Abstract
The CB1 cannabinoid receptor mediates many of the psychoactive effects of Δ9THC, the principal active component of cannabis. However, ample evidence suggests that additional non-CB1/CB2 receptors may contribute to the behavioral, vascular, and immunological actions of Δ9THC and endogenous cannabinoids. Here, we provide further evidence that GPR55, a G protein-coupled receptor, is a cannabinoid receptor. GPR55 is highly expressed in large dorsal root ganglion neurons and, upon activation by various cannabinoids (Δ9THC, the anandamide analog methanandamide, and JWH015) increases intracellular calcium in these neurons. Examination of its signaling pathway in HEK293 cells transiently expressing GPR55 found the calcium increase to involve Gq, G12, RhoA, actin, phospholipase C, and calcium release from IP3R-gated stores. GPR55 activation also inhibits M current. These results establish GPR55 as a cannabinoid receptor with signaling distinct from CB1 and CB2.
Keywords: orphan, pain, CB3, G protein-coupled receptor
Cannabis has been used and abused for its therapeutic and psychoactive properties for millennia. The effects of cannabinoid compounds are largely mediated by cannabinoid receptors. CB1, cloned in 1990 (1), is widely and highly expressed in the CNS, where it likely mediates the majority of the psychotropic and behavioral effects of cannabinoids. CB2 is primarily expressed in peripheral tissues (2). Both CB1 and CB2 are 7-transmembrane G protein-coupled receptors that engage predominantly the Gi/o family of G proteins. However, ample evidence suggests that additional receptors may contribute to the behavioral, vascular, and immunological actions of Δ9tetrahydrocannabinol (THC) and endogenous cannabinoids (3).
It has been suggested that GPR55 is a novel cannabinoid receptor (reviewed in ref. 4). GPR55 is only 13.5% identical to CB1 and 14.4% identical to CB2, and its mRNA is present in the brain and periphery (5–7). A recent study found that a variety of cannabinoid compounds stimulated GTPγS binding in cells stably expressing GPR55 (6). Here, we report GPR55 activation by THC, JWH015, and anandamide increases intracellular calcium by activating signaling pathways quite distinct from those used by CB1 and CB2.
Results
Activation of GPR55 by Cannabinoids Increases Intracellular Calcium.
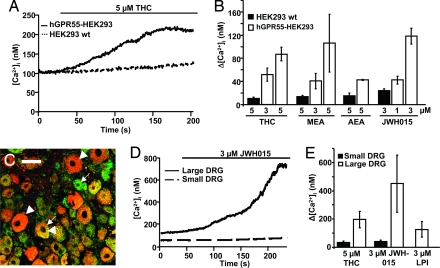
We first examined the signaling pathways activated by GPR55 in HEK293 cells transiently expressing human GPR55 (hGPR55). Perfusion with 5 μM THC evoked a calcium increase (Δ[Ca2+]i) averaging ≈100 nM (n = 7, Fig. 1 A and B). Perfusion with 3 μM THC evoked a more modest increase (n = 5, 50 nM; Fig. 1B). The agonist-induced calcium response was present in all cells tested, but because it varied in magnitude and time course, concurrent controls were always conducted. GPR55 was essential for the THC-evoked calcium rise because there was minimal calcium rise in nontransfected HEK293 cells exposed to 5 μM THC (n = 6, Fig. 1 A and B). A similar calcium increase was seen in CHO cells stably expressing hGPR55 (data not shown). In these cells (n = 5) 5 μM THC increased intracellular calcium by 240 ± 100 nM, insignificantly different from hGPR55-HEK293 cells (n = 6, 254 ± 80 nM).
Fig. 1.
GPR55 activation by several cannabinoids increases intracellular calcium in HEK293 cells and DRG neurons. (A) The time course of changes in [Ca2+]i in HEK293 cells loaded with fura-2 and perfused with THC. The lines above the figure indicate drug application. The solid line indicates a representative HEK293 cell transiently expressing the human GPR55 (hGPR55-HEK293) perfused with THC, whereas the black dashed line indicates a representative nontransfected HEK293 cell (HEK293 wt). (B) Summary of calcium responses upon perfusion of cannabinoid agonists. Responses in HEK293 wt cells (n = 6, black bars) and hGPR55-HEK293 cells (open bars) were perfused with THC, methanandamide (MEA), anandamide (AEA), or JWH015. (C) Immunostaining of mouse DRGs with a rabbit anti-GPR55 antibody (1:2,000, red) and a guinea pig anti-CB1 antibody (1:2,000, green) show that GPR55 is preferentially expressed in large-diameter DRG neurons (arrowheads) whereas CB1 is more widely expressed (arrows). See SI Text for full methods. (Scale bar, 50 μm.) (D) Representative JWH015-induced calcium rises in a large (solid line) and small (dashed line) DRG neuron. (E) Summary of calcium responses in small and large DRG neurons upon perfusion of THC, JWH015, and LPI.
Other cannabinoid agonists also activated hGPR55 to increase intracellular calcium. The endogenous cannabinoid anandamide (AEA), and its metabolically stable analog methanandamide (MEA), both increased intracellular calcium. On average, 5 μM MEA increased calcium by 100 nM (n = 7, Fig. 1B), and 5 μM AEA increased calcium by 45 nM (n = 6, Fig. 1B). The aminoalkylindole JWH015 is considered an efficacious, specific CB2 agonist (8). Surprisingly, JWH015 also stimulates GPR55, with 3 μM giving an average calcium rise of 100 nM in hGPR55-HEK293 cells (n = 6, Fig. 1B). In all instances, the agonist-induced calcium rise in hGPR55-HEK293 cells was significantly greater than in nontransfected HEK293 cells (Fig. 1B), indicating that it required GPR55. Notably, not all cannabinoid agonists activated GPR55. There was no calcium response in hGPR55-HEK293 cells perfused with the potent cannabinoid agonists WIN55,212–2 and CP55,940, the endogenous cannabinoids 2-AG and virodhamine, the phytocannabinoids cannabidiol and abnormal cannabidiol (abn-CBD), or the endogenous cannabinoid-like compound palmitoylethanolamide (PEA). Thus, GPR55's pharmacology is distinct from CB1 and CB2.
hGPR55 differs from mouse GPR55 (mGPR55) by a nine-residue deletion in the third intracellular loop and a single-residue insertion in the amino terminus; otherwise the two sequences are 76% identical. Despite the difference in the third intracellular loop, a region often important for G protein signaling, stimulation of mGPR55 by THC or JWH015 increased intracellular calcium similarly to hGPR55. Thus, activation of mGPR55 by 5 μM THC increased intracellular calcium by 77 ± 17 nM (n = 8) compared with 84 ± 17 nM (n = 6, P > 0.7) in hGPR55-expressing cells. Similarly, 3 μM JWH015 increased intracellular calcium by 185 ± 65 nM (n = 9) and 118 ± 14 (n = 6) in mGPR55 and hGPR55-expressing cells, respectively (P > 0.3).
Dorsal root ganglion (DRG) neurons with diameters >35 μm (large DRG neurons) express high levels of GPR55, whereas those with diameters <35 μm (small DRG neurons) do not [Fig. 1C and supporting information (SI) Fig. 6 B and E2]. Perfusion of either 5 μM THC or 3 μM JWH015 increased intracellular calcium in large DRG neurons. The 5 μM THC-induced calcium rise in large DRG neurons was 200 ± 60 nM (n = 10, Fig. 1E), compared with 30 ± 10 nM in small DRG neurons (n = 5, Fig. 1E, P < 0.05). In large DRG neurons (n = 13) 3 μM JWH015 increased intracellular calcium by 400 ± 200 nM, compared with 40 ± 10 nM in small neurons (n = 13) (Fig. 1 D and E, P < 0.05). Hence, DRG neurons respond similarly to hGPR55-HEK293 cells, and the calcium response correlates with GPR55 immunoreactivity. Lysophosphatidyl inositol (LPI) was suggested as an endogenous GPR55 agonist (9). Consistent with this report, 3 μM LPI increased intracellular calcium by 125 ± 50 nM in large DRG neurons (n = 6, Fig. 1E) with kinetics similar to JWH015.
SR141716A Can Act as a GPR55 Antagonist.
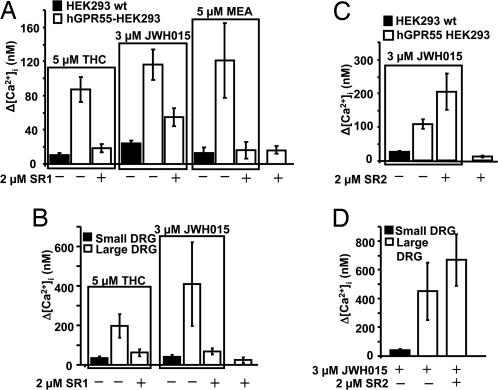
The CB1 antagonist SR141716A (SR1 or rimonabant) apparently binds to GPR55 (6), but its actions are uncertain (5, 6). To clarify this issue, hGPR55-HEK293 cells were coperfused with SR1 and GPR55 agonists. SR1 caused negligible calcium changes by itself (n = 10, Fig. 2A), but when coperfused with THC (n = 14), JWH015 (n = 9), or MEA (n = 7), it attenuated the agonist-induced calcium rise. Coperfusion with 2 μM SR1 reduced the calcium rise induced by 5 μM THC, 3 μM JWH015, and 5 μM MEA by 79%, 52%, and 87%, respectively (Fig. 2A; P < 0.05, 0.01, and 0.01, respectively).
Fig. 2.
The CB1 antagonist SR141716A, but not the CB2 antagonist SR144528, is a GPR55 antagonist. (A) Summary calcium responses in HEK293 wt cells or hGPR55-HEK293 cells treated with different combinations of agonist (THC, JWH015, or MEA) alone or coperfused with SR141716A (SR1). (B) Summary calcium responses in small or large DRG neurons treated with either THC or JWH015 alone or coperfused with SR1. (C) Summary calcium responses in HEK293 wt or hGPR55-HEK293 cells perfused with JWH015 or SR144528 (SR2) alone or together. (D) Summary calcium responses in small or large DRG neurons treated with JWH015 alone or coperfused with SR2.
Similarly, SR1 attenuated THC- and JWH015-induced calcium rises in large DRG neurons. In these experiments, 2 μM SR1 reduced the calcium rise induced by 5 μM THC (n = 5) and 3 μM JWH015 (n = 7) by 83% and 68%, respectively (Fig. 2B, P < 0.05). These data show that low micromolar concentrations of SR1 effectively antagonize GPR55 in neurons and transfected cells.
In contrast, the CB2 antagonist SR144528 (SR2, 2 μM) did not attenuate the 3 μM JWH015-induced calcium rise (n = 6, Fig. 2C, P > 0.1), nor did it affect intracellular calcium when perfused alone on hGPR55-HEK293 cells (n = 4, Fig. 2C). Similarly, 2 μM SR2 had no significant effect on the 3 μM JWH015-induced calcium rise in large DRG neurons (n = 7, Fig. 2D, P > 0.1).
GPR55 Activation Releases Calcium from Intracellular Stores.
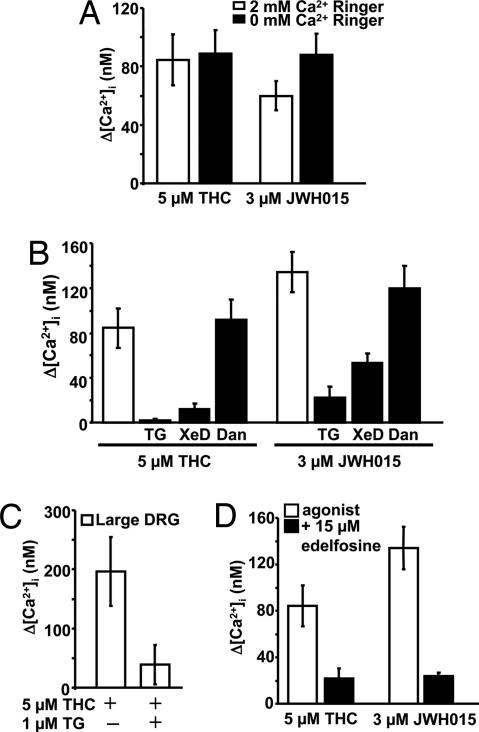
The source of the GPR55-stimulated cytoplasmic calcium rise could be extra- or intracellular. The persistence of the THC- and JWH015-induced calcium rise in 0 Ca Ringer's solution indicates that extracellular calcium is not required (Fig. 3A, P > 0.4, for both). Next, the contribution of calcium release from the endoplasmic reticulum (ER) was assessed with the SERCA inhibitor thapsigargin (TG). A 20-min pretreatment with 1 μM TG in 0 Ca Ringer's empties TG-sensitive ER stores (10) and attenuated the calcium rises induced by 5 μM THC (n = 6) or 3 μM JWH015 (n = 11) in hGPR55-HEK293 cells by 98% and 85%, respectively (Fig. 3B, P < 0.005 and 0.0001, respectively). In large DRG neurons, TG-pretreatment attenuated the 5 μM THC-induced calcium rise by 80% (n = 9, Fig. 3C, P < 0.05). Thus, GPR55 releases calcium from TG-sensitive intracellular stores.
Fig. 3.
GPR55 activation releases calcium from IP3R-mediated calcium stores via phospholipase C activation. (A) A subset of hGPR55-HEK293 cells was perfused with either THC or JWH015 in 0 Ca2+ Ringer's solution, and the changes in [Ca2+]i were compared with those in cells perfused with THC or JWH015 in control Ringer's solution (2 mM Ca2+). (B) Summary calcium responses in hGPR55-HEK293 cells pretreated with 1 μM thapsigargin (TG), or coperfused with either THC or JWH015, in combination with either the IP3R inhibitor xestospongin D (XeD, 1 μM) or the RyR inhibitor dantrolene (Dan, 10 μM). These calcium rises were compared with those seen in control cells. (C) Summary calcium responses in large DRG neurons pretreated with 1 μM thapsigargin (TG) and perfused with THC. (D) Calcium changes in a subset of hGPR55-HEK293 cells treated with the PI-specific PLC inhibitor, ET-18-OCH3 (edelfosine), relative to controls, upon perfusion of THC or JWH015.
Release from TG-sensitive calcium stores is mediated by inositol 1,4,5-trisphosphate receptors (IP3R) and ryanodine receptors (RyR). Xestospongin D (XeD) potently inhibits IP3Rs (11), whereas dantrolene inhibits RyR subtypes 1 and 3 (12). XeD pretreatment (1 μM for 30 s) produced an 85% reduction in the hGPR55-mediated calcium rise induced by THC (n = 8, Fig. 3B, P < 0.01) and reduced by 60% the JWH015-induced calcium rise (n = 8, Fig. 3B, P < 0.05). Dantrolene did not attenuate the agonist-induced calcium rise (Fig. 3B, P > 0.5). Thus, GPR55 releases calcium from intracellular stores via IP3-sensitive, but not Ry-sensitive, channels.
GPR55 Increases Intracellular Calcium via Gq and Phospholipase C (PLC).
Given the role of IP3-sensitive calcium release channels, we suspected PLC involvement. Pretreatment with 3 μM PLC inhibitor U73122 for 160 s, followed immediately by agonist suppressed 90% of the THC- and 50% of the JWH015-induced calcium rise (P < 0.05, data not shown). Treatment with the same concentration of the inactive analog of U73122, U73343, did not significantly affect the hGPR55-mediated calcium rise. To test for a role of phosphatidylinositol (PI)-specific PLC, hGPR55-HEK293 cells were pretreated for 20 min with 15 μM PI-PLC inhibitor ET-18-OCH3. This attenuated the calcium increase by 76% in cells perfused with 5 μM THC (n = 6, Fig. 3D, P < 0.005) and by 70% in cells perfused with 3 μM JWH015 (n = 6, Fig. 3D, P < 0.001), indicating that GPR55 activates a PI-specific PLC.
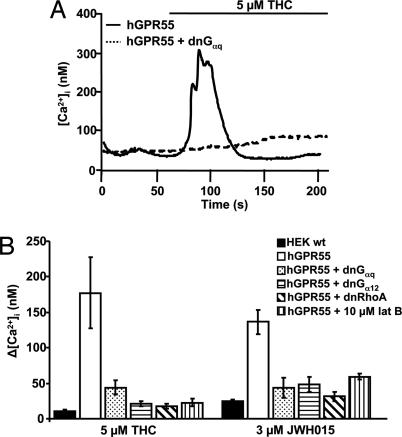
PLCβ can be activated by either Gαq or Gi/o βγ subunits (13). A role for Gi/o-derived βγ subunits was eliminated because overnight treatment with 500 ng/ml pertussis toxin (PTX) failed to eliminate the 5 μM THC-induced calcium transient (data not shown). To test for the involvement of Gq G proteins, hGPR55-HEK293 cells were cotransfected with a dominant-negative Gαq (dnGαq) (14). Cells expressing dnGαq had reduced calcium responses upon perfusion of either 5 μM THC (81% reduction, n = 10, Fig. 4 A and B, P < 0.05) or 3 μM JWH015 (65% reduction, n = 10, Fig. 4B, P < 0.0005). As a positive control for dnGαq, it reduced the M1 muscarinic receptor (M1R)-mediated calcium transient by 40% (n = 5). These data suggest that GPR55 couples to PLCβ via Gαq.
Fig. 4.
Gq/11 is involved in the GPR55-induced increase in [Ca2+]i. (A) The change in calcium upon THC application in a cell expressing hGPR55 alone or hGPR55 and dnGαq. (B) Summary calcium changes in response to perfusion of either THC or JWH015 in HEK293 wt, hGPR55-HEK293, or hGPR55-HEK293 cells also expressing dnGαq, dnGα12, or dnRhoA or coperfused with latrunculin B.
G12, RhoA, and an Intact Actin Cytoskeleton Are Required for the Calcium Increase.
GPR55 has been reported to couple to G12 G proteins (5), and these G proteins can mediate calcium transients via the small GTPase Rho and the actin cytoskeleton (15). To determine whether Gα12 is required for the GPR55-mediated calcium rise, hGPR55-HEK293 cells were cotransfected with a dominant-negative, GTPase-deficient Gα12 mutant (dnGα12). The calcium rises induced by 5 μM THC (n = 13) and 3 μM JWH015 (n = 12) were reduced by 88% and 65%, respectively (Fig. 4B, P < 0.01 for both). To rule out the possibility that dnGα12 might additionally perturb Gq signaling, we checked its effect on signaling through M1Rs, which signal solely through Gq/11. In M1R-HEK293 cells cotransfected with dnGα12, the 5 μM oxotremorine-M (Oxo-M) induced calcium rise (100 ± 20 nM) did not differ significantly from control cells expressing only M1R (90 ± 20 nM). Thus, the expression of dnGα12 in HEK293 cells inhibited Gα12, but not “pure” Gq/11, signaling; therefore Gα12 is required for GPR55 to release calcium from intracellular stores.
RhoA, a member of the Rho family of GTPases, is activated by several G proteins, including G12 (16). Cotransfection of hGPR55-HEK293 cells with a dominant-negative RhoA mutant (dnRhoA) suppressed GPR55-mediated calcium transients, reducing the 5 μM THC-induced rise by 90% (n = 8) and the 3 μM JWH015-induced rise by 77% (n = 10, Fig. 4B, P < 0.01 for both). As expected for a purely Gq-mediated pathway, expression of dnRhoA did not affect the M1R-mediated calcium rise: The 5 μM Oxo-M-induced calcium rise in M1R-HEK293 cells (175 ± 40 nM) did not differ significantly from that in M1R-HEK293 cells expressing dnRhoA (140 ± 25 nM) (n = 6, P > 0.1).
The actin cytoskeleton has been shown to play a role in the generation of calcium transients in some cells (15, 17). Because the Rho family of proteins regulates the organization of the actin cytoskeleton, and RhoA is involved in the GPR55-mediated calcium rise, we investigated the requirement for an intact actin cytoskeleton. Latrunculin B (lat B) prevents actin polymerization by sequestering actin monomers. Treatment of hGPR55-HEK293 cells with lat B reduced the 5 μM THC (n = 14) calcium rise by 78% and the 3 μM JWH015 (n = 16)-induced rise by 52% (Fig. 4B, P < 0.01 for both). In contrast, the 5 μM Oxo-M-induced calcium rise in lat B-treated M1R-HEK293 cells was insignificantly different from control (data not shown). Taken together, these data reveal a GPR55-mediated signaling pathway requiring G12, RhoA, and the actin cytoskeleton.
GPR55 Does Not Activate the ERK1/2 Kinase Pathway Robustly.
Because both CB1 and CB2 activate ERK1/2 (18–20), we considered that GPR55 might also activate ERK1/2. However, incubation of hGPR55-HEK293 cells with either 5 μM THC or 5 μM MEA failed to increase ERK1/2 phosphorylation above basal levels (data not shown).
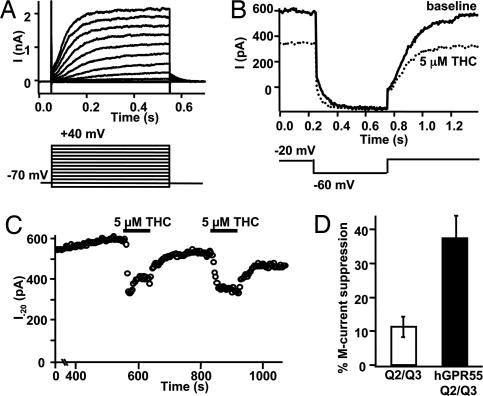
GPR55 Inhibits M-Type Potassium Current.
To examine possible physiological consequences of GPR55 activation, we looked for GPR55-induced suppression of a Gq-modulated and PIP2-requiring potassium current (21, 22), the M current (23). hGPR55-HEK293 cells were cotransfected with KCNQ2 and KCNQ3 subunits that form the heteromeric channels underlying neuronal M current (24). Reconstituted M current showed characteristic voltage dependence and slow activation and deactivation (Fig. 5 A and B). THC (5 μM) suppressed M current by 38 ± 7% in a reversible and repeatable manner (n = 5, Fig. 5 C and D). Analysis of slowly deactivating tail currents (data not shown) confirms that observed changes in current reflect modulation of M-type potassium channels. As a control, in cells lacking GPR55, 5 μM THC suppressed M current by only 11 ± 3% (n = 7, P < 0.01 compared with cells expressing hGPR55). In comparison, 1 μM Oxo-M suppressed M current by 88 ± 6% in tsA 201 cells transfected with M1R (n = 6).
Fig. 5.
GPR55 inhibits M-type potassium current. (A) Voltage activation of M current in a representative hGPR55-HEK293 cell expressing KCNQ2 and KCNQ3. Depolarization elicits noninactivating outward current with characteristic slow activation and deactivation. (B and C) Same cell as in A. (B) Deactivation protocol for M current before and during exposure to THC. (C) Time course of suppression of steady-state M current at −20 mV by repeated THC. (D) Summary of M current suppression by THC in cells expressing KCNQ2/KCNQ3 alone and in cells expressing hGPR55 and KCNQ2/KCNQ3.
Discussion
The status of GPR55 as a cannabinoid receptor is controversial (6, 9). We find that in HEK293 cells and large DRG neurons (where it is abundantly expressed), GPR55 activation gradually increased calcium upon exposure to the classical cannabinoid THC, the aminoalkylindole JWH015, the eicosanoids anandamide and MEA, and the endogenous lipid LPI (Fig. 1B). Our mechanistic studies showed that GPR55 releases calcium from intracellular stores in a manner requiring Gαq, PLC, Gα12, RhoA, and an intact actin cytoskeleton. In contrast, other cannabinoids and related compounds including 2-AG, WIN, CP, PEA, virodhamine, abn-CBD, and CBD did not increase intracellular calcium via GPR55. GPR55 also inhibited M current. Thus, GPR55 can be considered a cannabinoid receptor whose distinct signaling profile enlarges the cellular repertoire of cannabinoid action.
A recent report by Ryberg et al. (6) examined the role of GPR55 as a potential cannabinoid receptor, primarily by using GTPγS binding. They found that a number of cannabinoid compounds at low nanomolar concentrations stimulated GTPγS binding in HEK293 cells stably expressing GPR55. In addition, low micromolar concentrations of AEA and O-1602 stimulated RhoA activation. Of the compounds examined in both their study and ours, only two (THC and AEA) increased intracellular calcium. Specifically, CP, 2-AG, PEA, virodhamine, and abn-CBD increased GTPγS binding in the Ryberg study, but did not increase intracellular calcium in the present work. The inability of these latter compounds to increase intracellular calcium may be due to a difference between stably versus transiently transfected cells or to a functional selectivity (25) of different GPR55 agonists. Oka et al. (9) examined the signaling of GPR55 stably expressed in HEK293 cells and reported that a mixture of LPIs increased calcium in these cells. These investigators did not report whether any other potential GPR55 agonists increased intracellular calcium levels. We also found that LPI increased calcium in DRG neurons (Fig. 1E).
The development of potent, selective ligands will be important to define the role of GPR55. The aminoalkylindoles offer an intriguing lead because JWH015 is an agonist for GPR55, but the closely related compound WIN55,212–2 neither activates nor binds the receptor (Table 1) (6). The complex interaction of GPR55 with arylpyrazole CB1 antagonists such as SR1, AM251, and AM281 is noteworthy. We found SR1 to be a GPR55 antagonist, whereas Ryberg et al. (6) found the closely related compounds AM251 and AM281 to be an agonist and inactive, respectively. With the possible presence of GPR55 on adipocytes (5) and the clinical use of arylpyrazoles and related compounds, the interactions of these compounds with GPR55 and related receptors needs to be evaluated.
Table 1.
Cannabinoids and cannabimimetics that cause statistically insignificant calcium increases in hGPR55-HEK293 cells
| Drug | Δ[Ca2+]i, nM | n |
|---|---|---|
| 5 μM 2-AG | 14 ± 8 | 5 |
| 5 μM CP55,940 | 9 ± 2 | 5 |
| 5 μM PEA | 25 ± 4 | 6 |
| 3 μM virodhamine | 15 ± 2 | 6 |
| 3 μM abnormal-CBD | 10 ± 3 | 5 |
| 3 μM cannabidiol | 21 ± 11 | 5 |
| 5 μM WIN55,212-2 | 8 ± 3 | 9 |
GPR55 is abundantly expressed in large-diameter DRG neurons, but present only at low levels in small-diameter DRG neurons (Fig. 1C and SI Fig. 6). We find that GPR55 signals in large DRG neurons to release calcium from intracellular stores in a fashion similar to transfected HEK293 cells. Nonetheless, it is important to eliminate other mechanisms by which the agonists used in our study might increase intracellular calcium in DRG neurons. High concentrations of assorted cannabinoids have been reported to activate various transient receptor potential (TRP) channels (3). However, TRP channel activation is highly unlikely to account for the calcium transients observed here because of the lack of effect of GPR55 agonists on small-diameter neurons, and the attenuation of these calcium rises by thapsigargin and their antagonism by SR1. Similarly, the lack of antagonism by SR2 makes it very unlikely that CB2 receptors mediate the calcium transients. Finally, high (10 μM) concentrations of THC fail to activate CB1 to increase intracellular calcium (26) and CB1 is expressed in all sizes of DRG neurons (e.g., Fig. 1C), making it unlikely that CB1 receptors mediate the DRG calcium transients.
CB1 suppresses neuronal excitability by activating some potassium channels (27, 28). We have found that GPR55 activation has the opposite effect and inhibits potassium current through M-type potassium channels (Fig. 5). This result, plus the observation that GPR55 increases intracellular calcium, suggests that GPR55 activation enhances neuronal excitability. These findings, together with the preferential expression of GPR55 on large-diameter DRG neurons, which can be involved in nociception, particularly in neuropathic or inflammatory pain states (29–31), suggest that GPR55 may have a pronocioceptive role.
We have found that GPR55 is activated by THC, AEA, MEA, JWH015, and LPI but not by other cannabinoids including CP, WIN, and 2-AG. Its activation releases calcium from IP3-sensitive ER stores. SR1 at 2 μM suppressed the calcium rise, suggesting that this class of CB1 antagonist interacts with GPR55. The GPR55-mediated calcium increase was PTX-insensitive and required Gαq, PLC, Gα12, RhoA, and an intact actin cytoskeleton. GPR55 activation also inhibited M current, but did not affect the ERK1/2 pathway. Together these results establish GPR55 as an additional cannabinoid receptor that activates signaling pathways distinct from CB1 or CB2, and that may increase neuronal excitability and have additional effects through its action on RhoA.
Methods
Materials.
SR141716A, THC, and CP55,940 were supplied by the National Institute on Drug Abuse (NIDA) Research Resources Drug Supply System. Dominant-negative Gαq (Q209L/D277N), dominant-negative Gα12 (Q231L/D299N), and dominant-negative RhoA (T19N) plasmids were obtained from University of Missouri, Rolla, MO, cDNA Resource Center (www.cdna.org). Mouse M1 receptor, human KCNQ2, and rat KCNQ3 were obtained as before (32). LPI, cannabidiol, and JWH015 were generously donated by Nephi Stella (University of Washington).
Cloning and Expression Constructs.
Human GPR55 was amplified from a human brain cDNA library (Invitrogen) by using primers based on the published GPR55 sequence (7) and subcloned into HA-modified pcDNA3 or pEF4. Mouse GPR55 was amplified from a GPR55-containing BAC (RP24-172B20) by using primers based on the gm218 ORF derived from the C57BL/6 chromosome 1 data in GenBank and subcloned into a HA-modified pcDNA3. The amplified sequences corresponded to the genomic sequences in GenBank. Other constructs were subcloned by using PCR into the pcDNA3 expression vector (32).
Cells.
HEK293 and CHO cells were grown as described (32). CHO cells stably expressing hGPR55 (hGPR55-CHO) were generated (27) by using Zeocin (250 μg/ml) selection. For photometry experiments, cells were plated onto poly-d-lysine-coated coverslips and, when required, transfected the day after plating and used 24–36 h later. For electrophysiological experiments, cells were plated 1 day after transfection and used 36–48 h later. cDNA for additional genes (such as M1R, GPR55, and dominant-negative Gα constructs) were transiently transfected with Lipofectamine 2000. Transfected cells were identified by expression of cotransfected ds-Red.
DRG Cultures.
DRG were dissected from CO2-asphyxiated BALB/c mice (33). Dissociated DRGs were resuspended in F12 medium plus 10% FBS with penicillin/streptomycin and NGF (100 ng/ml). Neurons were plated onto laminin/poly-d-lysine-coated glass coverslips. After 2 h, neurons were immersed in fresh medium plus 10 μM cytoarabinoside. Medium was changed daily, and neurons were used for photometry 16–48 h after dissection.
Dye Loading and Photometry.
HEK293 cells expressing GPR55 were loaded for 40–45 min with 2 μM fura-2 AM, and CHO cells and DRG neurons were loaded for 20–25 min with 8 μM. Cytosolic Ca2+ was monitored as described (26). Photometric measurements were analyzed in Igor (Wavemetrics) and statistical analyses performed in Excel (Microsoft). All results are presented as mean± SEM.
ERK1/2 Kinase Assay.
ERK1/2 kinase activation was as described (34).
M Current Recording.
The whole-cell configuration of the patch–clamp technique at room temperature was used to record M currents (32). Test and control solutions were applied by local perfusion, and solution exchange was complete within 10 s. The KCNQ2/KCNQ3 currents were studied by using a standard deactivation protocol: Cells were held at −20 mV and a 500-ms hyperpolarizing step to −60 mV was applied every 4 s. The steady-state current at −20 mV was plotted. Data acquisition and analysis used the Pulse/Pulse Fit software in combination with an EPC-9 patch–clamp amplifier (HEKA). Further data processing was performed with Excel and Igor.
Solutions.
The Ringer's bath solution contained 160 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM Hepes and 8 mM glucose adjusted to pH 7.4 with NaOH. Ca2+-free Ringer's solution “0 Ca Ringer's” was as above without CaCl2 and with 0.5 mM EGTA. THC was dissolved in ethanol, and all other cannabinoids were dissolved in DMSO to make stock solutions that were stored at −20°C. Final dilutions were made in Ringer's solution (ethanol final concentration was 0.16% in the 5 μM THC solution; DMSO final concentration did not exceed 0.05%). BSA (1 mg/ml) was added as a carrier to all solutions. For patch–clamp recording a KCl-based internal was used (32), and the whole-cell configuration was achieved before washing in Ringer's solution containing 1 mg/ml BSA and 0.16% ethanol.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dr. Carmen Ufret-Vincenty, Dr. Sharona Gordon, James Wager-Miller, Carlos Ballester Rosado, and the Baylor Medicine Mental Retardation Developmental Disabilities Research Center core facility [National Institutes of Health (NIH) Grant HD24064] for technical assistance. This work was supported by NIH grants DA07278 (to J.E.L.), GM007108 (to J.B.J.), NS048884 (to H.-C.L.), NS08174 and AR17803 (to B.H.), and DA21696 and DA11322 (to K.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711278105/DC1.
References
- 1.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 2.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 3.Begg M, et al. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: Evidence for new players. Am Assoc Pharm Sci J. 2006;8:E298–E306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown A, Wise A, inventors. GlaxoSmithKline, assignee. 0,113,814. US Patent. 2003 inventors. assignee.
- 6.Ryberg E, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawzdargo M, et al. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res Mol Brain Res. 1999;64:193–198. doi: 10.1016/s0169-328x(98)00277-0. [DOI] [PubMed] [Google Scholar]
- 8.Huffman JW. The search for selective ligands for the CB2 receptor. Curr Pharm Des. 2000;6:1323–1337. doi: 10.2174/1381612003399347. [DOI] [PubMed] [Google Scholar]
- 9.Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Koh DS, Hille B. Dynamics of calcium clearance in mouse pancreatic beta-cells. Diabetes. 2003;52:1723–1731. doi: 10.2337/diabetes.52.7.1723. [DOI] [PubMed] [Google Scholar]
- 11.Gafni J, et al. Xestospongins: Potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 12.Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene—A review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59:364–373. doi: 10.1111/j.1365-2044.2004.03658.x. [DOI] [PubMed] [Google Scholar]
- 13.Fields TA, Casey PJ. Signalling functions and biochemical properties of pertussis toxin-resistant G-proteins. Biochem J. 1997;321(Pt 3):561–571. doi: 10.1042/bj3210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goel R, Phillips-Mason PJ, Gardner A, Raben DM, Baldassare JJ. Alpha-thrombin-mediated phosphatidylinositol 3-kinase activation through release of Gbetagamma dimers from Galphaq and Galphai2. J Biol Chem. 2004;279:6701–6710. doi: 10.1074/jbc.M308753200. [DOI] [PubMed] [Google Scholar]
- 15.Rey O, Young SH, Yuan J, Slice L, Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase C/inositol 1,4,5-trisphosphate-independent pathway that requires G12, rho, filamin-A, and the actin cytoskeleton. J Biol Chem. 2005;280:22875–22882. doi: 10.1074/jbc.M503455200. [DOI] [PubMed] [Google Scholar]
- 16.Dhanasekaran N, Dermott JM. Signaling by the G12 class of G proteins. Cell Signal. 1996;8:235–245. doi: 10.1016/0898-6568(96)00048-4. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro CM, Reece J, Putney JW., Jr Role of the cytoskeleton in calcium signaling in NIH 3T3 cells. An intact cytoskeleton is required for agonist-induced [Ca2+]i signaling, but not for capacitative calcium entry. J Biol Chem. 1997;272:26555–26561. doi: 10.1074/jbc.272.42.26555. [DOI] [PubMed] [Google Scholar]
- 18.Bouaboula M, et al. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312(Pt 2):637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouaboula M, et al. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi Y, Arai S, Waku K, Sugiura T. Activation by 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, of p42/44 mitogen-activated protein kinase in HL-60 cells. J Biochem (Tokyo) 2001;129:665–669. doi: 10.1093/oxfordjournals.jbchem.a002904. [DOI] [PubMed] [Google Scholar]
- 21.Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, et al. PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 23.Haley JE, et al. The alpha subunit of Gq contributes to muscarinic inhibition of the M-type potassium current in sympathetic neurons. J Neurosci. 1998;18:4521–4531. doi: 10.1523/JNEUROSCI.18-12-04521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Xia J, Kass RS. MinK-KvLQT1 fusion proteins, evidence for multiple stoichiometries of the assembled IsK channel. J Biol Chem. 1998;273:34069–34074. doi: 10.1074/jbc.273.51.34069. [DOI] [PubMed] [Google Scholar]
- 25.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 26.Lauckner JE, Hille B, Mackie K. The cannabinoid agonist WIN55,212–2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc Natl Acad Sci USA. 2005;102:19144–19149. doi: 10.1073/pnas.0509588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34:787–796. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- 29.Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: Systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 30.Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 31.Ruscheweyh R, Forsthuber L, Schoffnegger D, Sandkuhler J. Modification of classical neurochemical markers in identified primary afferent neurons with Abeta-, Adelta-, and C-fibers after chronic constriction injury in mice. J Comp Neurol. 2007;502:325–336. doi: 10.1002/cne.21311. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro MS, et al. Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3 K(+) channels that underlie the neuronal M current. J Neurosci. 2000;20:1710–1721. doi: 10.1523/JNEUROSCI.20-05-01710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daigle TL, Kearn CS, Mackie K. Rapid CB (1) cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology. 2008;54:36–44. doi: 10.1016/j.neuropharm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.