Abstract
Tuberculous glycolipid (TBGL) antigen is a cell wall component of Mycobacterium tuberculosis and has been used for the serodiagnosis of tuberculosis. We investigated correlations between the levels of anti-TBGL antibodies and a variety of laboratory markers that are potentially influenced by tuberculous infection. Comparisons between patients with cavitary lesions and those without cavitary lesions were also made in order to determine the mechanism underlying the immune response to TBGL. Blood samples were obtained from 91 patients with both clinically and microbiologically confirmed active pulmonary tuberculosis (60 male and 31 female; mean age, 59 ± 22 years old). Fifty-nine patients had cavitary lesions on chest X-rays. Positive correlations were found between anti-TBGL immunoglobulin G (IgG) and C-reactive protein (CRP) (r = 0.361; P < 0.001), between anti-TBGL IgA and soluble CD40 ligand (sCD40L) (r = 0.404; P < 0.005), between anti-TBGL IgG and anti-TBGL IgA (r = 0.551; P < 0.0000005), and between anti-TBGL IgM and serum IgM (r = 0.603; P < 0.00000005). The patients with cavitary lesions showed significantly higher levels of anti-TBGL IgG (P < 0.005), anti-TBGL IgA (P < 0.05), white blood cells (P < 0.01), neutrophils (P < 0.005), basophils (P < 0.0005), natural killer cells (P < 0.05), CRP (P < 0.0005), KL-6 (sialylated carbohydrate antigen KL-6) (P < 0.0005), IgA (P < 0.05), and sCD40L (P < 0.01). The observed positive correlations between the anti-TBGL antibody levels and inflammatory markers indicate the involvement of inflammatory cytokines and NKT cells in the immunopathogenesis of pulmonary tuberculosis.
There were an estimated 8.8 million new tuberculosis (TB) cases in 2005. TB incidence reached a peak worldwide, but the total number of new TB cases is still rising. The numbers of human immunodeficiency virus (HIV)-positive and multidrug-resistant TB patients diagnosed and treated are increasing (22). To develop new drugs and vaccines against TB, it is essential to study its immunopathogenesis. Lipoarabinomannnan (LAM), a complex glycolipid, is a major cell wall component of Mycobacterium tuberculosis. It has been researched extensively as an immunomodulator (4, 7, 9, 24, 26). LAM has also been used as a glycolipid antigen in the serodiagnostic method for TB. In addition to LAM, there are many glycolipids constituting the mycobacterial cell wall, such as trehalose 6,6-dimycolate (TDM). We used TDM, a glycolipid antigen purified from Mycobacterium tuberculosis H37Rv, in an enzyme-linked immunosorbent assay (ELISA) and reported that its sensitivity was 81% and its specificity was 96% (14). Subsequently, by combining TDM with more hydrophobic glycolipids, a new tuberculous glycolipid (TBGL) antigen was designed and a more sensitive serodiagnostic kit for TB, an anti-TBGL immunoglobulin G (IgG) test, was developed (11). Although TBGL has been used as a serodiagnostic antigen for TB and its clinical evaluations have been reported in several studies, how TBGL is involved in tuberculous pathogenesis has not been studied. Since TBGL is one of the cell wall components of Mycobacterium tuberculosis, like LAM, it may have some important roles in the immunopathogenesis of TB, as does LAM. In order to determine the mechanism underlying the immune response to TBGL, we measured plasma IgA, IgM, and IgG titers specific for TBGL and investigated correlations between those antibody titers and laboratory markers potentially influenced by TB infection in patients with active pulmonary TB. The measured markers were the numbers of white blood cells with differential counts, CD3-positive lymphocytes (T cells), CD20-positive lymphocytes (B cells), and CD56-positive lymphocytes (natural killer cells) and levels of serum IgG, serum IgA, serum IgM, serum albumin, serum creatinine, serum C-reactive protein (CRP), plasma soluble CD40 ligand (sCD40L), and plasma KL-6 (sialylated carbohydrate antigen KL-6). KL-6 is a mucinous high-molecular-weight glycoprotein expressed on type II pneumocytes, and it was reported to be elevated in the sera of patients with interstitial pneumonia (13). We used plasma samples, but the level of KL-6 is known to show no significant difference between serum and plasma. We measured KL-6 because TB patients with extensive radiographic changes were also reported to have higher KL-6 values (8).
CD40L is expressed on the surfaces of activated CD4+ T cells, basophils, and mast cells. The binding of CD40L to its receptor, CD40, on the surfaces of B cells stimulates B-cell proliferation, adhesion, and differentiation. A soluble isoform of CD40L has been shown to exist in the circulation, exhibiting full activity in B-cell proliferation and differentiation assays (16). It was reported that the treatment of wild-type CD40 mice with sCD40L fusion protein elicited a pulmonary inflammatory response that was not observed in identically treated CD40 knockout mice (21). Based on these reports, we measured sCD40L as a possible marker of pulmonary inflammation.
Furthermore, it was reported that the positive rate and the titers of anti-TBGL IgG were higher in pulmonary TB patients with cavitary lesions than in those without cavitary lesions (15). Considering this result, we subdivided the patients into two groups, those with cavitary lesions (cavity+ group) and those without cavitary lesions (cavity− group), and compared multiple laboratory markers to determine associations.
In addition, we categorized the patients into three groups based on chest X-ray findings, namely, minimal, moderately advanced, and far advanced, according to the classification of the National Tuberculosis and Respiratory Disease Association of the USA (NTA) (6), and compared all the measured laboratory markers, including anti-TBGL antibodies, among the three groups to determine if there were any parameters related to disease progression and severity.
MATERIALS AND METHODS
Subjects.
We designed a cross-sectional study using 121 patients at Tokyo Metropolitan Fuchu Hospital between May 2004 and August 2005. These patients were clinically diagnosed as having active TB and admitted to the hospital for treatment. Medical histories were taken from the enrolled patients, and all of them underwent physical examination, chest X-rays, blood test and culture test for acid-fast bacilli, and/or TB-PCR test of sputum samples. Ninety-one subjects were selected (60 males and 31 females; mean age [± standard deviation], 59 ± 22 years old) for analysis according to the following criteria: (i) diagnosed as having pulmonary TB by positive culture or positive PCR for Mycobacterium tuberculosis in sputum, (ii) untreated or undergoing less than 2 weeks of TB treatment, (iii) negative for Mycobacterium avium complex infection, (iv) negative for HIV infection, (v) no malignancy, and (vi) no other active pulmonary diseases. The remaining 30 patients were excluded for the following reasons: 4 for both negative culture and a negative PCR test for Mycobacterium tuberculosis in sputum, 5 for more than 2 weeks of TB treatment, 2 for Mycobacterium avium complex infection, 4 for HIV infection, 3 for malignancy, 2 for interstitial pneumonia, and 10 for insufficient data collection. We enrolled patients with less than 2 weeks of treatment based on a report that anti-TBGL IgG did not decrease until 1 month after the commencement of chemotherapy (15). The study was approved by the Ethics Committee of Tokyo Metropolitan Fuchu Hospital. We obtained written informed consent from all the enrolled patients.
TBGL antibody.
Anti-TBGL antibodies were measured using a Determiner TBGL antibody ELISA kit (Kyowa Medex, Tokyo, Japan), an in vitro ELISA for the quantitative measurement of anti-TBGL IgG antibody in serum or plasma. This assay employs glycolipid antigens purified from Mycobacterium tuberculosis H37Rv (TBGL antigen) coated on a 96-well plate. The details of the assay were described in our previous studies (2, 11), but briefly, plasma was diluted 41-fold and added to wells that bound TBGL antigen. The wells were washed, and horseradish peroxidase-conjugated rabbit anti-human IgG, IgA, and IgM, all of which are specific to each heavy chain (Dako Japan, Kyoto, Japan), were added, followed by 60 min of incubation at room temperature. The plates were washed three times with washing buffer, 100 μl of TMBZ (3,3′,5,5′-tetramethylbenzidine) solution was added to each well, and the plates were incubated for 15 min at room temperature. To stop the enzyme reaction, 100 μl of 1 M H2SO4 was added, and the absorbance at 450 nm was measured with an MTP-120 plate reader (Corona Electric Co., Tokyo, Japan). The antibody titer was expressed according to a cutoff index. We scored the sample as positive when the titer was above the cutoff index for anti-TBGL IgG of 2.0 U/ml, the cutoff point proposed by Kishimoto et al. for the screening of patients with TB based on the diagnostic efficiency by receiver operating characteristic curve analysis (12). The cutoff values for anti-TBGL IgA and IgM are not available.
Measured laboratory markers.
We investigated the correlations between anti-TBGL antibodies and laboratory markers of TB infection, including immunocompetent cells. We measured the number of white blood cells with differential counts and the numbers of lymphocytes positive for CD3, CD20, and CD56 by FACSCalibur flow cytometry (Becton Dickinson and Company, NJ), using phycoerythrin-conjugated Leu-4 monoclonal antibody (MAb), fluorescein isothiocyanate-conjugated Leu-16 MAb, and phycoerythrin-conjugated Leu-19 MAb, respectively (Becton Dickinson and Company, NJ). Serum albumin and serum creatinine were measured because malnutrition and chronic renal failure are major risk factors for TB infection. We also measured IgA, IgG, IgM, and CRP by using serum and sCD40L and KL-6 by using plasma. The rationales for measuring sCD40L and KL-6 were stated in the introduction. sCD40L and KL-6 ELISA kits were purchased from Medsystems Diagnostics (Vienna, Austria) and from Sanko-Junyaku (Tokyo, Japan), respectively. The titers were measured according to the manufacturers’ protocols.
Radiographic classification.
We subdivided the patients into two groups, the cavity+ group and the cavity− group. We also categorized the patients into three groups based on chest X-ray findings, namely, minimal, moderately advanced, and far advanced, according to the classification of the NTA. Minimal lesions include those that are of slight to moderate density but do not contain demonstrable cavitation. They may involve a small part of one or both lungs, but the total extent, regardless of distribution, should not exceed the volume of lung on one side which is present above the second chondrosternal junction and the spine of the fourth or the body of the fifth thoracic vertebra. Moderately advanced lesions may be present in one or both lungs, but the total extent should not exceed the following limits: disseminated lesions of slight to moderate density may extend throughout the total volume of one lung or the equivalent in both lungs; dense and confluent lesions must be limited in extent to one-third the volume of one lung; and the total diameter of cavitation, if present, must be less than 4 cm. Far advanced lesions are more extensive than moderately advanced lesions (6).
Statistical analysis.
Laboratory data were analyzed using Stat Flex, version 5 (Artec, Osaka, Japan), and Statcel 2 (OMS Publishing Inc., Saitama, Japan). Correlations between levels of each parameter were evaluated by Spearman's rank correlation coefficient. The significances of differences were evaluated by the Mann-Whitney test. P values of <0.05 were considered significant. Bonferroni adjustment was used for multiple comparisons.
RESULTS
Health status of patients and positive rate.
The health status of the included patients is shown in Table 1. Among the 91 patients in this study, there were 20 patients with histories of TB, 3 patients with histories of gastrectomy, 14 patients with diabetes mellitus, 5 patients with chronic renal failure, and 1 patient with both diabetes mellitus and chronic renal failure. Fifty-nine patients had cavitary lesions on chest X-rays. The positive rate for the anti-TBGL IgG test, a commercialized diagnostic test for TB, was 79.7% (47/59 patients) for the cavity+ group, 50% (16/32 patients) for the cavity− group, and 69.2% (63/91 patients) overall. No patient was on steroid therapy.
TABLE 1.
Health status of patients in the study
| Parameter | Value for group
|
||
|---|---|---|---|
| Total | Cavity+ | Cavity− | |
| No. of patients | 91 | 59 | 32 |
| Age (yrs [mean ± SD]) | 59 ± 22 | 56 ± 21 | 64 ± 22 |
| Gender (no. of males/no. of females) | 60/31 | 42/17 | 18/14 |
| No. of patients with history of: | |||
| TB | 20 | 14 | 6 |
| Gastrectomy | 3 | 3 | 3 |
| Diabetes mellitus | 14 | 7 | 7 |
| Chronic renal failure | 5 | 2 | 3 |
| Diabetes mellitus and chronic renal failure | 1 | 0 | 1 |
Correlations between anti-TBGL antibodies.
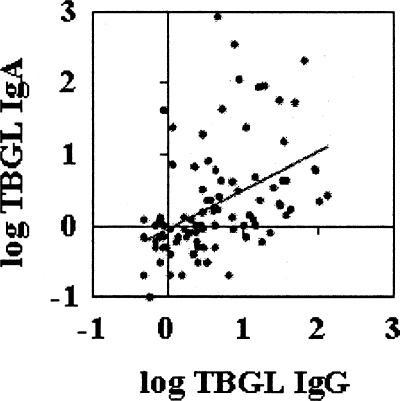
We sought correlations between each of the anti-TBGL antibodies. Anti-TBGL IgG had a positive correlation with anti-TBGL IgA (r = 0.551; P < 0.0000005) (Fig. 1). No other correlations were shown between the anti-TBGL antibodies.
FIG. 1.
Correlation between anti-TGBL IgG (TBGL IgG) and anti-TBGL IgA (TBGL IgA) (r = 0.551; P < 0.0000005).
Correlations between anti-TBGL antibodies and influential laboratory markers.
Anti-TBGL IgG had positive correlations with IgA (r = 0.228; P < 0.05), CRP (r = 0.361; P < 0.001), and KL-6 (r = 0.275; P < 0.01) and negative correlations with creatinine (r = −0.249; P < 0.05) and albumin (r = −0.240; P < 0.05). Anti-TBGL IgA had positive correlations with IgG (r = 0.285; P < 0.01), IgA (r = 0.300; P < 0.005), KL-6 (r = 0.225; P < 0.05), and sCD40L (r = 0.404; P < 0.005). Anti-TBGL IgM had positive correlations with IgM (r = 0.603; P < 0.00000005) and albumin (r = 0.251; P < 0.05).
Comparison between patients with and without cavitary lesions.
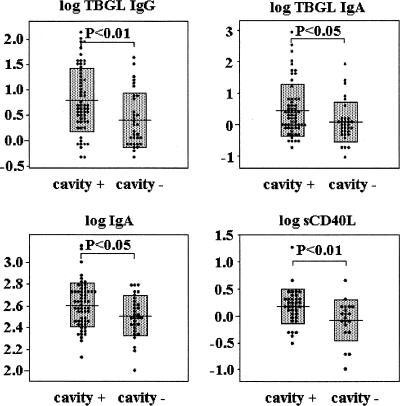
We compared all the measured laboratory markers between the patients with cavitary lesions (cavity+ group) and those without cavitary lesions (cavity− group) in order to determine new differences apart from that of the anti-TBGL IgG level (15). As shown in Table 2, both anti-TBGL IgG and anti-TBGL IgA levels were significantly higher in the cavity+ group (P < 0.005 and P < 0.05, respectively), but the anti-TBGL IgM titers showed no difference between the two groups. The numbers of white blood cells (P < 0.001), neutrophils (P < 0.005), basophils (P < 0.0005), and natural killer cells (CD56+) (P < 0.05) were significantly higher in the cavity+ group. The levels of CRP (P < 0.0005), KL-6 (P < 0.0005), IgA (P < 0.05), and sCD40L (P < 0.01) were also significantly higher in the cavity+ group (Table 2; Fig. 2 and 3).
TABLE 2.
Measured parameters (mean ± SD) and comparison between cavity+ group and cavity− group
| Parameter | Value for group
|
P valuea | ||
|---|---|---|---|---|
| Total | Cavity+ | Cavity− | ||
| TBGL-IgG (U/ml) | 13.2 ± 23.5 | 17.1 ± 27.6 | 6.0 ± 9.9 | <0.005* |
| TBGL-IgA (U/ml) | 22.6 ± 95.4 | 32.0 ± 117.2 | 5.3 ± 15.7 | <0.05* |
| TBGL-IgM (U/ml) | 6.0 ± 5.6 | 5.9 ± 5.8 | 6.2 ± 5.3 | NS |
| IgG (mg/dl) | 1,518 ± 471 | 1,523 ± 510 | 1,509 ± 395 | NS |
| IgA (mg/dl) | 416 ± 213 | 451 ± 236 | 348 ± 140 | <0.05* |
| IgM (mg/dl) | 106 ± 57 | 103 ± 58 | 111 ± 55 | NS |
| White blood cells/μl | 7,236 ± 2,706 | 7,830 ± 3,020 | 6,141 ± 1,513 | <0.01* |
| Neutrophils/μl | 5,567 ± 2,532 | 6,192 ± 2,798 | 4,415 ± 1,362 | <0.005* |
| Monocytes/μl | 397 ± 223 | 424 ± 243 | 347 ± 172 | NS |
| Eosinophils/μl | 115 ± 119 | 126 ± 135 | 94 ± 80 | NS |
| Basophils/μl | 24 ± 45 | 22 ± 46 | 29 ± 43 | <0.0005* |
| Lymphocytes/μl | 1,128 ± 740 | 1,061 ± 766 | 1,253 ± 685 | NS |
| CD3+ cells/μl | 751 ± 509 | 715 ± 517 | 815 ± 496 | NS |
| CD20+ cells/μl | 131 ± 115 | 114 ± 93 | 161 ± 143 | NS |
| CD56+ cells/μl | 208 ± 197 | 197 ± 223 | 231 ± 140 | <0.05* |
| CRP (mg/dl) | 4.5 ± 5.2 | 5.7 ± 5.8 | 2.2 ± 2.9 | <0.0005* |
| KL-6 (U/ml) | 564 ± 459 | 662 ± 518 | 382 ± 241 | <0.0005* |
| sCD40L (ng/ml) | 1.8 ± 2.5 | 2.1 ± 3.0 | 1.2 ± 1.0 | <0.01* |
| Creatinine (mg/ml) | 1.0 ± 1.5 | 0.8 ± 0.6 | 1.5 ± 2.4 | NS |
| Albumin (g/dl) | 3.4 ± 0.8 | 3.3 ± 0.7 | 3.6 ± 0.8 | NS |
Asterisks show significant differences between the cavity+ group and the cavity− group. NS, no significant difference. The significances of differences were evaluated by the Mann-Whitney test. P values of <0.05 were considered significant.
FIG. 2.
Anti-TBGL IgG (TBGL IgG), anti-TBGL IgA (TBGL IgA), IgA, and sCD40L levels in cavity+ and cavity− groups.
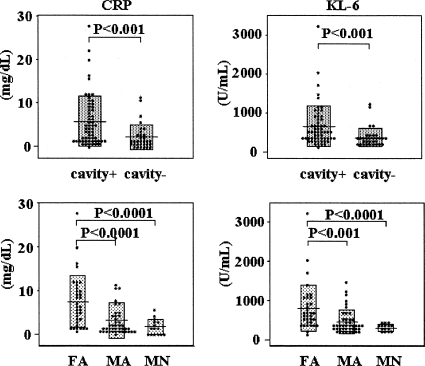
FIG. 3.
CRP and KL-6 levels in cavity+ and cavity− groups and NTA classification groups. FA, far advanced; MA, moderately advanced; MN, minimal.
Radiographic changes and inflammatory markers.
We compared the levels of the inflammatory markers CRP and KL-6 among the three groups and found that the far advanced group had significantly higher levels of CRP and KL-6 than did the moderately advanced group (P < 0.0001 and P < 0.0005, respectively) or the minimal group (P < 0.0001 and P < 0.00005, respectively). Although no statistical significance appeared, CRP and KL-6 had a tendency to be higher in the moderately advanced group than in the minimal group (Fig. 3). However, there was no correlation between the levels of CRP and those of KL-6. No other parameters, including anti-TBGL antibodies, showed significant differences between the groups.
DISCUSSION
We report for the first time that the anti-TBGL IgG level correlates with the CRP level. This may not be surprising because CRP is a well-known inflammatory marker and inflammation is generally involved in antibody synthesis. However, the mechanism underlying the association between the anti-TBGL IgG level and CRP was not readily understandable. Trehalose 6,6′-dimycolate (“cord factor”) is one of the principal antigens in TBGL, and cord factor has mycolic acid side chains. Mycolic acids are long-chain fatty acids that constitute the lipid-rich cell wall framework of mycobacteria, and their recognition is known to be mediated by CD1. Enomoto et al. discovered a CD1-restricted human T-cell line specific for glucose monomycolate, a glycosylated species of mycolic acids (5), and most CD1-restricted T cells are known to be natural killer T cells (NKT cells). Historical studies showed granuloma formation in the lungs of mice after intravenous administration of emulsified trehalose-6,6′-dimycolate (“cord factor”) (23). The role of NKT cells in granuloma formation was also confirmed by the fact that granulomas were actually formed in wild-type mice injected with cell walls from Mycobacterium tuberculosis but not in Jα281− mice, which lack NKT cells (1). On the other hand, Mempel et al. demonstrated that NKT cells migrate to and accumulate at inflammatory sites and behave like inflammatory cells independently of the CD1 molecules (17), which could lead to the production of inflammatory markers such as CRP. The possibility of NKT-cell involvement in anti-TB immunity was also suggested in a recent study describing that NKT cells are selectively lower in the peripheral blood mononuclear cells of individuals with pulmonary TB (19). More extensive studies are necessary to clarify the relationship between TBGL and NKT cells in tuberculous granuloma formation.
The anti-TBGL IgA level was correlated with sCD40L. Wiley et al. reported that the treatment of wild-type CD40 mice with sCD40L fusion protein elicited a pulmonary inflammatory response that was not observed in identically treated CD40 knockout mice and that CD40 ligation could play an important role in the establishment of the inflammatory response (21). On the other hand, the expression of CD40L was reported to have a direct correlation with Mycobacterium tuberculosis-stimulated gamma interferon production by peripheral blood mononuclear cells (18). Since sCD40L is involved in both pulmonary inflammation and TB infection, it could play a role as an inflammatory marker in pulmonary TB. The correlation between the anti-TBGL IgA level and sCD40L may also reflect the following immunopathogenesis of Mycobacterium tuberculosis infection. In the cavity+ group, sCD40L and IgA were significantly elevated. It is known that CD40 engagement by CD40L induces the production of endogenous transforming growth factor beta (TGF-β) and IgA secretion (25) and that TGF-β may be involved in the development and/or consequences of tuberculous granuloma formation (3). Therefore, the higher levels of sCD40L and IgA in the cavity+ group may reflect the intense granuloma formation in cavitary lesions, and these immune responses may have led to the correlation of anti-TBGL IgA level and the sCD40L level.
The level of anti-TBGL IgG had a correlation with that of anti-TBGL IgA. This correlation was not due to cross-reaction of the secondary antibodies because unwanted antibodies had been removed by solid-phase absorption. Julián et al. conducted a comparative study of IgG, IgM, and IgA antibody responses to four trehalose-containing glycolipids, including cord factor, purified from Mycobacterium tuberculosis in the sera from 92 TB patients. They concluded that IgG antibody was more sensitive, IgA antibody was more specific, and IgM reactivity was negligible for all the glycolipid antigens used (10). Since TBGL is a glycolipid antigen containing cord factor, anti-TBGL IgA may yield a higher specificity than does anti-TBGL IgG, and the detection of both anti-TBGL IgG and anti-TBGL IgA may improve the diagnostic value. A prospective controlled study on anti-TBGL IgA will be necessary to confirm this possibility.
There was a strong correlation between the levels of anti-TBGL IgM and serum IgM. However, we concluded that this did not reflect specific immunity in TB infection because IgM has a low affinity and cross-reactivity in addition to its pentameric structure (20).
Inoue et al. reported that the serum levels of KL-6 in 57 patients with active pulmonary TB rose significantly according to the increase in the extent of radiographic findings based on the classification of the NTA, but there was no significant difference between those with cavities and those without cavities (8). In our study, the far advanced group had significantly higher levels of KL-6 than did the moderately advanced group and the minimal group, and although the difference was not statistically significant, the moderately advanced group had higher levels of KL-6 than did the minimal group. In contrast to Inoue's data, KL-6 was significantly higher in the cavity+ group. The same results were shown for the level of CRP (Fig. 3), but no correlation was seen between KL-6 and CRP. Based on these findings, the level of KL-6 or CRP may reflect a different component of disease progression and could be used to evaluate the severity of pulmonary TB.
Although we found interesting correlations between anti-TBGL antibody levels and inflammatory markers, suggesting the involvement of inflammatory cytokines and NKT cells, confirmatory experiments have not been done, which is a major limitation of this study. Demonstrating specific immune responses to glycolipid antigens by using T cells from TB patients and their characterization would help to elucidate the immunopathogenesis of pulmonary TB.
Acknowledgments
This work was supported by a special educational grant from the Ministry of Education, Culture Sports, Science and Technology and by a grant-in-aid from the Scientific Expenses for Health and Welfare Program from the Ministry of Education, Culture Sports, Science and Technology.
We are grateful to T. Masunari for statistical analysis.
Footnotes
Published ahead of print on 9 January 2008.
REFERENCES
- 1.Apostolou, I., Y. Takahama, C. Belmant, T. Kawano, M. Huerre, G. Marchal, J. Cui, M. Taniguchi, H. Nakauchi, J. J. Fournie, P. Kourilsky, and G. Gachelin. 1999. Murine natural killer T (NKT) cells [correction of natural killer cells] contribute to the granulomatous reaction caused by mycobacterial cell walls. Proc. Natl. Acad. Sci. USA 96:5141-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashino, J., Y. Ashino, H. Guio, H. Saitoh, M. Mizusawa, and T. Hattori. 2005. Low antibody response against tuberculous glycolipid (TBGL) in elderly gastrectomised tuberculosis patients. Int. J. Tuberc. Lung Dis. 9: 1052-1053. [PubMed] [Google Scholar]
- 3.Aung, H., Z. Toossi, S. M. McKenna, P. Gogate, J. Sierra, E. Sada, and E. A. Rich. 2000. Expression of transforming growth factor-beta but not tumor necrosis factor-alpha, interferon-gamma and interleukin-4 in granulomatous lung lesions in tuberculosis. Tuber. Lung Dis. 80:61-67. [DOI] [PubMed] [Google Scholar]
- 4.Chan, J., X. Fan, S. W. Hunter, P. J. Brennan, and B. R. Bloom. 1991. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect. Immun. 59:1755-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enomoto, Y., M. Sugita, I. Matsunaga, T. Naka, A. Sato, T. Kawashima, K. Shimizu, H. Takahashi, Y. Norose, and I. Yano. 2005. Temperature-dependent biosynthesis of glucose monomycolate and its recognition by CD1-restricted T cells. Biochem. Biophys. Res. Commun. 337:452-456. [DOI] [PubMed] [Google Scholar]
- 6.Falk, A., J. B. O'Connor, and P. C. Pratt. 1969. Classification of pulmonary tuberculosis, p. 68-76. In Diagnostic standards and classification of tuberculosis, 12th ed., vol. 6. National Tuberculosis and Respiratory Disease Association, New York, NY. [Google Scholar]
- 7.Hunter, S. W., H. Gaylord, and P. J. Brennan. 1986. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J. Biol. Chem. 261:12345-12351. [PubMed] [Google Scholar]
- 8.Inoue, Y., K. Nishimura, M. Shiode, H. Akutsu, H. Hamada, S. Fujioka, S. Fujino, A. Yokoyama, N. Kohno, and K. Hiwada. 1995. Evaluation of serum KL-6 levels in patients with pulmonary tuberculosis. Tuber. Lung Dis. 76:230-233. [DOI] [PubMed] [Google Scholar]
- 9.Juffermans, N. P., A. Verbon, S. J. H. van Deventer, W. A. Buurman, H. Van Deutekom, P. Speelman, and T. van der Poll. 1998. Serum concentrations of lipopolysaccharide activity-modulating proteins during tuberculosis. J. Infect. Dis. 178:1839-1842. [DOI] [PubMed] [Google Scholar]
- 10.Julián, E., L. Matas, A. Perez, J. Alcaide, M. A. Laneelle, and M. Luquin. 2002. Serodiagnosis of tuberculosis: comparison of immunoglobulin A (IgA) response to sulfolipid I with IgG and IgM responses to 2,3-diacyltrehalose 2,3,6-triacyltrehalose and cord factor antigens. J. Clin. Microbiol. 40:3782-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawamura, M., N. Sueshige, K. Imayoshi, I. Yano, R. Maekura, and H. Kohno. 1997. Enzyme immunoassay to detect antituberculous glycolipid antigen (anti-TBGL antigen) antibodies in serum for diagnosis of tuberculosis. J. Clin. Lab. Anal. 11:140-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishimoto, T., O. Moriya, J. Nakamura, T. Matsushima, and R. Soejima. 1999. Evaluation of the usefulness of a serodiagnosis kit, the determiner TBGL antibody for tuberculosis: setting reference value. Kekkaku 74:701-706. [PubMed] [Google Scholar]
- 13.Kobayashi, J., and S. Kimura. 1995. KL-6: a serum marker for interstitial pneumonia. Chest 108:311-315. [DOI] [PubMed] [Google Scholar]
- 14.Maekura, R., M. Nakagawa, Y. Nakamura, T. Hiraga, Y. Yamamura, M. Ito, E. Ueda, S. Yano, H. He, and S. Oka. 1993. Clinical evaluation of rapid serodiagnosis of pulmonary tuberculosis by ELISA with cord factor (trehalose-6,6′-dimycolate) as antigen purified from Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 148:997-1001. [DOI] [PubMed] [Google Scholar]
- 15.Maekura, R., Y. Okuda, M. Nakagawa, T. Hiraga, S. Yokota, M. Ito, I. Yano, H. Kohno, M. Wada, C. Abe, T. Toyoda, T. Kishimoto, and T. Ogura. 2001. Clinical evaluation of anti-tuberculous glycolipid immunoglobulin G antibody assay for rapid serodiagnosis of pulmonary tuberculosis. J. Clin. Microbiol. 39:3603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzei, G. J., M. D. Edgerton, C. Losberger, S. Lecoanet-Henchoz, P. Graber, A. Durandy, J. Gauchat, A. Bernard, B. Allet, and J. Bonnefoy. 1995. Recombinant soluble trimetric CD40 ligand is biologically active. J. Biol. Chem. 31:7025-7028. [DOI] [PubMed] [Google Scholar]
- 17.Mempel, M., C. Ronet, F. Suarez, M. Gilleron, G. Puzo, L. V. Kaer, A. Lehuen, P. Kourilsky, and G. Gachelin. 2002. Natural killer T cells restricted by the monomorphic MHC class 1b CD1d1 molecules behave like inflammatory cells. J. Immunol. 168:365-371. [DOI] [PubMed] [Google Scholar]
- 18.Samten, B., E. K. Thomas, J. Gong, and P. F. Barnes. 2000. Depressed CD40 ligand expression contributes to reduced gamma interferon production in human tuberculosis. Infect. Immun. 68:3002-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder-Cappione, J. E., D. F. Nixon, C. P. Loo, J. M. Chapman, D. A. Meiklejohn, F. F. Melo, P. R. Costa, J. K. Sandberg, D. S. Rodrigues, and E. G. Kallas. 2007. Individuals with pulmonary tuberculosis have lower levels of circulating CD1d-restricted NKT cells. J. Infect. Dis. 195:1361-1364. [DOI] [PubMed] [Google Scholar]
- 20.Vollmers, H. P., and S. Brändlein. 2006. Natural IgM antibodies: from parias to parvenas. Histol. Histopathol. 21:1355-1366. [DOI] [PubMed] [Google Scholar]
- 21.Wiley, J. A., R. Geha, and A. G. Harmsen. 1997. Exogenous CD40 ligand induces a pulmonary inflammation response. J. Immunol. 158:2932-2938. [PubMed] [Google Scholar]
- 22.World Health Organization. 2007. Global tuberculosis control: surveillance, planning, financing: WHO report 2007. World Health Organization, Geneva, Switzerland.
- 23.Yarkoni, E., and H. J. Rapp. 1977. Granuloma formation in lungs of mice after intravenous administration of emulsified trehalose-6,6′-dimycolate (cord factor): reaction intensity depends on size distribution of the oil droplets. Infect. Immun. 18:552-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu, W., E. Soprana, G. Cosentino, M. Volta, H. S. Lichenstein, G. Viale, and D. Vercelli. 1998. Soluble CD141-152 confers responsiveness to both lipoarabinomannan and lipopolysaccharide in a novel HL-60 cell bioassay. J. Immunol. 161:4244-4251. [PubMed] [Google Scholar]
- 25.Zan, H., A. Cerutti, P. Dramitinos, A. Schaffer, and P. Casali. 1998. CD40 engagement triggers switching to IgA1 and IgA2 in human B cells through induction of endogenous TGF-beta: evidence for TGF-beta but not IL-10-dependent direct S mu→S alpha and sequential S mu→S gamma S gamma→S alpha DNA recombination. J. Immunol. 161:5217-5225. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, Y., M. Doerfler, T. C. Lee, B. Guillemin, and W. N. Rom. 1993. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by Mycobacterium tuberculosis components. J. Clin. Investig. 91:2076-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]