Abstract
The PD-1 costimulatory receptor inhibits T cell receptor signaling upon interacting with its ligands PD-L1 and PD-L2. The PD-1/PD-L pathway is critical in maintaining self-tolerance. In this study, we examined the role of PD-1 in a mouse model of acute infection with Histoplasma capsulatum, a major human pathogenic fungus. In a lethal model of histoplasmosis, all PD-1-deficient mice survived infection, whereas the wild-type mice died with disseminated disease. PD-L expression on macrophages and splenocytes was up-regulated during infection, and macrophages from infected mice inhibited in vitro T cell activation. Of interest, antibody blocking of PD-1 significantly increased survival of lethally infected wild-type mice. Thus, our studies extend the role of the PD-1/PD-L pathway in regulating antimicrobial immunity to fungal pathogens. The results show that the PD-1/PD-L pathway has a key role in the regulation of antifungal immunity, and suggest that manipulation of this pathway represents a strategy of immunotherapy for histoplasmosis.
Keywords: costimulation, fungal infection, programmed death-1
Histoplasmosis, caused by Histoplasma capsulatum (HC) is the most prevalent fungal respiratory disease in the US, affecting ≈500,000 individuals each year (1). Infection typically results in a mild, often asymptomatic respiratory illness but may progress to life-threatening systemic disease, particularly in immunocompromised individuals. Upon inhalation, Hc is ingested by resident pulmonary macrophages, where the fungus replicates and subsequently disseminates to other organs. Macrophages are considered the most important effector cells in host resistance against histoplasmosis by functioning in both innate and cell-mediated immunity (2). However, resolution of histoplasmosis depends on the activation of cell-mediated immunity, in particular effective T cell responses (1). Both CD4+ and CD8+ T cells contribute to host resistance in primary Hc infection. Reduction of CD4+ T cells results in fatal histoplasmosis in naïve mice and adoptive transfer of Hc reactive CD4+ T cells confers protection (3, 4). In mice that lack CD8+ T cells, clearance of Hc from organs is impaired (3, 4). Sublethal infection with Hc evokes a Th1-like response in mice, characterized by the dominance of IL-12, TNF-α, and IFN-γ during the acute phase of infection (5). Upon induction of cell-mediated immunity and the production of cytokines, macrophages are activated, and the fungus is eliminated. The importance of B cells in primary histoplasmosis is less critical (3), however, in B cell-deficient animals the progression toward lethal infection is accelerated in reactivation disease (6).
Programmed cell death-1 (PD-1, CD279) is an immune inhibitory receptor belonging to the CD28:B7 family of costimulatory molecules, which is expressed on activated T cells, B cells, and myeloid cells (7). PD-1 binds to two ligands, PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273). PD-L2 has higher affinity to PD-1 and is expressed on activated dendritic cells and macrophages whereas PD-L1 is expressed on T cells, B cells, dendritic cells (DC), and a variety of nonhematopoietic cell types (8–10). Engagement of PD-1 by its ligands simultaneously with TCR or BCR cross-linking induces negative signaling by recruitment of phosphatases such as SHP-2 and dephosphorylation of effector molecules involved in downstream TCR or BCR signaling (11). PD-1 has a crucial role in initiating and maintaining peripheral tolerance, consistent with the finding that PD-1-deficient mice (Pdcd1−/−) develop various spontaneous autoimmune diseases depending on the genetic background (12).
Mounting evidence suggests that the PD-1–PD-L pathway plays a central role in the interaction between host and pathogenic microbes that evolved to resist immune responses (13). Functional impairment (exhaustion) of virus-specific CD8+ T cells in chronic LCMV infection of mice is associated with elevated PD-1 expression on the exhausted cells (14). PD-1 expression is up-regulated on T cells after HIV infection, and it is associated with T cell exhaustion and disease progression (15–17). Blockade of the PD-1–PD-L interactions reverses the exhaustion of virus-specific T cells and restores effector functions, cytokine production, and cell proliferation. The PD-1–PD-L pathway is also exploited by parasites, such as Schistosoma mansoni and Taenia crassiceps, which suppress effective immune responses by up-regulating PD-Ls on host macrophages (18, 19). Of further interest, the pathogenic bacteria Helicobacter pylori has been found to up-regulate PD-L1 on gastric epithelial cells inducing host unresponsiveness and blockade of PD-L1 results in enhanced T cell proliferation and cytokine production (20).
Although the importance of the PD-1–PD-L pathway has been studied in several infection models, there are no data available concerning the role of this pathway in fungal infections. In this study, we report the crucial role of the PD-1–PD-L pathway in a fungal infection using a mouse model of histoplasmosis. Most strikingly, PD-1-deficient mice are resistant to lethal challenge with Hc. During infection, PD-L1 is up-regulated on alveolar and peritoneal macrophages as well as on all mononuclear cells in the lungs and on total splenocytes, and PD-L2 is up-regulated on macrophages and DCs in the lung. The macrophages expressing elevated PD-L1 levels inhibit proliferation and cytokine production upon interaction with CD4+ and CD8+ T cells in vitro, suggesting that these macrophages similarly suppress T cell activation in infected hosts. Furthermore, blockade of the PD-1 pathway in wild-type mice by administration of monoclonal antibody to PD-1 increased survival by 70%. Our data demonstrate the importance of the PD-1–PD-L pathway in experimental histoplasmosis and suggest that this pathway is a target for immunotherapy in histoplasmosis and, perhaps, in other fungal diseases.
Results
PD-1-Deficient Mice Survive Lethal Hc Challenge.
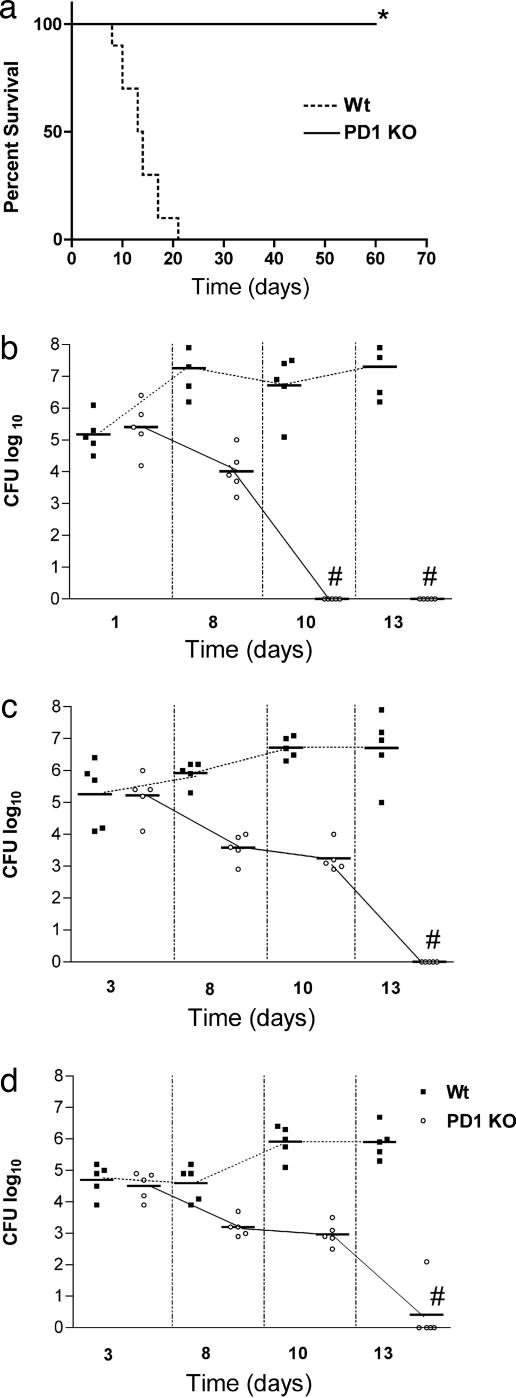
To study the importance of the PD-1/PD-L pathway in histoplasmosis, groups of PD-1-deficient and control C57BL/6 mice were infected with 1.25 × 107 Hc yeast cells and disease was monitored. In this model of histoplasmosis, all wild-type mice died by day 25 after infection. In contrast, 100% of PD-1−/− mice survived, and they were disease free for >90 days after infection (Fig. 1a). Moreover, PD-1-deficient mice also survived challenge with a dose 10 times higher than the standard lethal dose (data not shown). Initial colony-forming unit (cfu) values obtained after infection with sublethal 5 × 106 Hc yeast cells were similar between wild-type and PD-1-deficient mice, showing that the same inoculum was delivered to both PD-1−/− and wild-type mice. However, in contrast to a steady increase in the wild-type mice, the pathogen burden rapidly decreased in the lungs of PD-1−/− mice, and it could not be detected by day 10 after infection (Fig. 1b). Notably, PD-1-deficient mice also developed disseminated disease, as shown by the cfu values obtained from the liver and spleen 3 days after infection (Fig. 1 c and d). However, by day 13 after infection, PD-1-deficient mice totally eradicated the Hc.
Fig. 1.
PD-1-deficient mice survive lethal Hc challenge. (a) Survival curves of C57BL/6 (n = 10) and PD1−/− mice (n = 10) infected intranasally with 1.25 × 107 Hc yeast cells monitored during a 70-day period, *, P = 0.0002 (log-rank test). (b–d) Cfu in the lung (b), liver (c), and spleen (d) from C57BL/6 and PD1−/− mice infected with a sublethal inoculum (5 × 106) of Hc yeast cells. Each symbol represents one mouse, and horizontal bars represent median values for each group. P ≤ 0.0049 (Kruskal–Wallis test). #, no detectable cfu. Data are representative of two independent experiments.
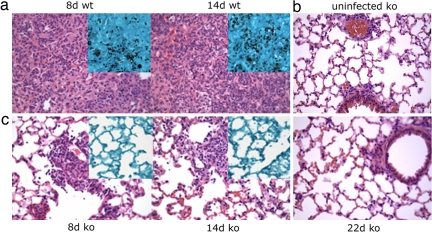
Histological analysis shows that wild-type mice develop progressive pneumonia, whereas the alveolar spaces of PD-1−/− mice are largely intact during the observed time intervals. At day 8, wild-type mice have bronchointerstitial pneumonia, manifested by edema and perivascular inflammation with thickened alveolar walls, as well as some vascular thrombosis (Fig. 2a). By day 10, the disease in wild-type mice progresses to necrotizing inflammation with thickened alveolar walls and substantial neutrophil infiltration. After day 14, persisting necrotizing pneumonia and fibrosis, as well as loss of airways due to edema and congestion, likely lead to the death of the wild-type mice. GMS staining specific for fungal cells shows an abundance of Hc yeast cells present in the lungs of wild-type mice (Fig. 2a Insets). In contrast, lungs of the PD-1−/− mice show rare areas of perivascular inflammation at day 8, GMS staining reveals no visible organisms (Fig. 2c). At days 10 and 14, multifocal sites of inflammation were present peribronchially, and lymphocytic infiltrates were found around some blood vessels, and cfu assays revealed that the lungs were sterile by day 10. At day 22 after infection, the lungs of the PD-1-deficient mice look similar to those of the mock-infected mice (Fig. 2b), indicating resolution of disease. Although minor adventitial vasculitis can be detected around some of the pulmonary vessels, similar findings were present in the lungs of the mock-infected PD-1-deficient mice. These data clearly show that, although Hc can cause a mild form of disease in PD-1-deficient mice, they can efficiently clear the pathogen and survive high-inoculum histoplasmosis.
Fig. 2.
PD-1-deficient mice develop less severe histoplasmosis than wild-type mice. Histological analysis of the lungs of Hc-infected C57BL/6 mice at days 8 and 14 after infection (a) and mock-infected PD-1−/− (b) and Hc-infected PD-1−/− mice 8, 14, and 22 days after infection (c). H&E-stained sections of the lungs are shown with the Insets representing GMS staining specific for Hc organisms (shown in black). (Original magnification, ×400.)
Hc Causes Up-Regulation of PD-L1 and PD-L2 Expression on Macrophages and Other Cell Types.
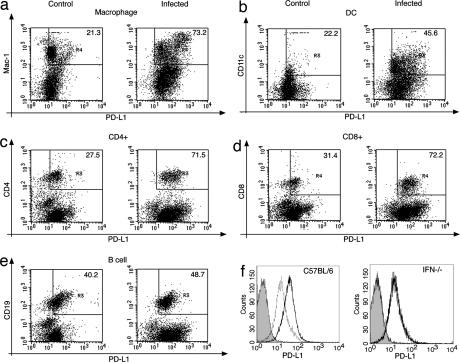
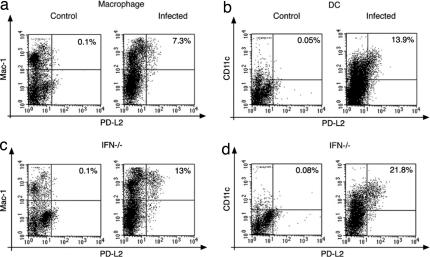
Because our findings showed that the lack of a functional PD-1/PD-L pathway abrogated the capacity of Hc to damage the host, we investigated whether the fungus can manipulate this inhibitory pathway by influencing the expression levels of the PD-1 receptor or its ligands, PD-L1 and PD-L2. FACS analysis showed that PD-L1 was markedly up-regulated on lung macrophages and dendritic cells, but also on CD4+ and CD8+ T cells and, to a lesser extent, on B cells [Fig. 3 a–e and supporting information (SI) Table 1]. Interestingly, PD-L2 expression was also up-regulated on a relatively small subset of macrophages (7.3% of lung macrophages; Fig. 4a) and dendritic cells (13.9% of lung DCs; Fig. 4b) in the lungs of Hc-infected animals. In contrast, PD-1 expression did not change upon Hc infection on any cell types examined at this time point. The expression levels of other costimulatory ligands such as B7–1, B7–2, and ICOS-ligand were not significantly different in Hc-infected versus control mice (data not shown).
Fig. 3.
PD-L1 is up-regulated on lung mononuclear cells and splenocytes of Hc-infected mice. (a–e) FACS analysis of lung macrophages (a), dendritic cells (b), CD4+ (c) and CD8+ T cells (d), and B cells (e) shows higher levels of PD-L1 expression after Hc infection [uninfected control mice (Left) and infected mice (Right)]. Values shown represent geometric mean fluorescence intensities for PD-L1, measured for the gated double-positive cells. Gates were set up by using the unstained and the appropriate isotype controls. Representative data of five independent experiments are shown. (f) PD-L1 up-regulation on splenocytes of Hc-infected mice depends on the presence of IFN-γ. PD-L1 expression on splenocytes of control mice (thin line) and Hc-infected mice (bold line) are shown, compared with isotype control antibody (shaded histogram). (Left) C57BL/6 mice. (Right) IFN-γ-deficient mice. Data are representative of two independent experiments.
Fig. 4.
PD-L2 is up-regulated on lung macrophages and dendritic cells in Hc-infected mice. (a and b) PD-L2 expression is increased in the lungs of Hc-infected mice on macrophages (a) and dendritic cells (b) of C57BL/6 mice. (c and d) Higher numbers of macrophages (c) and dendritic cells (d) express PD-L2 in the lungs of IFN-γ-deficient mice infected with Hc. (Left) Data from control mice. (Right) Data from infected mice. Values shown are percentages of PD-L2+ macrophages from the total macrophage population. Quadrants were set up by using the appropriate isotype controls so that the lower left quadrant contains cells that are negative for both antigens. Data are representative of two independent experiments performed with 2–5 mice in each group.
Flow-cytometric analysis of the spleen showed a broad up-regulation of PD-L1 on total splenocytes in the infected mice (Fig. 3f). In the spleen, PD-L1 was markedly up-regulated on DCs, macrophages, and CD4+ and CD8+ T cells, B cells, and natural killer (NK) cells as well (SI Fig. 7). When PD-L1 expression levels were compared on mononuclear cells from the lungs and the spleen, there was a tendency toward higher PD-L1 levels at the primary site of the infection. CD8+ and CD4+ T cells in the lungs of infected mice had 30–40% higher PD-L1 expression, compared with the CD8+ and CD4+ T cells in the spleen (P = 0.049 and 0.007, SI Table 1).
IFN-γ is known to be the most important regulator of PD-L1 expression (21). To test its involvement in Hc-induced PD-L up-regulation, IFN-γ-deficient mice were infected and examined for PD-L expression. CD4+ and CD8+ T cells in the lungs of infected IFN-γ-deficient mice showed 20–40% lower PD-L1 expression (P = 0.009 and 0.049) compared with wild-type mice, and there was no increase of PD-L1 expression on B cells (P = 0.009, SI Table 2). In contrast, there was no increase of PD-L1 expression on splenocytes of the IFN-γ-deficient mice infected with Hc, suggesting that the up-regulation of PD-L1 on splenocytes depends on the presence of IFN-γ (Fig. 3f Right). The ratio of cells expressing PD-L2 was ≈2-fold higher in infected IFN-γ deficient mice compared with wild-type mice: 13% of macrophages (Fig. 4c) and 21.8% of DCs (Fig. 4d) expressed PD-L2. These data show that infection with Hc induces an increased expression of PD-Ls that is also modulated by the availability of cytokines, such as IFN-γ.
Macrophages from Hc-infected Mice Suppress T Cell Activation in Vitro.
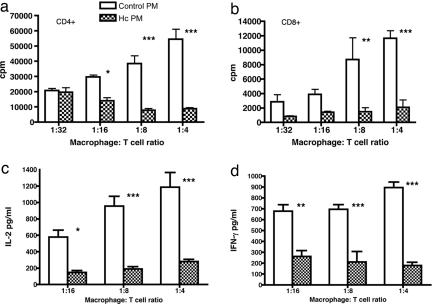
To investigate whether increased PD-L1 expression on cells from Hc-infected mice can induce immunosuppression, in vitro T cell assay was performed. Naïve CD4+ or CD8+ T cells purified from spleens of C57BL/6 mice were activated with low-dose anti-CD3, and macrophages from control or Hc infected mice were added to them in different ratios, keeping the T cell number constant. Peritoneal macrophages that have been shown to up-regulate PD-L1 expression after Hc infection (SI Fig. 8) were used for this assay because they can be obtained easily and in reasonable numbers. Fig. 5 a and b shows that the presence of naïve macrophages greatly increased proliferation of CD4+ and CD8+ T cells in response to anti-CD3 activation. In contrast, addition of the same number of macrophages from Hc-infected mice failed to increase proliferation of CD4+ or CD8+ T cells. CD4+ T cell proliferation was inhibited by as much as 53% compared with the control when infected macrophages were added at a ratio of 1:16 to T cells, the strongest inhibition being >80% at the ratio of 1:4. Moreover, CD8+ T cell proliferation was inhibited by >60% at a 1:32 macrophage-to-T cell ratio, and the highest inhibition was 80% at a 1:4 ratio. Next we looked at the early and late cytokine responses elicited by anti-CD3-activated T cells in the presence of macrophages from naïve or Hc-infected mice. Macrophages from naïve mice increased anti-CD3-activated secretion of cytokines such as IL-2 and IFN-γ from CD4+ T cells (Fig. 5 c and d). However, addition of macrophages from infected mice substantially decreased cytokine production. IL-2 levels were decreased by 75% and IFN-γ levels by 60–80% at 1:16 and 1:4 macrophage-to-T cell ratios. Macrophages from Hc-infected mice markedly inhibited cytokine secretion of CD8+ T cells as well (SI Fig. 9). These data suggest that Hc induces a suppressive phenotype of macrophages possibly through the up-regulation of PD-L1, which, in turn, inhibits activated CD4+ and CD8+ T cells that could potentially eliminate the pathogen.
Fig. 5.
Macrophages from Hc-infected mice inhibit T cell activation. (a and b) In vitro proliferation of CD4+ (a) and CD8+ (b) T cells cocultured with macrophages from control (open histograms) or Hc infected mice (hatched histograms). Mean values and SEM are shown. (c and d) Cytokine production of in vitro-activated CD4+ T cells: IL-2 (c) and IFN-γ (d) are significantly reduced in the presence of macrophages from Hc-infected mice compared with macrophages from control mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (ANOVA, Bonferroni posttest).
Blockade of the PD-1 Pathway Increases Survival of Lethally Infected Wild-Type Mice.
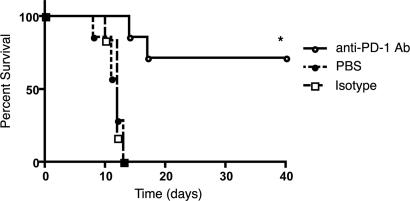
Based on our data showing that the absence of PD-1 renders mice resistant to experimental histoplasmosis, we hypothesized that blockade of this pathway in wild-type mice could be beneficial to enhance host immune responses and possibly facilitate the elimination of the infectious agent. To verify this hypothesis, we infected groups of mice with 1.25 × 107 Hc yeast cells and treated them with either PD-1-blocking antibody or isotype control. The treatment was started 1 day after infection and consisted of a total of three doses, one dose given every 3 days. All of the untreated mice and those receiving the isotype control antibody were dead by day 12 after infection. However, 70% of the mice treated with the monoclonal antibody to PD-1 (clone 29F.1A12) survived (Fig. 6). Importantly, in a repeated experiment continued administration of the antibodies up to a total of five doses increased survival to 90% (data not shown). Histological analysis of the lungs of mice from each group performed 7 days after infection confirmed that, whereas untreated or isotype control treated mice developed severe generalized bronchointerstitial pneumonia with edema and necrosis, anti-PD-1-treated mice had more contained, focal pneumonia that ultimately resolved (SI Fig. 10). Anti-PD-1-treated mice survived and were disease free for the follow-up period (>6 months).
Fig. 6.
Blockade of PD-1 protects mice from lethal histoplasmosis. Survival curves of C57BL/6 mice (n = 10 for each group) lethally infected with Hc treated with blocking monoclonal antibody to PD-1, isotype control antibody, or saline. *, P = 0.005 (log-rank test).
Discussion
Pathogens have developed diverse mechanisms to resist host immune responses. Recent findings suggest that the PD-1/PD-L pathway plays an important role in the complex interactions between host and pathogenic microbes. The PD-1/PD-L pathway critically regulates T cell responses during chronic viral infections in mice and humans. PD-1 expression is up-regulated on exhausted virus-specific T cells causing reversible immune dysfunction and disease progression in both chronic lymphocytic choriomeningitis virus (LCMV) infection in mice (14) and HIV infection in humans (15–17). Blockade of the PD-1/PD-L pathway efficiently restored the virus-specific effector functions of the exhausted T cells (14–17). In addition, pathogens such as certain bacteria, protozoa, and some worms can exploit this pathway to evade host immune responses (18, 19, 22). Although there is some evidence that the PD-1/PD-L pathway influences immune responses after acute infections, this question has not been addressed for fungal disease.
Using a murine model of histoplasmosis, our studies show that the PD-1/PD-L pathway is crucial in fungal pathogenesis. Strikingly, mice that are deficient in PD-1 show 100% survival after high-inoculum Hc challenge, demonstrating that, in the absence of a functional PD-1/PD-L activity, the fungus is unable to overcome host effector responses (Fig. 1a). Importantly, pulmonary and disseminated disease occur in the PD-1-deficient mice, but the subsequent immunological responses in these mice and wild-type animals differ dramatically. The lungs of both wild-type and PD-1−/− mice have similar cfu values 1 day after infection (Fig. 1b); however, in the PD-1−/− mice, cfu values rapidly decrease, whereas they steadily increase in the wild-type mice. Histological studies of the lungs show that infected PD-1-deficient mice develop pathological findings that are similar but less severe compared with wild-type mice and that the inflammatory responses in the PD-1-deficient mice resolve, and the mice survive.
This study shows that the absence of a T cell costimulatory molecule such as PD-1 confers protection against Hc infection in mice. In similar studies that used mice deficient in CD40L, another costimulatory molecule involved in regulating T cell responses and production of Th1 cytokines there were no differences in survival or fungal burden (4).
Our findings show that a functional PD-1 pathway is essential for this fungal pathogen to progressively invade and kill the host. It is also extremely relevant that the pathogen itself can modulate this pathway. Our data show a substantial up-regulation of PD-L1 on primary alveolar and peritoneal macrophages infected with Hc (Fig. 3a and SI Fig. 8). Moreover, Hc infection also induces up-regulation of PD-L1 on other cell types, such as DCs, CD4+, and CD8+ T cells and B cells in the lung and spleen (Fig. 3 b–f and SI Table 1). IFN-γ is essential for PD-L1 up-regulation on splenocytes (Fig. 3f) but not in the lungs of Hc-infected mice (SI Table 2), suggesting that other mechanisms are involved, such as a direct effect of the pathogen or stimulation from other cytokines released by the infected macrophages. PD-L2 up-regulation was also detected on a subset of macrophages and DCs in the lungs of Hc-infected wild-type mice (Fig. 4). When IFN-γ-deficient mice were infected, there was a twofold increase in the number of PD-L2-expressing macrophages and DCs, suggesting that IFN-γ has a negative effect on PD-L2 expression (24). However, IFN-γ is a key effector cytokine in host resistance against histoplasmosis, and absence or blockade of IFN-γ enhances the severity of Hc infection (25, 26). Because of the complexity of the role of IFN-γ in Hc infection, it remains to be clarified whether the effects observed in the IFN-γ-deficient mice are due to the direct effects of IFN-γ or secondary changes in the pathogenesis of the infection in the absence of this critical cytokine.
Unlike in other infection models, such as chronic viral infections, we were not able to detect up-regulation of PD-1 on any cell types in Hc-infected mice in this acute experimental model at 1 week after infection. It remains to be determined whether this is due to the low percentage of antigen-specific cells or to the transient kinetics of PD-1 expression in acute Hc infection.
Our data suggest that Hc is able to induce suppression of T cell responses facilitating its survival within the host. Peritoneal macrophages from Hc-infected mice expressing high levels of PD-L1 (SI Fig. 8) significantly inhibited in vitro proliferation and cytokine production of CD4+ and CD8+ T cells, whereas control macrophages from uninfected mice dose-dependently enhanced anti-CD3-induced T cell activation (Fig. 5). The inhibition observed here could be the direct effect of the increased expression of PD-L1 on macrophages from the infected mice. However, we cannot exclude the role of other mediators, such as nitric oxide, that has been shown to be involved in inducing a suppressive phenotype in macrophages (27). Similar studies using other parasites such as Taenia crassiceps and Schistosoma mansoni have also shown induction of anergy of naïve T cells through the selective up-regulation of PD-L1 and PD-L2 on macrophages (18, 19). Okazaki et al. (12) suggested a model in which expression of PD-L versus other costimulatory ligands on DCs would decide the fate of T cell activation, resulting in either inactivation/anergy or efficient activation. In accordance with this, in our model, selective increase of PD-L1 or PD-L2, but not B7–1, B7–2, or ICOSL, on Hc-infected macrophages induces a suppressive phenotype, in which PD-L expression will predominate over other “positive” costimulatory ligands. Macrophages expressing this suppressive phenotype triggered by the presence of the pathogen will, in turn, inhibit activated T cells aimed to eliminate the pathogen, favoring its survival.
PD-L1 up-regulation on other cell types such as CD4+ and CD8+ T cells could lead, through an as yet to be identified mechanism (perhaps involving T cell–T cell signaling; possibly through the recently identified PD-L1/B7–1 interaction), to a decrease in the number of CD4+ or CD8+ T cells that will again facilitate the survival of the pathogen (28). Based on this model, blockade of the PD-1 pathway in wild-type mice would enhance clearance of the pathogen and promote survival of the host, similar to what occurs in the PD-1−/− mice.
Indeed, our data show that administration of monoclonal antibody to PD-1 can efficiently prevent Hc-induced lethality in wild-type mice resulting in >70% survival (Fig. 6). Importantly, our data strongly suggest that therapeutic targeting of the PD-1 pathway could be beneficial in the management of histoplasmosis. The significance of our findings is highlighted by the fact that, despite intensive therapy with amphotericin B, mortality rates in disseminated histoplasmosis range from 5% to 10% in immunologically intact individuals (29, 30) and 46–70% in patients with HIV infection (31, 32). Hc is an opportunistic pathogen highly associated with severe disease in individuals infected with HIV (in fact it is an AIDS-defining infection). Because PD-1 was shown to be up-regulated on exhausted CD8+ T cells in chronic viral infections such HIV, it is reasonable to suggest that blockade of the PD-1 pathway would benefit the host immune response against both pathogens through restoration of the antiviral function of CD8+ T cells and abolishing T cell suppression mediated through Hc-infected antigen-presenting cells (APCs). This supposition is supported by the finding that sublethal Hc infection associated with persistent infection of LCMV clone 13 resulted in reduced immunity leading to increased fungal burdens and high mortality (33). Because LCMV has been shown to cause exhaustion of CD8+ T cells through up-regulation of PD-1 (14), it is intriguing to consider that Hc can cause fatal disease in these mice by efficiently avoiding host immune responses in the setting of high expression of both PD-Ls and PD-1.
Overall, our studies extend the role of the PD-1/PD-L pathway in regulating antimicrobial immunity to fungal pathogens by showing that the PD-1/PD-L costimulatory pathway dramatically affects immune responses against Hc. Blockade of this pathway by antibodies or other pharmacological agents could offer therapeutic tools in the treatment of histoplasmosis and perhaps other fungal diseases.
Materials and Methods
Mice.
C57BL/6 mice (6–12 weeks old) were purchased from NCI. PD-1-deficient mice in the C57BL/6 background were kindly provided by Tasuku Honjo (Kyoto University) and were used in the experiments at 6–12 weeks of age. IFN-γ-deficient mice (6–8 weeks old) were purchased from The Jackson Laboratory. All mice were maintained in pathogen-free conditions in the animal facility at Albert Einstein College of Medicine (AECOM). All animal work was performed according to the guidelines set by the AECOM Institutional Animal Care and Use Committee.
Fungus and Infections.
Hc var. capsulatum ATCC 26032 (G217B) was obtained from the American Type Culture Collection. Infections, cfu counts, and histological analysis were performed as described in SI Experimental Procedures.
Cell Preparation.
Alveolar and peritoneal lavage was used to isolate alveolar and peritoneal macrophages, from infected and control mice 7 days after infection. Single-cell suspensions were prepared by collagenase digestion from lungs (34) and spleens as described in SI Experimental Procedures.
Flow Cytometry.
Anti-CD11b-APC, anti-CD19-PE-, FITC-, and APC-conjugated anti-CD11c, anti-F4/80-APC, anti-CD49b-APC, anti-PD-L1-PE, and biotinylated antibodies for PD-1, PD-L1, and PD-L2 were purchased from eBiosciences, anti-CD4-PE and anti-CD8-FITC from BD Biosciences. FACS staining and analysis were performed as described in SI Experimental Procedures.
In Vitro T Cell Assays.
T cell assays were performed as described in SI Experimental Procedures.
Blocking Antibody Treatment.
For blocking PD-1 in vivo, 200 μg of rat anti-mouse PD-1 monoclonal antibody (clone 29F.1A12) (35) in PBS was administered i.p. every third day. Rat IgG2b isotype control (Bioexpress) was similarly administered. Ten mice were used for each group.
Statistical Analysis.
Statistical analysis was performed as described in SI Experimental Procedures.
Supplementary Material
ACKNOWLEDGMENTS.
We thank the Flow Cytometry Core Facility supported by National Cancer Institute Cancer Center Grant P30CA013330; the Histopathology Shared Resource of the Albert Einstein Cancer Center, especially Rani Sellers, for help with the histopathological analysis; and Teresa DiLorenzo for critical reading of the manuscript. We thank T. Honjo (Kyoto University, Kyoto, Japan) and L. Chen (The Johns Hopkins University) for providing the PD-1/1 mice. E.L.-M. was supported by a postdoctoral fellowship from the Cancer Research Institute. A.G. and J.D.N. were supported in part by National Institutes of Health (NIH) Grant AI056070-01A2. J.D.N. was also partially supported by a Wyeth Vaccine Young Investigator Research Award from the Infectious Disease Society of America and the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH Grant AI-51519). S.C.A. and S.G.N. were supported by NIH Grant AI07289.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711918105/DC1.
References
- 1.Deepe GS., Jr Immune response to early and late Histoplasma capsulatum infections. Curr Opin Microbiol. 2000;3:359–362. doi: 10.1016/s1369-5274(00)00104-1. [DOI] [PubMed] [Google Scholar]
- 2.Newman SL. Macrophages in host defense against Histoplasma capsulatum. Trends Microbiol. 1999;7:67–71. doi: 10.1016/s0966-842x(98)01431-0. [DOI] [PubMed] [Google Scholar]
- 3.Allendorfer R, Brunner GD, Deepe GS., Jr Complex requirements for nascent and memory immunity in pulmonary histoplasmosis. J Immunol. 1999;162:7389–7396. [PubMed] [Google Scholar]
- 4.Zhou P, Seder RA. CD40 ligand is not essential for induction of type 1 cytokine responses or protective immunity after primary or secondary infection with Histoplasma capsulatum. J Exp Med. 1998;187:1315–1324. doi: 10.1084/jem.187.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cain JA, Deepe GS., Jr Evolution of the primary immune response to Histoplasma capsulatum in murine lung. Infect Immun. 1998;66:1473–1481. doi: 10.1128/iai.66.4.1473-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen HL, Deepe GS., Jr B cells and CD4−CD8− T cells are key regulators of the severity of reactivation histoplasmosis. J Immunol. 2006;177:1763–1771. doi: 10.4049/jimmunol.177.3.1763. [DOI] [PubMed] [Google Scholar]
- 7.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 8.Dong H, Zhu G, Tamada K, Chen L. B7–H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 9.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 11.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki T, Honjo T. The PD-1–PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 14.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 15.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 16.Petrovas C, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trautmann L, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 18.Smith P, et al. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J Immunol. 2004;173:1240–1248. doi: 10.4049/jimmunol.173.2.1240. [DOI] [PubMed] [Google Scholar]
- 19.Terrazas LI, Montero D, Terrazas CA, Reyes JL, Rodriguez-Sosa M. Role of the programmed death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int J Parasitol. 2005;35:1349–1358. doi: 10.1016/j.ijpara.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Das S, et al. Expression of B7–H1 on gastric epithelial cells: Its potential role in regulating T cells during Helicobacter pylori infection. J Immunol. 2006;176:3000–3009. doi: 10.4049/jimmunol.176.5.3000. [DOI] [PubMed] [Google Scholar]
- 21.Mazanet MM, Hughes CC. B7–H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 22.Liang SC, et al. PD-L1 and PD-L2 have distinct roles in regulating host immunity to cutaneous leishmaniasis. Eur J Immunol. 2006;36:58–64. doi: 10.1002/eji.200535458. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 24.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci USA. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou P, Miller G, Seder RA. Factors involved in regulating primary and secondary immunity to infection with Histoplasma capsulatum: TNF-alpha plays a critical role in maintaining secondary immunity in the absence of IFN-gamma. J Immunol. 1998;160:1359–1368. [PubMed] [Google Scholar]
- 26.Allendoerfer R, Deepe GS., Jr Intrapulmonary response to Histoplasma capsulatum in gamma interferon knockout mice. Infect Immun. 1997;65:2564–2569. doi: 10.1128/iai.65.7.2564-2569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 28.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assi MA, Sandid MS, Baddour LM, Roberts GD, Walker RC. Systemic histoplasmosis: A 15-year retrospective institutional review of 111 patients. Medicine (Baltimore) 2007;86:162–169. doi: 10.1097/md.0b013e3180679130. [DOI] [PubMed] [Google Scholar]
- 30.Tobon AM, et al. Disseminated histoplasmosis: A comparative study between patients with acquired immunodeficiency syndrome and non-human immunodeficiency virus-infected individuals. Am J Trop Med Hyg. 2005;73:576–582. [PubMed] [Google Scholar]
- 31.Wheat J. Histoplasmosis in the acquired immunodeficiency syndrome. Curr Top Med Mycol. 1996;7:7–18. [PubMed] [Google Scholar]
- 32.Wheat LJ, et al. Factors associated with severe manifestations of histoplasmosis in AIDS. Clin Infect Dis. 2000;30:877–881. doi: 10.1086/313824. [DOI] [PubMed] [Google Scholar]
- 33.Wu-Hsieh BA, et al. Distinct CD8 T cell functions mediate susceptibility to histoplasmosis during chronic viral infection. J Immunol. 2001;167:4566–4573. doi: 10.4049/jimmunol.167.8.4566. [DOI] [PubMed] [Google Scholar]
- 34.Rivera J, Zaragoza O, Casadevall A. Antibody-mediated protection against Cryptococcus neoformans pulmonary infection is dependent on B cells. Infect Immun. 2005;73:1141–1150. doi: 10.1128/IAI.73.2.1141-1150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keir ME, Latchman YE, Freeman GJ, Sharpe AH. Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J Immunol. 2005;175:7372–7379. doi: 10.4049/jimmunol.175.11.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.