Abstract
Cells that have evolved to produce large quantities of secreted proteins to serve the integrated functions of complex multicellular organisms are equipped to compensate for protein misfolding. Hepatocytes and plasma cells have well developed chaperone and proteasome systems to ensure that secreted proteins transit the cell efficiently. The number of neurodegenerative disorders associated with protein misfolding suggests that neurons are particularly sensitive to the pathogenic effects of aggregates of misfolded molecules because those systems are less well developed in this lineage. Aggregates of the amyloidogenic (Aβ1–42) peptide play a major role in the pathogenesis of Alzheimer's disease (AD), although the precise mechanism is unclear. In genetic studies examining protein–protein interactions that could constitute native mechanisms of neuroprotection in vivo, overexpression of a WT human transthyretin (TTR) transgene was ameliorative in the APP23 transgenic murine model of human AD. Targeted silencing of the endogenous TTR gene accelerated the development of the neuropathologic phenotype. Intraneuronal TTR was seen in the brains of normal humans and mice and in AD patients and APP23 mice. The APP23 brains showed colocalization of extracellular TTR with Aβ in plaques. Using surface plasmon resonance we obtained in vitro evidence of direct protein–protein interaction between TTR and Aβ aggregates. These findings suggest that TTR is protective because of its capacity to bind toxic or pretoxic Aβ aggregates in both the intracellular and extracellular environment in a chaperone-like manner. The interaction may represent a unique normal host defense mechanism, enhancement of which could be therapeutically useful.
Keywords: protein interaction, protein misfolding, amyloidosis, dementia
A relationship between Alzheimer's disease (AD) and transthyretin (TTR) has been hypothesized on the basis of reports of physical interaction between amyloidogenic (Aβ) peptides and TTR proteins in vitro, prevention of Aβ aggregation in Caenorhabditis elegans transgenic for both mutant Aβ and TTR, increased cerebral transcription of the TTR gene in murine AD models, immunohistochemically detectable TTR in the vicinity of Aβ plaques in Aβ transgenic mice, and more aggressive histologic disease in such mice after local treatment with anti-TTR antibody (1–5). However, none of those studies demonstrated functional effects of the putative TTR–Aβ interaction. We performed genetic experiments designed to determine whether TTR has an effect on the development of the neuropathologic and behavioral phenotypes in a well characterized murine model of human AD.
Results
APP23 mice, carrying the Swedish autosomal dominant AD mutation and displaying the neuropathologic (Congophilic plaques, gliosis, neuronal death, Congophilic angiopathy) and behavioral (defined cognitive deficits) features associated with human AD, were mated with mice overexpressing WT human TTR (hTTR) and animals in which both copies of the endogenous TTR gene had been silenced by targeted disruption (6–8). The transgenics carried ≈90 copies of the human gene with serum concentrations of hTTR between 1 and 3 mg/ml and cerebrospinal fluid concentrations between 0.007 and 0.019 mg/ml.
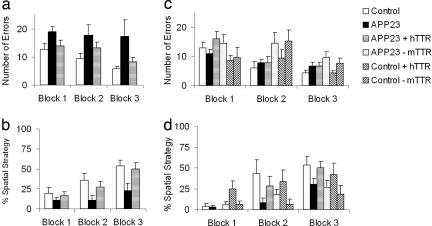
Results of Barnes maze testing (to assess cognitive function and spatial learning) of 15-month-old APP23, APP23 overexpressing hTTR (APP23/hTTR), and control mice are shown in Fig. 1a. In the APP23/hTTR and control mice the number of errors decreased across blocks [F (2,64) = 5.2, P < 0.01], demonstrating the effect of training. There was a significant effect of group (genotype) on the number of errors across the three blocks [(F(2,32) = 4.1, P < 0.05] caused by group differences in blocks 2 [F(2,32) = 3.0, P = 0.05] and 3 [F(2,32) = 3.7, P < 0.05]. APP23 mice made more errors in these blocks relative to control mice (P < 0.05). APP23/ hTTR mice were not significantly different from age-matched WT controls, whereas the performance of APP23/hTTR animals was superior to that of APP23 mice lacking the hTTR transgene (P < 0.05). The percentage of trials in each block in which mice used a spatial strategy to locate the escape tunnel is shown in Fig. 1b. There were significant effects of group [F(2,32) = 4.3, P < 0.05] and block [F(2,64) = 17.7, P < 0.001], but no significant group by block interaction. Spatial strategy utilization increased across blocks. Both control and APP23/ hTTR mice used this strategy more than APP23 mice in block 3 (P < 0.05). There was a significant effect of APP23 on numbers of errors made and strategy utilization in the 15-month-old animals. The presence of hTTR resulted in virtually normal performance in both measures, indicating amelioration of the APP23 behavioral phenotype.
Fig. 1.
Results of behavioral testing of control and APP23 mice. Separate mixed-sex groups of mice were tested. For younger mice, group sizes were: control WT, two male, five female; mTTR−/−, three male, five female; hTTR+, two male, four female; APP23, nine male, nine female; APP23/mTTR−/−, nine male, eight female; APP23/hTTR+, five male, four female. For older mice, group sizes were: control WT, seven male, seven female; APP23, six male, six female; APP23 httr, five male, seven female. Mice were tested once daily for 12 days. Sessions were videotaped and scored by an experimenter blind to the mouse genotype. Number of errors per session and strategy used to locate the escape tunnel were recorded. Errors included nose pokes and head deflections over any hole not having the tunnel beneath it. Search strategies were divided into operationally defined categories: (i) random, localized hole searches separated by crossings through the maze center, (ii) serial, systematic hole searches (every or every other hole) in a clockwise or counterclockwise direction, or (iii) spatial, reaching the escape tunnel with both error and distance (number of holes between the first hole visited and the escape tunnel) scores of 3 or less. Data were analyzed in four session blocks using two-way ANOVA with genotype and blocks as variables.
There were no significant group differences in anxiety-like behavior, rearing, or locomotor activity. There were trends for the older APP23 mice to show increased anxiety-like behavior and decreased activity (horizontal and vertical). Visual cliff analysis revealed that all mice had intact vision. The results (data not shown) are consistent with previous analyses of APP23 mice (9).
To determine whether absence of the endogenous murine TTR (mTTR) gene would have an effect on the development of disease in APP23 mice we carried out behavioral studies on 5.5-month-old mice expressing the APP23 gene in the presence (APP23) or absence of mTTR (APP23/mTTR−/−) and in mice lacking both an active endogenous TTR gene and the human AD gene (mTTR−/−). The number of errors is shown in Fig. 1c and the percent spatial strategy is shown in Fig. 1d. Because we had specific questions concerning the role of mTTR and hTTR in the behavior of APP23 mice, we performed three general analyses. We first examined whether APP23 influenced the measures of behavior. There was no effect of APP23 on errors [F(1,23) = 0.152, P > 0.05], but there was an impact on percent spatial strategy [F(1,23) = 7.1, P < 0.05], suggesting that at a younger age differences do exist, albeit they are less global. Second, performance was examined in mice not carrying the hTTR transgene (WT murine genotype) to determine whether mTTR ± APP23 behaved differently. There were significant interactions between mTTR and blocks in both errors [F(2,92) = 5.6; P < 0.01] and percentage spatial strategy [F(2,92) = 3.5; P < 0.05], suggesting a mild learning delay in mice lacking mTTR. The mTTR decrement was more prominent in WT mice lacking the human AD gene, as revealed by an APP23 by mTTR interaction on percentage spatial strategy [F(1,47) = 5.2; P < 0.05]. Finally, the effects of hTTR in the presence or absence of APP23 were examined in mTTR+/+ mice (WT genotype). APP23 reduces the percentage spatial strategy utilization and there was as a complex three-way interaction of blocks, APP, and hTTR [F(2,72) = 4.2; P < 0.05]. Further analysis revealed a reversal of the APP-associated deficits in spatial strategy utilization by hTTR. This result is consistent with the findings in the 15-month-old animals.
APP23/mTTR−/− mice were more active than APP23 mice with an intact TTR gene (P < 0.05), suggesting an effect of mTTR silencing on activity in the presence of the APP23 gene; however, examination of the Barnes maze behavior (data not shown) revealed no difference between the groups in the distances traveled across the trials, indicating that the cognitive deficits were independent of the differences in activity. Thus in 5.5-month-old mice, we detected a significant effect of APP23 on the progressive use of spatial strategy and a general slowing of learning in this test in mTTR null mutant mice.
As in the older animals, results of the light/dark transfer test of anxiety-like behavior showed no significant effect of group, nor did the genotype have an effect on the rearing often correlated with anxiety (data not shown), and the mice were not visually impaired.
Despite the absence of significant differences in behavior between mTTR−/−mice with or without a human AD gene, comparison of immunohistochemical staining of the frontal cortex and the hippocampus of the 5.5-month-old APP23 mice with an active or silenced mTTR gene revealed that 7 of the 11 animals without TTR had cortical or hippocampal Aβ deposition (Table 1 and Fig. 2 A and D). In the presence of the mTTR gene only one animal had detectable deposits. The quantitation of SDS and formic acid soluble Aβ1–40 and Aβ1–42 showed no significant differences in the concentrations or absolute amounts of the peptides in the extracted hemi-brains of the two groups, although the concentrations of formic acid-extractable peptides in the mTTR−/− mice were higher with a trend toward statistical significance. These findings suggested that immunohistochemistry was the more sensitive of the two methods.
Table 1.
Aβ deposition in brains of APP23 transgenic animals
| Parameter | APP23 mTTR | APP23 without mTTR | APP23 + hTTR | P value* |
|---|---|---|---|---|
| Animals at 5.5 months | ||||
| No. animals with deposits | 1/10 | 7/11 | 0.004 | |
| Mean deposition cortex, % | 0.033 ± 0.1 | 0.09 ± 0.1 | 0.1 | |
| Mean deposition hippocampus, % | 0.02 ± 0.08 | 0.11 ± 0.11 | 0.04 | |
| SDS soluble Aβ1–42, Pmol/g | 6.35 ± 2.5 | 5.5 ± 2.4 | 0.35 | |
| Formic acid-soluble Aβ1–42, Pmol/g | 2.8 ± 0.31 | 3.5 ± 3 | 0.15 | |
| SDS-soluble Aβ1–40, Pmol/g | 22 ± 11.6 | 20 ± 9.4 | 0.68 | |
| Formic acid-soluble Aβ1–40, Pmol/g | 5.1 ± 0.7 | 9.9 ± 17 | 0.17 | |
| Animals at 16 months | ||||
| No. animals with deposits | 8/8 | 21/24 | 0.55 | |
| Mean deposition cortex, % | 4.4 ± 3.5 | 1.8 ± 1.3 | 0.009 | |
| Mean deposition hippocampus, % | 3.0 ± 3.6 | 1.1 ± 0.8 | 0.05 | |
| SDS-soluble Aβ1–42, Pmol/g | 198 ± 121 | 53 ± 60 | 0.02 | |
| Formic acid-soluble Aβ1–42, Pmol/g | 28 ± 25 | 14 ± 14 | 0.07 | |
| SDS-soluble Aβ1–40, Pmol/g | 343 ± 168 | 129 ± 154 | 0.04 | |
| Formic acid-soluble Aβ1–40, Pmol/g | 429 ± 273 | 199 ± 158 | 0.05 | |
Immunohistochemically the mean amounts of Aβ deposition were measured as percentage of neuropil in the cortex and hippocampus replaced by Aβ-stained material.
*Frequencies analyzed by Fisher Exact Test. Means compared by Mann–Whitney.
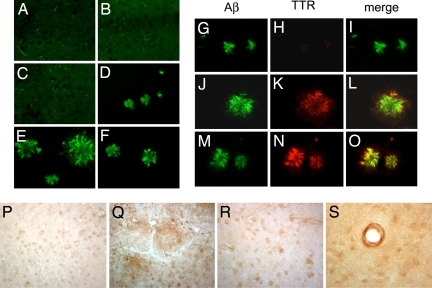
Fig. 2.
Aβ deposition in AD, APP23, and TTR mouse models. Vibratome sections of frontal cortex were treated with formic acid, immunostained with a polyclonal antibody identifying mTTR and hTTR or antibody to Aβ (4G8), and imaged with the confocal microscope (31). (A and B) No amyloid plaques were detected in nontransgenic mice (A) or mTTR−/− mice (B). (C) Five-month-old APP23 mice with an intact TTR gene show no plaques. (D) In APP23, mTTR−/− mice of the same age plaques are readily detectable. (E and F) Fifteen-month-old APP23 animals (mTTR+/+) show large and abundant amyloid plaques (E), which are reduced in APP23 mice expressing hTTR (F). (G–O) Brain sections of APP23 transgenic mice double labeled with antibodies against Aβ (green) and TTR (red) were imaged with the laser scanning confocal microscope. Aβ accumulation in plaques of APP23 (mTTR−/−) mice is shown in G–I. There is colocalization of Aβ and mTTR in APP23 (mTTR+/+) transgenics (J–L), which is increased in APP23, hTTR transgenics (M–O). (P and Q) There is mild TTR immunolabeling in neuronal cytoplasm of nontransgenic mice (P) and diffuse TTR staining of the plaques and neurons of APP23 transgenic mice (Q). (R) Brains from control nondemented humans show TTR localization in neuronal cell bodies. (S) In the AD brains TTR immunoreactivity was predominantly in vascular amyloid but also seen in neuronal cell bodies. (Magnifications: ×400.)
Examination of the cortex and hippocampus of 15- to 16-month-old APP23 mice with an antibody to human Aβ showed staining in both the cortex and hippocampus (Table 1 and Fig. 2E). Quantitative analysis of the images revealed that APP23/hTTR mice had significantly less Aβ staining in the cortex and hippocampus (Fig. 2F and Table 1). Staining with the anti-TTR antibody showed reactivity in neuronal cell bodies of all of the mice except the TTR knockouts (mTTR−/−) (data not shown). APP23 mice showed anti-TTR reactivity in neuronal cell bodies as well as extracellularly in areas of Aβ deposition (Fig. 2 P–R). Colocalization of TTR and Aβ is revealed in Fig. 2 L and O with lesser amounts of Aβ staining and smaller plaques in the APP23/hTTR brains. Using the same antibody brains from age-matched control animals, bearing neither the human Aβ nor the hTTR construct showed staining (as in the 5-month-old animals; data not shown). Determination of SDS and formic acid-soluble Aβ1–40 and Aβ1–42 showed significantly lower amounts of Aβ peptides in the hemi-brains of the APP23/hTTR animals than in the APP23 mice (Table 1) (10).
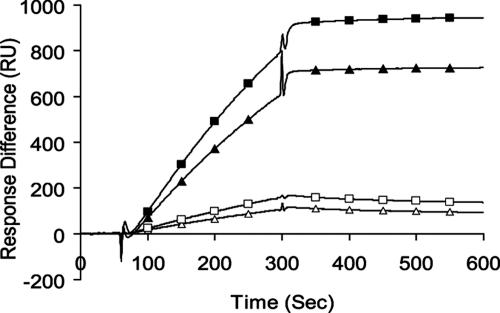
To determine whether we could demonstrate a direct interaction between TTR and Aβ we elected to use surface plasmon resonance (SPR), a methodology that did not involve interference with, or acceleration of, fibril formation. Previous liquid-phase studies using that approach were complicated by the fibril-forming properties of both components and the possible effects of preformed aggregates on the measurements. In our SPR experiments binding to aggregated Aβ was always better than to soluble monomers of either type independent of the TTR species of origin (Fig. 3). The interaction was always stronger for Aβ1–42 than for Aβ1–40, although it was not possible to determine an accurate KD for the interactions because of the size heterogeneity in the Aβ fibril preparations. Interactions among any combination of TTR and Aβ molecular species, i.e., TTR monomers, TTR tetramers, Aβ1–40 or Aβ1–42 monomers, or fibrils were greater at 37°C than 25°C (data not shown). The mTTR molecule appeared to have much greater affinity for any of the Aβ forms than did the human protein.
Fig. 3.
Purified recombinant hTTR or mTTR was applied to the Biacore M5 chip surface and exposed to either purified monomer Aβ1–40 or Aβ1–42 or aggregates formed from the same peptides over 7 days before analysis (31). The flow rate was 5 ml/min at 37°C. Ligands were Tetramer TTR Surface (human 4658RU; mouse 5122RU). Analytes were 20 ml of 20 mM Aβ1–42. In reverse experiments the Aβ preparations were applied to the chip and either mTTR or hTTR was allowed to interact in the flow cell. ■, Aβ fibrils on mTTR; ▴, Aβ fibrils on hTTR; □, Aβ monomer on mTTR; ▵, Aβ monomer on hTTR.
Discussion
We demonstrate markedly improved cognitive function of older APP23 transgenic mice when they carry multiple copies of the human WT TTR gene. The effect of endogenous TTR was confirmed when the APP23 gene was crossed onto a mTTR−/− background. Those mice showed immunohistochemical evidence of increased Aβ deposition relative to APP23 animals with an active endogenous TTR gene. The improved behavioral performance of older APP23 animals carrying the hTTR gene was associated with less severe Alzheimer-like brain pathology and smaller amounts of extractable Aβ than in the brains of control APP23 carriers.
Our use of the Barnes maze, rather than the more frequently reported Morris water maze, reflects current thinking that the Barnes test eliminates the swimming aspect of the behavior and that the murine anxiety response to swimming may reduce the sensitivity of the Morris assay in mice (11, 12).
Antibody staining of brain sections appears to have greater sensitivity than immunochemical quantitation of soluble Aβ, presumably because of its capacity to recognize localized deposits. The extraction procedures, although more quantitative, may be less sensitive because they pool affected and nonaffected regions of the brain, thus they are subject to dilutional effects, particularly in younger animals when deposits are smaller and more localized.
A recent report (13) indicated that hemizygous mTTR knockouts crossed with APPswe/PS1ΔE9 AD model mice had significantly greater amounts of Aβ deposition in the cortex and hippocampus after 7 months of age. In those studies, at 5 months of age the animals did not show Aβ staining or statistically significant differences in the amounts of extractable Aβ peptides. Hence detectable AD-like pathology appears earlier in the homozygous TTR knockouts, suggesting that mTTR demonstrates a gene dose-dependent protective effect.
Young animals lacking TTR gene expression displayed a defect in spatial learning even in the absence of the human AD-associated gene, confirming the recently reported observation that mTTR has a behavioral function independent of its interaction with Aβ (14).
The immunohistochemical findings in both the young and old animals argue against a primary role for the genetic background of the carrier strains on the effect. The APP23 animals had been extensively back-crossed to B6, and hTTR transgenics are carried on a B6/D2 background. Both the hTTR transgenics and the mTTR knockouts were crossed with the APP23's for six generations.
The SPR analyses show that both hTTR and mTTR bind Aβ1–40 and Aβ1–42. Binding of mTTR to any form of Aβ has not been previously investigated. The murine protein has much greater affinity for Aβ aggregates, fibrils, and monomers than does the human protein. Both hTTR and mTTR bind the aggregates better than they bind soluble monomers, and the genetic experiments show that both have an effect on Aβ toxicity. The SPR experiments indicate that the affinity of both TTRs for monomeric Aβ is low. We were unable to define the precise molecular nature of the TTR-binding species of Aβ by using this method. Light-scattering experiments have indicated that tetrameric hTTR binds Aβ1–40 aggregates but not fibrils, and it has been suggested that the findings are most consistent with TTR preventing protofibril elongation and subsequent lateral association (15). It is likely, given the molecular heterogeneity of our fibril preparations, that the binding activity is a property of subfibrillar Aβ aggregates present in the incubated samples rather than in mature fibrils. It has also been reported that mTTR coimmunoprecipitates with Aβ in brain extracts from AD model mice, but the molecular species of Aβ was not examined in those experiments (16).
The simplest interpretation of our findings, and one consistent with a physical interaction between TTR and Aβ1–40, Aβ1–42, is that TTR binds Aβ in a manner that prevents both toxicity and plaque formation, presumably by interfering with aggregation of some Aβ species larger than monomers (15). The results are consistent with previous reports of interactions between hTTR and various Aβ preparations (1, 2). The greater affinity of the murine protein for human Aβ was unexpected, but the marked physiologic effect of both proteins in the two experiments, i.e., suppression of the APP23 phenotype by the human protein, albeit in relatively high copy number transgenic animals, and acceleration of deposition in the absence of mouse protein were unequivocal. The human and mouse proteins are 80% homologous in amino acid sequence but have different biophysical properties, the murine protein being much more kinetically stable (17). That difference per se does not explain the higher affinity of mTTR for the Aβ aggregates.
Previous immunopathologic analyses of human AD brains with antisera for TTR have yielded conflicting results (18, 19). Our studies clearly show intraneuronal staining for TTR in both human and murine brains with no staining in the TTR knockout animals, TTR staining of the plaques in the mouse brains but predominant vascular staining in human AD brain (Fig. 2 P–S). These observations by themselves are consistent with either neuronal TTR synthesis or uptake of TTR synthesized elsewhere, presumably the choroid plexus (20, 21). The notion of neuronal synthesis of TTR, in addition to the well documented production of the protein in choroid plexus and leptomeningeal epithelial cells, is supported by the Allen brain atlas and other microarray studies that show TTR mRNA in other areas of carefully dissected mouse brains (22, 23). The question of whether the intraneuronal TTR is synthesized in neurons or only in the choroid plexus and taken up by neurons is not answered by our observations. However, the intraneuronal localization of the protein suggests that hTTR and mTTR are available to bind Aβ intraneuronally and/or extraneuronally.
A behavioral, presumably neuronal function for TTR was proposed on the basis of studies of TTR knockouts that showed “reduced signs of depressive activity and increased exploratory activity” (24). Our data with respect to activity levels in the animals are consistent with those findings. Others have suggested that the TTR effect on the behavioral phenotype is mediated through its role in thyroid or retinoic acid function, an interpretation not consistent with earlier physiologic studies (25–27). The SPR interaction studies also argue that the effect is direct; however, it is possible that the impact of TTR on behavior is bimodal.
The morphologic studies show differences in TTR localization between human AD and transgenic mouse model AD brains. In the hTTR/APP23 mice TTR is seen in proximity to the plaques, whereas in the human brains there is little TTR immunoreactivity in the plaques. The most prominent TTR staining in the human brains was in vessels showing Congophilic angiopathy (Fig. 2S). These observations may reflect differences in pathogenesis in the two species. Alternatively, in the double transgenic mice TTR and Aβ are overproduced and available to interact throughout life, which may be critical in reducing plaque density. Perhaps in the human brain plaque formation only occurs when there is insufficient TTR available to inhibit aggregation, i.e., late in life, and the presence of plaques is a marker for insufficient protective TTR. This would not be the case in blood vessels where the codeposited TTR could originate in the serum. It is likely that the transgenic overproduction of the hTTR is sufficient to overcome the relatively weak binding seen in vitro. Perhaps endogenously produced mTTR is sufficient to inhibit Aβ aggregation when the fragment is generated from endogenous murine or a low copy number human Aβ transgene but cannot be produced in sufficient quantities to overcome its production by Aβ transgenes integrated in high copy numbers and transcribed and translated to produce large quantities of aggregate precursor.
In studies of the regulation of TTR synthesis evidence suggesting transcription in the cerebral cortex was dismissed as artifactual (28). It is possible that the observation of cortical expression was not an artifact and that regulation in the cortex is similar to that in the liver and may actually decline in the face of cytokine expression. Local cytokine production has been shown in AD brain (29). If TTR regulation in the brain proper, apart from that in choroid plexus, is regulated in a fashion similar to that in the liver, the local inflammatory response to Aβ aggregates could suppress TTR transcription. Hence with aging TTR production may decrease, Aβ1–40 and Aβ1–42 production may increase, or both. The observed increase in TTR transcription in murine AD model brains argues in favor of exhaustion or inadequate production rather than suppression.
These data provide genetic, immunohistochemical, and biochemical evidence for an interaction between TTR and Aβ playing a role in resistance to the development of the neuropathologic and behavioral manifestations in a murine model of AD and suggest that a similar relationship may exist in the human brain. It appears that the interaction is physical and that TTR may behave in a chaperone-like manner for molecular species of Aβ larger than monomers. The observations support the novel notion that increasing cerebral TTR synthesis is a potential therapeutic/prophylactic approach to human AD.
Materials and Methods
Behavioral.
Mice were group-housed in a temperature-controlled room in which the lights were on a 12-h light/dark cycle (lights off at 6 a.m.). All behavioral testing was performed in The Scripps Research Institute mouse behavior assessment core facility during the dark (active) phase. Food and water were available ad libitum. All procedures were conducted in accordance with the guidelines established by the Department of Agriculture and the National Institutes of Health in the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
In the Barnes maze test we used an opaque Plexiglas disk 75 cm in diameter elevated 58 cm above the floor by a tripod. Twenty holes, 5 cm in diameter, were located 5 cm from the perimeter, and a black Plexiglas escape box (19 × 8 × 7 cm) was placed under one of the holes. Distinct spatial cues were located all around the maze and were kept constant throughout the study. On the first day of testing, a training session was performed, which consisted of placing the mouse in the escape box and leaving it there for 1 min. One minute later, the first session was started. At the beginning of each session, the mouse was placed in the middle of the maze in a 10-cm-high cylindrical black start chamber. After 10 s the start chamber was removed, a buzzer (80 dB) and a light (400 lux) were turned on, and the mouse was set free to explore the maze. The session ended when the mouse entered the escape tunnel or after 5 min elapsed. When the mouse entered the escape tunnel, the buzzer was turned off and the mouse was allowed to remain in the dark for 1 min. When the mouse did not enter the tunnel by itself it was gently put in the escape box for 1 min. The tunnel was always located underneath the same hole (stable within the spatial environment), which was randomly determined for each mouse. The platform and escape box were cleaned with 70% ethanol between mice.
Additional behaviors, including activity levels, anxiety states, and visual acuity, that may influence findings in Barnes maze testing, were also examined. Locomotor activity was measured in polycarbonate cages (42 × 22 × 20 cm) placed into frames (25.5 × 47 cm) mounted with two levels of photocell beams at 2 and 7 cm above the bottom of the cage (San Diego Instruments). These two sets of beams allowed for the recording of both horizontal (locomotion) and vertical (rearing) behavior. A thin layer of bedding material was applied to the bottom of the cage. Mice were tested for 30 min.
The light/dark transfer procedure has been used to assess anxiety-like behavior in mice by capitalizing on the conflict between exploration of a novel environment and the avoidance of a brightly lit open field (11). The apparatus is a rectangular box made of Plexiglas divided by a partition into two environments. One compartment (14.5 × 27 × 26.5 cm) is dark (8–16 lux), and the other compartment (28.5 × 27 × 26.5 cm) is highly illuminated (400–600 lux) by a 60-W light source located above it. The compartments are connected by an opening (7.5 × 7.5 cm) located at floor level in the center of the partition. The time spent in the light compartment was used as a predictor of anxiety-like behavior, i.e., a greater amount of time in the light compartment was indicative of decreased anxiety-like behavior. The mice were placed in the dark compartment to initiate the test session, and behavior was recorded with a camera mounted above the apparatus. The test duration was 5 min.
The visual cliff test provides a measure of visual acuity. It evaluates the ability of the animal to see a drop-off at the edge of a horizontal surface. In this apparatus there is the visual appearance of a cliff, but, in fact, the Plexiglas provides a solid horizontal surface. If the animal sees the cliff, it will step down onto the “safe” side (the horizontal checkered surface) in most trials. A blind animal will just as often step down onto the “negative” side (the vertical appearing surface), i.e., making 50% correct and 50% incorrect choices. Each mouse was placed onto the center ridge, and the side onto which the animal stepped down was recorded. Six consecutive trials were used for each mouse, and the percentage of correct choices was calculated for the individual animals.
Neuropathology.
Brain preparation.
Animals were anesthetized with isofluthane, the skull was opened, and the brain was removed intact. The hemispheres were separated with one half being placed in 4% paraformaldehyde, and the other snap-frozen and stored at −80°C for biochemical analysis.
Immunohistochemistry.
All brains were examined for Aβ and TTR with antibodies specific for those proteins. Vibratome sections were incubated overnight at 4°C with the mouse mAb 4G8 (1:600; Senetek), which specifically recognizes Aβ or the DAKO anti-TTR antibody, which recognizes both mTTR and hTTR. Two methods were used to detect primary antibody binding. We used either a Vector ABC Elite kit and DAB/H2O2 or FITC-conjugated anti-mouse IgG (Vector Laboratories). Sections treated with DAB/H2O2 were examined with a ×2.5 objective for the Olympic Vanox light microscope. The percentage area of the hippocampus or cortex covered by 4G8-immunoreactive material was assessed with a Quantimet 570C microscope (Leica). Digitized images were analyzed with the NIH Image 1.43 program to determine the number of plaques per unit area and the plaque size. Three immunolabeled sections were analyzed per region per mouse, and the average of individual measurements was used to calculate group means.
Brain extraction to determine Aβ content.
Hemi-brains were dounce-homogenized in the cold in 2% SDS in water containing 0.7 mg/ml Pepstatin A and a mixture of protease inhibitors (Roche 1836153). Formic acid (70%) was added to part of each hemi-brain to fully solubilize fibrils that were present. The homogenates were sonicated to shear DNA and centrifuged at 100,000 × g for 1 h. The supernates were analyzed by a capture ELISA with antibodies specific for Aβ1–40 and Aβ1–42 (10).
Biacore experiments to analyze the interaction between TTR and Aβ1–40 and Aβ1–42.
Aβ1–40 and Aβ1–42 were obtained from Quality Controlled Biochemicals. The lyophilized powder was dissolved in aqueous NaOH to yield a final concentration of 2 mM, based on molecular weight. The pH was adjusted to 10.5 with aqueous NaOH (100 mM). The solution was sonicated (20 min, 25°C), then filtered sequentially through 0.2 μm and 10-kDa cutoff filters (14,000 × g, 30 min) (31). The concentration of protein was determined by UV absorption at 280 nm (ε = 1,280 M−1·cm−1). The peptide in the filtrate was diluted to 200 μM with aqueous NaOH (pH 10.5) and used immediately. We noted that approximately half of the Aβ sample was retained on the 10-kDa filter. After pretreatment, the final peptide solution was seed-free by atomic force microscopy analysis. Aβ fibrils were formed by incubating the monomer in PBS buffer for 7 days at 37°C with constant shaking.
hTTR and mTTR were overexpressed in Escherichia coli and purified as described (17, 30).
ACKNOWLEDGMENTS.
This work was supported by National Institute on Aging Grants R01 AG15916 (to J.N.B.) and R01 AG18440 (to E.M.), the W. M. Keck Foundation (J.N.B.), The Skaggs Foundation (Z.Y.), the Fidelity Foundation (J.N.B.), and The Stein Fund.
Footnotes
The authors declare no conflict of interest.
References
- 1.Schwarzman AL, et al. Transthyretin sequesters amyloid β protein and prevents amyloid formation. Proc Natl Acad Sci USA. 1994;91:8368–8372. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwarzman AL, Goldgaber D. In: The Nature and Origin of Amyloid Fibrils. Bock GR, Goode JA, editors. New York: Wiley; 1996. pp. 146–164. [Google Scholar]
- 3.Schwarzman AL, et al. Amyloidogenic and anti-amyloidogenic properties of recombinant transthyretin variants. Amyloid. 2004;11:1–9. doi: 10.1080/13506120410001667458. [DOI] [PubMed] [Google Scholar]
- 4.Link CD. Expression of human β-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci USA. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein TD, Johnson JA. Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein is associated with increased levels of transthyretin and the activation of cell survival pathways. J Neurosci. 2002;22:7380–7388. doi: 10.1523/JNEUROSCI.22-17-07380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturchler-Pierrat C, et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng MH, et al. Amyloid and nonfibrillar deposits in mice transgenic for wild-type human transthyretin: A possible model for senile systemic amyloidosis. Lab Invest. 2001;81:385–396. doi: 10.1038/labinvest.3780246. [DOI] [PubMed] [Google Scholar]
- 8.Episkopou V, et al. Disruption of the transthyretin gene results in mice with depressed levels of plasma retinol and thyroid hormone. Proc Natl Acad Sci USA. 1993;90:2375–2379. doi: 10.1073/pnas.90.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prut L, et al. Aged APP23 mice show a delay in switching to the use of a strategy in the Barnes maze. Behav Brain Res. 2007;179:107–110. doi: 10.1016/j.bbr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Levites Y, et al. Anti-Aβ42- and anti-Aβ40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model. J Clin Invest. 2006;116:193–201. doi: 10.1172/JCI25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawley JN. Behavioral phenotyping of rodents. Comp Med. 2003;53:140–146. [PubMed] [Google Scholar]
- 12.McLay RN, Freeman SM, Zadina JE. Chronic corticosterone impairs memory performance in the Barnes maze. Physiol Behav. 1998;63:933–937. doi: 10.1016/s0031-9384(97)00529-5. [DOI] [PubMed] [Google Scholar]
- 13.Choi SH, et al. Accelerated Aβ deposition in APPswe/PS1deltaE9 mice with hemizygous deletions of TTR (transthyretin). J Neurosci. 2007;27:7006–7010. doi: 10.1523/JNEUROSCI.1919-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sousa JC, et al. Transthyretin influences spatial reference memory. Neurobiol Learn Mem. 2007;88:381–385. doi: 10.1016/j.nlm.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Murphy RM. Kinetics of inhibition of β-Amyloid aggregation by transthyretin. Biochemistry. 2006;45:15702–15709. doi: 10.1021/bi0618520. [DOI] [PubMed] [Google Scholar]
- 16.Carro E, Trejo JL, Gonez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-β levels. Nat Med. 2002;8:1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- 17.Reixach N, et al. Human-murine transthyretin heterotetramers are kinetically stable and nonamyloidogenic: A lesson in the generation of transgenic models of diseases involving oligomeric proteins. J Biol Chem. 2008;283:2098–2107. doi: 10.1074/jbc.M708028200. [DOI] [PubMed] [Google Scholar]
- 18.Shirahama T, et al. Senile cerebral amyloid: Prealbumin as a common constituent in the neuritic plaque, in the neurofibrillary tangle, and in the microangiopathic lesion. Am J Pathol. 1982;107:41–50. [PMC free article] [PubMed] [Google Scholar]
- 19.Stein TD, et al. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPsw mice resulting in Tau phosphorylation and loss of hippocampal neurons: Support for the amyloid hypothesis. J Neurosci. 2004;24:7707–7717. doi: 10.1523/JNEUROSCI.2211-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber G. The evolution of transthyretin synthesis in the choroid plexus. Clin Chem Lab Med. 2002;40:1200–1210. doi: 10.1515/CCLM.2002.210. [DOI] [PubMed] [Google Scholar]
- 21.Sousa JC, Cardoso I, Marques F, Saraiva MJ, Palha JA. Transthyretin and Alzheimer's disease: Where in the brain? Neurobiol Aging. 2006;28:713–718. doi: 10.1016/j.neurobiolaging.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Lein ES, et al. Genomewide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 23.Zapala MA, et al. Adult mouse brain gene expression patterns bear an embryologic imprint. Proc Natl Acad Sci USA. 2005;102:10357–10362. doi: 10.1073/pnas.0503357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sousa JC, et al. Transthyretin is involved in depression-like behavior and exploratory activity. J Neurochem. 2004;88:1052–1058. doi: 10.1046/j.1471-4159.2003.02309.x. [DOI] [PubMed] [Google Scholar]
- 25.Brouillette J, Quirion R. Transthyretin: A key gene involved in the maintenance of memory capacities during aging. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Goodman AB. Retinoid receptors, transporters, and metabolizers as therapeutic targets in late-onset Alzheimer disease. J Cell Physiol. 2006;209:598–603. doi: 10.1002/jcp.20784. [DOI] [PubMed] [Google Scholar]
- 27.Palha JA, et al. Thyroid hormone metabolism in a transthyretin-null mouse strain. J Biol Chem. 1994;269:33135–33139. [PubMed] [Google Scholar]
- 28.Yan C, Costa RH, Darnell JE, Jr, Chen JD, Van Dyke TA. Distinct positive and negative elements control the limited hepatocyte and choroid plexus expression of transthyretin in transgenic mice. EMBO J. 1990;9:869–878. doi: 10.1002/j.1460-2075.1990.tb08184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin WS, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reixach N, Deechongkit S, Jiang X, Kelly JW, Buxbaum JN. Tissue damage in the amyloidoses: Transthyretin monomers and non-native oligomers are the major cytotoxic species in tissue culture. Proc Natl Acad Sci USA. 2004;101:2817–2822. doi: 10.1073/pnas.0400062101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teplow DB. Preparation of amyloid β-protein for structural and functional studies. Methods Enzymol. 2006;413:20–33. doi: 10.1016/S0076-6879(06)13002-5. [DOI] [PubMed] [Google Scholar]