Abstract
Mast cells degranulate and release the contents of intracellular secretory granules in response to the cross-linking of FcεRI by multivalent antigens. These granules contain a variety of biologically active inflammatory mediators; however, it is not clear whether these granules are homogenous or whether there is heterogeneity within the secretory granule population in mast cells. By using genetically altered mice lacking specific vesicle-associated SNARE membrane fusion proteins, we found that VAMP-8-deficient mast cells exhibited defects in FcεRI-regulated exocytosis, whereas synaptobrevin 2- or VAMP-3-deficient mast cells did not. Surprisingly, the defect in secretion in VAMP-8-deficient mice was limited to the subpopulation of mast cell secretory granules containing serotonin and cathepsin D, whereas regulated exocytosis of secretory granules containing histamine and TNF-α was normal. Confocal microscopy confirmed that serotonin and histamine were present in distinct intracellular granules and that most serotonin-containing granules were VAMP-8-positive. Thus, this study demonstrates that mast cells do indeed possess distinct subsets of secretory granules and that these subsets use different SNARE isoforms for exocytosis.
Keywords: serotonin, histamine, VAMP-8
Mast cells are specialized secretory cells that play an important role in host defense by discharging a variety of proinflammatory mediators, chemotactic factors, and immunoregulatory cytokines (1, 2). After stimulation of their surface Fc receptors with IgE, mast cells release their large population of secretory granules containing histamine, serotonin, and other inflammatory mediators. These mediators play central roles in both the immediate and late-phase inflammatory reactions. In addition, these secreted products also regulate the acquired immune response by altering the polarization of T lymphocytes (3).
As in other hematopoietic cells, the secretory granules of mast cells possess a number of proteins present almost exclusively in lysosomes in nonhematopoietic cells, and for this reason, these organelles have been referred to as “secretory lysosomes” (4). For example, FcεRI stimulation induces the release of lysosomal hydrolases such as β-hexosaminidase and cathepsin D, in addition to the more conventional secretory granule cargo molecules serotonin and histamine (1). Furthermore, in addition to lysosome-like secretory granules, mast cells possess conventional lysosomes that can fuse with the plasma membrane when mast cells receive a strong calcium flux after addition of calcium ionophore or after extensive cross-linking of FcεRI (5). Despite the clear difference between lysosome-like secretory granules and conventional lysosomes, it is not known whether there is heterogeneity within the secretory granule population in mast cells.
Granule exocytosis in mast cells is controlled by membrane–membrane fusion proteins termed SNAREs. There are distinct SNARE proteins on different intracellular organelles in all cell types, and these proteins function by linking specific SNAREs on vesicles (termed vesicle SNAREs, or v-SNAREs) with cognate SNAREs on target membranes (termed t-SNAREs). SNAREs are a class of proteins that mediate membrane–membrane fusion in eukaryotic cells (6). Although there have been numerous studies examining the repertoire of granule-associated SNARE proteins present in mast cells (7–10), there have been no conclusive data revealing a role for specific v-SNARE isoforms in regulated exocytosis of secretory granules from these cells.
We now report the surprising finding that mast cells do indeed possess distinct subsets of secretory granules that can be defined based on the requirement for the vesicle fusion protein VAMP-8 for their release. Whereas histamine release from mast cells derived from VAMP-8-deficient mice is normal, we observed profound perturbations in the release of serotonin and cathepsin D from secretory lysosomes in these same mast cells. To our knowledge, these results reveal for the first time the presence of functionally distinct mast cell secretory granule subsets and indicate that VAMP-8 is a key regulator of serotonin and cathepsin D release from mast cells.
Results and Discussion
Characterization of Mast Cells in v-SNARE-Deficient Mice.
The liberation of all mast cell inflammatory mediators is regulated by signaling after aggregation of FcεRI by multivalent antigens (1, 2). For this reason, it has been difficult to know whether all mast cell secretory granules are the same or whether there is heterogeneity within the secretory granule population of mast cells. Furthermore, although the release of β-hexosaminidase is a widely used indicator of FcεRI-mediated secretory lysosome exocytosis from mast cells, it is unclear whether the release of this single granule marker accurately reflects the behavior of all secretory granules in mast cells. To determine the extent to which genetic deletion of specific SNARE proteins affects regulated exocytosis from mast cells, we analyzed mice deficient in the major granule-associated v-SNAREs present in these cells (7, 8). Mice deficient in synaptobrevin 2 (11) and VAMP-3 (12) have been described previously. We also obtained mice in which the VAMP-8 gene was disrupted via gene trapping approach [supplementary information (SI) Fig. 6]. Although VAMP-7 has been reported to be present on secretory granules of mast cells (7–10), mice deficient in this v-SNARE isoform are not available. Flow cytometry revealed that mast cells derived from synaptobrevin 2-deficient, VAMP-3-deficient, and VAMP-8-deficient mice expressed normal levels of FcεRI and c-kit on their surface (SI Fig. 7), grew at the same rate and possessed similar amounts of granule-associated β-hexosaminidase, serotonin, and histamine as their wild-type littermates (data not shown), confirming normal development of mast cells from each mouse strain.
Pancreatic acinar cells and platelets isolated from VAMP-8-deficient mice have slightly increased expression of the v-SNARE synaptobrevin 2 (13, 14). For this reason, we performed immunoblot analyses to determine whether compensatory changes in VAMP-expression occurred in mast cells derived from each knockout animal used in this study. As expected, the v-SNAREs synaptobrevin 2, VAMP-3, and VAMP-8 were absent in the respective null mouse-derived mast cells, and these proteins were expressed at half the wild-type levels in mast cells derived from the respective heterozygous mice (Fig. 1). Unlike results obtained in pancreas and in platelets (13–15), we did not observe dramatic compensatory increases in expression of the v-SNAREs synaptobrevin 2, VAMP-3, VAMP-7, and VAMP-8 in any of the mice used in our study.
Fig. 1.
Expression of v-SNAREs in synaptobrevin 2−/−, VAMP-3−/−, and VAMP-8−/− mast cells. Whole-cell lysates from 4 × 106 mast cells derived from synaptobrevin 2−/−, VAMP-3−/−, and VAMP-8−/− mice (and corresponding wild-type and heterozygous littermate controls) were analyzed by reducing SDS/PAGE and immunoblotting by using antibodies recognizing synaptobrevin 2, VAMP-3, VAMP-7, VAMP-8, SNAP-23, and β-tubulin.
VAMP-8, but Not Synaptobrevin 2 or VAMP-3, Is Important for Regulated Secretion from Mast Cells.
Because mast cells appeared phenotypically normal in v-SNARE-deficient mice, we examined the requirement for these proteins in regulated exocytosis from mast cells. For these initial studies, we measured regulated β-hexosaminidase release. There was no significant difference in β-hexosaminidase release from mast cells isolated from synaptobrevin 2- or VAMP-3-deficient mice in response to FcεRI cross-linking or direct stimulation with PMA/ionomycin (Fig. 2 A and B). Control experiments confirmed that PMA/ionomycin treatment caused significant release of β-hexosaminidase without affecting mast cell viability or plasma membrane integrity (SI Fig. 8). Mast cells generated from bone marrow of lethally irradiated mice reconstituted with fetal liver stem cells isolated from synaptobrevin 2-deficient embryos also showed normal exocytosis in response to FcεRI cross-linking (data not shown), demonstrating that mast cells generated from either bone marrow- or fetal liver-derived stem cells from synaptobrevin 2-deficient mice behaved identically. By contrast, FcεRI-dependent secretion of β-hexosaminidase was significantly inhibited in VAMP-8-deficient mast cells (Fig. 2A) and partial inhibition of β-hexosaminidase release was observed even when PMA/ionomycin (Fig. 2B) were used as triggers, demonstrating that bypassing FcεRI still resulted in diminished secretory granule exocytosis in VAMP-8-deficient mast cells. Basal release of β-hexosaminidase was not affected in VAMP-8-deficient mast cells and FcεRI-dependent exocytosis of β-hexosaminidase was inhibited in both VAMP-8 heterozygous and VAMP-8-deficient mast cells at all doses of antigen used to crosslink FcεRI (Fig. 2C). Kinetic studies showed that the partial inhibition of β-hexosaminidase was observed at all time points examined after FcεRI cross-linking (Fig. 2D). The defect in exocytosis in VAMP-8-deficient mast cells was not a consequence of defective FcεRI signaling, because protein tyrosine phosphorylation and FcεRI down-regulation in response the FcεRI cross-linking were normal in these mice (SI Fig. 9). These results demonstrate that whereas deletion of synaptobrevin 2 or VAMP-3 does not affect secretory granule exocytosis from mast cells, deletion of VAMP-8 leads to either (i) partial inhibition of exocytosis of all secretory granules or (ii) complete inhibition of exocytosis from a subset of secretory granules in mast cells.
Fig. 2.
Loss of VAMP-8, but not synaptobrevin 2 or VAMP-3, partially inhibits regulated β-hexosaminidase release from mast cells. (A and B) Mast cells derived from synaptobrevin 2−/−, VAMP-3−/−, and VAMP-8−/− mice (and corresponding wild-type littermate controls) were sensitized overnight with anti-DNP-specific IgE and induced for secretion the next day by cross-linking FcεRI by using 10 ng/ml DNP–BSA for 30 min (A) or by using PMA and ionomycin together for 15 min (B). The data shown are means ± SEM of at least five independent experiments. (C) Mast cells from VAMP-8+/+, VAMP-8+/−, and VAMP-8−/− mice were triggered for secretion by cross-linking FcεRI-associated IgE with different concentrations of DNP–BSA for 30 min. The data shown are means ± SEM of at least eight independent experiments. (D) Mast cells from VAMP-8+/+ or VAMP-8−/− mice sensitized overnight with anti-DNP-specific IgE were stimulated for various times by IgE cross-linking by using 10 ng/ml DNP–BSA. The release of β-hexosaminidase into the cell supernatant was assayed as described in Materials and Methods. The data shown are means ± SEM of two independent experiments. In all experiments shown, asterisks indicate statistically significant differences between wild-type and knockout mast cells (*, P < 0.01; **, P < 0.001).
Deletion of VAMP-8 Inhibits Serotonin and Cathepsin D Release from Mast Cells.
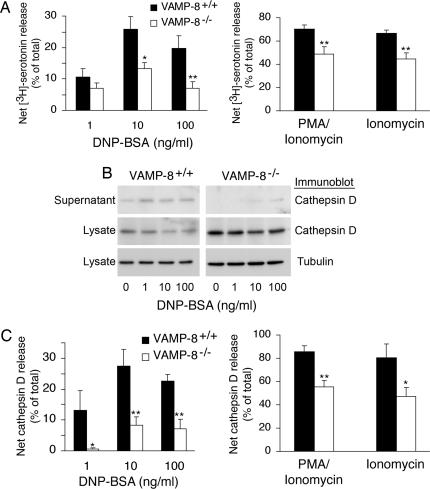
To determine whether release of β-hexosaminidase faithfully indicates exocytosis of all secretory granules in mast cells and whether only a subpopulation of secretory granules is affected in VAMP-8-deficient mast cells, we analyzed secretion of the secretory granule cargo serotonin from these cells. VAMP-8-deficient mast cells showed a partial inhibition in regulated secretion of [3H]serotonin in response to FcεRI cross-linking, PMA and ionomycin together, or ionomycin alone (Fig. 3A), and the extent of inhibition was similar to that observed when analyzing β-hexosaminidase release. In agreement with our results measuring β-hexosaminidase release, there was no defect in FcεRI-dependent exocytosis of serotonin from mast cells isolated from either synaptobrevin 2- or VAMP-3-deficient mice (data not shown). These data demonstrate that exocytosis of serotonin-containing secretory granules from mast cells is regulated, in part, by the v-SNARE VAMP-8.
Fig. 3.
VAMP-8 regulates serotonin and cathepsin D release from mast cells. (A) Mast cells from VAMP-8−/− mice (and their wild-type littermates) were incubated with [3H]serotonin overnight. The cells were then induced for secretion by IgE cross-linking by using the indicated concentrations of DNP–BSA for 30 min or by treatment with PMA/ionomycin or ionomycin alone for 15 min. The net [3H]serotonin secretion in each condition (triggered release minus control release) is shown. The data shown are means ± SEM of seven independent experiments. (B) Mast cells isolated from VAMP-8−/− mice (and their wild-type littermates) were induced for secretion by IgE cross-linking by using different doses of DNP–BSA for 30 min. The concentrated supernatants and lysates from 8 × 105 cells were analyzed by SDS/PAGE and immunoblotting by using cathepsin D antibodies. Lysates were also blotted for β-tubulin as a loading control. The shown blots are representative of at least three independent experiments. (C) The intensity of the bands corresponding to mature cathepsin D were quantitated by densitometry, and the amount of cathepsin D released after IgE cross-linking with the indicated concentrations of DNP–BSA for 30 min or after treatment of the cells with PMA/ionomycin or ionomycin alone for 15 min was expressed as a percentage of the total amount of cathepsin D present in the supernatant and cells. The data shown are means ± SEM of three independent experiments. In all experiments shown, asterisks indicate statistically significant differences between wild-type and VAMP-8-deficient mast cells (*, P < 0.05; **, P < 0.005).
In addition to monitoring β-hexosaminidase and serotonin release from mast cells, we measured the release of mature cathepsin D from mast cells after FcεRI stimulation. Like β-hexosaminidase, cathepsin D is present in lysosome-like secretory granules, as well as conventional lysosomes in mast cells (5). We observed a profound (> 80%) reduction of cathepsin D release in response to the FcεRI cross-linking in VAMP-8-deficient mast cells (Fig. 3 B and C). It is interesting to note that at the lowest dose of FcεRI cross-linking used in our studies, we observed the most profound inhibition of cathepsin D exocytosis in VAMP-8-deficient mast cells, suggesting that more robust signaling could partially bypass the requirement for VAMP-8 in secretory granule exocytosis.
Because strong calcium signals are known to mobilize both secretory granules and conventional lysosomes in a variety of cell types (16), we set out to examine whether all cathepsin D release from mast cells utilizes a VAMP-8-dependent mechanism or whether only FcεRI-stimulated secretory granule release was affected. Stimulation with PMA and ionomycin together or ionomycin alone resulted in almost complete release of cathepsin D from wild-type mast cells, and deletion of VAMP-8 resulted in a partial (albeit statistically significant) inhibition of exocytosis even in response to these strong stimuli (Fig. 3C). Curiously, ≈30% of the total pool of cathepsin D was retained intracellularly after ionomycin stimulation of VAMP-8-deficient mast cells. This value was similar to amount of cathepsin D released from the secretory granules of wild-type mast cells stimulated by FcεRI cross-linking, suggesting that VAMP-8 only regulates lysosome-like secretory granule exocytosis and does not play a role in ionomycin-stimulated conventional lysosome release from mast cells.
Deletion of VAMP-8 Does Not Affect Histamine or TNF-α Release from Mast Cells.
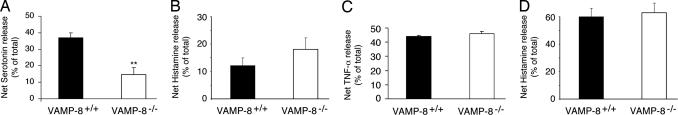
Having observed that FcεRI-induced secretion of serotonin was inhibited in VAMP-8-deficient mast cells, we set out to determine whether regulated exocytosis of other secretory granule cargo was also defective in VAMP-8-deficient mast cells. In marked contrast to the results obtained by examining the FcεRI-mediated release of either [3H]serotonin (Fig. 3A) or endogenous serotonin (Fig. 4A) from wild-type and VAMP-8-deficient mast cells, we were surprised to find that there was no defect in the release of either histamine (Fig. 4B) or TNF-α (Fig. 4C) in these same supernatants. It can also be noted that the percentage of release of histamine after 30 min of stimulation was considerably less than the release of serotonin or TNF-α, a finding that has been observed by others and attributed to the decreased ability of histamine to dissociate from the proteoglycan core of mast cell secretory granules (17). Nevertheless, even bypassing FcεRI-signaling by directly stimulating mast cells with PMA and ionomycin showed that histamine release is VAMP-8-independent (Fig. 4D). These unexpected findings demonstrate that histamine/TNF-α and serotonin are present in distinct secretory granule subtypes in mast cells and that exocytosis of these distinct granules is regulated by distinct SNAREs. Whether histamine and TNF-α are present in the same granules or distinct granules remains to be determined.
Fig. 4.
Loss of VAMP-8 does not affect histamine and TNF-α release from mast cells. Mast cells isolated from VAMP-8−/− mice (and their wild-type littermates) were induced for secretion by IgE cross-linking by using 10 ng/ml DNP–BSA for 30 min (A–C) or with PMA/ionomycin for 15 min (D). The release of endogenous serotonin (A), histamine (B and D), or TNF-α (C) was determined and was expressed as a percentage of the total amount present in the supernatant as well as remaining in the cells. The data shown are means ± SEM of nine independent experiments (serotonin), five independent experiments (histamine), or three independent experiments (TNF-α). Asterisks indicate statistically significant differences between wild-type and VAMP-8-deficient mast cells (**, P < 0.005).
VAMP-8 Is Present on Serotonin-Containing Lysosome-Like Secretory Granules.
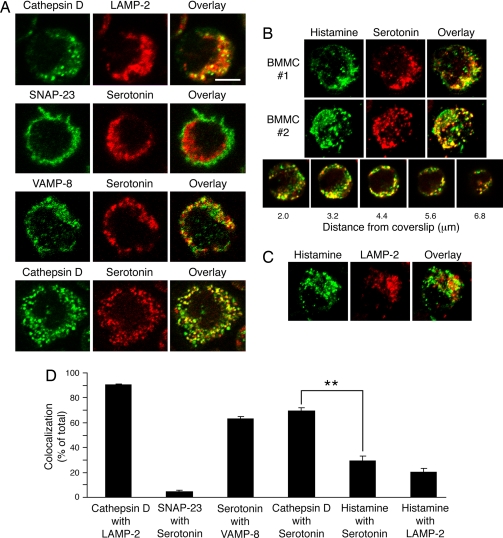
The different effects on serotonin and histamine exocytosis from VAMP-8-deficient mast cells could be explained if VAMP-8 were present on serotonin-containing secretory granules and if histamine were not present on these same granules. Confocal microscopy and digital image analysis of individual confocal planes from >20 individual cells revealed the percentage of colocalization between various markers of secretory granules, lysosomes, and the plasma membrane in mast cells (Fig. 5). Cathepsin D colocalized very well with the lysosome-associated membrane protein LAMP-2 (90.4 ± 0.8%), whereas the plasma membrane SNARE SNAP-23 colocalized poorly with the secretory granule-associated cargo serotonin (4.7 ± 0.9%). Serotonin colocalized with VAMP-8 (62.8 ± 2.4%) and with cathepsin D (69.5 ± 2.3%), indicating that most serotonin-containing secretory granules are VAMP-8+ and contain the lysosomal enzyme cathepsin D (Fig. 5 A and D). Examination of multiple cells by using quantitative analyses of a variety of focal planes of cells stained with anti-histamine, anti-serotonin, and anti-LAMP-2 antibodies revealed little colocalization of histamine with either serotonin (29.1 ± 4.3%) or LAMP-2 (20.6 ± 2.6), indicating that most histamine-containing secretory granules in mast cells do not contain serotonin and are not lysosomal in nature (Fig. 5 B–D). These data are consistent with our finding that VAMP-8 regulates release of serotonin but not histamine from secretory granules of mast cells and support the hypothesis that mast cells possess distinct secretory granules that possess histamine but not serotonin (and VAMP-8).
Fig. 5.
Histamine and serotonin are present in distinct secretory granule subsets in mast cells. Mast cells isolated from wild-type mice were stained with the indicated antibodies and analyzed by confocal immunofluorescence microscopy. (A) Single 0.4-μm-thick optical sections of representative images are shown. (Scale bar: ≈6 μm.) (B) Three-dimensional reconstruction of serial sections from two representative mast cells stained with histamine (green) and serotonin (red) antibodies and an overlay (yellow) are shown. An overlay of individual serial sections of a mast cell stained with histamine (green) and serotonin (red) antibodies reveals heterogeneity within the secretory granule population. (C) Three-dimensional reconstruction of serial sections from a representative mast cell stained with histamine (green) and LAMP-2 (red) antibodies and an overlay (yellow) are shown. (D) The percentage of colocalization was calculated for each combination of markers by voxel analysis of each confocal plane of >20 cells. There was a statistically significant difference in the colocalization of cathepsin D with serotonin as compared with colocalization of histamine with serotonin (**, P < 0.001).
Mast cells are major regulators of allergy and inflammation whose soluble secretory granule contents mediate most of their functions (1, 2). Because the secretory granules of mast cells and other hematopoietic cells contain a variety of lysosomal enzymes, many investigators have conveniently relied on the release of enzymes such as β-hexosaminidase as an indicator of regulated exocytosis from mast cells. The underlying assumption in such an approach is that there is not significant functional heterogeneity in the secretory granule population in these cells and that β-hexosaminidase release accurately reflects the behavior of all FcεRI-dependent secretory granules in mast cells. In this study, we demonstrate that this assumption is incorrect and show that mast cells do indeed possess distinct subsets of secretory granules that can be defined based on the requirement of the v-SNARE VAMP-8 in exocytosis. We found that deletion of VAMP-8 profoundly inhibited FcεRI-mediated exocytosis of serotonin and cathepsin D from mast cells but had no effect on either histamine or TNF-α release. In addition, VAMP-8 does not appear to play a significant role in ionomycin-induced exocytosis of conventional cathepsin D+ lysosomes from mast cells. For technical reasons (because of the requirement for different fixation conditions for anti-histamine and anti-VAMP-8 immunoreactivity), we were unable to examine the colocalization of histamine and VAMP-8 in mast cells. Nevertheless, our functional data demonstrate that histamine-containing secretory granule exocytosis is not VAMP-8-dependent, suggesting that either VAMP-8 is not present on histamine-granules or that additional SNARE isoforms can compensate for the lack of VAMP-8 during exocytosis of these granules. Subcellular fractionation data have shown that histamine-containing secretory granules cofractionate with granules that contain β-hexosaminidase (but not cathepsin D) in the RBL mast cell line (5). Our finding that FcεRI-dependent exocytosis of histamine, but not β-hexosaminidase, is normal in VAMP-8-deficient mast cells argues that the subcellular fractionation data show that histamine-containing secretory granules have a buoyant density that is similar to that of β-hexosaminidase-containing lysosome-like secretory granules. Characterizing the nature of these granules will be important to help understand the mechanisms regulating histamine release from mast cells.
Our study demonstrates that mast cells must now be added to the list of hematopoietic cells that possess distinct secretory granule subsets, each with different cargo, different functions, and in some cases distinct SNARE proteins regulating their fusion with the plasma membrane. Neutrophils possess secondary/specific granules and gelatinase-rich tertiary granules, and exocytosis of these granules is inhibited by interfering with VAMP-2 function (18). By contrast, inhibition of VAMP-7 prevents azurophilic granule release from these cells, whereas inhibition of VAMP-2 does not affect this granule subset (18). However, the differential regulation of discrete secretory granules by distinct SNAREs is not universally true, because VAMP-8 regulates release of all three major secretory granule subtypes in platelets (14). Clearly, this must be addressed on a cell type-specific basis, although our findings are consistent with the hypothesis that, like neutrophils, mast cells also possess functionally distinct secretory granule subsets whose function is regulated by different SNARE proteins. Knowing that such granule heterogeneity exists in mast cells will allow us to investigate the machinery regulating the fusion of each distinct granule subtype, potentially allowing one to specifically target one granule subtype in these cells in an attempt to regulate the side-effects of allergic reactions.
Materials and Methods
Antibodies.
The synaptobrevin 2 mAb clone 69.1 and VAMP-8 rabbit serum were obtained from Synaptic Systems. VAMP-3 rabbit antiserum was from Abcam. The VAMP-7 mAb clone 158.2 was a generous gift from Thierry Galli (Institut National de la Santé et de la Recherche Médicale, Paris). Rabbit antiserum recognizing the SNAP-23 carboxyl terminus has been described (19). The β-tubulin mAb was obtained from Sigma Chemical Co.. Goat anti-cathepsin D antibody C20 (Santa Cruz Biotechnology) was used for immunoblotting, and rabbit anti-cathepsin D serum was used for immunofluorescence (a gift from Stuart Kornfeld, Washington University, St. Louis). Rabbit anti-histamine serum was obtained from Immunostar, Inc. Rat mAb recognizing LAMP-2 was obtained from the Developmental Studies Hybridoma Bank. Anti-serotonin mAb was obtained from DAKO. Anti-2,4-dinitrophenyl (DNP) IgE (clone TIB-142) was obtained from the American Type Culture Collection. Alexa dye-conjugated secondary antibodies were obtained from Molecular Probes.
Mice and Genotyping.
Synaptobrevin 2+/− mice, on a C57BL/6 background (11), were from Thomas Sudhof (University of Texas Southwestern Medical Center, Dallas, TX). VAMP-3+/− mice, on a mixed C57BL/6–129Sv background (12), were from Dr. Jeffery Pessin (State University of New York, Stony Brook, NY). VAMP-8+/− mice, on a mixed C57BL/6–129Sv background, were obtained from The Jackson Laboratory. Because VAMP-8−/− mice have not been used in the literature, complete characterization of these mice is described in SI Text. To lessen the effects of the genetic backgrounds, all mice were obtained by heterozygous mouse matings, and littermate controls were used for all experiments.
Generation of Mast Cells.
Femurs and tibias were isolated aseptically from 8- to 12-week-old VAMP-8−/− and VAMP-3−/− mice (and their littermates), and bone marrow cells were isolated by repeated flushing with mast cell medium (RPMI medium 1640, 20% FBS, 4 mM l-glutamine, 5 × 10−5 M β-mercaptoethanol, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and antibiotics). The cultures were maintained in mast cell medium at 37°C (5% CO2) in the presence of murine IL-3 (20 ng/ml; Peprotech) and murine stem cell factor (SCF) (20 ng/ml; Peprotech), with adherent cells being discarded. After 4 weeks, the cultures were >90% positive for both c-kit and FcεRI, as determined by flow cytometry.
Mast cells were generated from stem cells derived from spleen and liver of 18-day embryos isolated from synaptobrevin 2+/− matings. The spleen and liver cells were cultured at 1.0 × 106 per milliliter in complete medium [DMEM containing 20% FBS, 4 mM l-glutamine, 5 × 10−5 M β-mercaptoethanol, 10% NCTC 109 medium (Sigma Chemical Co.), 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, and antibiotics]. The medium was supplemented with 50 ng/ml IL-3 and 50 ng/ml SCF, and the cells were cultured as described above.
Stimulation of Mast Cells.
Mast cell exocytosis was triggered by using 1 μM ionomycin alone, 10 nM PMA and 1 μM ionomycin together, or by cross-linking FcεRI as described previously (20). Aliquots of the culture supernatant were saved at the indicated times after induction of exocytosis, and at the end of the assay, the cells were lysed by using 1% Triton X-100 in RPMI medium 1640. Release of β-hexosaminidase was determined by using an enzymatic assay (20); release of serotonin (Fitzgerald Industries), histamine (Immunotech), and TNF-α (R&D Systems) was measured by ELISA. In some experiments, mast cells were incubated with 1 μCi/ml [3H]serotonin (New England Nuclear) overnight, washed, and incubated for 1 h at 37°C before inducing them for secretion as above. Cell-associated and -released [3H]serotonin was measured in a scintillation counter. Cathepsin D was assayed by concentrating culture supernatants by using Microcon centrifugal filter devices (10-kDa cutoff; Millipore) and analyzing concentrated supernatants and remaining cell extracts by SDS/PAGE and immunoblotting with an anti-cathepsin D serum. The amount of the fully processed (43-kDa) form of mature cathepsin D present in cells or released into the medium was determined by densitometry. In all experiments, the amount of release was expressed as a percentage of the total amount of each marker secreted into the medium as compared with the total amount of each marker secreted into the medium plus that remaining cell associated. The significance of any differences was calculated by using a one-tailed distribution in a two-sample equal variance Student's t test.
SDS/PAGE and Immunoblotting.
Immunoblot analysis of cell lysates, concentrated culture supernatants, and lysates from stimulated cells was performed as described previously (9). The bands were revealed with Western Lightening Chemiluminescence Reagent Plus (PerkinElmer Life Sciences Inc.). The intensity of the bands was determined by using a Molecular Dynamics densitometer, and the results obtained by at least three exposures of identical blots were averaged. The significance of any differences was calculated by using a one-tailed distribution in a two-sample equal variance Student's t test.
Confocal Microscopy.
Mast cells were allowed to adhere to poly(l-lysine)-coated coverslips and fixed with 4% paraformaldehyde in PBS. For examination of histamine distribution, mast cells were fixed by using 40 mg/ml 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide HCl (EDC) (Pierce Biotechnology, Inc.) before addition of PFA (Sigma). The cells were then permeabilized in PBS containing either 1% Nonidet P-40/0.05% saponin/3% normal goat serum or 0.1% saponin/3% normal goat serum (for cathepsin D), stained with the appropriate primary and secondary antibodies, and analyzed by confocal microscopy (9). Confocal images were collected with a Zeiss 510 META laser-scanning microscope with a ×100 Plan-Apochromat (N.A. 1.4) lens, 100 nm per pixel xy sampling, and a pinhole diameter set to provide an optical slice thickness of 1.0 μm. Image z-stacks were collected through the depth of the cell by using a 0.4-μm step size. The extent of colocalization of two labels was measured by using the Colocalization module of Imaris 4.2.0 (Bitplane AG), as described in SI Text.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dave Segal, Steve Shaw, and Reuben Siraganian for critical reading of the manuscript and helpful discussions. We are grateful to Sidney Whiteheart (University of Kentucky College of Medicine, Lexington, KY) for providing us with synaptobrevin 2+/− and VAMP-3+/− mice and Thierry Galli and Stuart Kornfeld for the generous gift of antibodies. We also thank Mike Kruhlak of the Experimental Immunology Branch Microscopy Facility for help with image analysis. This work was supported by the Intramural Program of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707854105/DC1.
References
- 1.Metcalfe DD, Baram D, Mekori YA. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ, Nakae S, Tsai M. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 3.Mazzoni A, Siraganian RP, Leifer CA, Segal DM. J Immunol. 2006;177:3577–3581. doi: 10.4049/jimmunol.177.6.3577. [DOI] [PubMed] [Google Scholar]
- 4.Blott EJ, Griffiths GM. Nat Rev Mol Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- 5.Baram D, Adachi R, Medalia O, Tuvim M, Dickey BF, Mekori YA, Sagi-Eisenberg R. J Exp Med. 1999;189:1649–1658. doi: 10.1084/jem.189.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jahn R, Sudhof TC. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 7.Guo Z, Turner C, Castle D. Cell. 1998;94:537–548. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- 8.Paumet F, Le Mao J, Martin S, Galli T, David B, Blank U, Roa M. J Immunol. 2000;164:5850–5857. doi: 10.4049/jimmunol.164.11.5850. [DOI] [PubMed] [Google Scholar]
- 9.Puri N, Kruhlak MJ, Whiteheart SW, Roche PA. J Immunol. 2003;171:5345–5352. doi: 10.4049/jimmunol.171.10.5345. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Verdeaux S, Pombo I, Iannascoli B, Roa M, Varin-Blank N, Rivera J, Blank U. J Cell Sci. 2003;116:325–334. doi: 10.1242/jcs.00216. [DOI] [PubMed] [Google Scholar]
- 11.Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 12.Yang C, Mora S, Ryder JW, Coker KJ, Hansen P, Allen LA, Pessin JE. Mol Cell Biol. 2001;21:1573–1580. doi: 10.1128/MCB.21.5.1573-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CC, Ng CP, Lu L, Atlashkin V, Zhang W, Seet LF, Hong W. Dev Cell. 2004;7:359–371. doi: 10.1016/j.devcel.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Ren Q, Barber HK, Crawford GL, Karim ZA, Zhao C, Choi W, Wang CC, Hong W, Whiteheart SW. Mol Biol Cell. 2007;18:24–33. doi: 10.1091/mbc.E06-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schraw TD, Rutledge TW, Crawford GL, Bernstein AM, Kalen AL, Pessin JE, Whiteheart SW. Blood. 2003;102:1716–1722. doi: 10.1182/blood-2003-01-0331. [DOI] [PubMed] [Google Scholar]
- 16.Andrews NW. Trends Cell Biol. 2000;10:316–321. doi: 10.1016/s0962-8924(00)01794-3. [DOI] [PubMed] [Google Scholar]
- 17.Travis ER, Wang YM, Michael DJ, Caron MG, Wightman RM. Proc Natl Acad Sci USA. 2000;97:162–167. doi: 10.1073/pnas.97.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mollinedo F, Calafat J, Janssen H, Martin-Martin B, Canchado J, Nabokina SM, Gajate C. J Immunol. 2006;177:2831–2841. doi: 10.4049/jimmunol.177.5.2831. [DOI] [PubMed] [Google Scholar]
- 19.Low SH, Roche PA, Anderson HA, van Ijzendoorn SC, Zhang M, Mostov KE, Weimbs T. J Biol Chem. 1998;273:3422–3430. doi: 10.1074/jbc.273.6.3422. [DOI] [PubMed] [Google Scholar]
- 20.Vaidyanathan VV, Puri N, Roche PA. J Biol Chem. 2001;276:25101–25106. doi: 10.1074/jbc.M103536200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.