Abstract
IL-33, a new member of the IL-1 family, signals through its receptor ST2 and induces T helper 2 (Th2) cytokine synthesis and mediates inflammatory response. We have investigated the role of IL-33 in antigen-induced hypernociception. Recombinant IL-33 induced cutaneous and articular mechanical hypernociception in a time- and dose-dependent manner. The hypernociception was inhibited by soluble (s) ST2 (a decoy receptor of IL-33), IL-1 receptor antagonist (IL-1ra), bosentan [a dual endothelin (ET)A/ETB receptor antagonist], clazosentan (an ETA receptor antagonist), or indomethacin (a cyclooxygenase inhibitor). IL-33 induced hypernociception in IL-18−/− mice but not in TNFR1−/− or IFNγ−/− mice. The IL-33-induced hypernociception was not affected by blocking IL-15 or sympathetic amines (guanethidine). Furthermore, methylated BSA (mBSA)-induced cutaneous and articular mechanical hypernociception depended on TNFR1 and IFNγ and was blocked by sST2, IL-1ra, bosentan, clazosentan, and indomethacin. mBSA also induced significant IL-33 and ST2 mRNA expression. Importantly, we showed that mBSA induced hypernociception via the IL-33 → TNFα → IL-1β → IFNγ → ET-1 → PGE2 signaling cascade. These results therefore demonstrate that IL-33 is a key mediator of immune inflammatory hypernociception normally associated with a Th1 type of response, revealing a hitherto unrecognized function of IL-33 in a key immune pharmacological pathway that may be amenable to therapeutic intervention.
Keywords: cytokines, inflammation, pain, rheumatoid arthritis, hyperalgesia
IL-33 is a recently described member of the IL-1 family that includes IL-1β and IL-18. Like IL-1β and IL-18, IL-33 was found to have strong immunomodulatory functions (1). However, unlike IL-1β and IL-18, which mainly promote T-helper 1 (Th1)-associated responses, IL-33 predominantly induces the production of Th2 cytokines (IL-5 and IL-13) and increases the levels of serum Ig. IL-33 was recently found to be the ligand for the orphan receptor ST2 (1), which also is known as T1, DER4, or Fit (2–5). The interaction between IL-33 and ST2 was sufficient to trigger the activation of NF-κB and all three MAPKs (p38, ERK1/2, and JNK1/2) in a mast cell line. In vitro, IL-33 enhanced IL-5 and IL-13 production by polarized Th2, but not Th1, cell lines. In addition, the administration of human IL-33 into naïve mice provoked innate type 2 cytokine, IgE production, and eosinophilia (1).
The ST2 gene encodes two isoforms of ST2 protein: ST2L, a transmembrane form, and soluble ST2 (sST2) (6), a secreted form that can serve as a decoy receptor of IL-33 (1). ST2L is preferentially expressed on Th2, but not Th1, cells (7, 8) and can profoundly suppress innate and adaptive immunity (9, 10). ST2 also is expressed on mast cells, macrophages, and fibroblasts. We have previously shown that sST2 is a potent inhibitor of collagen-induced arthritis in mice (11), suggesting that the IL-33–ST2 signaling pathway is a key mediator of rheumatoid arthritis (RA). Arthritic lesions are accompanied by movement limitation secondary to articular hyperalgesia, and there is consistent evidence that cytokines induce hypernociception (hyperalgesia and/or allodynia in experimental animals) (12–15). Therefore, we have investigated the role of IL-33 in immune inflammatory hypernociception in mice.
We report here that IL-33 is a key mediator of methylated BSA (mBSA)-induced cutaneous and articular mechanical hypernociception. IL-33 mediates hypernociception via the sequential TNFα → IL-1β → IFNγ → endothelin 1 (ET-1) → prostaglandin (PG) E2 signaling cascade. These results therefore reveal a hitherto uncharacterized important pathway of antigen-induced hypernociception normally associated with Th1 response. Furthermore, our results suggest that IL-33 may be a potential therapeutic target for immune inflammatory hypernociception.
Results
IL-33 Mediates Cutaneous Mechanical Hypernociception.
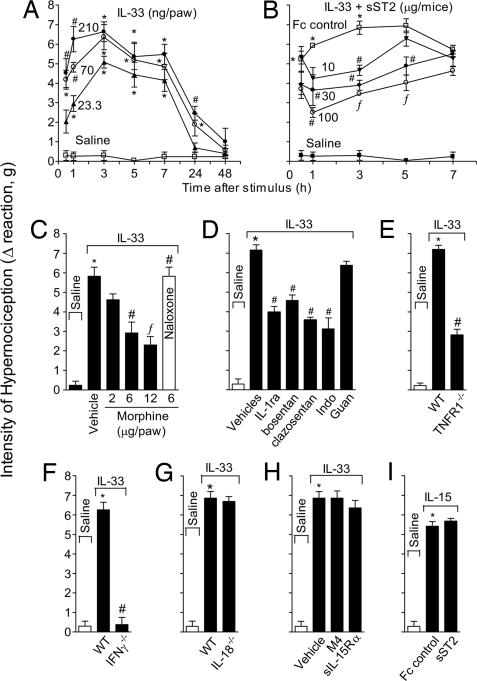
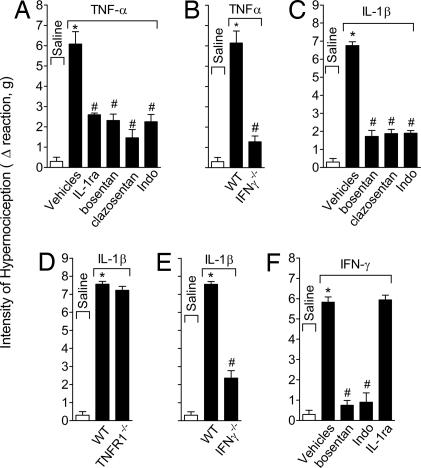
IL-33 intraplantar (i.pl.) injection induced mechanical hypernociception in mice in a dose- and time-dependent manner (Fig. 1A). The response was significant from 0.5 h, reaching maximal response between 3 and 5 h, decreasing thereafter to a control level at 48 h. The IL-33-induced hypernociception was restricted to the ipsilateral paw at the doses used (data not shown), indicating a local effect. A 70 ng per paw dose and readout at 3 h were chosen for subsequent experiments. IL-33-induced cutaneous hypernociception was significantly inhibited by the treatment with sST2-Fc, but not by the Fc control (Fig. 1B), indicating the specificity of the IL-33-induced effect. Furthermore, IL-33-induced cutaneous hypernociception was inhibited by local morphine treatment, and the effects of morphine were reversed by naloxone (opioid receptor antagonist) (Fig. 1C), confirming that the reaction observed was related to hypernociception. IL-33-induced hypernociception also was significantly inhibited by IL-1 receptor antagonist (IL-1ra), bosentan, clazosentan, or indomethacin, but was not affected by guanethidine (Fig. 1D). IL-33-induced hypernociception was markedly reduced in TNFR1−/− (Fig. 1E) and IFNγ−/− mice (Fig. 1F). In contrast, IL-33-induced hypernociception was normal in IL-18−/− mice (Fig. 1G) and was not affected by a blocker of IL-15 (sIL-15Rα) (Fig. 1H). Conversely, IL-15-induced hypernociception (16) was not affected by sST2 (Fig. 1I). Thus, IL-33-induced hypernociception depends on IL-1β, TNFα, IFNγ, ET, and prostanoids, but is independent of IL-15, IL-18, and sympathetic amines.
Fig. 1.
IL-33 induces mechanical cutaneous hypernociception. (A) Mice received i.pl. injection of IL-33 (23.3–210 ng per paw). (B) Mice were treated with sST2 (30–300 μg per mouse) 30 min before IL-33 (70 ng per paw) injection. (C) Mice were treated with morphine (2–12 μg per paw) 2 h after IL-33 injection. Another group of mice was treated with naloxone (1 mg/kg) 1 h before morphine (6 μg per paw) treatment, and hypernociception was evaluated 3 h after IL-33 injection. (D) IL-33 (70 ng) was injected i.pl. in mice pretreated with IL-1ra (30 mg/kg i.v.,15 min before IL-33), bosentan (100 mg/kg p.o., 1 h), clazosentan (10 mg/kg s.c.,15 min), indomethacin (Indo, 5 mg/kg i.p., 30 min), and guanethidine (guan, 30 mg/kg s.c.,1 h). (E–G) IL-33 induced minimal hypernociception in TNFR1−/− (E) or IFNγ−/− (F) mice, compared with WT mice but normal response in IL-18−/− mice (G). (H and I) sIL-15Rα or M4-control protein (30 μg per mouse i.p., 30 min) did not affect IL-33-induced hypernociception (H) nor did sST2 (100 μg per mouse i.p., 30 min) affect IL-15 (50 ng per paw)-induced hypernociception (I). Data are means ± SEM (n = 4–6), representative of two independent experiments. *, P < 0.05, compared with saline group. #, P < 0.05, compared with vehicles group; ƒ, P < 0.05, compared with the lowest dose tested.
IL-33 Mediates Antigen-Induced Cutaneous Mechanical Hypernociception.
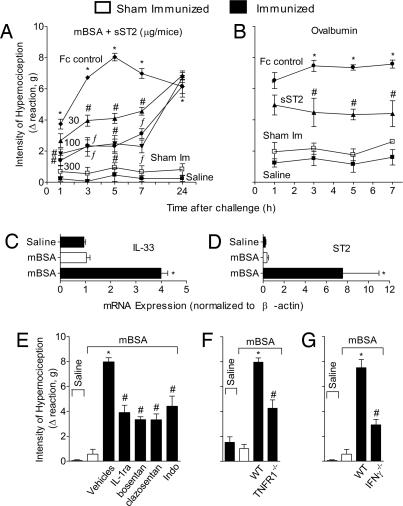
We next investigated the role of IL-33 in immune inflammatory hypernociception. Mice were immunized s.c. and boosted 7 days later with mBSA or ovalbumin (OVA) in complete Freund's adjuvant (CFA) and challenged i.pl. on day 21 with mBSA or OVA, respectively. The mBSA challenge induced considerable hypernociception that was attenuated by sST2 in a dose- and time-dependent manner (Fig. 2A). The OVA-induced hypernociception also was significantly inhibited by sST2 (Fig. 2B). Furthermore, the mBSA challenge induced markedly enhanced expression of IL-33 (Fig. 2C) and ST2 (Fig. 2D) in the injection site. These results suggest that IL-33/ST2 signaling play a significant role in the antigen-induced hypernociception. Consistent with this finding, mBSA-induced mechanical hypernociception was significantly inhibited by treatment with IL-1ra, bosentan, clazosentan, or indomethacin (Fig. 2E). Moreover, mBSA failed to induce hypernociception in TNFR1−/− (Fig. 2F) or IFNγ−/− (Fig. 2G) mice.
Fig. 2.
Role of IL-33 in mBSA-induced cutaneous hypernociception. (A) mBSA-immunized or sham-immunized (sham-Im) mice were treated with sST2 (30–300 μg per mouse/i.p.) or Fc control (300 μg) 15 min before challenged i.pl. with mBSA (90 μg per paw) or saline (25 μl per paw). (B) OVA-immunized mice were treated with sST2 (100 μg per mouse/i.p.) or Fc control (100 μg) 30 min before challenged i.pl. with OVA (3 μg) or saline (25 μl). (C and D) mBSA-immunized mice were challenged i.pl. with saline or mBSA, and sham-Im mice received mBSA. The s.c. plantar tissue samples were collected 2 h after challenge for qPCR analysis of IL-33 (C) and ST2 (D) mRNA expression. (E–G) mBSA was injected i.pl. in mice pretreated with IL-1ra, bosentan, clazosentan, or indomethacin at doses described in Fig. 1 (E), and mBSA induced significantly reduced response in TNFR1−/− (F) or IFNγ−/− (G) mice compared with WT mice. Cutaneous hypernociception was evaluated 3 h after antigen challenge otherwise indicated. Data are means ± SEM (n = 4–6), representative of two independent experiments. *, P < 0.05, compared with saline group; #, P < 0.05, compared with vehicles group; ƒ, P < 0.05, compared with the lowest dose tested.
IL-33 Induces Mechanical Articular Hypernociception.
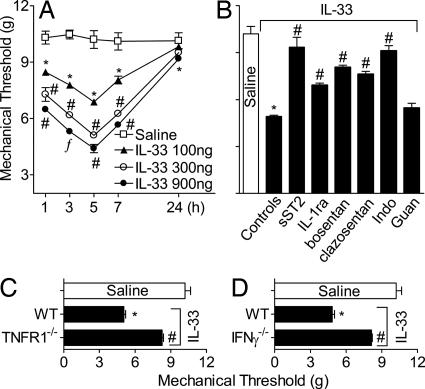
To further confirm the role of IL-33 in inflammatory hypernociception, we used a recently described articular mechanical hypernociception method (17). IL-33 administrated in the tibiotarsal joint induced dose- and time-dependent articular hypernociception (Fig. 3A). The IL-33-induced articular hypernociception was inhibited by treatment with sST2, IL-1ra, bosentan, clazosentan, or indomethacin, but not by guanethidine (Fig. 3B). IL-33 also failed to induce articular hypernociception in TNFR1−/− (Fig. 3C) or IFNγ−/− (Fig. 3D) mice. Thus, IL-33 induces cutaneous and articular hypernociception by the same mechanism.
Fig. 3.
IL-33 induces mechanical articular hypernociception. (A) Mice were injected i.a. with IL-33 (100–900 ng per joint), and articular hypernociception was evaluated over 1–24 h. (B) IL-33 (300 ng per joint) was injected in mice pretreated with sST2, IL-1ra, bosentan, clazosentan, indomethacin, or guanethidine as described in Fig. 1. (C and D) TNFR1−/− (C) and IFNγ−/− (D) mice showed significantly reduced IL-33-induced articular hypernociception, compared with WT mice. Articular hypernociception was evaluated 5 h after IL-33 injection unless indicated otherwise. Data are means ± SEM (n = 5), representative of two independent experiments. *, P < 0.05, compared with saline group; #, P < 0.05, compared with controls/WT groups; ƒ, P < 0.05, compared with the lowest dose tested.
IL-33 Mediates mBSA-Induced Articular Mechanical Hypernociception.
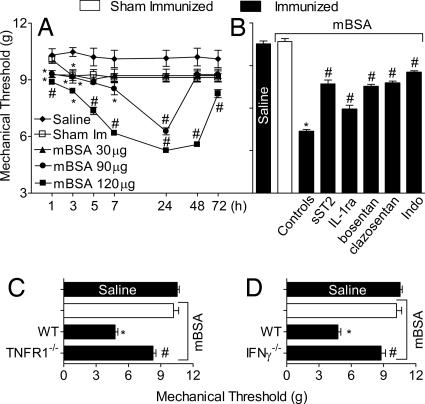
We then investigated whether IL-33 also mediates antigen-induced articular hypernociception, a disease model akin to RA. Mice were immunized and boosted with mBSA, as in Fig. 2, and challenged intraarticularly (i.a.) in the tibiotarsal joint on day 21 with mBSA. Mice immunized and challenged with mBSA showed marked hypernociception in an antigen dose- and time-dependent manner, peaking at 24 h and largely subsiding by 72 h after the challenge (Fig. 4A). The mBSA-induced articular hypernociception was significantly reduced by the treatment with sST2 (Fig. 4B), suggesting the involvement of the IL-33/ST2 pathway. Furthermore, the antigen-induced articular hypernociception also was significantly reduced by IL-1ra, bosentan, clazosentan, or indomethacin (Fig. 4B). Consistent with the previous finding, mBSA did not induce articular hypernociception in TNFR1−/− (Fig. 4C) or IFNγ−/− (Fig. 4D) mice.
Fig. 4.
Role of IL-33 in mBSA-induced articular hypernociception. (A) mBSA-immunized or sham-Im mice were challenged i.a. with 30–120 μg of mBSA or 25 μl of saline. Articular hypernociception was evaluated after 1–72 h. (B) mBSA-immunized mice were treated with sST2, IL-1ra, bosentan, clazosentan, or indomethacin as described in Fig. 1 and then challenged i.a. with 120 μg of mBSA. (C and D) mBSA failed to induce hypernociception in TNFR1−/− (C) or IFNγ−/− (D) mice. Articular hypernociception was evaluated 24 h after mBSA challenge unless indicated otherwise. Data are means ± SEM (n = 5), representative of two independent experiments. *, P < 0.05, compared with saline group; #, P < 0.05, compared with control/WT groups.
Molecular Events of IL-33-Mediated Hypernociception.
We then investigated the molecular mechanism by which IL-33 mediates mechanical hypernociception. We began with TNFα. Data in Fig. 5A show that TNFα-induced hypernociception was markedly inhibited by IL-1ra, bosentan, clazosentan, or indomethacin. Furthermore, TNFα failed to induce hypernociception in IFNγ−/− mice (Fig. 5B). These results demonstrate that TNFα acts upstream of IL-1β, IFNγ, ET, and PG. We then determined the association of IL-1β in this cascade of events. We found that IL-1β-induced hypernociception was significantly blocked by bosentan, clazosentan, or indomethacin (Fig. 5C). However, IL-1β induced normal hypernociception in TNFR1−/− mice (Fig. 5D), but not in IFNγ−/− mice (Fig. 5E). These results show that IL-1β acts upstream of IFNγ and further confirm that IL-1β acts downstream of TNFα. We then determined the relationship of IFNγ, ET, and PG. IFNγ-induced hypernociception was completely blocked by bosentan or indomethacin, but was not affected by IL-1ra (Fig. 5F). These results confirm that IFNγ acts downstream of IL-1β and further show that IFNγ acts upstream of ET-1, which is a well known inducer of PG (18, 19).
Fig. 5.
Molecular mechanism of hypernociception. (A) Mice were pretreated with IL-1ra, bosentan, clazosentan, or indomethacin as described in Fig. 1 and then injected i.pl. with 100 pg of TNFα. Hypernociception was determined 3 h after TNFα injection. (B) TNFα failed to induce hypernociception in IFNγ−/− mice. (C) Mice were pretreated with bosentan, clazosentan, or indomethacin before injected i.pl. with 1 ng of IL-1β, and hypernociception was determined as above. (D and E) IL-1β induced normal hypernociception in TNFR1−/− mice (D), but failed to do so in IFNγ−/− mice (E). (F) IFNγ (3 ng)-induced hypernociception was blocked by bosentan and indomethacin, but not by IL-1ra. Data are means ± SEM (n = 5), representative of two independent experiments. *, P < 0.05, compared with saline group; #, P < 0.05, compared with the vehicle/WT groups.
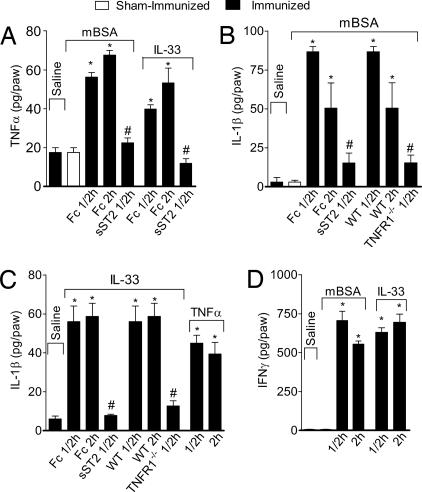
To further confirm these results, we ascertained the role of IL-33 in the induction of TNFα, IL-1β, and IFNγ in the mBSA- or IL-33-induced hypernociception. Mice were immunized, boosted, and challenged i.pl. with mBSA as above. Some mice also were injected i.pl. with IL-33. Then 0.5 or 2 h after i.pl. injection, mice were killed and the plantar tissue was removed and homogenized, and the concentrations of cytokine were determined by ELISA. Mice challenged with mBSA produced significant amounts of TNFα, which was markedly inhibited by prior treatment with sST2, but not by the Fc control (Fig. 6A). Consistent with these results, IL-33 also induced significant amounts of TNFα, which also was abolished by pretreatment with sST2 (Fig. 6A). The mBSA challenge also induced significant amounts of IL-1β, which was not evident when the mice were pretreated with sST2 (Fig. 6B). Furthermore, the mBSA challenge failed to induce IL-1β production in TNFR1−/− mice (Fig. 6B). The i.pl. injection of IL-33 induced significant amounts of IL-1β, which was again abolished by pretreatment with sST2 (Fig. 6C). IL-33 failed to induce IL-1β in TNFR1−/− mice, whereas i.pl. injection of TNFα induced significant amounts of IL-1β (Fig. 6C). Finally, substantial amounts of IFNγ were induced by i.pl. challenge of mBSA or IL-33 (Fig. 6D). Confirming the leading role of IL-33 in antigen-induced hypernociception, mBSA induced IL-33 and ST2 expression (Fig. 2 C and D), whereas i.pl. injection of TNFα, IL-1β, or IFNγ failed to induce IL-33 or ST2 message as determined by quantitative PCR (qPCR) (data not shown). Therefore, this series of experiments established the following cascade of events in immune inflammatory hypernociception: antigen → IL-33 → TNFα → IL-1β→ IFNγ → ET-1 → PG.
Fig. 6.
IL-33 and mBSA induce proinflammatory cytokine production. (A) TNFα synthesis in the plantar skin tissue induced by mBSA immunized and challenged or IL-33 injection was markedly inhibited by pretreatment with 100 μg of sST2. (B) IL-1β synthesis also was inhibited by sST2, and mBSA failed to induce significant amount of IL-1β in TNFR1−/− mice. (C) IL-1β synthesis was blocked by sST2. IL-33 failed to induce IL-1β synthesis in TNFR1−/− mice, whereas TNFα induced IL-1β production. (D) Both mBSA and IL-33 induced IFNγ synthesis. Paw skin samples were collected 0.5–2 h after i.pl. injection. Doses of antigen and cytokines used were the same as in Figs. 2 and 5. Results are mean ± SEM (n = 5), representative of two independent experiments. *, P < 0.05, compared with saline group; #, P < 0.05, compared with the Fc or WT groups.
IL-33 Induces PreproET-1 mRNA Expression and PGE2 Production.
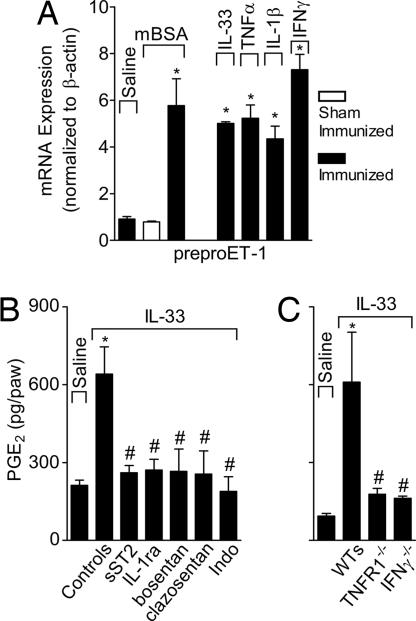
To further confirm the effector mechanism of the IL-33-mediated hypernociception, we determined the ability of IL-33 to induce preproET-1 and PGE2. Mice were immunized, boosted, and challenged i.pl. with mBSA or injected i.pl. with IL-33, TNFα, IL-1β, or IFNγ as described for experiments presented in Fig. 6. Mice were killed 2 h later, and plantar tissues were harvested. The mRNA of preproET-1 was assayed by the qPCR and PGE2 level determined by an RIA. preproET-1 mRNA expression was induced by mBSA, IL-33, TNFα, IL-1β, and IFNγ (Fig. 7A). PGE2 also was induced by IL-33, and this induction was completely abolished by the pretreatment of the mice with sST2, IL-1ra, bosentan, clazosentan, or indomethacin (Fig. 7B). Furthermore, IL-33 failed to induce PGE2 synthesis in TNFR1−/− or IFNγ−/− mice (Fig. 7C). Therefore, these results clearly demonstrate the pivotal role of IL-33 in the immune inflammatory hypernociception via the TNFα → IL-1β → IFNγ → ET-1 → PGE2 signaling cascade.
Fig. 7.
IL-33 induces preproET-1 mRNA expression and PGE2 production. (A) mBSA-immunized or sham-Im mice were challenged i.pl. with saline, mBSA, IL-33, TNFα, IL-1β, or IFNγ (doses as in Fig. 5). Paw skin samples were collected 2 h after challenge and analyzed for preproET-1 (ET-1 precursor) mRNA expression by qPCR. (B) Mice were pretreated with sST2, IL-1ra, bosentan, clazosentan, or indomethacin (as in Fig. 1) and then injected i.pl. with IL-33. PGE2 present in the plantar skin was determined 2 h later by RIA. (C) IL-33 failed to induce significant amount of PGE2 in TNFR1−/− or IFNγ−/− mice. Data are means ± SEM (n = 5), representative of two independent experiments. Vehicle group is the mean for all vehicle controls plus Fc control groups. *, P < 0.05, compared with saline group; #, P < 0.05, compared with the control/WT groups.
Discussion
IL-33 is a newly discovered cytokine that acts through the ST2/IL–1RAcP (IL-1 receptor accessory protein) receptor complex (1, 20, 21) and is closely associated with Th2-type immune functions (1, 7, 8) and mast cell activation (1, 22, 23). We have previously demonstrated that Th1 cytokines induce hypernociception, whereas Th2 cytokines such as IL-4 and IL-13 inhibit hypernociception (24–27). Thus, data presented here showing a pronociceptive role of IL-33 may seem contraintuitive. Furthermore, IL-33 also can induce the production of IFNγ, albeit indirectly via TNFα and IL-1β, suggesting that IL-33 is a pleiotropic cytokine with functions beyond its dominant role in the induction of Th2 cell activities. This finding parallels the pleiotropic role of IL-18 (28–30), whose receptor was found to be selectively expressed on Th1, and not Th2, cells (31). Interestingly, both IL-33 and IL-18 receptors consists of a common IL-1RAcP chain, which is essential to induce cell activation and cytokine production (20, 21, 32) and may confer the pleiotropic nature of the cytokines. Thus, although IL-33 and IL-18 predominantly act on Th2 and Th1 cells, respectively, these two cytokines are able to amplify the functions of other cell types depending on the cytokine milieu and the activation state of the cells.
sST2, a decoy receptor of IL-33, can attenuate experimental asthma (33) and RA (11). Treatment with sST2 also diminished LPS-induced production of inflammatory cytokines such as TNFα, IL-1β, and IFNγ (9, 34). IL-33 also has been detected in asthma (35) and in the synovial membrane of patients with RA (D.X. and F.Y.L., unpublished data). These reports strongly suggest a proinflammatory role of IL-33. Data reported here provide the hitherto unrecognized pronociceptive role of IL-33, a key feature of inflammation exemplified by cutaneous and articular responses induced by mBSA.
Our results demonstrate that antigen-induced articular hypernociception can be mediated by IL-33, which in turn triggers the TNFα → IL-1β → IFNγ → ET-1 → PGE2 signaling cascade. We have previously demonstrated the role of these mediators in hypernociception (12, 16, 18, 19, 27) and that antigen-induced hypernociception also could be mediated by IL-15 (12) and IL-18 (19). IL-15-induced hypernociception was independent of IL-18 or TNFα, but was completely dependent on IFNγ (12). IL-18-mediated hypernociception was independent of IL-15 and TNFα, but was also completely dependent on IFNγ (19). In contrast, IL-33-mediated mechanical hypernociception reported here was independent of IL-15 or IL-18, but was largely dependent on TNFα and IFNγ. Furthermore, considering the demonstrations of IFNγ-independent effects of IL-15 and IL-18 (36), it is conceivable that IFNγ production also can be triggered independently of IL-15 and IL-18, as we observed for IL-33. It is likely that IL-15, IL-18, and IL-33 act in parallel and via distinct initial pathways, but collectively transmit the hypernociceptive signal through IFNγ, which in turn induces the production of ET-1 and PGE2 (12, 19). These findings are in line with the multifactorial nature of immune inflammatory hypernociception, which is mediated by a network of different cytokines often acting in parallel, in sequence, or maybe in synergy.
Data presented here demonstrate that IL-33 is a hitherto unrecognized cytokine that plays a key role in mediating antigen-induced mechanical hypernociception. Therefore, IL-33 could be a major contributor to articular movement limitation in RA. Our demonstration that IL-33 induces hypernociception via the TNFα → IL-1β → IFNγ → ET-1 → PGE2 pathway not only reveals a previously uncharacterized signaling pathway but also suggests targets for potential therapeutic intervention for a range of inflammatory diseases.
Materials and Methods
Animals.
Adult BALB/c, C57BL/6, IL-18−/−, TNFR1−/−, and IFNγ−/− mice of both sexes were used in this study. IL-18−/− mice were generated in C57BL/6 mice and back-crossed to BALB/c mice for eight generations (37). IFNγ−/− and TNFR1−/− mice were obtained from The Jackson Laboratory. All mice were bred and maintained in the Faculty of Medicine of Ribeirao Preto (FMRP) at the University of São Paulo (USP). Animal care and handling procedures were in accordance with the International Association for the Study of Pain guidelines and with the approval of the FMRP Ethics Committee of the USP.
Reagents.
The following materials were obtained from the sources indicated: murine IL-15 and IFNγ (Peprotech); guanethidine, OVA, and mBSA (Sigma–Aldrich); IL-1β and TNFα (National Institute of Biological Standards and Control); and indomethacin (Prodome). IL-33 (38); sIL-15Rα, a soluble fragment of IL-15Rα-chain lacking transmembrane and cytoplasmic domains; M4 mutant control protein (39); and sST2, a Fc fusion protein (32), were generated as previously described. The doses of reagents used in vivo were chosen based on previous reports (11, 16, 40–43): 100 mg/kg bosentan (p.o., given 1 h before treatment), 10 mg/kg clazosentan (s.c., 15 min), 30 mg/kg guanethidine (s.c., 1 h), 30 mg/kg IL-1ra (i.v., 15 min), 5 mg/kg indomethacin (i.p., 30 min), 2–12 μg per paw of morphine (2 h), 1 mg/kg naloxone (i.p., 1 h), 30–300 μg per mouse of sST2 (i.p., 30 min), 30 μg per mouse of sIL-15Rα or M4 control protein (i.p., 30 min), 23.3–210 ng per paw or 100–900 ng per joint of IL-33, and 90 μg per paw or 30–120 μg per joint of mBSA. In the mBSA-induced articular hypernociception model, the inhibitors were given at the time indicated above, plus a reinforcing dose at 21 h except sST2 (one treatment only as indicated above). Drug treatments, genetic deficiencies, and the immunization procedure did not affect the baseline response of the mice to mechanical stimulation (data not shown).
Electronic Pressure Meter Test.
Cutaneous hypernociception.
The mechanical hypernociception test (44) consisted of evoking a hindpaw flexion reflex with a hand-held force transducer (electronic anesthesiometer, IITC; Life Science) adapted with a 0.5-mm2 polypropylene tip. The endpoint was the removal of the paw, followed by clear flinching movements. After the paw withdrawal, the intensity of the pressure was automatically recorded, and the value for the response was obtained by averaging three measurements. The animals were tested before and after treatments. The results are expressed by Δ withdrawal threshold (in grams) calculated by subtracting the mean measurements 3 h after stimulus from the zero-time mean measurements. Withdrawal threshold was 8.6 ± 0.5 g (mean ± SEM, n = 40) before injection of the hypernociceptive agents.
Articular hypernociception.
Experiments were performed as described previously (17). Similar to cutaneous hypernociception measurement, an increasing perpendicular force was applied to the central area of the plantar surface, inducing the flexion of the tibiotarsal joint. A nonnociceptive tip probe with area size of 4.15 mm2 was used. The results were expressed as the flexion-elicited withdrawal threshold (in grams).
Immunization Procedure.
Mice were injected s.c. with 500 μg of mBSA or 100 μg of OVA emulsified in CFA on day 0. The mice were boosted with the same preparations on day 7. Control sham-immunized mice received the same emulsions without mBSA or OVA. Mice were challenged on day 21 by i.pl. or i.a. injection of 90 μg of mBSA or 3 μg of OVA (16, 43).
Cytokine Measurements.
At indicated times after the i.pl. injection of inflammatory stimuli, animals were terminally anesthetized, and the plantar skin tissues were removed and homogenized in 500 μl of buffer containing protease inhibitors. TNFα, IL-1β, and IFNγ concentrations were determined by ELISA using paired antibodies (BD Bioscience).
Real-Time PCR.
qPCR was performed as described (45). Briefly, mice were killed 2 h after i.pl. injection, and the plantar cutaneous hind paw tissues were harvested. Samples were homogenized in TRIzol reagent, and total RNA was extracted by using the SV Total RNA Isolation System (Promega). qPCR was performed in an ABI Prism 7500 sequence detection system by using the SYBR-green fluorescence (Applied Biosystems). The primers used were: preproET-1 (16), sense: 5′-TGT GTC TAC TTC TGC CAC CT-3′, antisense: 5′-CAC CAG CTG CTG ATA GAT AC-3′; IL-33, sense: 5′-TCC TTG CTT GGC AGT ATC CA-3′, antisense: 5′-TGC TCA ATG TGT CAA CAG ACG-3′; ST2, sense: 5′-GCA ATT CTG ACA CTT CCC ATG-3′, antisense: 5′-ACG ATT TAC TGC CCT CCG TA-3′; and β-actin, sense: 5′-AGC TGC GTT TTA CAC CCT TT-3′, antisense: 5′-AAG CCA TGC CAA TGT TGT CT-3′. The expression of β-actin mRNA was used as a control for tissue integrity in all samples.
Determination of PGE2 Production.
Two hours after i.pl. injection of IL-33 or mBSA, paw skin tissue samples were collected in 0.5 ml of a mixture of acetone, 1 M HCl, and water (10:1:5, vol/vol/vol). After homogenizing with a polytron, the samples were centrifuged at 2,000 × g for 20 min at 4°C, and the supernatant was decanted before drying the pellet in a centrifugal evaporator at 37°C. The pellet was reconstituted in 500 μl of Tris·HCl buffer (1.22% of Tris, pH adjusted with HCl to 7.8–8.0). The concentrations of PGE2 were determined by RIA (Amersham) (16).
Statistical Analyses.
Results are presented as means ± SEM. Each experiment was performed at least twice. Two-way ANOVA was used to compare the groups and doses at all times when the hypernociceptive responses were measured at different times after the stimulus injection. The analyzed factors were treatments, time, and time versus treatment interaction. One-way ANOVA followed by Bonferroni's t test was performed for each time. P < 0.05 was considered significant.
ACKNOWLEDGMENTS.
We thank Ieda Schivo, Sérgio Rosa, and Giuliana Francisco for technical assistance and Actelion Pharmaceutical (Allschwil, Switzerland) for providing bosentan and clazosentan. This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and PRONEX (Brazil); the Chief Scientist Office (Scotland); and the Welcome Trust and the Medical Research Council (United Kingdom).
Footnotes
The authors declare no conflict of interest.
References
- 1.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989;258:301–304. doi: 10.1016/0014-5793(89)81679-5. [DOI] [PubMed] [Google Scholar]
- 3.Werenskiold AK, Hoffmann S, Klemenz R. Induction of a mitogen-responsive gene after expression of the Ha-ras oncogene in NIH 3T3 fibroblasts. Mol Cell Biol. 1989;9:5207–5214. doi: 10.1128/mcb.9.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanahan A, Williams JB, Sanders LK, Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992;12:3919–3929. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergers G, Reikerstorfer A, Braselmann S, Graninger P, Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994;13:1176–1188. doi: 10.1002/j.1460-2075.1994.tb06367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gachter T, Werenskiold AK, Klemenz R. Transcription of the interleukin-1 receptor-related T1 gene is initiated at different promoters in mast cells and fibroblasts. J Biol Chem. 1996;271:124–129. doi: 10.1074/jbc.271.1.124. [DOI] [PubMed] [Google Scholar]
- 7.Xu D, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohning M, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci USA. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brint EK, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 10.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 11.Leung BP, Xu D, Culshaw S, McInnes IB, Liew FY. A novel therapy of murine collagen-induced arthritis with soluble T1/ST2. J Immunol. 2004;173:145–150. doi: 10.4049/jimmunol.173.1.145. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 13.Watkins LR, et al. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- 14.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verri WA, Jr, et al. Nociceptive effect of subcutaneously injected interleukin-12 is mediated by endothelin (ET) acting on ETB receptors in rats. J Pharmacol Exp Ther. 2005;315:609–615. doi: 10.1124/jpet.105.089409. [DOI] [PubMed] [Google Scholar]
- 16.Verri WA, Jr, et al. IL-15 mediates immune inflammatory hypernociception by triggering a sequential release of IFN-gamma, endothelin, and prostaglandin. Proc Natl Acad Sci USA. 2006;103:9721–9725. doi: 10.1073/pnas.0603286103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerrero AT, et al. Hypernociception elicited by tibio-tarsal joint flexion in mice: A novel experimental arthritis model for pharmacological screening. Pharmacol Biochem Behav. 2006;84:244–251. doi: 10.1016/j.pbb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira SH, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol. 1989;13(Suppl 5):S220–222. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- 19.Verri WA, Jr, et al. Antigen-induced inflammatory mechanical hypernociception in mice is mediated by IL-18. Brain Behav Immun. 2007;21:535–543. doi: 10.1016/j.bbi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Chackerian AA, et al. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 21.Ali S, et al. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc Natl Acad Sci USA. 2007;104:18660–18665. doi: 10.1073/pnas.0705939104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 23.Iikura M, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 24.Cunha FQ, Poole S, Lorenzetti BB, Veiga FH, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-4. Br J Pharmacol. 1999;126:45–50. doi: 10.1038/sj.bjp.0702266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vale ML, et al. Antinociceptive effects of interleukin-4, −10, and −13 on the writhing response in mice and zymosan-induced knee joint incapacitation in rats. J Pharmacol Exp Ther. 2003;304:102–108. doi: 10.1124/jpet.102.038703. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzetti BB, Poole S, Veiga FH, Cunha FQ, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-13. Eur Cytokine Netw. 2001;12:260–267. [PubMed] [Google Scholar]
- 27.Verri WA, Jr, et al. Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol Ther. 2006;112:116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Xu D, et al. IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. Eur J Immunol. 2000;30:3147–3156. doi: 10.1002/1521-4141(200011)30:11<3147::AID-IMMU3147>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 29.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/s1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 31.Xu D, et al. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med. 1998;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Born TL, Thomassen E, Bird TA, Sims JE. Cloning of a novel receptor subunit, AcPL, required for interleukin-18 signaling. J Biol Chem. 1998;273:29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- 33.Coyle AJ, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweet MJ, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol. 2001;166:6633–6639. doi: 10.4049/jimmunol.166.11.6633. [DOI] [PubMed] [Google Scholar]
- 35.Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 36.Verri WA, Jr, et al. IL-15 mediates antigen-induced neutrophil migration by triggering IL-18 production. Eur J Immunol. 2007;37:3373–3380. doi: 10.1002/eji.200737488. [DOI] [PubMed] [Google Scholar]
- 37.Wei XQ, Leung BP, Arthur HM, McInnes IB, Liew FY. Reduced incidence and severity of collagen-induced arthritis in mice lacking IL-18. J Immunol. 2001;166:517–521. doi: 10.4049/jimmunol.166.1.517. [DOI] [PubMed] [Google Scholar]
- 38.Komai-Koma M, et al. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779–2786. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- 39.Ruchatz H, Leung BP, Wei XQ, McInnes IB, Liew FY. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: A role for IL-15 in development of antigen-induced immunopathology. J Immunol. 1998;160:5654–5660. [PubMed] [Google Scholar]
- 40.Valerio DA, et al. Anti-inflammatory and analgesic effects of the sesquiterpene lactone budlein A in mice: Inhibition of cytokine production-dependent mechanism. Eur J Pharmacol. 2007;562:155–163. doi: 10.1016/j.ejphar.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 41.Cunha TM, et al. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verri WA, Jr, et al. Interleukin-18 induces mechanical hypernociception in rats via endothelin acting on ETB receptors in a morphine-sensitive manner. J Pharmacol Exp Ther. 2004;310:710–717. doi: 10.1124/jpet.103.063990. [DOI] [PubMed] [Google Scholar]
- 43.Santodomingo-Garzon T, et al. Atorvastatin inhibits inflammatory hypernociception. Br J Pharmacol. 2006;149:14–22. doi: 10.1038/sj.bjp.0706836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunha TM, et al. An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res. 2004;37:401–407. doi: 10.1590/s0100-879x2004000300018. [DOI] [PubMed] [Google Scholar]
- 45.Cunha TM, et al. TNF-alpha and IL-1beta mediate inflammatory hypernociception in mice triggered by B (1) but not B (2) kinin receptor. Eur J Pharmacol. 2007;573:221–229. doi: 10.1016/j.ejphar.2007.07.007. [DOI] [PubMed] [Google Scholar]