeding assay, we verified the surfaces derived from our capillary-feeding trials in relation to traditional demography cage techniques. In each cage (n = 10 cages per treatment), 130 mated female flies were provided with access to one of the three key diets: P:C 1:16, 1:4, or 1:2, each at a total of 180 g·liter−1. Longevity and egg production were recorded daily within cages. The patterns for LS, LEP, and REP were precisely as predicted from Fig. 1; namely, LS was highest on 1:16, REP on 1:2, and LEP on the intermediate ratio, 1:4 (SI Table 3). We measured the lipid content of such flies, which was found to be highest for the longest-lived flies: 30.0 ± 1.1 (mean % dry mass ± SE, n = 20, flies at age 7 days after being placed onto diet), 15.1 ± 0.7, and 14.0 ± 0.9 for P:C 1:16, 1:4, and 1:2, respectively. Starvation trials indicated a mean difference of 26.4 h and a maximal difference of 39.2 h between these flies (SI Fig. 8 and SI Table 4), which could not account for longevity differences between the treatments, either in the demography cages or for capillary-fed flies. The fact that flies in demography cages lived less long on high percentage protein diets also indicates that shorter lifespans for such flies in the capillary-feeding assay were not the result of egg-laden flies being unable to access the capillary tubes, because access to food in the demography trials did not require such feats of climbing.
Discussion
Caloric restriction was not responsible for extending lifespan in our flies. Rather, lifespan declined from the maximum as protein intake increased and as carbohydrate intake fell. Given that protein and carbohydrate are closely similar in caloric density, isoclines for energy consumption on the intake surfaces in Fig. 1 are lines with a slope of −1 (i.e., parallel to the isocaloric line, A, on Fig. 1). The contours of the longevity surface run almost orthogonally to such isocaloric lines. Our results robustly dissociate lifespan from caloric intake even if protein and carbohydrate do not yield equal amounts of available energy to flies (e.g., because of extra energetic costs of processing protein), such that the slope of net isocaloric lines in Fig. 1 would be less than −1. Matching LS isoclines to isoenergetic lines would require that ingesting 1 unit of protein causes a net loss of multiple units of energy; and even then, CR would have shortened, not lengthened lifespan. The most parsimonious explanation of the LS surface is that reduced lifespan is a direct result of incorporating an increasing proportion of protein in the diet (25), although, because yeast contains micronutrients (totaling ≈2% by mass), the possibility exists that these too could play a role.
Longevity is commonly expected to trade off against reproductive effort (14, 17, 26, 27). A comparison of the lifespan and egg production surfaces offers insights into this relationship. Our results provide some support for such a trade-off, because diets at which fitness components are maximized differ, and because the measure closest to fitness—lifetime egg production—is maximized at an intermediate P:C ratio. However, any such trade-off, whether because of allocation of resources between somatic repair and maintenance and reproduction, or a direct longevity cost associated with reproduction (14), does not explain the major pattern that we found. To illustrate, consider isocaloric line A in Fig. 1. Lifetime egg production remains near constant as P:C increases up to P:C 1:4, then falls. Egg production rate rises shallowly until P:C = 1:2, then falls. Lifespan, however, falls steeply and continuously as P:C rises along the isocaloric line, from left to right. The lack of direct evidence that egg production affects reduced longevity is consistent with recent manipulative evidence that ablation of the Drosophila germ line does not lead to increased longevity (28).
Data from the choice experiment indicated that flies behaved like small “nutrient-seeking missiles,” converging on an intake trajectory that led them inexorably toward maximal lifetime egg production. These data indicate that flies have the capacity to regulate intake of both protein and carbohydrate separately, as has been found by using the Geometric Framework in a number of other animal species (19, 20), and that they select a diet composition that maximises fitness. Just as in other animals, we found that flies had limitations in their capacity to track their intake target. Flies' ability to compensate for sugar dilution was limited—rather than ingesting substantially more of a diluted sugar solution, they preferred to gain limiting sugar by eating more yeast, thereby incidentally eating too much protein. It is likely that volumetric constraints on total intake were a contributing factor in this response. For example, maintaining the P:C 1:4 intake target ratio would have required flies provided with the pairing of 45g·liter−1 sugar and 45 g·liter−1 yeast solutions ingesting nearly twice the total volume as those flies provided with 180 g·liter−1 sugar and 45 g·liter−1 yeast solutions.
Just as previously published data on Drosophila could be reconciled within our experimental paradigm, when viewed in light of the Geometric Framework existing data on rodents leave open the possibility that CR is also not responsible for the life-extending effects of dietary restriction in these animals (15). A study based on the current fly experiment is needed on rodents, albeit somewhat restricted in scope to be logistically feasible. The way is also open to begin to compare the dietary responses of different fly strains and to address specific life-history and mechanistic questions. Similar approaches can also be taken to investigate the role of micronutrients. A key question that arises is what are the life-shortening consequences of ingesting excess protein? Candidates include, inter alia, increased mitochondrial generation of radical oxygen species (29), changes in the relationship between insulin/IGF-1 and amino acid signaling (e.g., TOR) pathways (30), and damage to organs arising from nitrogenous breakdown products.
Materials and Methods
Fly Stock.
Wild-type Canton-S flies (Bloomington Stock Centre) were maintained at 25°C under a 12:12 L:D photoregime and were cultured on standard sugar-yeast medium (250 g semolina, 500 g treacle, 170 g yeast, 24 g agar, 20 g Nipagin in 2.1 liters of water).
Experimental Protocol.
Three balanced runs were conducted of the no-choice diet experiment, yielding data for 1,008 individually housed flies. Several hundred freshly emerged flies of both sexes were collected over a 24-h period, transferred to fresh medium, and kept together for another 24 h to mate. Females were then collected under light CO2 anesthesia and placed into individual 6-ml plastic vials, kept in racks of 64 housed within a 6-liter sealed plastic box with a paper towel floor moistened with 40 ml of sterile water containing 1% Nipagin to maintain high RH. Each rack contained representatives of all 28 dietary treatments within the experiment (see below) along with two control vials for each yeast and sugar concentration used. The experimental room was maintained at 25°C under a 12:12 L:D photoregime. Within each vial, a fly was presented with food (see below) and an oviposition site (a 1-cm diameter black paper disk, moistened with distilled water). Food eaten and egg production were recorded for 3-day periods, and survivorship was recorded daily. Pilot trials in which eggs were counted daily indicated that counting eggs and empty egg shells every 3 days provided an accurate measure of eggs laid.
In a second choice experiment, the capacity of flies to maintain a target P-C intake trajectory was measured by providing flies with two capillaries, one containing yeast at 45, 90, or 180 g·liter−1and the other sugar at 45, 90, or 180 g·liter−1 (i.e., nine choice treatments, n = 25 per treatment).
We chose to use 24-h premated female flies to make our data comparable to other key articles in the field of Drosophila aging research. Drosophila mate soon after adult eclosion in nature. Flies from different treatments would have commenced the experiment with the same average sperm load. The rate of sperm depletion would have been a function of egg production; hence, were sperm to have run short, it might have dampened the elevation of the peak in LEP, but it would not have been expected to change the position of the peak on the P-C intake plane.
Liquid Foods and Measurement of Intake.
Twenty-eight liquid foods were prepared in sterile, distilled water. Foods varied in sucrose (S) and hydrolyzed yeast (Y) content (no-choice treatments, n = 36 per treatment). The seven Y:S ratios used were 0:1, 1:7, 1:3.4, 1:1.6, 1:0.7, 1:0.2, or 1:0; yielding protein (P) to carbohydrate (C) ratios of P:C 0:1, 1:16, 1:8, 1:4, 1:2, 1:1, and 1.9:1, respectively. The four Y+S concentrations were 45, 90, 180, or 360 g·liter−1. Macronutrient compositions were calculated based on autolyzed yeast (MP Biomedicals, catalogue no. 103304), containing 45% protein, 24% carbohydrate (as glucose equivalents), 21% indigestible fiber, 8% water, and the remaining 2% fatty acids, minerals, and vitamins. Each diet contained 0.01% phosphoric acid and 0.1% proprionic acid as antimould agents. To measure intake, flies were provided with 5-μl microcapillary tubes (Drummond Microcaps) filled with liquid diet. Intake was measured against a scale bar by height difference in the column of liquid within the microcapillary. Because RH was kept high in the environment, evaporation was minimal, but it was nonetheless measured for each solution type and used to correct volume consumed and nutrient concentration. There was some expansion of volume for the highest concentration (360 g·liter−1), which was also taken into account when calculating nutrient intake.
Measuring Lifespan and Egg Production.
Lifespan (LS) was measured as the time from when an individual fly entered its experimental vial (at 2 days of age) until its death, measured to within 24 h. Lifetime egg production (LEP) was the total number of eggs laid throughout the life of an individual fly from the time it entered its experimental vial until death. Eggs were counted at 3-day intervals until death. Egg production rate was LEP/LS.
Data Analysis.
We used Lande–Arnold regression approaches to estimate parametric nonlinear response surfaces (22). These comprise linear and quadratic components for protein and carbohydrate intake and the cross-product of P and C. Response surfaces for lifespan, lifetime egg production, and egg production rate were fitted over protein-carbohydrate (P-C) intake arrays and compared by using partial F tests (31) based on each pair of fitness components. To ensure that differences in scaling of the fitness components did not contribute to detected differences, we standardized each dependent measure before fitting models used for partial F tests. Fitness surfaces are best visualized by using nonparametric techniques that do not constrain the shape of the surface (22). We fitted nonparametric thin-plate splines using the fields package in R (version 2.5.1). Surfaces were fitted onto total intake over days 0–6; closely similar patterns were obtained for daily intake rate calculated over this period or the entire lifetime.
A three-parameter Gompertz equation [y = a exp(−exp((x0 − x)/b))] was fitted to cumulative proportional mortality data for each of the 28 no-choice treatments (see SI Fig. 6) by using an iterative least-squares procedure in SigmaPlot 8.0. Independent measures of lifespan from 36 individual flies within each treatment provided extremely good model fits. R2 values exceeded 0.955 for all 28 treatments, and estimates for x0 (point of inflection) and b (age-specific rate of mortality) had P < 0.0001 in all cases. These values were used to construct response surfaces over mean nutrient intake arrays for each diet treatment, as above. Coefficient a indicates the asymptote and was near 1, reflecting the fact that the proportion of mortality accumulated to 1.0.
Demography Cage and Starvation Trials.
The three diets (P:C 1:16, 1:4, or 1:2, each at a total of 180 g·liter−1) were prepared as for the liquid diets with the addition of 1.5 g of agar per 100 ml to set the media. Diets were boiled to dissolve the agar before being poured into 2-cm diameter circular molds to form blocks. Each cage (n = 10 cages per diet treatment) was provided with two food blocks, which were changed daily when any dead flies were removed. For the starvation trial, food blocks were replaced after 7 days with cotton wool moistened with ultrafiltered distilled water. The moistened cotton wool was changed every 12 h. Dead flies were removed and counted every 4 h. Fly lipid content was determined as the difference in weight of freeze-dried flies after three chloroform washes from two groups of 10 randomly selected flies from each cage (n = 5 cages per treatment).
Supplementary Material
ACKNOWLEDGMENTS.
This work was supported by the Australian Research Council, the University of Sydney, and an Australian Research Council Federation Fellowship (to S.J.S.); a QEII fellowship (to R.B.); and National Science Foundation Grant DEB-0444766 and National Institutes of Health Grant RO1 GM067862-01 (to J.W.O.B.). Canton S flies were provided by Assoc. Prof. Steve McKechnie, Monash University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710787105/DC1.
References
- 1.Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C Thomas; 1988. [Google Scholar]
- 2.Partridge L, Brand MD. Dietary restriction, longevity and ageing–the current state of our knowledge and ignorance. Mech Ageing Dev. 2005;126:911–912. [Google Scholar]
- 3.Nemoto S, Finkel T. Ageing and the mystery at Arles. Nature. 2004;429:149–152. doi: 10.1038/429149a. [DOI] [PubMed] [Google Scholar]
- 4.Merry BJ. Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol. 2002;34:1340–1354. doi: 10.1016/s1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 5.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 6.Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–552. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- 7.Heilbronn LK, Ravussin E. Calorie restriction and aging: Review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 8.Masoro EJ. Caloric restriction and aging: Controversial issues. J Gerontol A Biol Sci Med Sci. 2006;61:14–19. doi: 10.1093/gerona/61.1.14. [DOI] [PubMed] [Google Scholar]
- 9.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: Understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol. 2003;38:47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]
- 11.Mair W, Piper MDW, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:1305–1311. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piper MDW, Mair W, Partridge L. Counting the calories: The Role of specific nutrients in extension of life span by food restriction. J Gerontol A Biol Sci Med Sci. 2005;60:549–555. doi: 10.1093/gerona/60.5.549. [DOI] [PubMed] [Google Scholar]
- 13.Min K-J, Flatt T, Kulatos I, Tatar M. Counting calories in Drosophila diet restriction. Exp Gerontol. 2007;42:247–251. doi: 10.1016/j.exger.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatar M. In: Mechanisms of Caloric Restriction in Aging. Interdisciplinary Topics in Gerontology. Mobbs CV, Yen K, Hof PR, editors. Basel: Karger; 2007. pp. 115–136. [Google Scholar]
- 15.Simpson SJ, Raubenheimer D. Caloric restriction and aging revisited: the need for a geometric analysis of the nutritional bases of aging. J Gerontol A Biol Sci Med Sci. 2007;62:707–713. doi: 10.1093/gerona/62.7.707. [DOI] [PubMed] [Google Scholar]
- 16.Raubenheimer D, Lee KP, Simpson SJ. Does Bertrand's rule apply to macronutrients? Proc R Soc London Ser B. 2005;272:2429–2434. doi: 10.1098/rspb.2005.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piper MDW, Partridge L. Dietary restriction in Drosophila: Delayed aging or experimental artefact? PLoS Genet. 2007;3:461–466. doi: 10.1371/journal.pgen.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat Method. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raubenheimer D, Simpson SJ. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr Res Rev. 1997;10:151–179. doi: 10.1079/NRR19970009. [DOI] [PubMed] [Google Scholar]
- 20.Simpson SJ, Sibly RM, Lee KP, Behmer ST, Raubenheimer D. Optimal foraging when regulating intake of multiple nutrients. Anim Behav. 2004;68:1299–1311. [Google Scholar]
- 21.Ja WW, et al. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blows MW, Brooks R. Measuring non-linear selection. Am Nat. 2003;162:815–820. doi: 10.1086/378905. [DOI] [PubMed] [Google Scholar]
- 23.Chapman T, Partridge L. Female fitness in Drosophila melanogaster: An interaction between the effect of nutrition and of encounter rate with males. Proc R Soc London Ser B. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 24.Min K-J, Tatar M. Drosophila diet restriction in practice: Do flies consume fewer nutrients? Mech Ageing Dev. 2006;127:93–96. doi: 10.1016/j.mad.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Min K-J, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech Ageing Dev. 2006;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Hunt J, et al. High quality male field crickets invest heavily in sexual display but die young. Nature. 2004;432:1024–1027. doi: 10.1038/nature03084. [DOI] [PubMed] [Google Scholar]
- 28.Barnes AI, Boone JM, Jacobson J, Partridge L, Chapman T. No extension of lifespan by ablation of germ line in Drosophila. Proc R Soc London Ser B. 2006;273:939–947. doi: 10.1098/rspb.2005.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanz A, Caro P, Barja G. Protein restriction without strong caloric restriction decreases mitochondrial oxygen radical production and oxidative DNA damage in rat liver. J Bionenerg Biomembr. 2004;36:545–552. doi: 10.1007/s10863-004-9001-7. [DOI] [PubMed] [Google Scholar]
- 30.Kapahi P, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signalling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chenoweth SF, Blows MW. Contrasting mutual sexual selection on homologous signal traits in Drosophila serrata. Evolution (Lawrence, Kans) 2005;165:281–289. doi: 10.1086/427271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.