Abstract
Digitoxin and other cardiac glycosides are important, centuries-old drugs for treating congestive heart failure. However, the mechanism of action of these compounds is still being elucidated. Calcium is known to potentiate the toxicity of these drugs, and we have hypothesized that digitoxin might mediate calcium entry into cells. We report here that digitoxin molecules mediate calcium entry into intact cells. Multimers of digitoxin molecules also are able to form calcium channels in pure planar phospholipid bilayers. These digitoxin channels are blocked by Al3+ and La3+ but not by Mg2+ or the classical l-type calcium channel blocker, nitrendipine. In bilayers, we find that the chemistry of the lipid affects the kinetics of the digitoxin channel activity, but not the cation selectivity. Antibodies against digitoxin promptly neutralize digitoxin channels in both cells and bilayers. We propose that these digitoxin calcium channels may be part of the mechanism by which digitoxin and other active cardiac glycosides, such as digoxin, exert system-wide actions at and above the therapeutic concentration range.
Keywords: cardiac glycoside, cytotoxicity
Despite long experience with cardiac glycosides, the exact mechanism of action for these drugs has remained an enigma. Operationally, the observed action of these drugs on the failing myocardium is to cause an increase in intracellular calcium, resulting in more efficient contraction (1, 2). Because toxic concentrations of these drugs block NaKATPase activity, it has been hypothesized that the enzyme may be partially blocked even at the lower drug concentrations found in the therapeutic range (3). A consequence of this slight depolarization process is that voltage-dependent calcium channels may open, thereby permitting calcium entry into the cell. However, this reasoning has been challenged by the fact that nitrendipine, a classical voltage-dependent calcium channel blocker, fails to reverse cardiac glycoside-induced side effects in healthy human volunteers (4). An alternative mechanism has been a slight increase in intracellular Na, followed by exchange for extracellular Ca2+ by the NCX sodium/calcium exchanger (5). However, very high concentrations of cardiac glycosides are needed to clearly observe NCX activation. Finally, at the upper end of the therapeutic concentration window, digitoxin toxicity is potentiated by elevations in circulating calcium concentrations (6). However, the mechanism by which calcium potentiates cardiac glycoside toxicity is not known either.
A further puzzling aspect of the NaKATPase rationale is the fact that, although the complex between cardiac glycosides and NaKATPase is virtually irreversible (7), toxicity in humans can be reversed in minutes by i.v. administration of Digibind (8–10). Digibind is an inactivating Fab fragment antibody against the digitalis pharmacophore, which is widely available in all poison control centers. In fact, the NaKATPase hypothesis for the regulation of positive inotropic effects of cardiac glycosides has been under continuous questioning by investigators for many years without compelling resolution (11–16). Non-NaKATPase effects also have been observed. For example, quite low subnanomolar concentrations of the cardiac glycoside digitoxin also are able to block TNF-α/NF-κB signaling (17, 18). Thus, the mechanism for calcium-specific actions of digitoxin and other medicinal cardiac glycosides remains to be fully understood.
Our approach to this problem has been to build on previous studies by ourselves and others showing that a variety of small molecules are able to form multimeric, transmembrane ion-conducting channels (19–25). On this basis, we hypothesized that digitoxin also might mediate calcium entry into cells by forming ion channels. Experiments to test this hypothesis are described in the present article.
Results
Digitoxin Induces Ca2+ Uptake into Cells.
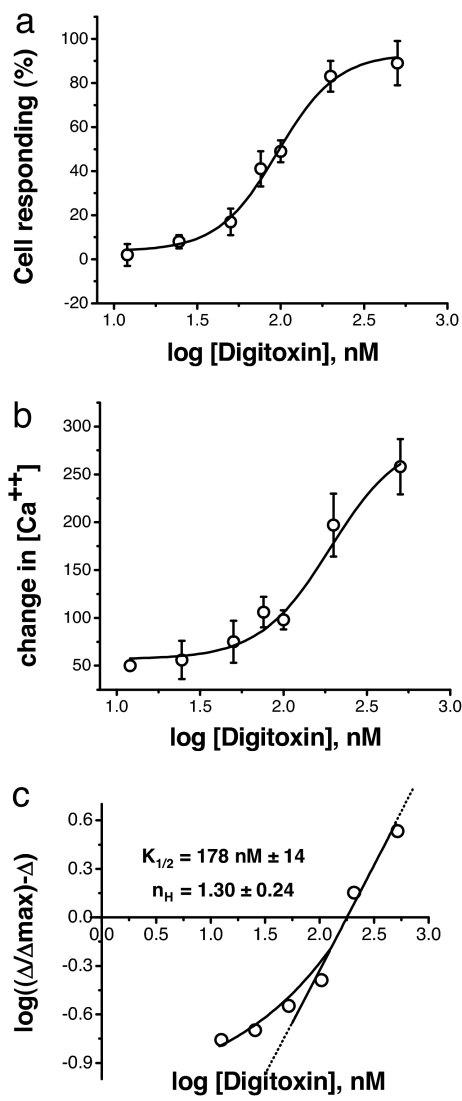
As shown in Fig. 1a, the addition of digitoxin to cells results in a dose-dependent increase in the number of cells responding with changes in intracellular calcium concentration. These data were derived from averaging three independent experiments featuring 50–80 cells each. The criterion for identifying a cell as responsive was taken to be a change of at least 25 nM from the baseline value. In parallel, we also measured the exact changes in intracellular calcium concentration as a function of digitoxin concentration in the extracellular medium. Fig. 1b shows the peak change in intracellular calcium as a function of digitoxin concentration. A Hill plot based on these data (see Fig. 1c) indicates that the k1/2,app. is 178 nM, with a Hill coefficient nH of 1.3. Based on a conventional cooperativity interpretation of the Hill coefficient, more than one digitoxin molecule may thus be responsible for the calcium uptake process. In addition, the sigmoid shape of the uptake curve also supports the concept that a multimer of digitoxin molecules may be responsible for calcium uptake from the medium into cells.
Fig. 1.
Digitoxin induces cells to take up calcium. (a) Fraction of cells taking up calcium as a function of digitoxin concentration. GT1–7 cells were grown to near confluence and loaded with the calcium-sensitive probe FURA-2 a.m. Groups of 50–80 cells were optically marked, and cells responding to digitoxin were enumerated. The data shown are the averages of a series of experiments performed on three separate cultures, indicating that ≈90% of the cells can be induced to take up calcium. The solvent, 0.01% ETOH, was without effect on any of the parameters. A change of at least 25 nM calcium was taken to be a response. (b) Change in peak intracellular calcium concentration as a function of digitoxin concentration. The changes in calcium concentration were measured over time (see the example in SI Fig. 5 in SI Appendix). The peak values for changes in calcium are plotted here as a function of extracellular calcium concentration. The data are representative of three independent experiments. (c) Hill plot for analysis of change in peak intracellular calcium concentration as a function of digitoxin concentration. The data from b were used for Hill plot analysis. The value of Δmax was estimated analytically from an inverse plot of the data and used to calculate the Hill plot term (nH) on the vertical axis. The digitoxin values are k1/2,app = 178 ± 14 nM and nH = 1.3 ± 0.2.
Above a threshold of ca. 40 nM, most of the responsive cells begin uptake after a lag of only a few seconds [see supporing information (SI) Fig. 5a in SI Appendix]. Furthermore, based on an EGTA experiment, the source of the calcium increment appears to be extracellular (see SI Fig. 5b in SI Appendix). In addition, as shown in SI Fig. 5c in SI Appendix, digitoxin-induced calcium uptake is blocked by a monoclonal antibody against digitoxin. These latter data suggest that the digitoxin channel is accessible from the outside of the cell and that functional digitoxin channels are not internalized, but remain in the plasma membrane.
Digitoxin Compromises Cell Survival.
The acute effects of digitoxin on calcium uptake suggested that longer term experiments might elicit evidence of a cytotoxic effect. To test this hypothesis, we incubated cells for 3 days with different concentrations of digitoxin. On the third day, we measured the release of lactic dehydrogenase (LDH), a soluble cytosolic enzyme (see SI Fig. 6 in SI Appendix). Simultaneously, we measured the remaining mitochondrial function by XTT (see SI Fig. 6 Inset in SI Appendix). Digitoxin is known to bind to serum proteins, and the inclusion of serum in the culture medium slightly reduced potency. Thus, the effects of digitoxin on cell survival appear to have become significant above ca. 60 nM. Relevantly, this concentration is well into the toxic range (namely, >40 nM) for humans treated chronically with digitoxin (see also a calculation from the primary literature in SI Fig. 5 in SI Appendix) (2).
Digitoxin Is Sensitive to Phosphatidylserine (PS) on the Cell Surface.
An important question is what digitoxin might be interacting with or responding to on the plasma membrane of the target cell. As shown in SI Fig. 7a in SI Appendix, the potency of digitoxin cytotoxicity is optimal when the target cell expresses PS on the extracellular leaflet of the plasma membrane. Using the mitochondrial XTT assay as an endpoint for cytotoxicity, SI Fig. 7b in SI Appendix shows that PS-positive cells are statistically more sensitive to 10 nM, 100 nM, and 500 nM digitoxin, respectively, than PS-negative cells. The control culture, consisting of both PS-negative and PS-positive cells, appears to represent contributions from both cell types and is statistically more sensitive than the PS-negative culture. SI Fig. 7c SI Appendix shows data supporting similar conclusions using % LDH release as the criterion for cytotoxicity. LDH release is a measure of membrane integrity. Thus, both types of assays support the conclusion that externally oriented PS enhances digitoxin-dependent cytotoxicity.
Digitoxin Forms Calcium Channels in Planar Lipid Bilayers.
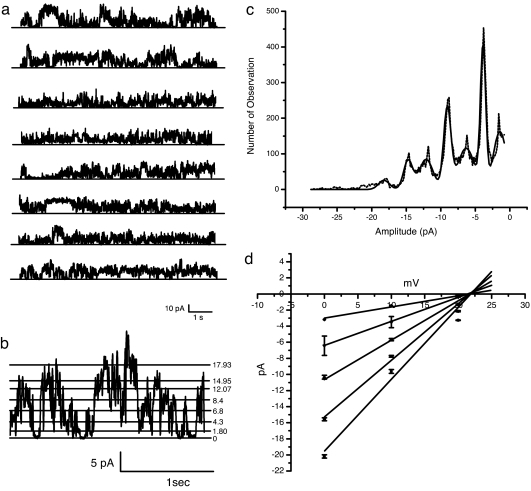
The rapidity by which digitoxin initiated calcium uptake into cells suggested the possibility that digitoxin might play a direct role in the transport process. To test this hypothesis directly, we added digitoxin to pure planar lipid bilayers. These bilayers were enriched in the acidic phospholipid PS [namely, palmitoyloleoyl PS (POPS)/palmitoyloleoyl phosphatidylethanolamine (POPE), 1:1]. By convention, Cs+ was used for most channel experiments as a faithful surrogate for Ca2+. Furthermore, we initially imposed a chemical gradient of CsCl on the system to avoid the complication of an electrical driving force. Fig. 2a shows a stable, continuous record of digitoxin-induced channel activity. The activity exhibited rapid transitions between levels and was uniformly maintained for very long time periods. We also observed that when digitoxin was added to the chamber, current activity was detected after a concentration-dependent delay (data not shown). We conjecture that this delay is related to the time it takes for digitoxin molecules to find their way to the membrane and assemble into a channel structure. To determine the charge of the conducted ion, we prepared a current–voltage (I–V) curve to estimate the reversal potential. As shown in Fig. 2b, we were able to expand portions of the digitoxin current trace and to identify individual levels. From large amounts of current data, we prepared a complete analysis of the frequency of different amplitudes (see Fig. 2c). Finally, we replicated this analysis at three different voltages. As shown in Fig. 2d, graphical analysis reveals a common reversal potential of ca. +24 mV. This potential closely compares with a reversal potential of +35 mV, which can be calculated from the Nernst equation assuming a perfectly selective cationic channel.
Fig. 2.
Digitoxin forms ion-conducting channels in acidic phospholipid planar lipid bilayers. (a) Electrical activity generated by the flow of ions through digitoxin channels formed in a POPS/POPE planar lipid bilayer. A single continuous record of an ionic current of nearly 60 s is shown. No electrical potential difference was imposed. The membrane separates two chambers with asymmetric contents. One chamber contained 200 mM CsCl, whereas the other contained 50 mM CsCl. Digitoxin was directly added to the chamber containing 200 mM CsCl, to a final concentration of 43 nM. (b) Multiple conductance states of digitoxin in a POPS/POPE planar lipid bilayer. Two seconds of electrical recording from the experiment described in a illustrate the presence of different single-channel current levels. The current amplitudes are identified by numbers next to each of the calculated stable current values. The bilayer electrical potential is zero. An asymmetric CsCl gradient (200/50 mM) is the sole driving force. (c) Amplitude histogram of digitoxin channels in a POPS/POPE planar lipid bilayer. Conductance events, collected over a 2-s period, were fit to a multi-Gaussian function and analyzed as a current–amplitude histogram. Marquardt least squares fitting of this histogram indicated that the current values distributed mainly into seven Gaussians. (d) I–V curve for digitoxin channel in POPS/POPE planar lipid bilayer. The current–events amplitudes from the Gaussian-fitted histograms were plotted for different bilayer potential values. The slopes from the linear regression lines through the data estimate the main subconductance levels to be 150.20 ± 0.01, 305.300 ± 0.007, 450.000 ± 0.009, 672.00 ± 0.06, and 845.00 ± 0.12 pS. The line intercepts generate an average equilibrium potential of 23.13 ± 1.51 mV.
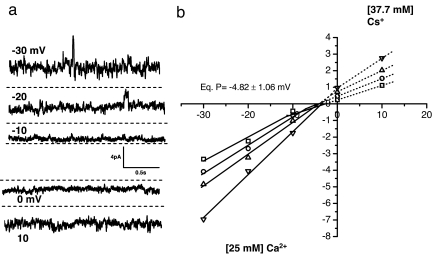
To directly test the permeability of the digitoxin channel for calcium, we made a direct comparison of Cs+ and Ca2+ conductance (see Fig. 3 and Materials and Methods). The trans side of the chamber contains 25 mM CaCl2, and the cis side contained 37.5 mM CsCl. Therefore, the traces showing upward-going currents, driven by membrane potentials of 10, 20, and 30 mV, respectively, show calcium ions moving from trans to cis across the digitoxin channels. Under these biionic conditions, the equilibrium potential is approximately −4 mV. From this potential, we calculate that the permeability ratio PCa/PCs is ≈0.6 (19, 26). Thus, the data strongly support the hypothesis that digitoxin plays a direct role in calcium transport processes.
Fig. 3.
Digitoxin channels conduct calcium ions. (a) Traces of digitoxin channel activity in POPE/POPS planar lipid bilayer under biionic conditions. The trans side of the chamber contains 25 mM CaCl2, and the cis side contains 37.5 mM CsCl. Therefore, the traces showing upward-going currents, driven by membrane potentials of 10, 20, and 30 mV, show calcium ions moving from trans to cis across the digitoxin channels. At zero membrane potential, the current is generated by the movement of cesium ions. (b) I–V curve for the digitoxin channel under biionic conditions. The four major currents peaks obtained from the current–amplitude histogram are fitted to a multi-Gaussian function and plotted at different membrane potentials. Under these conditions, a graphical analysis reveals a common reversal potential of approximately −4 mV, and the permeability ratio (PCa/PCs) is computed to be ≈0.6 (19, 26).
Digitoxin Channel Kinetics Are Influenced by the Phospholipid Head Group.
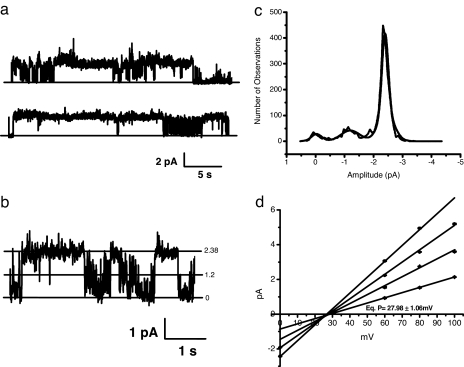
To test whether the choice of phospholipid might influence the kinetics or selectivity of the digitoxin channel, we examined the behavior of digitoxin channels in membranes enriched in the neutral phosphatidylcholine (PC). As shown in Fig. 4, the digitoxin channels enriched in PC (Fig. 4a) exhibit open and closed states that are longer lived than those in PS and exhibit amplitudes that are approximately 5-fold less than of those observed in PS. A direct comparison can be made with equivalent PS data in Fig. 2. As shown in Fig. 4b, the difference in kinetics can be readily observed on an expanded time scale. Fig. 4c shows that there is preferred amplitude in PC, although other conductances can be readily detected. Finally, the I–V curve in Fig. 4d reveals a reversal potential of +27 mV. This value is somewhat closer to the calculated reversal potential of +35 mV than that calculated in POPS membranes. Thus, although the ion selectivity of the digitoxin channel is similar in either PS or PC, the kinetics are significantly different. The importance of this distinction between high levels of activity in PS membranes and significantly lower levels of activity in PC membranes may be relevant to the fact that, in vivo, proapoptotic cells preferentially express PS on their outer leaflets.
Fig. 4.
Digitoxin forms ion-conducting channels in neutral phospholipid planar lipid bilayers. (a) Digitoxin channels formed in a neutral planar lipid bilayer. A single continuous record of ionic current of >60 s is shown. The planar lipid bilayer was prepared from a 1:1 mixture of POPE and POPC. No electrical potential difference was imposed. The membrane separates two chambers with asymmetric contents. One chamber contained 200 mM CsCl, whereas the other contained 50 mM CsCl. Digitoxin was directly added to the chamber containing 200 mM CsCl to a final concentration of 42 nM. The pattern of current activity exhibits well defined current levels and long time-duration openings. The amplitudes of the main conductances are ≈10 times smaller than the one observed from digitoxin channels formed in POPS/POPE bilayers. These data are representative of many hours of recording the activity from digitoxin channels. (b) Multilevel conductance activity of digitoxin channels in a neutral (POPC/POPE) planar lipid bilayer. A current trace of 4.5 s from the experiment described in a illustrates the presence of two clearly defined single-channel current levels. The current amplitudes are identified by numbers (in pA) next to each of the calculated stable current values. The bilayer electrical potential is zero. An asymmetric CsCl gradient (200/50 mM) is the sole driving force. (c) Current–amplitude histogram of digitoxin channels in a neutral (POPC/POPE) planar lipid bilayer. The current amplitude histogram shows the distribution of the current events from a representative 60-s current segment taken at zero bilayer potential. The histogram was fitted by the Marquardt least squares-fitting algorithm to a multi-Gaussian function. (d) I–V curve for digitoxin channels in neutral (POPC/POPE) planar lipid bilayer. The current–events amplitudes obtained from the Gaussian-fitted histograms were plotted for different bilayer electrical potential values. The slopes from the linear regression lines through the data indicate that the main subconductance levels are 30.20 ± 2.16, 51.170 ± 0.005, 70.480 ± 0.001, and 92.10 ± 0.09 pS. The line-intercepts generate an average equilibrium potential of 27.98 ± 1.06 mV.
Digitoxin Channels Are Blocked by Anti-Digitoxin Antibodies.
As mentioned in the Introduction, an overdose of digitoxin or digoxin in humans treated for heart failure can be quickly reversed over a 15- to 30-min time period by administering specific anti-digitoxin/digoxin antibodies (14–16). Therefore, we hypothesized that if digitoxin channel activity contributed to cytotoxic adverse events, anti-digitoxin antibodies might quickly block both channel activity in vitro and digitoxin-dependent calcium uptake into cells. As shown in SI Fig. 8a in SI Appendix, digitoxin channel amplitudes are reduced by nearly 50% by 10 min after the addition of the antibody. After 14 min, the channel activity is barely detectable. SI Fig. 8b in SI Appendix (“After 14 min”) shows that the distribution of amplitudes is very close to zero. However, the I–V curves in SI Fig. 8c in SI Appendix show that the antibody has virtually no effect on the reversal potential of the digitoxin channel. Thus, as hypothesized, the antibody quickly reduces the rate with which digitoxin channels conduct ions. Nonetheless, the selectivity of the digitoxin ion channel remains intact.
Digitoxin Is Differentially Sensitive to Conventional Calcium Channel Blockers.
The data so far collected suggested that digitoxin channels conduct cations, including calcium. To further test this hypothesis, we examined the effect of various known calcium channel inhibitors on digitoxin channel activity in a bilayer enriched in PS. These inhibitors included trivalent LaCl3 and AlCl3, which are known to block most known calcium channels; MgCl2, which inhibits most known cellular calcium channels; and nitrendipine, a specific inhibitor of voltage-dependent l-type calcium channels. As shown in SI Fig. 9 Upper in SI Appendix, 8.3 μM LaCl3 promptly suppresses digitoxin channel activity after only 20 s of exposure. Activity is nearly completely abolished by raising the LaCl3 to 16.7 μM (54 s after the addition of blocker). A washout experiment (after 1 min) shows that the inhibition is partially reversible. The amplitude histogram of these different conditions quantitatively validates the effects suggested by the trace segments.
SI Fig. 10 in SI Appendix shows similar inhibition data for digitoxin channels exposed to AlCl3. SI Fig. 10 Upper in SI Appendix shows the activity of digitoxin channels in POPS lipids after 1.3 min in 8.3 μM AlCl3. The second trace is channel activity in 16.7 μM AlCl3 after 2.4 min of exposure. The third trace is 25 μM AlCl3 after 1.3 min of exposure. The amplitude histograms for these data are shown in SI Fig. 10 Lower in SI Appendix. These data indicate that, although both LaCl3 andAlCl3 inhibit digitoxin channels in PS bilayers, LaCl3 is clearly more potent. Tests with 33.3 μM MgCl2 (SI Fig. 11 in SI Appendix) and 33.3 μM nitrendipine (SI Fig. 12 in SI Appendix) show that both agents are inactive. However, LaCl3 inhibits digitoxin activity alone or in the simultaneous presence of either of these inactive agents. Thus, Mg2+ does not appear to protect the digitoxin channel either. The failure of nitrendipine to block the digitoxin channel is consistent with the previously mentioned lack of protection for humans suffering in vivo from cardiac glycoside toxicity (4).
Other Cardiac Glycosides Form Calcium Channels.
Cardiac glycosides such as digitoxin have different chemical moeities that could theoretically contribute to channel activity. SI Fig. 13 in SI Appendix shows chemical structures (Upper) and channel activities (43 nM each) (Lower) for oleandrin (SI Fig. 13a in SI Appendix), digitoxin (SI Fig. 13b in SI Appendix), and digoxigenin 3, 12-diAc (SI Fig. 13c in SI Appendix). At a common concentration of 43 nM, all three cardiac glycosides were able to form long-lived multiconductance pathways in planar lipid bilayers enriched in PS. Thus, digitoxin-like channel activity is preserved in the absence of sugars at position 3 or by substitution of –OH or acetyl groups for –H at C-12. We conclude that the remaining common structures in this class of compounds permits them to behave as cation channels in planar lipid bilayers.
Discussion
In conclusion, these studies indicate that digitoxin forms calcium-conductance pathways in cultured cells and cation-selective calcium channels in planar lipid bilayers in vitro. Similar channels are formed by homologues such as digoxin and oleandrin. Based on the kinetic data, it is likely that the digitoxin channel is formed from multiple digitoxin molecules. The high k1/2 values suggest that the channels form preferentially at concentrations associated with toxic concentrations in the human circulation. This article reports on the intrinsic calcium channel activity for digitoxin and related cardiac glycosides. We suggest that this property may provide a possible explanation for the previously well documented mystery of the mechanism behind calcium-enhanced cytotoxicity for this important class of drugs.
The physiological relevance of digitoxin calcium channels to toxic effects of digitoxin in vivo is supported by at least five lines of evidence. First, digitoxin induces prompt and dose-dependent increases in calcium uptake from the medium into cells. Consistently, increased calcium in the human circulation is known to be responsible for increased digitoxin toxicity in humans (1). Second, the threshold digitoxin concentration for induction of calcium uptake into cells is ≈40 nM. This value also is the top end of the therapeutic window for the treatment of heart failure in humans with digitoxin (see calculation in SI Text 1 in SI Appendix). Thus, the onset of digitoxin calcium channel activity at a physiologic calcium concentration coincides with the toxic digitoxin effects in vivo. Third, anti-digitoxin antibodies block digitoxin channel activity in planar lipid bilayers and calcium uptake into cells within minutes of addition. Consistently, i.v. administration of the Digibind antibody reverses digitoxin toxicity in humans within the same limited time frame (14–16). We interpret the latter data to mean that the toxic mechanism for digitoxin action is equally available to an extracellular inhibitory antibody in a cultured cell system, a planar lipid bilayer system, and in vivo in humans. Fourth, the threshold concentration for digitoxin-dependent cell death begins to become statistically significant above ca. 60 nM digitoxin. This value also is the range in which significant toxicity has been documented to occur in vivo to human patients (see calculation in SI Text 1 in SI Appendix). Finally, nitridipine fails to block either digitoxin channels or digitoxin-mediated calcium entry into cells. This failure also is paralleled by clinical experience (4). Although other entry mechanisms cannot be excluded, these data together constitute strong evidence that intrinsic calcium channel activity constitutes a biologically important, calcium-dependent toxicity mechanism for digitoxin and other clinically important cardiac glycosides.
The data also are consistent with the concept that the digitoxin structure is directly responsible for the fundamental channel property of selectivity. For example, digitoxin forms biophysically classical channels for which the amplitude and kinetics of digitoxin channels are dependent on whether the bilayer is composed of acidic PS or neutral PC. However, in both cases, digitoxin channels exhibit the same cation selectivity. This behavior stands in contrast to the multimeric triterpines (avicins) that form highly cation-selective channels in acidic phospholipid bilayers, but show no selectivity whatsoever in neutral phospholipids (20). The avicins, unlike digitoxin, have been interpreted to follow the torroidal model, typical of pore-forming peptides, such as the magainins, melittin, and protegrins (21–23). Thus, it would seem that digitoxin channels are more like the classical channel former alamethacin, which assembles as a multimeric “barrel stave” structure for which the ion selectivity is independent of phospholipid composition (24, 25). Many other types of sterols are altogether electrically silent (27), further supporting the structural specificity of the digitoxin channel.
As summarized in SI Fig. 14 in SI Appendix, the calcium transport/channel functions of digitoxin can be clearly distinguished from other digitoxin functions that distribute over a wide concentration range. These other functions appear to be nonexclusive. For example, digitoxin blockade of proinflammatory TNF-α/NF-κB signaling occurs at subnanomolar concentrations (17, 18, 28). This function is therefore unlikely to be dependent on digitoxin calcium channels, which only become active above a threshold of ca. 40 nM. Both digitoxin and digoxin have similar calcium channel activities, suggesting that channel activity does not provide an explanation for the reported clinical advantages of digitoxin over digoxin (29, 30). The inhibition of both NaKATPase (13) and the NaKATPase-linked Na/Ca exchanger (5) occur at much higher cardiac glycoside concentrations. Accordingly, a possible role for digitoxin calcium channels in these processes, although less likely, cannot be ruled out. Therefore, we suggest that this insight into the occurrence and function of digitoxin-based calcium channels will serve to promote a better translational understanding of how to manage therapeutic use of these old cardiac glycoside drugs at bedsides.
Materials and Methods
Cells and Cell Culture.
The immortalized human neuronal cell line GT1–7 (a gift from M. Kawahara, Kyushu University of Health and Welfare, Kyushu, Japan) was cultured in DMEM/Ham's F-12 medium (mixed 1:1), 10% FBS, 2 mM l-glutamine, and 1.5 g/liter Na2HCO3. Rat neuronal PC12 cells were a gift from Gordon Guroff (deceased; National Institutes of Health, Bethesda, MD) and have been continuously maintained in our laboratory since their arrival.
Reagents.
Oleandrin (I) was obtained from Indofine. Digitoxin (II), digoxin (III), and digoxigenin 3,12-diAcetyl (VIII) were obtained from Sigma–Aldrich (see ref. 17). Rabbit polyclonal anti-digitoxin antisera (lot no. P4050312) was obtained from Fitzgerald and used at a dilution of 1:200.
Assays of Intracellular-Free Calcium Measurements.
To measure the intracellular-free calcium after the addition of digitoxin, cells were plated at a density of 3 × 105 cells per milliliter on glass coverslips coated with Vitrogen collagen (1:100 in PBS) in 24-well plastic plates (Corning). Two days later, cells (when at ≈80% confluence) were loaded with the calcium-sensitive probe FURA-2 a.m. at 4 μM (Molecular Probes) at 37°C for 25 min in a serum-free BSS medium [135 mM NaCl, 10 mM glucose, 5 mM KCl, 2.5 mM CaCl2, 10 mM Na-Hepes, 1.2 mM MgCl2 (pH 7.2)]. Excess FURA-2 a.m. was removed by rinsing twice with serum-free BSS medium, and intracellular FURA-2 a.m. was allowed to be hydrolyzed for 15 min at 37°C. Cells were optically marked for analysis, and the [Ca2+]i followed over time after the addition of different concentrations of digitoxin or other cardiac glycoside. Changes in fluorescence of at least 50 cells were measured simultaneously at 340-nm and 380-nm excitation wavelengths and 510-nm emission wavelength by using an inverted epifluorescence/phase contrast microscope equipped with a low-light level-integrating CCD camera plus microphotometer assembly (InCyt I/P-2 TM Imaging and Photometry System; Intracellular Imaging). The solvent, 0.01% EtOH, was without effect on any of the parameters.
Assays of Cell Viability.
To measure the cytotoxicity of digitoxin, cells were incubated in either normal or serum-free medium, to which were added different concentrations of digitoxin. Cell survival was measured 24 h later. Cell viability was measured by using a colorimetric XTT assay for mitochondria (cell proliferation kit II; Roche Molecular Biochemicals) or by using an LDH release kit for plasma membrane integrity (Roche Molecular Biochemicals).
FACS.
For flow-cytometric analysis and cell sorting, PC12 cells were incubated in Annexin V-FITC and binding buffer (Annexin V-FITC apoptosis detection kit; Sigma–Aldrich) for 20 min. The manufacturer's directions were followed in every detail. Cells with different Annexin V-FITC affinity were separated by FacsAria (BD Biosciences). All single cells were included. Low-pressure (30 psi) and low-speed (3,000 events per second) cell-sorting techniques were used for all preparative work.
Planar Lipid Bilayer Methodology.
Planar lipid bilayers and ionic current analysis were made as described previously (19, 31). Briefly, a suspension of POPS or palmitoyloleoyl PC (POPC) and POPE (1:1) in n-decane was prepared. This suspension was applied to an orifice of ≈100–120 μm in diameter in a Teflon film separating two compartments, 1.2-ml volume each. The ionic solutions in the compartments contained asymmetrical concentrations of CsCl (200cis/50trans mM) or asymmetrical concentrations of CsCl and CaCl2 (37.5 mM CsClcis/25 mM CaCl2trans). The two ionic compartments were electrically connected via agar bridges and Ag/AgCl pellet electrodes to the input of a voltage clamp amplifier. Current was recorded by using a patch-clamp amplifier [Axopatch-1D equipped with a low-noise (CV-4B) headstage; Axon Instruments], and data were stored on computer disk memory. Off-line analysis of the channel activity was carried out by using the software package pClamp (Axon Instruments).
Statistics.
All data are presented as the means ± SEM of three to five experiments. Origin scientific analysis software was used for fitting lines on experimental points and for determining significance levels using Student's t test. A single asterisk denotes P < 0.05 or P < 0.001 as indicated.
Supplementary Material
ACKNOWLEDGMENTS.
This work was supported by National Institutes of Health Grants RO1-DK-53051 and NO-1-HV-28187 (to H.B.P.), the Cystic Fibrosis Foundation (H.B.P.), the Institute for the Study of Aging (H.B.P.), and the Alzheimer's Association of America (N.A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712270105/DC1.
References
- 1.Kelly RA, Smith TW. In: Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th Ed. Hardman JG, et al., editors. New York: McGraw–Hill; 1996. pp. 809–838. [Google Scholar]
- 2.Roden DM. In: Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th Ed. Hardman JG, et al., editors. New York: McGraw–Hill; 1996. p. 864. [Google Scholar]
- 3.Schwartz A. Is the cell membrane Na+K+-ATPase enzyme system the pharmacological receptor for digitalis? Circ Res. 1976;39:2–7. doi: 10.1161/01.res.39.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Kirch W, Logemann C, Heidemann H, Santos SR, Ohnhaus EE. Effect of two different doses of nitrendipine on steady state plasma digoxin level and systolic time intervals. Eur J ClinPharmacol. 1986;31:391–395. doi: 10.1007/BF00613512. [DOI] [PubMed] [Google Scholar]
- 5.Altamirano J, et al. The inotropic effect of cardioactive glycosides in ventricular myocytes requires Na+/Ca2+ function. J Physiol (London) 2006;575:845–854. doi: 10.1113/jphysiol.2006.111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bower JO, Mengle HAK. The additive effects of calcium and digitalis: A warning with a report of two deaths. J Am Med Assoc. 1936;106:1151–1153. [Google Scholar]
- 7.Clark AF, Swanson PD, Stahl WL. Increase in dissociation rate constants of cardiotonic steroid-brain (Na+K+)-ATPase complexes by reduction of the unsaturated lactone. J Biol Chem. 1975;250:9355–9359. [PubMed] [Google Scholar]
- 8.Spiegel A, Marchlinski FE. Time course for reversal of digoxin toxicity with digoxin-specific antibody fragments. Am Heart J. 1985;109:1397–1399. doi: 10.1016/0002-8703(85)90376-x. [DOI] [PubMed] [Google Scholar]
- 9.Wenger T, et al. Treatment of sixty three severely digitalis toxic patients with digoxin-specific antibody fragments. J Am Coll Cardiol. 1985;5:118A–123A. doi: 10.1016/s0735-1097(85)80471-x. [DOI] [PubMed] [Google Scholar]
- 10.Smith TW, Haber E, Yeatman L, Butler VP., Jr Reversal of advanced digoxin intoxication with Fab fragments of digoxin-specific antibodies. New Eng J Med. 1976;294:797–800. doi: 10.1056/NEJM197604082941501. [DOI] [PubMed] [Google Scholar]
- 11.Bachmaier A, Ebner F, Reiter M. Potassium changes the relationship between receptor occupancy and the inotropic effect of cardiac glycosides in guinae-pig myocardium. Br J Pharmacol. 1985;85:755–765. doi: 10.1111/j.1476-5381.1985.tb11073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebner F, Korth M, Kuhlkamp V. The reaction of ouabain with the sodium pump of guinae-pig myocardium in relation to its inotropic effect. J Physiol (London) 1986;379:187–203. doi: 10.1113/jphysiol.1986.sp016247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marban E, Tsien RW. Enhancement of calcium current during digitalis inotropy in mammalian heart: Positive feedback regulation by intracellular calcium? J Physiol (London) 1982;329:589–614. doi: 10.1113/jphysiol.1982.sp014321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weingart R, Kass RS, Tsien RW. Is digitalis inotropy associated with enhanced slow inward calcium current? Nature. 1978;273:389–392. doi: 10.1038/273389a0. [DOI] [PubMed] [Google Scholar]
- 15.Wasserstrom JA, Aistrup GL. Digitalis: New actions for an old drug. Am J Physiol Heart Circ Physiol. 2005;289:H1781–H1793. doi: 10.1152/ajpheart.00707.2004. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Wible BA, Wan X, Ficker E. Cardiac glycosides as novel inhibitors of human Ether-a-go-go-related gene channel trafficking. J Pharm Exp Therap. 2007;320:525–534. doi: 10.1124/jpet.106.113043. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava M, et al. Digitoxin mimics CFTR-gene therapy, and suppresses hypersecretion of proinflammatory Interleukin-8 (IL-8) from cystic fibrosis lung epithelial cells. Proc Natl Acad Sci USA. 2004;101:7693–7698. doi: 10.1073/pnas.0402030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Q-F, et al. Cardiac glycosides inhibit TNFα/NFκB signaling by blocking recruitment of TRADD to the TNF receptor. Proc Natl Acad Sci USA. 2005;102:9631–9636. doi: 10.1073/pnas.0504097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid β protein forms calcium channels in bilayer membranes: Blockade by tromethamine and aluminum. Proc Natl Acad Sci USA. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li XX, Davis B, Haridas V, Gutterman JU, Colombini M. Proapoptotic triterpene electrophiles (avicins) form channels in membranes: Cholesterol dependence. Biophys J. 2005;88:2577–2584. doi: 10.1529/biophysj.104.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruciani RA, et al. Magainin 2, a natural antibiotic from frog skin, forms ion channels in lipid bilayer membranes. Eur J Pharmacol. 1992;226:287–296. doi: 10.1016/0922-4106(92)90045-w. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shai Y, Oren Z. From “carpet” mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides. Peptides. 2001;22:1629–1641. doi: 10.1016/s0196-9781(01)00498-3. [DOI] [PubMed] [Google Scholar]
- 24.Boheim G. Statistical analysis of alamethicin channels in black lipid membranes. J Memb Biol. 1974;19:277–303. doi: 10.1007/BF01869983. [DOI] [PubMed] [Google Scholar]
- 25.Cafiso D. Alamethicin: A peptide model for voltage gating and protein-membrane interaction. Ann Rev Biophys Biomol Struct. 1994;23:141–165. doi: 10.1146/annurev.bb.23.060194.001041. [DOI] [PubMed] [Google Scholar]
- 26.Lakshminarayanaiah N. Equations of Membrane Biophysics. Orlando, FL: Academic; 1984. [Google Scholar]
- 27.Micelli S, Meleleo D, Picciarelli V, Gallucci E. Effects of sterols on β-amyloid peptide (AβP 1–40) channel formation and their properties in planar lipid bilayers. Biophys J. 2004;86:2231–2237. doi: 10.1016/S0006-3495(04)74281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabary O, et al. Calcium-dependent regulation of NF-κB activation in cystic fibrosis airway epithelial cells. Cell Signal. 2006;18:652–660. doi: 10.1016/j.cellsig.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Roever C, Ferrante J, Gonzalez EC, Naazneen P, Roetzheim RG. Comparing the toxicity of digoxin and digitoxin in a geriatric population: Should an old drug be rediscovered? Southern Med J. 2000;93:199–202. [PubMed] [Google Scholar]
- 30.Belz GG, Breithaupt-Grogler K, Osowski U. Treatment of congestive heart failure–current status of use of digitoxin. Eur J Clin Invest. 2001;31(Suppl 2):10–17. doi: 10.1046/j.1365-2362.2001.0310s2010.x. [DOI] [PubMed] [Google Scholar]
- 31.Wonderlin W, French RJ, Arispe N. In: Neuro-Methods Neurophysiological Techniques: Basic Methods and Concept. Boulton A, Baker G, Vanderwolf C, editors. Vol 14. Clifton, NJ: Humana; 1988. pp. 35–142. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.