Abstract
The innate immune system encodes cytosolic Nod-like receptors (NLRs), several of which activate caspase 1 processing and IL-1β and IL-18 secretion. Macrophages respond to Salmonella typhimurium infection by activating caspase 1 through the NLR Ipaf. This activation is mediated by cytosolic flagellin through the activity of the virulence-associated type III secretion system (T3SS). We demonstrate here that Pseudomonas aeruginosa activates caspase 1 and induces IL-1β secretion in infected macrophages. While live, virulent P. aeruginosa activate IL-1β secretion through caspase 1 and Ipaf, strains that have mutations in the T3SS or in flagellin did not. Ipaf-dependent caspase 1 activation could be recapitulated by delivering P. aeruginosa flagellin to the macrophage cytosol. We examined the role of Naip5 in P. aeruginosa-induced caspase 1 activation by using A/J (Naip5-deficient) compared with C57BL/6 and BALB/c (Naip5-sufficient) macrophages and observed that A/J macrophages secrete IL-1β in response to P. aeruginosa, S. typhimurium, and Listeria monocytogenes infection, as well as in response to cytosolic flagellin, but at slightly reduced levels. Thus, Ipaf-dependent detection of cytosolic flagellin is a conserved mechanism by which macrophages detect the presence of pathogens that use T3SS.
Keywords: flagellin, inflammation, type III secretion, macrophage
The innate immune system recognizes microorganisms via pattern-recognition receptors (PRRs). These receptors trigger the inflammation in response to conserved structures on pathogens [pathogen-associated molecular patterns (PAMPs)]. The Toll-like receptors (TLRs) are prototypic transmembrane PRRs (1). They respond to PAMPs that are present in the extracellular space or within phagocytic compartments by activating the transcription of numerous cytokine genes, including TNF, IL-6, and IL-12, as well as a variety of chemokine genes (1). By contrast, the secretion of IL-1β and IL-18 is regulated in a two-step process: The expression of the proforms of the cytokines is induced by TLRs, whereas processing and secretion depend on Nod-like receptor (NLR)-mediated caspase 1 activity (2).
NLRs are PRRs that detect cytosolic PAMPs (3, 4). They contain three domains: (i) an amino-terminal-signaling domain, (ii) a central oligomerization domain, and (iii) a carboxy-terminal sensor domain. Many NLRs, including Nalp3 and Ipaf, activate caspase 1 (3, 4). Ipaf activates caspase 1 through the interactions between its caspase activation and recruitment domain (CARD) and the CARD domain of caspase 1 (5). Nalp3 activates caspase 1 via a pyrin domain, but does so by using the adaptor protein ASC to recruit caspase 1 (3). The NLR Naip5 was identified as the Legionella pneumophila susceptibility locus in A/J mice (6, 7). Because Ipaf also provides resistance to L. pneumophila infection and Ipaf and Naip5 have been shown to interact with each other in overexpression assays, it has been suggested that the two work in concert to activate caspase 1 (8–11). However, a direct requirement for Naip5 in Ipaf signaling has not been demonstrated, and recent studies using L. pneumophila suggest that Naip5 is not required for Ipaf-dependent responses to L. pneumophila (12).
TLR5 and Ipaf act in dual-signaling pathways in response to bacterial flagellin. TLR5 signals in response to extracellular flagellin, whereas Ipaf signals in response to flagellin within the mammalian cell cytosol (8–10, 13–15). In the case of Salmonella typhimurium, the type III secretion system (T3SS) is required for the delivery of flagellin to the eukaryotic cell cytosol (4, 16). We wished to investigate whether the detection of the T3SS and flagellin through the Ipaf-signaling pathway represents a general innate immune strategy. We chose to study Pseudomonas aeruginosa, a flagellated Gram-negative bacterium that causes disease associated with significant inflammatory responses (17, 18). Much of the pathogenicity of P. aeruginosa can be traced to the expression of several virulence traits, including the synthesis of toxins, the modification of LPS (19), and the expression of the Psc T3SS. In particular, virulence factors injected into host cells by the Psc T3SS promote tissue damage and invasive disease (20).
The detection of P. aeruginosa by TLR4 and TLR5 has been described (21–23), but the ability of cytosolic NLRs to respond to P. aeruginosa has not been previously investigated. We demonstrate that macrophages respond to P. aeruginosa infection by activating caspase 1 and secreting IL-1β. Both T3SS and flagellin expression are required for Ipaf-mediated caspase 1 activation in response to P. aeruginosa. We further determined that strain differences between A/J macrophages (which are Naip5-deficient) and C57BL/6 macrophages slightly alter, but do not abrogate, the detection of cytosolic flagellin.
Results
P. aeruginosa Induces Caspase 1 Activation in Macrophages Through Ipaf.
P. aeruginosa infection of bone marrow-derived macrophages (BMMs) resulted in marked dose-dependent cytotoxicity within 1 h of infection (Fig. 1A). This phenotype was dependent on viable bacteria as heat-killed P. aeruginosa were not cytotoxic (Fig. 1A). Maximal cytotoxicity was observed when macrophages were infected with bacteria in the late logarithmic to the early stationary phase of growth (data not shown). Because of the rapid onset of loss of membrane integrity, we hypothesized that P. aeruginosa was killing the cells via pyroptosis (24), a type of cell death that is completely dependent on caspase 1 activity. This hypothesis was supported by the observation that caspase 1-null BMMs were resistant to P. aeruginosa-induced cell death (Fig. 1A).
Fig. 1.
P. aeruginosa activates caspase 1 through Ipaf. (A–C) BMMs were stimulated with LPS to promote proIL-1β production before infection with live or heat-killed P. aeruginosa. (B and C) Cytotoxicity was determined by LDH release (A), or IL-1β (B) and IL-18 (C) secretions were analyzed by ELISA. (D) Macrophages were infected with P. aeruginosa for 1 h and then treated with gentamicin before TNF secretion was determined after four additional hours. (E) IL-1β secretion by LPS primed WT, Ipaf-null, or Nalp3-null macrophages infected with P. aeruginosa for 1 h.
In addition to pyroptosis, caspase 1 activation also triggers IL-1β and IL-18 secretion. P. aeruginosa infection induced robust IL-1β secretion that was dependent on live bacteria as well as caspase 1 (Fig. 1B). This was also the case for IL-18 secretion (Fig. 1C). As a control, both WT and caspase 1-null macrophages secreted TNF at equivalent levels in response to P. aeruginosa (Fig. 1D). We also found that the NLR Ipaf was crucial for P. aeruginosa-induced caspase 1 processing (data not shown) and IL-1β secretion (Fig. 1E). By contrast, P. aeruginosa-induced IL-1β secretion was unimpaired in Nalp3-null macrophages (Fig. 1E).
P. aeruginosa T3SS Is Required to Induce IL-1β Secretion.
Caspase 1 activation by S. typhimurium is dependent on the activity of bacterial T3SS. Therefore, we examined the ability of T3SS mutant P. aeruginosa to induce IL-1β secretion and pyroptosis (see Fig. 2A for a schematic of mutant structures formed). A pscC mutation, which eliminates an operon of genes forming the T3SS apparatus that spans the bacterial inner and outer membranes, did not activate IL-1β secretion or pyroptosis (Fig. 2B and data not shown). Further, strains that lack the translocon genes popB or popD, which together form the pore in the eukaryotic cell membrane thorough which bacterial effector proteins pass, also did not activate caspase 1 as determined by IL-1β secretion and induction of pyroptosis (Fig. 2B and data not shown). Concordant with these observations, caspase 1 processing was absent in macrophages infected with pscC mutants (Fig. 2C). As a control, WT and pscC mutants stimulated equivalent amounts of IL-12 p40 secretion (Fig. 2D).
Fig. 2.
Ipaf responds to P. aeruginosa that expresses both T3SS and flagellin. (A) Schematic of T3SS mutants. (B) WT BMMs were stimulated with LPS to promote proIL-1β expression and infected with WT or mutant P. aeruginosa. IL-1β expression was determined in response to T3SS mutants lacking the Psc apparatus (pscC) or translocon (popB or popD). (C) Caspase 1 processing was determined by Western blot from macrophages infected with WT or pscC mutant P. aeruginosa. (D) IL-12 p40 secretion was determined by ELISA from macrophages infected with P. aeruginosa strains for 1 h, washed, and treated with gentamicin for four additional hours. The asterisk denotes a nonspecific band.
P. aeruginosa Flagellin Is Required to Induce IL-1β Secretion.
Macrophages activate caspase 1 in response to cytosolic S. typhimurium flagellin (14, 15). Therefore, we infected BMMs with a panel of P. aeruginosa flagellar mutants (see Fig. 3A for schematics of the flagellar structures synthesized by each mutant). A strain lacking multiple flagellar export apparatus components and that does not express the flagellin monomer, flgB, did not induce IL-1β secretion or pyroptosis in BMMs (Fig. 3B and data not shown). Similarly, fliC mutants, which synthesize an intact flagellar hook and basal body without expressing the flagellin monomer, also did not induce IL-1β secretion (Fig. 3B). In contrast, flgK mutants, which lack the hook-associated proteins but constitutively express unpolymerized monomeric flagellin, induced IL-1β secretion (Fig. 3B). These results were corroborated by experiments investigating caspase 1 processing. flgK mutants induced caspase 1 processing, whereas fliC and flgB mutants did not (Fig. 3C). To further verify the results obtained with these transposon mutants, we generated independent mutants in flgK and fliC where the entire ORF was replaced with a gentamicin cassette and found identical results (data not shown). As controls, flgK and fliC mutants induced equivalent amounts of IL-12 p40 secretion as WT bacteria (Fig. 3D). Interestingly, although flagellin monomer expression was required for caspase 1 activation, motility was not a factor. flgK mutants are amotile (Fig. 3E), but express flagellin monomers and activate caspase 1-dependent processing and secretion of IL-1β (Fig. 3 B and C).
Fig. 3.
Ipaf responds to P. aeruginosa expressing flagellin. (A) Diagram of flagellar structure formed by WT P. aeruginosa or the amotile flgK, fliC, and flgB mutants. (B) IL-1β secretion by LPS-primed WT macrophages infected with WT P. aeruginosa, amotile mutants expressing flagellin monomer (flgK), or amotile mutants not expressing flagellin monomer (fliC or flgB). (C) Caspase 1 processing in macrophages infected with P. aeruginosa mutants was examined by Western blot. (D) IL-12 p40 secretion was determined by ELISA from macrophages infected with P. aeruginosa strains for 1 h, washed, and treated with gentamicin for four additional hours. (E) The motility phenotype of strains was confirmed in soft agar. The asterisk denotes a nonspecific band.
Cytosolic Delivery of P. aeruginosa Flagellin Is Necessary for the Activation of IL-1β Secretion.
The results presented in Figs. 2 and 3 above imply that cytosolic flagellin, possibly delivered by T3SS, is necessary for Ipaf activation. In support of this hypothesis, cytosolic delivery of purified P. aeruginosa flagellin was required to induce induced IL-1β secretion (Fig. 4). Because BMMs do not express TLR5 (25), we could be certain that extracellular flagellin did not activate IL-1β secretion, and TLR5-null macrophages secreted IL-1β in response to cytosolic P. aeruginosa flagellin (Fig. 4). Moreover, Ipaf is required for the detection of cytosolic P. aeruginosa flagellin (Fig. 4).
Fig. 4.
Ipaf responds to cytosolic P. aeruginosa flagellin. WT, TLR5-null, or Ipaf-null BMMs were stimulated with LPS to promote pro-IL-1β expression before stimulation with P. aeruginosa flagellin with or without protein transfection reagent (Profect P1). IL-1β secretion was determined by ELISA 1 h later.
Comparison of Naip5-Sufficient and -Deficient Macrophages in Response to Infection with Flagellated Bacteria.
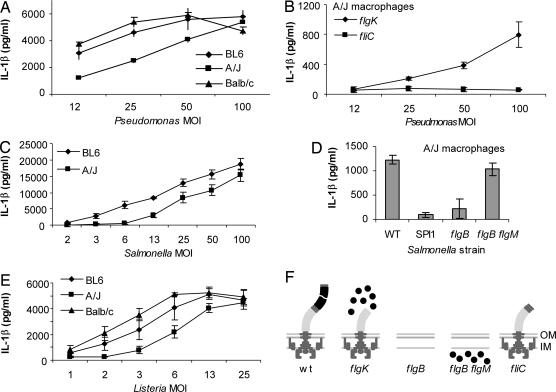
Naip5 is another NLR that may regulate Ipaf activation, as discussed above. Therefore, we investigated whether P. aeruginosa or S. typhimurium induced IL-1β secretion in BMMs derived from A/J mice, which have defective Naip5. BMMs from C57BL/6 and BALB/c mice were used as Naip5-sufficient controls (Fig. 5). BMMs from control as well as A/J mice secreted IL-1β when infected with P. aeruginosa (Fig. 5A) and S. typhimurium (Fig. 5C). This IL-1β secretion in A/J macrophages depended on bacterial expression of flagellin in both P. aeruginosa (Fig. 5B) and S. typhimurium (Fig. 5D). Reproducible decreases in IL-1β secretion were observed in A/J macrophages compared with C57BL/6 and BALB/c macrophages. To determine whether these changes were specific to bacterial pathogens that activate caspase 1 through Ipaf, we infected macrophages with L. monocytogenes, which activates caspase 1 through Nalp3. L. monocytogenes also induced IL-1β secretion in A/J BMM with slightly decreased sensitivity (Fig. 5E).
Fig. 5.
Contribution of Naip5 to caspase 1 activation. (A–D) BMMs derived from Naip-sufficient (C57BL/6 and BALB/c) or Naip5-deficient (A/J) mice were prestimulated with LPS to induce proIL-1β expression and infected with P. aeruginosa WT or flagellin-expressing nonmotile (flgK) or flagellin-nonexpressing nonmotile strains (fliC) (A and B), S. typhimurium WT, SPI1 T3SS mutant (ΔprgH-K), flagellin-nonexpressing nonmotile (flgB), or flagellin-expressing nonmotile (flgB flgM) strains (C and D), or L. monocytogenes strains (E) for 1 h before determination of IL-1β by ELISA. (F) Schematic of flagellar mutants used.
Comparison of Naip5-Sufficient and -Deficient Macrophages in Response to Cytosolic Flagellin.
We and others have previously shown that the delivery of purified flagellin protein to the cytosol of macrophages by liposomal delivery or membrane permeablization activates caspase 1 and induces IL-1β secretion through Ipaf (10, 14, 15). As a control, we analyzed the flagellar hook protein, which is 15% identical to flagellin, forms a similar tertiary structure, and functions to form a hollow tube integral to the flagellar structure (26, 27). Macrophages were stimulated with LPS to induce proIL-1β production, and IL-1β secretion was analyzed after the delivery of purified proteins to the cytosol by using a liposomal delivery reagent. Cytosolic delivery of purified flagellin induced robust IL-1β secretion that was Ipaf-dependent and TLR5-independent as described above (Fig. 4). Purified hook protein did not activate IL-1β secretion (Fig. 6A).
Fig. 6.
Contribution of Naip5 to Ipaf-dependent IL-1β secretion in response to cytosolic flagellin. BMMs were prestimulated with LPS to induce proIL-1β expression and stimulated with purified GST-flagellin or GST-hook protein delivered to the cytosol using the Profect P1 protein transfection reagent. IL-1β secretion was determined 1 h later by ELISA. (A) The BMMs derived from C57BL/6, isogenic TLR5-null, or isogenic Ipaf-null mice were compared. (B–D) Naip-sufficient (C57BL/6 and BALB/c) or Naip5-deficient (A/J) macrophages were analyzed.
We next compared Ipaf-activated IL-1β secretion in Naip5-sufficient (BALB/c and C57BL/6) and Naip5-deficient (A/J) backgrounds. All three strains responded to cytosolic flagellin and not to the hook control with similar dose–response curves (Fig. 6 B–D). As with the infection experiments above, A/J macrophages showed a decreased sensitivity to cytosolic flagellin.
Discussion
We show here that P. aeruginosa induces caspase 1 activation in macrophages. This results in the secretion of IL-1β and IL-18 and in rapid pyroptotic cell death. We have further defined the bacterial virulence traits (the Psc T3SS and flagellin) and the host response pathway (Ipaf) involved in the activation of caspase 1 in macrophages during P. aeruginosa infection. These results also reconcile a previous observation implicating the Psc T3SS apparatus, but not its effector proteins, in mediating macrophage cytotoxicity (28, 29). While this article was in preparation, other groups also showed that P. aeruginosa activates caspase 1 through Ipaf, T3SS, and flagellin. Some T3SS effectors may modulate this process (30–32).
More than 90% of environmental P. aeruginosa isolates express both flagellin and the Psc T3SS (33, 34). However, a substantial proportion of P. aeruginosa isolates from cystic fibrosis patients do not express these proteins (33, 34). Therefore, our results suggest that the lack of flagellin or a Psc T3SS represents an immune evasion mechanism for the bacterium because host macrophages will no longer activate caspase 1 and produce IL-1β and IL-18. The down-regulation of flagellin and the Psc T3SS also would have additional benefits for the bacterium because it would result in TLR5 evasion and reduced tissue damage, both of which are proinflammatory.
We also examined the contribution of Naip5 to Ipaf signaling. The possibility of a cooperative interaction between Ipaf and Naip5 has been investigated thoroughly in L. pneumophila infections, which we have recently reviewed in detail (4). Briefly, several lines of indirect evidence have suggested that Ipaf and Naip5 act in concert: (i) Ipaf and Naip5 coimmunoprecipitate in overexpression assays (9, 11), (ii) both Ipaf and Naip5 are L. pneumophila susceptibility genes in vitro in macrophages and in vivo in mice (6, 7, 9, 13), and (iii) both Ipaf and Naip5 modulate caspase 1 activity (5, 9). Other data indicate that Naip5-deficient macrophages retain their ability to activate caspase 1 and IL-1β secretion in response to L. pneumophila (8, 10, 12). Clearly, our experiments comparing A/J to C57BL/6 macrophages do not isolate any observed differences to the Naip5 locus. However, IL-1β secretion by A/J and C57BL/6 BMMs after P. aeruginosa and S. typhimurium infection are quite similar, and the detection of flagellin during bacterial infection and after transfection is apparent. Thus, we can conclude that Naip5 is not absolutely required for Ipaf-dependent caspase 1 activation. We observed slight decreases in IL-1β secretion in A/J BMMs, but we cannot conclude whether these are due to Naip5 deficiency or other strain differences. Our results are in agreement with recently published data examining L. pneumophila that show that Naip5 and Ipaf act independently and that reductions in IL-1β secretion seen in A/J strains are a result of decreased pro-IL-1β production (12).
Our results with P. aeruginosa parallel previous studies with S. typhimurium (14, 15). Other Gram-negative pathogens activate caspase 1 through the activity of T3SS, including Burkholderia pseudomallei (35), which also expresses flagellin. Moreover, it is known that L. pneumophila flagellin also activates Ipaf. However, in this case, the type IV secretion system is required (8–10, 13). Thus, it seems likely that an Ipaf-dependent response to flagellated bacteria expressing a virulence factor-associated secretion system represents a general innate immune defense mechanism by which macrophages incite increased inflammatory responses in the presence of virulent bacteria compared with nonpathogenic bacteria.
Materials and Methods
Strain Construction and Bacterial Growth Conditions.
P. aeruginosa PAO1 and S. typhimurium 14028s were grown in LB overnight, diluted 1:40, and grown 3 or 4 h for infections. L. monocytogenes was grown in brain heart infusion overnight and diluted 1:5 for 4 h at room temperature before infection. Predicted motility and swarm phenotypes of P. aeruginosa mutants were verified in 0.3% or 0.5% agar, respectively. P. aeruginosa PAO1 mutants were from C. Manoil (36). S. typhimurium mutants were described (15).
Tissue Culture and Mice.
BMMs were prepared from the femur of C57BL/6, BALB/c, A/J (Charles River Laboratories), caspase 1-null (37), Ipaf-null (38), Nalp3-null (39), and TLR5-null (40) mice by culturing with 20% FBS 30% L929 cell supernatant 50% DMEM. Mice were housed in a specific pathogen-free environment that was approved and supervised by the Institutional Animal Care and Use Committee.
Cytotoxicity and Cytokine Assays.
Assays were performed as described previously (15) with the following modifications. To induce proIL-1β expression, BMMs were stimulated for 3–5 h with 20–50 ng/ml ultra-pure S. minnesota LPS (List Biological Laboratories) that does not activate proIL-1β processing and secretion. Bacteria were added to the BMMs and centrifuged for 5 min at 233 × g. Infections were 60 min. Lactate dehydrogenase activity was determined with the CytoTox 96 assay (Promega). IL-1β, TNF, and IL12p40 secretion were determined by ELISA (R&D Systems). The proIL-1β release was controlled for as described (15).
Immunoblot.
BMMs in six-well plates were washed twice before infection in serum-free media for 1 h. Cells were lysed in 0.2 or 1% triton PBS and cleared by centrifugation. Culture media were precipitated with 10% TCA to collect exported caspase 1 protein. Cytosolic extract and TCA-precipitated proteins were combined, and caspase 1 was detected by immunoblot for p10 (Santa Cruz Biotechnology).
Protein Transfection.
Flagellin was purified from P. aeruginosa culture as described (15, 41). GST fusions were purified by using glutathione Sepharose. LPS contamination was undetectable by Limulus assay (Cambrex) after filtration through a 100-kDa filter (Chemicon). BMMs were stimulated with 50 ng/ml LPS for 3–5 h before protein transfection with Profect P1 as described (15) with the addition of a 15 × g 5-min centrifugation step before a 1-h incubation.
ACKNOWLEDGMENTS.
We thank Shizuo Akira (Osaka University, Osaka, Japan), Vishva Dixit (Genentech), and Richard Flavell (Yale University, New Haven, CT) for sharing mouse strains, and Colin Manoil (University of Washington) for sharing Pseudomonas mutant strains. This work was supported by National Institutes of Health Grants AI065878, AI052286, AI032972, and AI025032.
Footnotes
The authors declare no conflict of interest.
References
- 1.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann NY Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 3.Martinon F, Gaide O, Petrilli V, Mayor A, Tschopp J. NALP inflammasomes: A central role in innate immunity. Semin Immunopathol. 2007;29:213–229. doi: 10.1007/s00281-007-0079-y. [DOI] [PubMed] [Google Scholar]
- 4.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: Dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29:275–288. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 5.Poyet JL, et al. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 6.Diez E, et al. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat Genet. 2003;33:55–60. doi: 10.1038/ng1065. [DOI] [PubMed] [Google Scholar]
- 7.Wright EK, et al. Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr Biol. 2003;13:27–36. doi: 10.1016/s0960-9822(02)01359-3. [DOI] [PubMed] [Google Scholar]
- 8.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamboni DS, et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 10.Molofsky AB, et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damiano JS, Oliveira V, Welsh K, Reed JC. Heterotypic interactions among NACHT domains: Implications for regulation of innate immune responses. Biochem J. 2004;381:213–219. doi: 10.1042/BJ20031506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamkanfi M, et al. The Nod-like receptor family member Naip5/Birc1e restricts Legionella pneumophila growth independently of caspase-1 activation. J Immunol. 2007;178:8022–8027. doi: 10.4049/jimmunol.178.12.8022. [DOI] [PubMed] [Google Scholar]
- 13.Amer A, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 14.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in Salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 15.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 16.Sun YH, Rolan HG, Tsolis RM. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J Biol Chem. 2007;282:33897–33901. doi: 10.1074/jbc.C700181200. [DOI] [PubMed] [Google Scholar]
- 17.Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet. 2003;67:471–485. doi: 10.1046/j.1469-1809.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 18.Machen TE. Innate immune response in CF airway epithelia: Hyperinflammatory? Am J Physiol Cell Physiol. 2006;291:C218–C230. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- 19.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 20.Kurahashi K, et al. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest. 1999;104:743–750. doi: 10.1172/JCI7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feuillet V, et al. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci USA. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajjar AM, et al. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol. 2002;3:354–359. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 23.Andersen-Nissen E, et al. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci USA. 2005;102:9247–9252. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 26.Samatey FA, et al. Structure of the bacterial flagellar hook and implication for the molecular universal joint mechanism. Nature. 2004;431:1062–1068. doi: 10.1038/nature02997. [DOI] [PubMed] [Google Scholar]
- 27.Yonekura K, Maki-Yonekura S, Namba K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature. 2003;424:643–650. doi: 10.1038/nature01830. [DOI] [PubMed] [Google Scholar]
- 28.Dacheux D, et al. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect Immun. 2000;68:2916–2924. doi: 10.1128/iai.68.5.2916-2924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vance RE, Rietsch A, Mekalanos JJ. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun. 2005;73:1706–1713. doi: 10.1128/IAI.73.3.1706-1713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franchi L, et al. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 31.Galle M, et al. The Pseudomonas aeruginosa type III secretion system plays a dual role in the regulation of Caspase-1 mediated IL-1beta maturation. J Cell Mol Med. 2007 doi: 10.1111/j.1582-4934.2007.00190.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutterwala FS, et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahenthiralingam E, Campbell ME, Speert DP. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun. 1994;62:596–605. doi: 10.1128/iai.62.2.596-605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain M, et al. Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis. J Clin Microbiol. 2004;42:5229–5237. doi: 10.1128/JCM.42.11.5229-5237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun GW, Lu J, Pervaiz S, Cao WP, Gan YH. Caspase-1 dependent macrophage death induced by Burkholderia pseudomallei. Cell Microbiol. 2005;7:1447–1458. doi: 10.1111/j.1462-5822.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs MA, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 38.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC, Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 39.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 40.Uematsu S, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]