Abstract
Cerebral malaria (CM) in children is associated with a high mortality and long-term neurocognitive sequelae. Both erythropoietin (Epo) and vascular endothelial growth factor (VEGF) have been shown to be neuroprotective. We hypothesized that high plasma and cerebrospinal fluid (CSF) levels of these cytokines would prevent neurological sequelae in children with CM. We measured Epo, VEGF, and tumor necrosis factor in paired samples of plasma and CSF of Kenyan children admitted with CM. Logistic regression models were used to identify risk and protective factors associated with the development of neurological sequelae. Children with CM (n = 124) were categorized into three groups: 76 without sequelae, 32 with sequelae, and 16 who died. Conditional logistic regression analysis matching the 32 patients with CM and neurological sequelae to 64 patients with CM without sequelae stratified for hemoglobin level estimated that plasma Epo (>200 units/liter) was associated with >80% reduction in the risk of developing neurological sequelae [adjusted odds ratio (OR) 0.18; 95% C.I. 0.05–0.93; P = 0.041]. Admission with profound coma (adjusted OR 5.47; 95% C.I. 1.45–20.67; P = 0.012) and convulsions after admission (adjusted OR 16.35; 95% C.I. 2.94–90.79; P = 0.001) were also independently associated with neurological sequelae. High levels of Epo were associated with reduced risk of neurological sequelae in children with CM. The age-dependent Epo response to anemia and the age-dependent protective effect may influence the clinical epidemiology of CM. These data support further study of Epo as an adjuvant therapy in CM.
Keywords: anemia, Plasmodium falciparum, severe malaria, vascular endothelial growth factor
Cerebral malaria (CM) is the most severe neurological complication of Plasmodium falciparum infection. Even with appropriate antimalarial treatment, 18.6% of children with CM die, 11% have neurological deficits detected on discharge (1), and up to 24% children have neurocognitive impairment (2, 3) and epilepsy (4, 5) when assessed many years later.
Over a wide range of endemic areas severe malarial anemia is the most common manifestation of severe malaria in younger children, whereas CM occurs more commonly in older children (6). The pathogenesis of CM is not completely understood and the factors involved in the development of neurological sequelae remain unclear. A number of studies have consistently identified deep and prolonged coma, recurrent seizures, and hypoglycemia as independent risk factors associated with the development of neurological sequelae (reviewed in ref. 7). Protective factors are less well defined. Low levels of hemoglobin (Hb) were associated with neurological sequelae in the Gambian studies (8), but not in other African studies (9, 10).
We have hypothesized that the outcome of CM is modified by the cytokine response to hypoxia. Erythropoietin (Epo), principally produced in the kidney in response to hypoxia, is crucial for sustained proliferation and differentiation of erythroid cells (11). However, Epo and Epo receptors are also expressed in neurons and astrocytes (12, 13). Recombinant human Epo (rhEpo) is protective in animal models of brain injury (13, 14) and reduces vasoconstriction, neuronal apoptosis, and reperfusion injury (15–18). Indeed, preliminary clinical studies in patients with stroke have supported a neuroprotective role for Epo (19).
Recent studies have shown high levels of Epo in African children with malaria anemia (20–22). Malaria appears to induce Epo concentrations up to 30-fold higher than those found in anemia not associated with acute malaria infection (20). The peak levels of Epo are >1,000 units/liter in many cases and are in the range used therapeutically to reduce morbidity in neuronal injury. Furthermore, the administration of rhEpo in a murine model of malaria reduced mortality by 90% (23).
Vascular endothelial growth factor (VEGF) is also up-regulated by hypoxia (24) and is both neurotrophic and neuroprotective (25). It improves functional outcome in cerebral ischemia in rats, reducing motor and cognitive defects (26). However, VEGF can also increase expression of intercellular adhesion molecule-1 (ICAM-1) and macrophage inflammatory protein 1α (MIP1α) in endothelial and brain parenchymal cells (27) and increase the permeability of the brain–blood barrier (BBB) (28, 29). Other studies show the levels of the proinflammatory cytokine tumor necrosis factor (TNF) increase during acute stroke (30), and in an animal model, intraventricular administration of TNF enlarges infarct volume (31). In children with malaria, high TNF levels have been associated with poor outcome (32, 33).
The available evidence suggests that cytokines may modulate the outcome of CM. We hypothesized that high levels of Epo and VEGF protect children with CM from neurological sequelae or death. We studied a well defined group of children admitted with CM who were assessed for neurological damage on discharge from hospital. We therefore compared the levels of Epo, VEGF, and TNF in children who died and in those who survived with and without neurological deficits.
Results
A total of 426 children were admitted to Kilifi District Hospital with CM from January 1999 through December 2001. Nine had incomplete admission data and were excluded. Of the remaining 417, paired plasma and CSF samples were available for 179 cases. Of those, 65 received a blood transfusion. In 55 cases the samples had been obtained after a blood transfusion and were excluded from the study. Thus, we included in this study 124 children with paired CSF and plasma samples.
The median age of the children with a primary diagnosis of CM was 28.5 [interquartile range (IQR) 16–40] months, and the mean (SD) Hb concentration was 8.2 (1.91) g/dl. Fifteen of 32 (46%) children discharged with neurological deficits had multiple neurological sequelae. The major sequelae were visual impairment (n = 8), impairment of speech (n = 14), and motor impairment (hemiparesis, quadriparesis, and monoparesis) (n = 9).
Epo Levels and Development of Neurological Sequelae.
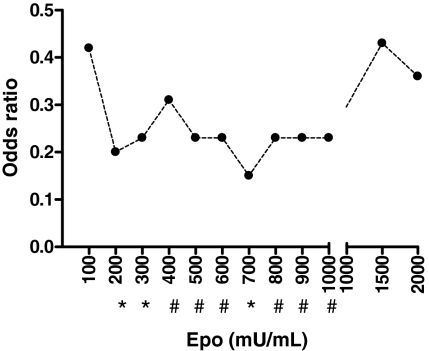
To test our hypothesis that high Epo levels were associated with protection from neurological sequelae in children with CM, we first examined different concentrations of Epo (ranging from 100 to 2,000 units/liter) associated with development of neurological sequelae adjusting for confounders, namely hypoglycemia, seizures during admission, and depth of coma. A protective effect [odds ratio (OR) from 0.15 to 0.43] was found for Epo concentrations ranging from 200 to 1,000 units/liter. We chose 200 units/liter as a cut-off as it was the lowest value of Epo associated with a significant protective effect (see Fig. 1).
Fig. 1.
The variation of OR for neurological sequelae with concentrations of plasma Epo. ORs represent values after adjusting for hypoglycemia, depth of coma, seizures during admission, and age in a multivariate conditional logistic regression analysis. *, P < 0.05; #, P 0.05–0.08
Logistic regression analysis identified plasma Epo concentration (OR 0.28; 95% C.I. 0.09–0.79), depth of coma (OR 7.74; 95% C.I. 2.50–23.98), and seizures during admission (OR 3.42; 95% C.I. 1.11–10.48) as the main factors independently associated with development of neurological sequelae (Table 1).
Table 1.
Factors associated with the development of neurological sequelae in 124 Kenyan children with CM
| Factor | Unmatched* |
Matched |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age† |
Hb level‡ |
|||||||||||
| OR | P | 95% C.I. | OR | P | 95% C.I. | OR | P | 95% C.I. | ||||
| EPO, >200 units/liter | 0.28 | 0.01 | 0.09 | 0.79 | 0.21 | 0.030 | 0.05 | 0.86 | 0.18 | 0.041 | 0.03 | 0.93 |
| Hypoglycemia | 3.11 | 0.11 | 0.76 | 12.71 | 4.51 | 0.152 | 0.57 | 35.65 | 9.27 | 0.057 | 0.93 | 91.73 |
| Seizures | 3.42 | 0.03 | 1.11 | 10.48 | 6.91 | 0.019 | 1.37 | 34.92 | 16.35 | 0.001 | 2.94 | 90.79 |
| Depth of coma | 7.74 | 0.00 | 2.50 | 23.98 | 18.60 | 0.002 | 2.91 | 118.80 | 5.47 | 0.012 | 1.45 | 20.67 |
*Hosmer-Lemeshow = 5.54, P = 0.476.
†Cases of CM with neurological sequelae (n = 32) are matched by age (within 18 months) to two cases of CM discharged without neurological sequelae (n = 64).
‡Cases of CM with neurological sequelae (n = 32) are matched by the Hb level (within groups Hb < 5, 5.1–7, 7.1–9, and >9.1 g/dl) to two cases of CM discharged without neurological sequelae (n = 64).
Age was associated with Epo concentration (r = −0.18; P = 0.04) and Blantyre coma score (BCS) on admission (r = −0.22; P = 0.01). We therefore performed the same analyses in a conditional logistic regression model matching by age (within 18 months). This analysis identified again plasma Epo (OR 0.21; 95% C.I. 0.05–0.86), seizures (OR 6.9; 95% C.I. 1.37–34.92), and depth of coma (OR 18.6; 95% C.I. 2.91–118.8) as variables independently associated with neurological sequelae (Table 1).
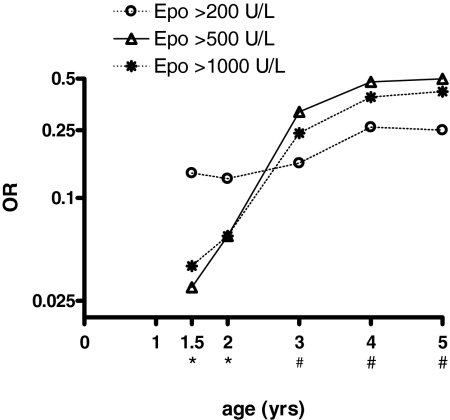
The risk of developing sequelae given a certain concentration of Epo was lower in the age-matched compared with the unadjusted model, suggesting an age-dependent dose effect. Indeed, the OR for neurological sequelae at different concentrations of plasma Epo, after adjusting for hypoglycemia, depth of coma, seizures during admission, and age in a multiple logistic regression analysis, was much lower in children <2 years of age at concentrations of Epo >500 units/liter (Fig. 2).
Fig. 2.
Effect of age on the OR for neurological sequelae at different concentrations of plasma Epo. ORs represent values after adjusting for hypoglycemia, depth of coma, seizures during admission, and age in a multiple logistic regression analysis using different cut-off values for age. *, P < 0.05; #, P 0.05–0.08 (P values are the significance of the OR in the logistic regression analyses for Epo >500 and >1,000 units/liter).
Plasma Epo was negatively associated with Hb concentrations (r = −0.59; P < 0.001). We introduced Hb level as an ordinal independent variable (categorized as Hb <5, 5–7, 7–9, and >9 g/dl) in the logistic regression model to predict sequelae and found that children with Hb concentrations between 5 and 7 g/dl were associated with a significant reduction in the risk of developing neurological sequelae (OR 0.17; 95% C.I. 0.03–0.87). The median Epo concentration for this subgroup was 2,097 (IQR 287–3,693). We therefore matched each case of CM with neurological sequelae and two CM controls within the same Hb range. Plasma Epo (OR 0.21; 95% C.I. 0.05–0.86), seizures (OR 6.9; 95% C.I. 1.37–34.92), and depth of coma (OR 18.6, 95% 2.91–118.8) remained as variables independently associated with neurological sequelae (Table 1).
Epo concentrations were also measured in cerebrospinal fluid (CSF) from the same patients. Levels of plasma Epo correlated with Epo in CSF (r = 0.40; P < 0.001) in children discharged without neurological sequelae (n = 76) but not in those who developed neurological sequelae (n = 32) (r = 0.21; P = 0.23). CSF Epo levels were not associated with neurological sequelae.
Effect of Plasma Epo on Mortality in CM.
We performed an ordinal logistic regression to measure the impact of Epo on poor outcome (death or neurological sequelae). In this model, mortality and discharge with and without sequelae were used as the dependent (ordinal) variable. In the subgroup of children with CM who died (n = 16) the median plasma concentration of Epo was 124 (IQR 30–1,726) units/liter. Blood transfusion, deep (acidotic) breathing, and hyperparasitemia were also more frequent in this group (Table 2). In addition to the independent variables used to identify risk factors for neurological sequelae, in this model we adjusted for deep breathing, hyperparasitemia, and papilloedema because these factors may be associated with an increased fatality rate in severe malaria.
Table 2.
Description of the study population
| Factor | Good outcome(n = 76) | Neurological sequelae(n = 32) | Dead(n = 16) |
|---|---|---|---|
| Age (months), median (IQR) | 27 (14.5–36.5) | 28 (21–46) | 36.5 (27–46) |
| Gender, male n (%) | 45 (59.2) | 14 (43.7) | 9 (56.2) |
| Weight for age Z-score, mean (SD) | −1.7 (1.1) | −2 (1.02) | −1.7 (1.6) |
| Fever, n (%) | 69 (90.7) | 29 (90.6) | 15 (93.7) |
| Seizures before admission, n (%) | 71 (93.4) | 29 (90.6) | 12 (75) |
| Seizures during admission, n (%) | 37 (48.6) | 26 (81.2) | 12 (75) |
| Profound coma, BCS = 0, n (%) | 14 (18.4) | 13 (40.6) | 6 (66.6) |
| Abnormal motor posturing during admission, n (%) | 21 (27.6) | 17 (53.1) | 8 (50) |
| Features of raised intracranial pressure on fundoscopy,*n (%) | 4 (7.8) | 8 (34.7) | 3 (27.2) |
| Coma duration (h), median (IQR) | 8 (4–23) | 78 (38–125) | 17.5 (7.5–36.5) |
| Deep (acidotic) breathing, n (%) | 15 (19.7) | 8 (25) | 12 (75) |
| Hypoglycemia, n (%) | 8 (10.5) | 6 (18.7) | 5 (31.2) |
| Parasite density/μl, geometric mean | 42,328 | 28,439 | 60,495 |
| (95% C.I. of mean) | (25,870–69,256) | (11,785–68,627) | (16,207–225,801) |
| Hb (g/dl), mean (SD) | 8.0 (1.8) | 8.6 (2.0) | 8.3 (1.9) |
| Severe anemia (Hb < 50 g/liter), n (%) | 3 (3.9) | 2 (6.2) | 0 (0) |
| Transfused, n (%) | 2 (3.8) | 3 (6.4) | 5 (31.2) |
| Platelets (103μl−1), median (IQR) | 101 (58–95) | 192 (76–284) | 93 (63–219) |
| Plasma Epo (milliunits/ml), median (IQR) | 278.6 (96.7–1,852) | 184.2 (23.9–694.4) | 123.5 (29.5–1,726.2) |
| Plasma VEGF (ng/ml), median (IQR) | 39.6 (23.2–77.2) | 53.1 (25.9–118.1) | 42.9 (18.1–122.7) |
| Plasma TNF (pg/ml), median (IQR) | 60.6 (24.8–137.4) | 80.6 (13.9–187.80) | 92.1 (63.0–487.8) |
| CSF Epo (milliunits/ml), geometric mean (95% C.I.) | 14.3 (3.8–52.6) | 14.3 (1.2–165.2) | 29.8 (1.6–534.9) |
| CSF VEGF (ng/ml), geometric mean (95% C.I.) | 42.9 (21.1–87.2) | 59.1 (16.1–216.8) | 37.7 (2.3–608.9) |
| CSF TNF (pg/ml), geometric mean (95% C.I.) | 361.3 (58.3–2,236.9) | 199.8 (2.8–1,4165.6) | 470.6† |
*n = 85 (n = 51, n = 23, n = 11, respectively).
†TNF was detectable only in one case, hence the 95% C.I. of the geometric mean cannot be calculated.
Here, the logistic regression model identified the following variables to be independently associated with poor outcome: seizures during admission (OR 6.77; 95% C.I. 2.18–20.98), depth of coma (OR 5.65; 95% C.I. 2.24–14.21), deep breathing (OR 6.09; 95% C.I. 2.08–17.79), and hyperparasitemia (OR 5.19; 95% C.I. 1.26–21.34). In this model, plasma Epo was also independently associated with a better outcome (OR 0.21; 95% C.I. 0.08–0.54).
Papilloedema was strongly associated with poor outcome (OR 5.69; 95% C.I. 1.09–29.5). This association remained significant when the model was adjusted for seizures during admission and plasma Epo (OR 6.06; 95% C.I. 1.07–34.13). However, this association was no longer significant when the analysis was adjusted for depth of coma (OR 4.90; 95% C.I. 0.73–32.63).
Effect of VEGF and TNF on Neurological Sequelae.
VEGF is both neuroprotective and proinflammatory in the brain. VEGF was not associated with the development of neurological sequelae in the univariate analysis and was therefore not included as an independent variable in any of the multiple logistic regression models. However, VEGF (>100 ng/ml) was strongly associated with seizures during admission (OR 4.1; 95% C.I. 1.75–9.60). Similarly, plasma VEGF (>100 ng/ml) was associated with a 4.5-fold increase in the risk signs of raised intracranial pressure by fundoscopy (95% C.I. 1.41–14.74) and a 12.1-fold increase in the risk of finding papilloedema (95% C.I. 1.84–79.37). VEGF concentrations in plasma were correlated with plasma TNF (r = 0.23; P < 0.001) and inversely correlated with plasma Epo (r = −0.17; P = 0.051). However, plasma VEGF was associated with higher concentrations of Epo in CSF (r = 0.24; P = 0.007) and TNF in CSF (r = 0.38; P < 0.001).
We measured the TNF in plasma and CSF to assess the role of inflammation in relation to the outcome of children with CM. Plasma levels of TNF were higher in children with neurological sequelae compared with those healthy at discharge (Table 2). TNF concentrations >100 pg/ml were associated with a 2.78-fold increase (95% C.I. 0.89–8.63) in the risk of developing neurological sequelae. This association was of borderline significance (P = 0.07). In the conditional logistic regression analysis, high levels of TNF were associated with a 4.2 (95% C.I. 0.98–17.9) and 5.1 (95% C.I. 0.96–27.9) increase in the risk of developing neurological sequelae after adjusting for age and anemia, respectively, with these associations falling just short of statistical significance. Furthermore, plasma TNF levels were correlated with TNF concentration in CSF (r = 0.35; P < 0.001).
Discussion
In this study, we report a strong association of high concentrations of plasma Epo and reduced risk of neurological sequelae in children with CM and the association of VEGF with seizures and signs of raised intracranial pressure.
The potential neuroprotective mechanisms of Epo and VEGF in response to hypoxia are related to their neurotrophic and proangiogenic activities (34). In the last decade a number of experimental and preclinical studies have investigated the tissue-protective activities of Epo (reviewed in ref. 35) and VEGF (reviewed in ref. 25).
In this study, we have found that high Epo levels in plasma are associated with a 70% reduction of the risk of being discharged with neurological sequelae and 79% and 82% reduction when the analyses are matched by age or level of Hb, respectively. Epo is thought to prevent neuronal apoptosis (17) and down-regulate the inflammatory response in the brain (36), which may explain differences in the clinical presentation and outcome in CM. Neuronal apoptosis has been recently reported in a mouse model with experimental CM (37). However, previous studies with a similar model suggested that protection is caused by the antiinflammatory (rather than the antiapoptotic) effect of Epo (23).
The neuroprotective activities of Epo are time- and dose-dependent. In a rodent model of stroke, neurons within the ischemic penumbra undergo apoptosis unless exposed to Epo within 3 h (14). In our study it was impossible to ascertain whether the patients were anemic (or had high levels of Epo) before the malaria episode. However, our previous study in 120 Kenyan children with acute malarial anemia (20) indicated that more than one-third of children with severe to moderate anemia had levels of Epo >200 units/liter a week after treatment. We have shown that Epo levels on admission >200 units/liter are independently associated with a reduced risk of sequelae.
In vitro studies suggest that concentrations ranging from 100 to 1,000 units/liter are associated with the neuroprotective activities of Epo (17). Similarly, clinical studies have suggested that endogenous Epo is associated with tissue protection at concentrations >20 units/liter (38). Although our data suggest that Epo concentrations up to 1,000 units/liter were significantly associated with protection, a larger study would be required to establish the range of protection with confidence. The biological relevance of plasma Epo is usually difficult to interpret in the absence of any direct measurement of Epo in the brain. Epo is known to cross the BBB by active translocation possibly via Epo receptors expressed in the brain vasculature (14). We found CSF and plasma Epo to be correlated in those cases discharged without neurological sequelae, but this association was moderately influenced by five cases with Epo concentrations >5,000 units/liter. Moreover, Epo in CSF was not associated with a reduced risk of neurological sequelae.
The association of younger age with higher Epo concentrations and a greater protective effect of Epo in children <2 years of age are intriguing but have been reported previously (39). There is no immediate biological explanation for these observations. However, these data do offer a possible reason for the widely observed and yet unexplained age-related presentation of severe malarial anemia and CM (6).
We have previously shown that malaria infection per se is associated with an increased level of Epo secretion for any Hb concentration (20). The specific mechanisms responsible for the increased Epo concentration in patients with acute malaria are unclear but may include tissue hypoxia, hypoglycemia, and possibly oxidative damage (7). These three conditions have been shown to induce the expression of hypoxia-inducible transcription factor (HIF-1), which up-regulates the production of Epo and other hypoxia-related proteins (40). Similarly, iron deficiency may also contribute to the up-regulation of Epo and other hypoxia-related proteins by inhibiting the function of HIF-prolyl 4-hydroxylases (41). A beneficial effect of iron chelation therapy in CM is unclear (42), although some studies suggest partial neuroprotection is associated with desferoxamine (43). It is possible that up-regulation of HIF-1 and Epo concentrations could have accounted for some of the tissue-protective effect of iron chelation.
The activation of VEGF in CM has been reported in patients with CM (44). VEGF is also up-regulated in response to tissue hypoxia (45), and we initially predicted that the angiogenic and neurotrophic properties of VEGF would have a protective role in CM. However, our data suggest that VEGF is not associated with neuroprotection but rather with features associated with a poor outcome. We have found that VEGF is associated with a 4-fold increase in the risk of seizures during admission. Pilocarpine-induced seizures in experimental animals are associated with a marked increase of VEGF levels in neuronal and glial cells and consequently vascular permeability and BBB leakage (27). Thus it is possible that increased VEGF concentrations may be the consequence rather than the cause of seizures. However, it is also likely that VEGF directly contributes to the pathophysiology of CM by increasing the permeability of the BBB (28). Indeed, intracranial hypertension is a common finding in CM in children (46). Here, we report that concentrations of plasma VEGF (>100 pg/ml) are associated with a 4.5-fold increase in the risk signs of raised intracranial pressure and a 12-fold increase in the risk of papilloedema (95% C.I. 1.84–79.37).
In this study, TNF was associated with an increase in the risk in neurological sequelae. However, this association was of borderline significance possibly because of wide variation of TNF concentrations in these patients. TNF (and other proinflammatory cytokines) have been shown to up-regulate VEGF, which may contribute to the development of neuropathological signs associated with inflammation in CM (47). However, some of these effects may be partially antagonized by the TNF-induced up-regulation of Epo receptor in the brain (48), and may explain the borderline significance of the association of TNF with poor outcome in this study. A larger study may be required to demonstrate the effect of TNF on outcome.
Our data suggest that in addition to the inflammatory response to the parasite, molecules that orchestrate adaptation to hypoxia may influence the clinical presentation and outcome of severe malaria syndromes. Moreover, this study supports the preliminary data from murine models of CM that indicate a neuroprotective role for Epo in CM (23). While rapidly effective antimalarial drugs would be used to clear sequestered and nonsequestered parasites, the use of Epo in CM would aim to protect potentially viable brain tissue to prevent development of neurological sequelae. The neuroprotective concentration of plasma Epo at different ages should be studied prospectively before the investigation of the potential use of Epo [and nonerythropoietic Epo derivatives (49)] as adjuvant treatment in CM to prevent neurological sequelae.
Methods
Study Design.
The study was conducted in children admitted to Kilifi District Hospital, in which all children were seen by research clinicians from the Kenya Medical Research Institute/Wellcome Trust Research Laboratories collaborative program. This was a retrospective study of children admitted with CM and classified by outcome: children who survived without neurological deficits, children who survived with neurological deficits, and children who died.
Study participants were identified from the hospital database admitted between 1999 and 2001. CM was defined as admission to hospital with coma [unable to localize a painful stimulus or BCS ≤2 (9), at least 1 h after termination of a seizure if present or correction of hypoglycemia], with asexual forms of P. falciparum malaria parasites on a Giemsa-stained blood smears, and with no evidence of pyogenic meningitis on examination of the CSF (50). All patients had a detailed neurological examination on admission, which included a fundoscopic examination of the retina for features of raised intracranial pressure. Lumbar punctures were performed to exclude pyogenic meningitis when the level of consciousness improved (BCS >2) or if the child did not have brainstem signs (within 48 h of admission in almost all cases) (46, 50). A portion of the CSF was stored at −20°C and later frozen at −80°C together with the corresponding specimen of plasma obtained at the time of the lumbar puncture. Patients with epilepsy, cerebral palsy, and sickle cell disease and those without stored paired samples or samples collected after a blood transfusion were excluded. The study was approved by the Kenyan National Scientific and Ethical Committee.
Procedures.
All patients had been treated with standard protocols developed for the management of severe falciparum malaria (51) and according to the World Health Organization (WHO) (52). At discharge all children were assessed for neurological sequelae by the attending physicians. Neurological assessment at follow-up was carried out in some of the children. Neurological sequelae were classified as motor sequelae (cranial nerve palsies, spasticity, and hypotonia), ataxia, movement disorders (tremors, dystonia, and choreoathetoid movements), speech (speech difficulties or aphasia), visual (blindness) and/or hearing impairments, epileptic seizures, and behavioral abnormalities (aggressive behavior and hyperactivity) (10).
Laboratory Procedures.
Plasma and CSF levels of Epo, VEGF, and TNF were measured by ELISA following the manufacturer's instructions (R&D Systems). The lower limit of detection for Epo was 2.5 units/liter, for VEGF it was 15.6 pg/ml, and for TNF it was 15.6 pg/ml. Ten percent of samples were tested in duplicate (the correlation of values was r > 0.90). Values above the standard detection range were diluted and reassayed.
Statistical Analysis.
Data were analyzed with STATA 9 (Stata Corporation). Binary logistic regression analysis was used to identify risk/protective factors independently associated with the development of neurological sequelae in children with CM. Goodness-of-fit was assessed by the Hosmer-Lemeshow test. Variables detected from previous studies in the unit and elsewhere (10, 53), known to be associated with the dependent variable (neurological sequelae), were included in the analysis, namely, hypoglycemia, depth of coma, and seizures. The independent variables were checked for interaction. Conditional logistic regression was used to match 32 children with neurological sequelae to 64 children with good outcome. The subgroup of children not used in the matching (n = 12) had similar age and Hb concentrations to those used in the regression. Matching was by age (within 18 months) and Hb level (within groups Hb <5, 5.1–7, 7.1–9, and >9.1 g/dl). Matching criteria were based on biologically meaningful cut-off points, but also on a value range that allowed inclusion of all children with sequelae. Each patient with CM who developed neurological sequelae (n = 32) was age-matched (within 18 months) with two patients with CM who did not develop sequelae (n = 64). In 92% of the cases age-matching was possible within 12 months and in 75% of the cases within 6 months. The average age for matching was 10 (SD 4.5) months. Ordinal logistic regression was used to identify risk factors associated with outcome (no sequelae at discharge, neurological sequelae, and death). For ordinal logistic regression analysis deviance (χ2 test) was used to assess goodness-of-fit.
ACKNOWLEDGMENTS.
This study was supported by The Wellcome Trust, the National Health Service, and the Kenya Medical Research Institute. D.J.R. is supported by the Howard Hughes Medical Institute and the National Blood Service. C.R.J.C.N. holds a Wellcome Trust Career Post in Clinical Tropical Medicine (no. 070114).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Newton CR, Krishna S. Severe falciparum malaria in children: Current understanding of pathophysiology and supportive treatment. Pharmacol Ther. 1998;79:1–53. doi: 10.1016/s0163-7258(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 2.Carter JA, et al. Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry. 2005;76:476–481. doi: 10.1136/jnnp.2004.043893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter JA, et al. Developmental impairments following severe falciparum malaria in children. Trop Med Int Health. 2005;10:3–10. doi: 10.1111/j.1365-3156.2004.01345.x. [DOI] [PubMed] [Google Scholar]
- 4.Carter JA, et al. Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004;45:978–981. doi: 10.1111/j.0013-9580.2004.65103.x. [DOI] [PubMed] [Google Scholar]
- 5.Ngoungou EB, et al. Epilepsy as a consequence of cerebral malaria in area in which malaria is endemic in Mali, West Africa. Epilepsia. 2006;47:873–879. doi: 10.1111/j.1528-1167.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 6.Snow RW, et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 7.Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–8240. doi: 10.1016/S1474-4422(05)70247-7. [DOI] [PubMed] [Google Scholar]
- 8.Brewster DR, Kwiatkowski D, White NJ. Neurological sequelae of cerebral malaria in children. Lancet. 1990;336:1039–1043. doi: 10.1016/0140-6736(90)92498-7. [DOI] [PubMed] [Google Scholar]
- 9.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in pediatric cerebral malaria: A study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]
- 10.Idro R, Carter JA, Fegan G, Neville BG, Newton CR. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child. 2006;91:142–148. doi: 10.1136/adc.2005.077784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carnot P, Deflandre C. Sur l'activité hémopoiétique des différents organes au cours de la régénération du sang. C R Acad Sci. 1906;143:384–386. [Google Scholar]
- 12.Masuda S, et al. A novel site of erythropoietin production: Oxygen-dependent production in cultured rat astrocytes. J Biol Chem. 1994;269:19488–19493. [PubMed] [Google Scholar]
- 13.Sakanaka M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brines ML, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grasso G, et al. Beneficial effects of systemic administration of recombinant human erythropoietin in rabbits subjected to subarachnoid hemorrhage. Proc Natl Acad Sci USA. 2002;99:5627–5631. doi: 10.1073/pnas.082097299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R, Suzuki A, Guo Z, Mizuno Y, Urabe T. Intrinsic and extrinsic erythropoietin enhances neuroprotection against ischemia and reperfusion injury in vitro. J Neurochem. 2006;96:1101–1110. doi: 10.1111/j.1471-4159.2005.03597.x. [DOI] [PubMed] [Google Scholar]
- 17.Siren AL, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen TC, et al. Erythropoietin protects neurons against chemical hypoxia and cerebral ischemic injury by up-regulating Bcl-xL expression. J Neurosci Res. 2002;67:795–803. doi: 10.1002/jnr.10166. [DOI] [PubMed] [Google Scholar]
- 19.Ehrenreich H, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 20.Casals-Pascual C, et al. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood. 2006;108:2569–2577. doi: 10.1182/blood-2006-05-018697. [DOI] [PubMed] [Google Scholar]
- 21.Kurtzhals JA, et al. Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparum malaria. Br J Haematol. 1997;97:169–174. doi: 10.1046/j.1365-2141.1997.82654.x. [DOI] [PubMed] [Google Scholar]
- 22.Newton CR, et al. Severe anemia in children living in a malaria ndemic area of Kenya. Trop Med Int Health. 1997;2:165–178. doi: 10.1046/j.1365-3156.1997.d01-238.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser K, et al. Recombinant human erythropoietin prevents the death of mice during cerebral malaria. J Infect Dis. 2006;193:987–995. doi: 10.1086/500844. [DOI] [PubMed] [Google Scholar]
- 24.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, et al. Vascular endothelial growth factor improves recovery of sensorimotor and cognitive deficits after focal cerebral ischemia in the rat. Brain Res. 2006;1115:186–193. doi: 10.1016/j.brainres.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 27.Croll SD, Goodman JH, Scharfman HE. Vascular endothelial growth factor (VEGF) in seizures: A double-edged sword. Adv Exp Med Biol. 2004;548:57–68. doi: 10.1007/978-1-4757-6376-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang ZG, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi OZ, Hunter C, Liu X, Weiss HR. Effects of VEGF and nitric oxide synthase inhibition on blood-brain barrier disruption in the ischemic and nonischemic cerebral cortex. Neurol Res. 2005;27:864–868. doi: 10.1179/016164105X49418. [DOI] [PubMed] [Google Scholar]
- 30.Intiso D, et al. Tumor necrosis factor alpha serum levels and inflammatory response in acute ischemic stroke patients. Neurol Sci. 2004;24:390–396. doi: 10.1007/s10072-003-0194-z. [DOI] [PubMed] [Google Scholar]
- 31.Barone FC, et al. Tumor necrosis factor-alpha: A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 32.Grau GE, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 33.Kwiatkowski D, et al. TNF concentration in fatal cerebral, nonfatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 34.Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207:3233–3242. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- 35.Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- 36.Villa P, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiese L, Kurtzhals JA, Penkowa M. Neuronal apoptosis, metallothionein expression and proinflammatory responses during cerebral malaria in mice. Exp Neurol. 2006;200:216–226. doi: 10.1016/j.expneurol.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Namiuchi S, et al. High serum erythropoietin level is associated with smaller infarct size in patients with acute myocardial infarction who undergo successful primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;45:1406–1412. doi: 10.1016/j.jacc.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell A, et al. Age-related changes in adaptation to severe anemia in childhood in developing countries. Proc Natl Acad Sci USA. 2007;104:9440–9444. doi: 10.1073/pnas.0703424104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fandrey J, et al. Cobalt chloride and desferrioxamine antagonize the inhibition of erythropoietin production by reactive oxygen species. Kidney Int. 1997;51:492–496. doi: 10.1038/ki.1997.68. [DOI] [PubMed] [Google Scholar]
- 41.Siddiq A, et al. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition: A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280:41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith HJ, Meremikwu M. Iron chelating agents for treating malaria. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD001474. [DOI] [PubMed] [Google Scholar]
- 43.Gordeuk V, et al. Effect of iron chelation therapy on recovery from deep coma in children with cerebral malaria. N Engl J Med. 1992;327:1473–1477. doi: 10.1056/NEJM199211193272101. [DOI] [PubMed] [Google Scholar]
- 44.Deininger MH, Winkler S, Kremsner PG, Meyermann R, Schluesener HJ. Angiogenic proteins in brains of patients who died with cerebral malaria. J Neuroimmunol. 2003;142:101–111. doi: 10.1016/s0165-5728(03)00250-9. [DOI] [PubMed] [Google Scholar]
- 45.Levy AP, Levy NS, Goldberg MA. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem. 1996;271:2746–2753. doi: 10.1074/jbc.271.5.2746. [DOI] [PubMed] [Google Scholar]
- 46.Newton CR, et al. Intracranial pressure in African children with cerebral malaria. Lancet. 1991;337:573–576. doi: 10.1016/0140-6736(91)91638-b. [DOI] [PubMed] [Google Scholar]
- 47.Ryuto M, et al. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells: Possible roles of SP-1. J Biol Chem. 1996;271:28220–28228. doi: 10.1074/jbc.271.45.28220. [DOI] [PubMed] [Google Scholar]
- 48.Nagai A, et al. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J Neuropathol Exp Neurol. 2001;60:386–392. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- 49.Leist M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 50.Berkley JA, et al. Diagnosis of acute bacterial meningitis in children at a district hospital in sub-Saharan Africa. Lancet. 2001;357:1753–1757. doi: 10.1016/S0140-6736(00)04897-2. [DOI] [PubMed] [Google Scholar]
- 51.Njuguna P, Newton C. Management of severe falciparum malaria. J Postgrad Med. 2004;50:45–50. [PubMed] [Google Scholar]
- 52.WHO Expert Committee on Malaria. Twentieth Report. Geneva: WHO; 2000. [Google Scholar]
- 53.Idro R, Aloyo J. Manifestations, quality of emergency care, and outcome of severe malaria in mulago hospital. Uganda Afr Health Sci. 2004;4:50–57. [PMC free article] [PubMed] [Google Scholar]