Abstract
Galanin is a neuropeptide with a wide range of effects in the nervous and endocrine systems, mediated through three G protein-coupled receptor subtypes (GalR1–3). Interestingly, galanin and its receptors are also expressed in certain tumors. Here we studied the effects of galanin in rat pheochromocytoma (PC12) cells stably transfected with GFP-tagged GalR2. Galanin at 100 nM inhibited cell proliferation in both nontransfected and transfected cells. Conversly, both galanin and the GalR2(R3)-agonist AR-M1896 induced caspase-dependent apoptotic cell death only in GalR2-transfected cells. Western-blot analyses of downstream mediators of the Gq/11-type G protein showed down-regulation of pAkt and pBad in galanin-exposed transfected cells. Also, the specific PI3 kinase inhibitor LY-294002 increased the level of pBad and decreased activation of caspases. In addition, p21cip1 levels were up-regulated in galanin-exposed PC12 cells and down-regulated in galanin-exposed GalR2-transfected cells. In agreement, FACS analyses of galanin exposed cells showed occurrence of cell cycle arrest in PC12 cells and cell death in transfected cells. Finally, as shown with real-time PCR, galanin and its receptors were expressed at very high levels in human pheochromocytoma tissues as compared with normal adrenal medulla. These findings point to GalR2 as a possible target for therapeuthic interventions in pheochromocytoma.
Keywords: adrenal medulla, galanin receptor, neuropeptide, pheochromocytoma, tumor
Galanin is a 29-aa neuropeptide with a widespread distribution in the nervous system (1) involved in nociception (2–4), metabolism and reproduction (5, 6), survival and regeneration (7), Alzheimer's disease (8), and epilepsy (9).
Recent findings suggest an involvement of galanin and its receptors in regulation of cell proliferation and survival of several types of tumors, including pheochromocytomas (10). Also, other peptides can influence tumor proliferation, and their levels have been correlated with tumor stage and prognosis (11). Both neuroendocrine (12) and nonendocrine tumors, such as meningiomas, gliomas, and several peripheral cancers (13), synthesize a variety of neuropeptides and express peptide receptors. The presence of peptides and their receptors in human cancers offers the possibility to develop diagnostic tools and therapeutic strategies based on the molecular targeting.
Galanin mediates its effects via at least three seven-transmembrane, G protein-coupled receptors (GPCRs), namely GalR1, -R2, and -R3 (14–17), which have a broad distribution as revealed with RT-PCR (18) and in situ hybridization (19). GalR1 is mainly expressed in central nervous system (CNS), whereas GalR3 is restricted to a number of brain regions and some peripheral tissues and is present at low levels. Both GalR1 and -R3 act through Gi/o receptor subtype, inhibiting adenyl cyclase, and causing hyperpolarization via opening K+ channels (14–17). GalR2 has a broader distribution and is present in the CNS, dorsal root ganglia, and many peripheral tissues (18, 19). Activation of GalR2 leads to accumulation of inositol phosphate, mobilization of intracellular Ca2+, and activation of a Ca2+-dependent Cl− channel via Gq/11-type G proteins. In addition, GalR2 may inhibit cAMP accumulation, through Gi/o-type receptor and stimulate mitogen-activated protein kinase mediated via Go (14–17).
Interestingly, recent findings have shown that the GalR2 mediates apoptosis in SH-SY5Y neuroblastoma cells (20) and initiates multiple signaling pathways in small cell lung cancer cells (21). In light of these data pointing to a critical role of GalR2 in tumor cell proliferation and survival, we have investigated the potential effects of galanin on rat pheochromocytoma PC12 and GFP-tagged GalR2-transfected PC12 cells. Pheochromocytomas are catecholamine-producing tumors originating from neural crest and arising from the adrenal medulla (22, 23). Up to a third of patients may have no symptoms, hence either being discovered incidentally or found postmortally at autopsy (22, 24–26). To check the possible relevance of our experimental findings on PC12 cells for human pathology, we also quantified galanin and its three receptors in human pheochromocytoma tumor and postmortem adrenal medulla tissues by real-time PCR.
Results
Effects of Galanin.
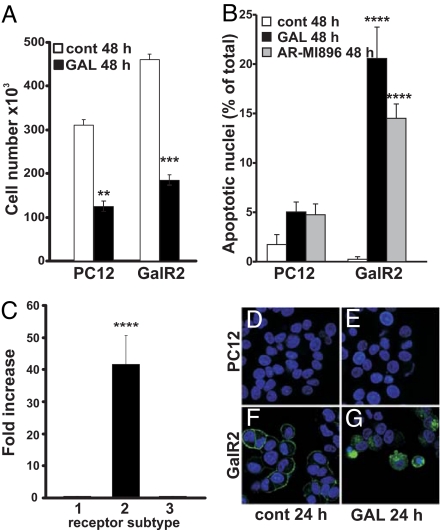
Exposure to 100 nM galanin alone significantly inhibited cell proliferation in both nontransfected and transfected cells as observed with trypan blue staining (Fig. 1A). To evaluate nuclear morphology, control and galanin-exposed cells were fixed and stained with Hoechst dye 33358. Galanin-exposed GalR2-transfected cells showed a significant increase in apoptotic nuclei, whereas PC12 cells showed a modest but not significant increase (Fig. 1 B and D–G). Moreover, exposure of cells to 100 nM AR-M1896, a GalR2 agonist, significantly increased the number of apoptotic nuclei only in GalR2-transfected cells, further supporting the hypothesis that galanin induces apoptosis mainly through GalR2 (Fig. 1B). We used real-time PCR to measure the expression levels of galanin and its receptors in transfected versus nontransfected cells. The expression levels of R1 (CT = 28.3), R2 (CT = 26.5), and R3 (CT = 28.3) in PC12 cells were used as controls. In GalR2-transfeced PC12 cells, the expression levels of R1 (CT = 28.5), and R3 (CT = 28.3), were not significantly different from the nontransfected cells; however, there was a 40-fold increase in the expression of GalR2 (CT = 20.1) in the transfected cells (Fig. 1C). No galanin expression could be detected in either cell type (data not shown). In agreement with a previous study, the fusion protein was detected on the surface of the cell membrane in control GalR2 cells (Fig. 1F) and internalized after galanin exposure (27), even in cells exhibiting apoptotic condensed nuclei (Fig. 1G).
Fig. 1.
Increased galanin-induced cytotoxicity in GalR2-transfected cells. Exposure of PC12 and GalR2-transfected cells to galanin (GAL) for 48 h reduces significantly the total cell number (A), whereas GAL induces a significant increase in apoptotic nuclei only in GalR2-transfected cells (B). Exposure to AR-MI896 also increases the apoptotic nuclei only in transfected cells (B). Real-time PCR quantification was performed for detection of mRNA levels of GalR1, GalR2, and GalR3 in PC12 and GalR2-transfected cells. The amount of target gene was normalized to GAPDH, and the values for PC12 cells were considered as control. The relative increase was equal to 2−Δ(ΔCTtarget − ΔCTcontrol). There was a significant increase in the expression level of GALR2 in the transfected cells (C). Fluorescence micrographs showing PC12 (D and E) and GalR2-transfected cells (F and G) after 24-h exposure to 100 nM GAL (E and G). Exposed transfected cells showed internalized GalR2 and condensed apoptotic nuclei, as shown with Hoechst dye 33358 (G). Values are means ± SEM of three determinations. **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
Activation of Caspases in GalR2-Mediated Cell Death.
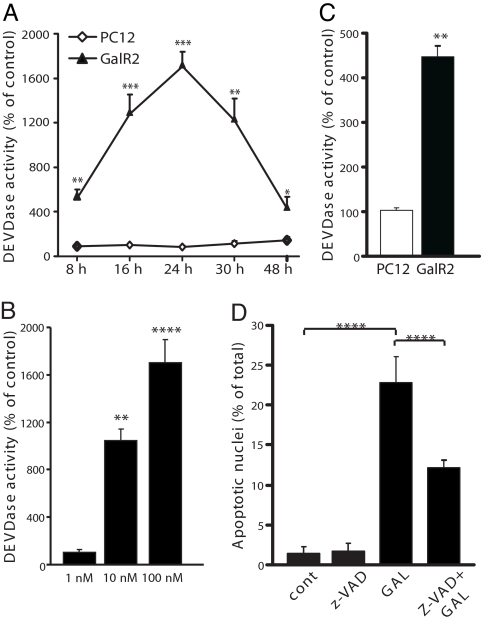
Caspases are a family of cysteine-proteases, which upon activation will cleave cellular protein targets, leading to the biochemical and morphological alterations typical of apoptosis (28). We measured the caspase 3-like activity in extracts obtained from control and galanin-exposed PC12 and GalR2-transfected cells. Exposure to 100 nM galanin for 8–48 h induced a significant increase in DEVDase activity, compared with control only in GalR2-transfected cells, with the strongest activation after exposure for 24 h (Fig. 2A). Also, exposure to 100 nM AR-M1896 for 24 h caused increased DEVDase activity only in the transfected cells (Fig. 2C). The induction of caspase activity in GalR2-transfected cells by galanin was dose-dependent (Fig. 2B). Preincubation of cells with the pan-caspase inhibitor z-VAD-fmk (20 μM) 30 min prior exposure to 100 nM galanin for 48 h, significantly reduced the number of apoptotic nuclei in GalR2-transfected cells (Fig. 2D). In addition, after exposure to 100 nM galanin for 24 h, only transfected cells showed loss of mitochondrial membrane potential and release of cytchrome c (cyt c) into the cystosol (data not shown). Mitochondrial membrane potential is a key indicator of cellular viability, and the release of cyt c from intermembrane space plays an essential role in the formation of the apoptosome complex with subsequent activation of the caspase cascade executing apoptosis (29, 30). Thus, these results point to the activation of caspases by galanin as a critical step in the process of apoptotic cell death occurring in GalR2-transfected cells.
Fig. 2.
Caspase activity after exposure to GAL was measured with the fluorimetric DEVDase assay. Exposure of transfected GalR2 cells to 100 nM GAL induced a time-dependent significant increase in DEVDase activity (A). Exposure to different concentrations of GAL for 24 h caused a dose-dependent increase (B). A significant DEVDase activity was also induced after 24 h exposure to AR-MI896 only in transfected cells (C). Transfected cells were preincubated with the pan-caspase inhibitor z-VAD-fmk for 30 min before exposure to GAL for 48 h and then fixed with methanol and stained with Hoechst dye 33358. z-VAD-fmk significantly decreased the number of apoptotic nuclei (D). Values are means ± SEM of three determinations. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
GalR2 Mediates its Actions Through the PI3-Kinase/AKT Pathway.
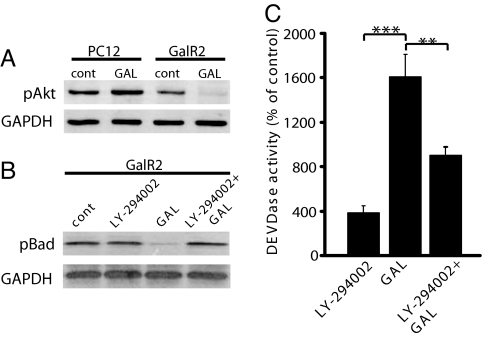
Activation of GalR2 leads to mobilization of intracellular Ca2+, via Gq/11-type G proteins (31–34). It has been shown that the GalR2 is capable of activating the phosphatidylinositol (PtdIns) signaling pathway (31–34). Galanin causes a concentration-dependent increase in intracellular Ca2+ levels in GalR2 cells but not in nontransfected cells (27). PtdIns 3-kinase (PI3K) mediates a variety of biological responses, including inhibition of apoptosis and stimulation of cellular growth. Using immunoblotting, we measured the levels of phosphorylated Akt/PKB, a known downstream target of PI3K, in protein extracts from cells exposed to 100 nM galanin for 24 h. There was a significant increase in pAkt in exposed PC12 cells, whereas this phosphorylated protein was hardly detectable in exposed transfected cells (Fig. 3A). The pro- and antiapoptotic Bcl-2 family proteins are key regulators of apoptosis (35). Inhibition of Akt/PKB leads to decreased phosphorylation of the proapoptotic protein Bad, which subsequently promotes apoptosis by antagonizing anti-apoptotic Bcl-Xl proteins (36). Therefore, we measured the protein levels of pBad in GalR2-transfected cells and, in accord with the very low pAkt expression, pBad was scarcely detected (Fig. 3B). Additionally, preincubation of cells with 25 μM LY-294002, a specific PI3-kinase inhibitor, 30 min before exposure to 100 nM galanin for 24 h, significantly increased pBad levels (Fig. 3B) and significantly decreased caspase 3-like activity (Fig. 3C) in GalR2-transfected cells. Thus, GalR2-mediated apoptotic cell death is probably PI3K-dependent.
Fig. 3.
Galanin inhibits pAkt and pBad in GalR2-transfected cells. Western blot analysis from total protein extracts showed increased levels of pAkt after 24 h of exposure to GAL in PC12 cells, but decreased levels in GalR2-transfected cells (A). Exposure for 24 h also resulted in decreased levels of pBad in transfected cells, whereas preincubation with the PI3-kinase inhibitor LY-294002 prior for 30 min before GAL exposure retrieved the pBad levels (B), as well as protected the GalR2-transfected cells against caspase activation (C). Values are means ± SEM of three determinations. **, P ≤ 0.01; ***, P ≤ 0.001.
Galanin Induces Apoptosis in GalR2-Transfected Cells and Cell Cycle Arrest in PC12 Cells.
p21cip1 was originally found to act as a cyclin-dependent kinase (CDK) inhibitor. Interestingly, immunoblot experiments in galanin-exposed cells showed a significant increase of p21cip1 levels in PC12 cells, whereas there was a significant decrease in GalR2 cells (Fig. 4A). Furthermore, using FACS analysis, we assessed the distribution of cells in different phases of the cell cycle. PC12 cells exposed to 100 nM galanin for 24 h (white background), displayed a bigger population in G1 phase and to some extent also in G2/M phase than the control (gray background) (Fig. 4B). However, there was no difference between control and exposed cells in the sub-G1 phase (Fig. 4C). On the other hand, 100 nM galanin-exposure for 24 h (white background) did not cause any differences in the populations found in the G1 or G2/M phases as compared with the control (gray background) (Fig. 4C). Notably, there was a bigger population of cells in the sub-G1 phase in the exposed GalR2-transfected cells than in the control (Fig. 4E). These results imply that galanin plays different roles depending on the expression of its receptors; inducing cell cycle arrest in PC12 cells and cell death in GalR2-transfected cells.
Fig. 4.
Galanin induces cell cycle arrest in PC12 cells and apoptosis in GalR2-transfected cells. Western blot analysis from total protein extracts showed increased levels of p21cip1 after 24 h of exposure to GAL in PC12 cells, but decreased levels in GalR2-transfected cells (A). FACS analysis showing cell cycle related changes in PC12 (B and C) and GalR2-transfected cells (D and E) (controls are shown in gray and exposed cells in white background). Exposure for 24 h to GAL increased the number in G1 in PC12 cells (B), but no differences were detected in the sub-G1 phase (C). There were no detectable changes between control and exposed GalR2-transfected cells in G1 (D), whereas there was increased cell population in the sub-G1 phase in exposed cells (E).
The Expression of Galanin, GalR1, and GalR2 Is Increased in Human Pheochromocytoma Tissues.
Using real-time PCR, we quantified the expression of galanin and its three receptors in 13 pheochromocytoma tumor tissues from patients and in control human samples obtained from a tissue bank (UK Human Tissue Bank). The average expression levels of R1 (CT = 33.9), R2 (CT = 35), and galanin (CT = 30.3) in medulla samples were used as controls. The expression of galanin (P < 0.0001), R1 (P < 0.0001) and R2 (P < 0.01) was significantly higher in the tumor samples with the exception of two patients (6 and 13) that had GalR2 levels equal to controls. GalR3 was not detectable in either tumor or control samples (Fig. 5).
Fig. 5.
Real-time PCR quantification was preformed for detection of mRNA levels of GAL, GalR1, and GalR2 in 13 tumor samples obtained from patients and 2 human control samples obtained from a tissue bank. The relative quantification expression values were calculated by the arithmetic equation 2−ΔΔCT. The amount of target gene was normalized to GAPDH and the relative increase was equal to 2−Δ(ΔCTtarget−ΔCTcontrol). There was a significant increase of galanin, GalR1, and GalR2 expression levels in the tumor samples, except for patients 6 and 13, who had GalR2 levels equal to the controls.
Discussion
In the present study, galanin induced a decrease in proliferation in both PC12 and GalR2-transfected cells. However, we detected a significant induction of apoptosis with characteristic morphological changes and caspase activation only in GalR2-transfected cells after exposure to galanin or to the GalR2 agonist AR-M1896. Recently, it has been described that AR-M1896 has affinity, albeit much lower, also for rat GalR3 (16, 37). Considering that we did not observe any difference in GalR3 transcript levels between the transfected and nontransfected cells, the galanin-induced effects are presumably mediated via GalR2. Our results are in agreement with the findings by Kofler and colleagues, who showed galanin-induced apoptosis in SH-SY5Y neuroblastoma cells transfected with the GalR2 (20).
GalR2 signals through several different classes of G proteins that activate diverse intracellular pathways. The most frequently reported pathway involves activation of the Gq/11-type G protein resulting in hydrolysis of inositol phosphate, activation of PKC and PI3K (31–34). We investigated the intracellular signaling pathway mediated through PI3k/Akt. Activation of the PI3K/Akt pathway plays a role in cell survival and growth; e.g., by preventing proteosomal degradation of cyclin D and Myc, and down-regulating transcriptional and posttranslational levels of p21cip1 and p27kip1. Both events lead to further promotion of cell proliferation (39). PI3K/Akt has also been shown to suppress apoptotic cell death by phosphorylation of Bad (36), or by phosphorylation of caspase 9 (39), which impedes the formation of the apoptosome complex upstream of caspase 3 activation. In our experiments, galanin induced an increase in pAkt-levels in PC12 cells, whereas there was a decrease in pAkt-levels in galanin-exposed GalR2-transfected cells. The decrease in pAkt- was followed by a decrease in pBad-levels, which was reversed by preincubation with the specific PI3K-inhibitor LY-294002. Preincubation with LY-294002 also decreased significantly activation of caspases in galanin-exposed GalR2 cells. These data suggest that GalR2-mediated signaling in our GFP-GalR2 transfected cells leads to inhibition of the PI3K/Akt pathway.
Activation of the Gq/11-type G protein can also result in release of Ca2+ from intracellular stores and opening of Ca2+-dependent chloride channels (31–33). Intracellular Ca2+ overload can trigger either apoptotic or necrotic cell death (40). In a previous study using the same cell lines, we have shown that activation of GalR2 leads to an increase in intracellular Ca2+ concentration (27). Increased intracellular Ca2+ leading to activation of calcineurin can dephosphorylate Bad and enhance its heterodimerization with Bcl-Xl, hence promoting apoptosis (41). In addition, high levels of intracellular Ca2+ can activate the Ca2+ dependent proteases calpains. We have preliminary data showing the presence of the 150-kDa fragment of α-fodrin, a specific cleavage product of calpains (R.T., unpublished data). These data point to Ca2+ as an additional player in apoptosis induced by galanin.
The Cip/Kip family (CDK interacting protein/kinase inhibitory protein), encompasses broad-range inhibitors of cyclin-dependent kinases (CDKs), containing three members, p21cip1, p27kip1, and p57kip2. In addition to the negative regulatory effects on cell cycle, p21kip1 can bind and inhibit PCNA's DNA synthesis function without disrupting the DNA-repair ability of PCNA (42, 43). Among the Cip/Kip family, p27kip1 has been identified as a tumor suppressor (44), but the involvement of the other two members of this family in the process of tumorgenesis is not clear yet. However, there is evidence showing that overexpression of p21cip1 suppresses the growth of several types of cancer (45–47). Furthermore, it has been demonstrated that p21cip1 can form a complex with procaspase 3 and suppress its activation (47), but can also be cleaved and inactivated by active caspase 3 (49). Seemingly, the different levels of p21cip1 give an indication about the propensity of a cell toward prevention of proliferation or progression of cell death. This was clearly reflected in our experimental models, where the different effects of galanin in control PC12 and in GalR2-transfected cells correlated with different expression levels of p21cip1. The data obtained from FACS analyses also confirmed that exposure to galanin arrests the cell cycle, mostly in G1, in PC12 cells, whereas it induces cell death in the GalR2-transfected cells, as shown by a significant cell population in sub-G1. The antiproliferate effects of GalR1 reported in oral squamous carcinoma (50, 51) suggest that the cell cycle arrest induced by galanin in nontransfected PC12 cells could be mediated via GalR1.
Another interesting finding with the p21cip1 immunoblotting was the equal levels of p21cip1 in galanin-exposed PC12 cells and in nonexposed GalR2-transfected cells. In our previous work, we demonstrated both ligand-dependent internalization of GalR2, but also constitutive ligand-independent endocytosis and recycling of this receptor (27). These phenomena have also been reported for other GPCRs (52–54). The significance of having constitutively internalized receptors in the absences of ligands is not clear, although it might play an important role for the maintenance of cellular homeostasis and/or in the onset of pathophysiological conditions; e.g., tumor cells escaping the actions of neuropeptides by having unresponsive receptors.
Our analyses of 13 pheochromocytomas revealed, as compared to control adrenals, very high expression levels of galanin, GalR1, and GalR2, with the exception of two patients where GalR2 levels were in the same range as in controls. Increased expression of galanin and/or its receptors has been reported in human pheochromocytoma (55) and neuroblastoma (56). The coexpression of galanin and its receptors in tumors is suggestive of an autocrine/paracrine mechanism. However, as shown by Kofler's group, higher galanin concentrations in neuroblastoma are associated with less functional GalRs indicating a negative feedback regulation of galanin on its receptors (56). A more recent study has reported that the most undifferentiated forms of neuroblastic tumors display high levels of galanin, GalR1 and GalR3. Conversely, in well differentiated tumor forms, GalR2 levels are highly increased, whereas the expression of galanin and its receptor 1 and 3 is reduced (57). As in other tumors, pheochromocytomas may have different clinical course and prognosis depending also on the degree of cell differentiation (58, 59). Unfortunately, we could not get access to the clinical records of the patients after surgery to correlate the expression levels of galanin and its receptors found in the respective tumor with the prognosis and clinical evolution.
Although galanin induces cell death in tumor cells, it is known that it can also exert trophic effects (60). It has been reported that galanin and galanin-like peptide can modulate neurite outgrowth via protein kinase C activation of ERK (61). It would therefore have been interesting also to study galanin-like peptide in this model. We have preliminary results showing protective effects of galanin against apoptotic cell death induced by the broad kinase inhibitor staurosporine in primary hippocampal neurons (R.T., unpublished data). These data are in agreement with previous results where galanin or GalR2 agonist AR-M1896 promotes survival of hippocampal neurons undergoing exicitotoxic injuries (62, 63), by activation of GalR2 and hence Akt and ERK pathway (62). Our present data, showing that galanin causes chromatin condensation, loss of membrane potential, release of cyt c into the cytosol, and activation of caspases only in GalR2 cells as a result of inhibition of PI3K/Akt pathway, enlighten the opposing roles of GalR2 in tumor and nontumor cells.
In conclusion, based on the high expression of GalR2 in most of the tumors we examined and in light of our in vitro results on the apoptotic pathways activated by galanin in GalR2-transfected cells, it is conceivable to propose GalR2 as a promising target for therapeutic intervention against pheochromocytoma.
Materials and Methods
Stable Tansfection and Experimental Treatments.
The rat pheochromocytoma PC12 cells were obtained from the American Type Culture Collection. The construction of the GFP-tagged GalR2 and culture procedures has been described in detail (27). Cell were exposed to 1–100 nM galanin (rat) or 100 nM AR M1896 (AstraZeneca) (64) for 8–48h. The caspase inhibitor z-VAD-fmk (20 μM) (Peptide Institute) and the PI3-kinase inhibitor LY-294002 (25 μM) (Sigma) were added 30 min before exposure to galanin and left in the culture for the entire exposure period.
Total Cell Number and Nuclear Staining.
Total cell number was scored with Trypan blue staining as described (65). Cells grown on coverslips were fixed with ice-cold methanol, washed in PBS and stained with Hoechst dye 33358 (1 μg/ml) and the percentage of apoptotic nuclei was determined as reported in ref. 65.
Caspase 3-Like Activity.
Caspase activity was detected by measuring the cleavage of the tetrapeptide DEVD-AMC substrate leading to the release of free AMC (Peptide Institute), as described in ref. 66.
Western Blot Analysis.
Total protein extracts were subjected to SDS/PAGE and immunoblotted as described in ref. 65. For details about the antibodies, see supporting information (SI) Text.
Cell Cycle Analysis.
The distribution of cells in different phases of the cell cycle was measured by FACS-analysis. Ice-cold ethanol-fixed cells were stained with a solution containing 0.5 μg propidium iodide/ml, 2.5 μg RNase A/ml in PBS, pH 7.4, and analyzed on a Becton Dickinson flow cytometer.
Patient and Control Samples.
Pheochromocytoma tissues were obtained from 13 patients. All procedures were approved by the ethical committee of our institution. Postmortem control samples (postmortal times <12 h), were obtained from a Tissue Bank (UK Human Tissue Bank, Leicester, U.K.). The control samples were analyzed histologically to detect tyrosine hydoxylase (TH)-immunoreactivity, and 5 TH-positive medulla pieces from two samples were dissected and used as controls.
Extraction of Total RNA, cDNA Synthesis, and Real-Time PCR.
Total RNA was isolated with Qiagen RNeasy Mini kit. First-strand cDNA was produced and subjected to PCR. For details, see SI Text.
Statistical Analysis.
Experiments were performed in triplicates and replicated three times, except for the analyses on human material, where experiments were performed in duplicates and repeated two times. ANOVA (Fisher's PLSD) was used for comparisons (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dr. Ali Moshfegh and Dr. Ghazal Zaboli (Karolinska Institutet) for helping with real-time PCR, Dr. R. Schmidt (AstraZeneca R&D, Montreal, QC, Canada) for the generous gift of AR-M1986, and The U.K. Human Tissue Bank for adrenal tissues through Dr. Rosamund Graves. This study was supported by Swedish Research Council Grants 62X-10815 and 04X-2887, European Commission Grant CT-2003-506143, the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning, and The Marianne and Marcus Wallenberg Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712300105/DC1.
References
- 1.Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, Mutt V. Galanin: A novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 2.Liu HX, Hokfelt T. The participation of galanin in pain processing at the spinal level. Trends Pharmacol Sci. 2002;23:468–474. doi: 10.1016/s0165-6147(02)02074-6. [DOI] [PubMed] [Google Scholar]
- 3.Wiesenfeld-Hallin Z, Xu XJ. Neuropeptides in neuropathic and inflammatory pain with special emphasis on cholecystokinin and galanin. Eur J Pharmacol. 2001;429:49–59. doi: 10.1016/s0014-2999(01)01305-x. [DOI] [PubMed] [Google Scholar]
- 4.Xu XJ, Hokfelt T, Bartfai T, Wiesenfeld-Hallin Z. Galanin and spinal nociceptive mechanisms: recent advances and therapeutic implications. Neuropeptides. 2000;34:137–147. doi: 10.1054/npep.2000.0820. [DOI] [PubMed] [Google Scholar]
- 5.Kalra SP, Horvath TL. Neuroendocrine interactions between galanin, opioids, and neuropeptide Y in the control of reproduction and appetite. Ann NY Acad Sci. 1998;863:236–240. doi: 10.1111/j.1749-6632.1998.tb10698.x. [DOI] [PubMed] [Google Scholar]
- 6.Leibowitz SF. Regulation and effects of hypothalamic galanin: Relation to dietary fat, alcohol ingestion, circulating lipids and energy homeostasis. Neuropeptides. 2005;39:327–332. doi: 10.1016/j.npep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Holmes FE, Mahoney SA, Wynick D. Use of genetically engineered transgenic mice to investigate the role of galanin in the peripheral nervous system after injury. Neuropeptides. 2005;39:191–199. doi: 10.1016/j.npep.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Counts SE, et al. Galanin: Neurobiologic mechanisms and therapeutic potential for Alzheimer's disease. CNS Drug Rev. 2001;7:445–470. doi: 10.1111/j.1527-3458.2001.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazarati A, Lu X. Regulation of limbic status epilepticus by hippocampal galanin type 1 and type 2 receptors. Neuropeptides. 2005;39:277–280. doi: 10.1016/j.npep.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Berger A, et al. Galanin and galanin receptors in human cancers. Neuropeptides. 2005;39:353–359. doi: 10.1016/j.npep.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Kogner P. Neuropeptides in neuroblastomas and ganglioneuromas. Progr Brain Res. 1995;104:325–338. doi: 10.1016/s0079-6123(08)61798-7. [DOI] [PubMed] [Google Scholar]
- 12.Barakat MT, Meeran K, Bloom SR. Neuroendocrine tumours. Endocrine-Related Cancer. 2004;11:1–18. doi: 10.1677/erc.0.0110001. [DOI] [PubMed] [Google Scholar]
- 13.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 14.Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- 15.Iismaa TP, Shine J. Galanin and galanin receptors. Results Problems Cell Differ. 1999;26:257–291. doi: 10.1007/978-3-540-49421-8_12. [DOI] [PubMed] [Google Scholar]
- 16.Lang R, Gundlach AL, Kofler B. The galanin peptide family: Receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Therapeutics. 2007;115:177–207. doi: 10.1016/j.pharmthera.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Hwa J, Varty G. Galanin receptors and their therapeutic potential. Emerging Drugs. 2000;5:415–440. [Google Scholar]
- 18.Waters SM, Krause JE. Distribution of galanin-1, -2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience. 2000;95:265–271. doi: 10.1016/s0306-4522(99)00407-8. [DOI] [PubMed] [Google Scholar]
- 19.O'Donnell DM, F., Hoffert C, Hulatsch D, Pelletier M, Walker P, Ahmad S. In: Handbook of Chemical Anatomy, Peptide Receptors. Quirion R, Bjorklund A, Hökfelt T, editors. Amsterdam: Elsevier; 2003. pp. 195–224. II. [Google Scholar]
- 20.Berger A, et al. Galanin receptor subtype GalR2 mediates apoptosis in SH-SY5Y neuroblastoma cells. Endocrinology. 2004;145:500–507. doi: 10.1210/en.2003-0649. [DOI] [PubMed] [Google Scholar]
- 21.Wittau N, et al. The galanin receptor type 2 initiates multiple signaling pathways in small cell lung cancer cells by coupling to G(q), G(i) and G(12) proteins. Oncogene. 2000;19:4199–4209. doi: 10.1038/sj.onc.1203777. [DOI] [PubMed] [Google Scholar]
- 22.Elder EE, Elder G, Larsson C. Pheochromocytoma and functional paraganglioma syndrome: No longer the 10% tumor. J Surg Oncol. 2005;89:193–201. doi: 10.1002/jso.20177. [DOI] [PubMed] [Google Scholar]
- 23.Hoehner JC, et al. Developmental gene expression of sympathetic nervous system tumors reflects their histogenesis. Lab Invest. 1998;78:29–45. [PubMed] [Google Scholar]
- 24.Edstrom Elder E, Hjelm Skog AL, Hoog A, Hamberger B. The management of benign and malignant pheochromocytoma and abdominal paraganglioma. Eur J Surg Oncol. 2003;29:278–283. doi: 10.1053/ejso.2002.1413. [DOI] [PubMed] [Google Scholar]
- 25.Stenstrom G, Svardsudd K. Pheochromocytoma in Sweden 1958–1981: An analysis of the National Cancer Registry data. Acta Medica Scand. 1986;220:225–232. [PubMed] [Google Scholar]
- 26.Sutton MG, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma: Review of a 50-year autopsy series. Mayo Clinic Proc. 1981;56:354–360. [PubMed] [Google Scholar]
- 27.Xia S, et al. Visualization of a functionally enhanced GFP-tagged galanin R2 receptor in PC12 cells: Constitutive and ligand-induced internalization. Proc Natl Acad Sci USA. 2004;101:15207–15212. doi: 10.1073/pnas.0406571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorman AM, Orrenius S, Ceccatelli S. Apoptosis in neuronal cells: Role of caspases. NeuroReport. 1998;9:R49–R55. doi: 10.1097/00001756-199807130-00001. [DOI] [PubMed] [Google Scholar]
- 29.Li P, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 30.Brecht S, Buschmann T, Grimm S, Zimmermann M, Herdegen T. Persisting expression of galanin in axotomized mamillary and septal neurons of adult rats labeled for c-Jun and NADPH-diaphorase. Brain Res. 1997;48:7–16. doi: 10.1016/s0169-328x(97)00070-3. [DOI] [PubMed] [Google Scholar]
- 31.Fathi Z, et al. Molecular characterization, pharmacological properties and chromosomal localization of the human GALR2 galanin receptor. Brain Res. 1998;58:156–169. doi: 10.1016/s0169-328x(98)00116-8. [DOI] [PubMed] [Google Scholar]
- 32.Fathi Z, et al. Cloning, pharmacological characterization and distribution of a novel galanin receptor. Brain Res. 1997;51:49–59. doi: 10.1016/s0169-328x(97)00210-6. [DOI] [PubMed] [Google Scholar]
- 33.Smith KE, et al. Expression cloning of a rat hypothalamic galanin receptor coupled to phosphoinositide turnover. J Biol Chem. 1997;272:24612–24616. doi: 10.1074/jbc.272.39.24612. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, et al. Differential intracellular signaling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry. 1998;37:6711–6717. doi: 10.1021/bi9728405. [DOI] [PubMed] [Google Scholar]
- 35.Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 36.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14–3-3 not BCL-X(L). Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 37.Lu X, Lundstrom L, Bartfai T. Galanin (2–11) binds to GalR3 in transfected cell lines: limitations for pharmacological definition of receptor subtypes. Neuropeptides. 2005;39:165–167. doi: 10.1016/j.npep.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Maddika S, et al. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Update. 2007;10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Cardone MH, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 40.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 41.Wang HG, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 42.Li R, Waga S, Hannon GJ, Beach D, Stillman B. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature. 1994;371:534–537. doi: 10.1038/371534a0. [DOI] [PubMed] [Google Scholar]
- 43.Waga S, Hannon GJ, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369:574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 44.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eastham JA, et al. In vivo gene therapy with p53 or p21 adenovirus for prostate cancer. Cancer Res. 1995;55:5151–5155. [PubMed] [Google Scholar]
- 46.Joshi US, et al. Inhibition of tumor cell growth by p21WAF1 adenoviral gene transfer in lung cancer. Cancer Gene Ther. 1998;5:183–191. [PubMed] [Google Scholar]
- 47.Shibata MA, Yoshidome K, Shibata E, Jorcyk CL, Green JE. Suppression of mammary carcinoma growth in vitro and in vivo by inducible expression of the Cdk inhibitor p21. Cancer Gene Ther. 2001;8:23–35. doi: 10.1038/sj.cgt.7700275. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki A, et al. Procaspase 3/p21 complex formation to resist fas-mediated cell death is initiated as a result of the phosphorylation of p21 by protein kinase A. Cell Death Differ. 2000;7:721–728. doi: 10.1038/sj.cdd.4400706. [DOI] [PubMed] [Google Scholar]
- 49.Los M, Stroh C, Janicke RU, Engels IH, Schulze-Osthoff K. Caspases: more than just killers? Trends Immunol. 2001;22:31–34. doi: 10.1016/s1471-4906(00)01814-7. [DOI] [PubMed] [Google Scholar]
- 50.Henson BS, et al. Galanin receptor 1 has anti-proliferative effects in oral squamous cell carcinoma. J Biochem. 2005;280:22564–22571. doi: 10.1074/jbc.M414589200. [DOI] [PubMed] [Google Scholar]
- 51.Kanazawa T, et al. Galanin and galanin receptor type 1 suppress proliferation in squamous carcinoma cells: Activation of the extracellular signal regulated kinase pathway and induction of cyclin-dependent kinase inhibitors. Oncogene. 2007;26:5762–5771. doi: 10.1038/sj.onc.1210384. [DOI] [PubMed] [Google Scholar]
- 52.Costa T, Herz A. Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci USA. 1989;86:7321–7325. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leterrier C, Bonnard D, Carrel D, Rossier J, Lenkei Z. Constitutive endocytic cycle of the CB1 cannabinoid receptor. J Biol Chem. 2004;279:36013–36021. doi: 10.1074/jbc.M403990200. [DOI] [PubMed] [Google Scholar]
- 54.Signoret N, et al. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139:651–664. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bauer FE, et al. Localization and molecular forms of galanin in human adrenals: elevated levels in pheochromocytomas. J Clin Endocrinol Metab. 1986;63:1372–1378. doi: 10.1210/jcem-63-6-1372. [DOI] [PubMed] [Google Scholar]
- 56.Tuechler C, Hametner R, Jones N, Jones R, Iismaa TP, Sperl W, Kofler B. Galanin and galanin receptor expression in neuroblastoma. Ann NY Acad Sci. 1998;863:438–441. doi: 10.1111/j.1749-6632.1998.tb10718.x. [DOI] [PubMed] [Google Scholar]
- 57.Perel Y, et al. Galanin and galanin receptor expression in neuroblastic tumours: correlation with their differentiation status. Br J Cancer. 2002;86:117–122. doi: 10.1038/sj.bjc.6600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bronshtein ME, Smirnova EA, Iur'eva NP. Catecholamine-producing tumors of the chromaffin tissue. Morphological and ultrastructural characteristics. Voprosy onkologii. 1988;34:826–832. [PubMed] [Google Scholar]
- 59.Raikhlin NT, et al. Ultrastructural criteria of malignancy of adrenal medullary tumor. Bull Exp Biol Med. 2002;134:64–68. doi: 10.1023/a:1020616923868. [DOI] [PubMed] [Google Scholar]
- 60.Wynick D, Bacon A. Targeted disruption of galanin: New insights from knock-out studies. Neuropeptides. 2002;36:132–144. doi: 10.1054/npep.2002.0888. [DOI] [PubMed] [Google Scholar]
- 61.Hawes JJ, Narasimhaiah R, Picciotto MR. Galanin and galanin-like peptide modulate neurite outgrowth via protein kinase C-mediated activation of extracellular signal-related kinase. Eur J Neurosci. 2006;23:2937–2946. doi: 10.1111/j.1460-9568.2006.04828.x. [DOI] [PubMed] [Google Scholar]
- 62.Elliott-Hunt CR, Pope RJ, Vanderplank P, Wynick D. Activation of the galanin receptor 2 (GalR2) protects the hippocampus from neuronal damage. J Neurochem. 2007;100:780–789. doi: 10.1111/j.1471-4159.2006.04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pirondi S, et al. The galanin-R2 agonist AR-M1896 reduces glutamate toxicity in primary neural hippocampal cells. J Neurochem. 2005;95:821–833. doi: 10.1111/j.1471-4159.2005.03437.x. [DOI] [PubMed] [Google Scholar]
- 64.Liu HX, et al. Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: Selective actions via GalR1 and GalR2 receptors. Proc Natl Acad Sci USA. 2001;98:9960–9964. doi: 10.1073/pnas.161293598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dare E, et al. Styrene 7,8-oxide induces caspase activation and regular DNA fragmentation in neuronal cells. Brain Res. 2002;933:12–22. doi: 10.1016/s0006-8993(02)02274-6. [DOI] [PubMed] [Google Scholar]
- 66.Gorman AM, Hirt UA, Zhivotovsky B, Orrenius S, Ceccatelli S. Application of a fluorometric assay to detect caspase activity in thymus tissue undergoing apoptosis in vivo. J Immunol Methods. 1999;226:43–48. doi: 10.1016/s0022-1759(99)00054-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.