Abstract
We studied the time course of immunological and virological markers after highly active antiretroviral therapy (HAART) interruption in chronically human immunodeficiency virus type 1 (HIV-1)-infected patients immunized with an HIV lipopeptide preparation. In a prospective open pilot study, 24 HIV-1-infected HAART-treated patients with undetectable plasma viral loads (pVLs) and CD4+ T-cell counts above 350/mm3 were immunized at weeks 0, 3, and 6 with a candidate vaccine consisting of six HIV lipopeptides. At week 24, patients with pVLs of <1.7 log10 copies/ml were invited to stop taking HAART. Antiretroviral therapy was resumed if the pVL rose above 4.47 log10 copies/ml and/or if the CD4+ cell count fell below 250/mm3. Immunological and virologic parameters were studied before and after HAART interruption. The median baseline and nadir CD4+ cell counts were 482 (interquartile range [IQR], 195 to 826) and 313 (IQR, 1 to 481)/mm3, respectively. New specific CD8+ cell responses to HIV-1 epitopes were detected after immunization in 13 (57%) of 23 assessable patients. Twenty-one patients were evaluated 96 weeks after HAART interruption. The median time to pVL rebound was 4 weeks (IQR, 2 to 6), and the median peak pVL was 4.26 (IQR, 3 to 5) log10 copies/ml. Thirteen of these 21 patients resumed HAART a median of 60 weeks after immunization (IQR, 9.2 to 68.4 weeks), when the median pVL was 4.8 (IQR, 2.9 to 5.7) log10 copies/ml and the median CD4+ cell count was 551 (IQR, 156 to 778)/mm3. Eight patients were still off therapy at 96 weeks, with a median pVL of 4 (IQR, 1.7 to 4.6) log10 copies/ml and a median CD4+ cell count of 412 (IQR, 299 to 832)/mm3. No clinical disease progression had occurred. Despite the lack of a control arm, these findings warrant a randomized study of therapeutic vaccination with HIV lipopeptides followed by long-term HAART interruption in AIDS-free chronically infected patients.
Highly active antiretroviral therapy (HAART) limits human immunodeficiency virus type 1 (HIV-1) replication and drastically reduces AIDS-related morbidity and mortality (35). However, HAART is not universally available, must be taken for life, and has clinical and/or metabolic adverse effects in 40 to 60% of patients.
Long-term HAART increases the number of naive immune T cells and improves the functional status of CD4+ and CD8+ T cells (31). However, HAART also drives the production of HIV antigens below the threshold required to efficiently stimulate HIV-specific T-cell effectors or HIV-specific naive cells (24). Approaches aimed at enhancing anti-HIV immunity include adoptive therapy, cytokine therapy, and therapeutic immunization combined with structured treatment interruption (STI) (6, 17).
Therapeutic immunization has the potential to improve immunologic control of HIV and, possibly, to permit at least temporary discontinuation of antiretroviral therapy. In chronically infected patients with full suppression of HIV replication by antiretroviral therapy, STI has been proposed as a strategy to conserve active drugs, reduce treatment costs, and avoid adverse effects. Evaluation of therapeutic vaccines for HIV infection has been hindered by the lack of surrogate markers of protective or beneficial HIV-specific immunity in patients with chronic infection (11, 33, 39). Therapeutic immunization has so far proven disappointing (2, 5, 7, 14-16, 18, 20, 25-29, 36, 44, 47) and is not currently recommended (4, 8, 9, 13, 42, 50), but immunization with an HIV lipopeptide vaccine combined with a recombinant canarypox vaccine (ALVAC-HIV1) and interleukin-2 (IL-2) before HAART cessation contributed to controlling viral replication in chronically HIV-1-infected patients (19).
Lipopeptides are promising vaccine components. In a randomized phase I trial, we showed that a mixture of six HIV-1 lipopeptides was well tolerated and induced HIV-specific B-cell and T-cell responses in healthy volunteers (3, 21, 38).
The aims of the LIPTHERA study were the following: (i) to evaluate lipopeptide immunogenicity in HIV-1-infected patients and (ii) to examine if lipopeptide immunization permits long-term STI.
MATERIALS AND METHODS
Immunization protocol and study design.
This was an open phase II pilot vaccine trial. Twenty-four patients meeting the following inclusion criteria were selected in two clinical centers: asymptomatic chronic HIV-1 infection for at least 18 months, 18 years of age or more, at least 1 year of HAART, and HIV RNA level of <1.7 log10 copies/ml and CD4+ T-cell count of >350/mm3 for at least 6 months. Patients were excluded if (i) they had received cytokines, immunomodulatory therapy, or other candidate HIV vaccines or (ii) they were pregnant or had a serious illness at enrollment. The study took place in the following three phases: an immunization phase (LIPTHERA I), a treatment interruption phase (LIPTHERA II), and a long-term follow-up phase (LIPTHERA III). The study was approved by our institutional ethical review board (CCPPRB), and all patients gave their written informed consent.
In LIPTHERA I, 24 patients received three intramuscular injections of a six-lipopeptide mixture (three Nef, two Gag, and one Env lipopeptide) at 0, 3, and 6 weeks and continued antiretroviral treatment until week 24 (W24). In LIPTHERA II (starting at W24 after the last immunization), patients with HIV RNA levels of <1.7 log10 copies/ml were invited to stop taking HAART. HAART was resumed, after at least 1 month of interruption, if the CD4+ cell count fell below 250/mm3, the plasma viral load (pVL) was >4.47 log10 copies/ml on two consecutive occasions, or a major illness occurred. In LIPTHERA III, patients with stable pVLs and CD4+ cell counts of >350/mm3 at W40 after immunization continued without HAART until the end of follow-up (W96).
Each injection delivered 500 μg of each of the six lipopeptides included in the vaccine. Blood samples were collected prior to vaccination (W0), at W2, W6, W10, W20, and W24, every 2 months until W52, and routinely thereafter. Blood samples were also collected 14 days after each injection to measure immunogenicity.
HIV-1 RNA genotyping was performed at W4 and W8 after HAART interruption. After HAART interruption (W24), genotyping was performed on proviral DNA, as pVL was undetectable. Proviral HIV DNA was extracted from peripheral blood mononuclear cells (PBMCs), and the RT and protease genes were amplified as previously described (37). Only mutations associated with antiretroviral resistance were considered (22).
The main end point was the percentage of patients who developed new HIV-specific CD4+ and/or CD8+ T-cell responses. A new T-cell response was defined as a positive specific T-cell response that was absent before immunization (W0). Secondary end points were the clinical and biological tolerability of vaccination and the subsequent immunological and virological outcome.
The lipopeptide vaccine is a mixture of six large HIV-1 peptides derived from Nef, Gag, and Env amino acid sequences, modified by the addition of a palmitoyl-lysylaminide group, as described elsewhere (21). Three lipopeptides were derived from the HIV LAI Nef protein and were designated L-N1 (Nef amino acids [aa] 66 to 97), L-N2 (Nef aa 117 to 147), and L-N3 (Nef aa 182 to 205); two lipopeptides were derived from the HIV LAI Gag protein and were designated L-G1 (Gag aa 183 to 214) and L-G2 (Gag aa 253 to 284); and one lipopeptide, designated L-E (Env aa 303 to 335), was derived from the V3-Env gp120 consensus sequence that is also found in the HIV BX08 strain.
Laboratory methods.
Immunological parameters were measured in the 24 patients at W0, W2, W4, W5, and W24. T-cell proliferative responses were determined prior to (W0) and after each vaccine injection with PBMCs purified using standard methods (12, 13, 20). Briefly, PBMCs or CD4+ or CD8+ T cells (105 cells/well) were cultured in medium alone (negative control) or with 1 μg/ml of one of the soluble large peptides similar to those used in the vaccine mixture (N1, N2, N3, G1, G2, and E). Proliferation was determined on day 7 by adding 1 μCi/well of [3H]thymidine. The results are reported as a stimulation index, calculated as the ratio of the level of proliferation with the peptide (in counts per minute) to proliferation without the peptide. The proliferative response was considered positive if the stimulation index was >3 (21).
HIV-1-specific CD8+ T cells producing gamma interferon were measured with an ex vivo enzyme-linked immunospot assay (21). Briefly, PBMCs (105 cells/well) were stimulated with CD8+ T-cell epitopes appropriate for each patient's HLA class I molecules. An average of 18 (range, 2 to 34) HIV-1 peptides were tested for each patient. Numbers of spot-forming cells (SFCs) were normalized to 106 PBMCs and averaged from duplicate wells. The number of specific SFCs per 106 PBMCs was calculated by subtracting the negative control value from the experimental SFC count. A CD8+ T-cell response was considered significant when (i) a minimum of 100 SFCs/106 PBMCs were present per well, (ii) this number was ≥2 times that obtained with the negative control (PBMCs alone), (iii) the number of SFCs in stimulated wells (with peptide) minus the number of SFCs in unstimulated wells was ≥100/106 PBMCs, and (iv) this was true for both duplicates, as described elsewhere (21, 51). Patients with new HIV-specific CD4+ or CD8+ T-cell responses were considered vaccine responders.
Statistical analysis.
SAS software, version 8.2, for Windows (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. The chi-square test or Fisher's exact test was used to compare categorical variables.
The Kaplan-Meier method was used to evaluate the risk of HAART resumption. Since few subjects remained off treatment at the end of the study, multivariate analyses were performed separately for CD4+ T-cell and viral load variables. Student's t test or the Wilcoxon test was used to compare continuous variables. The correlation between the CD4+ T-cell nadir and the time off therapy was evaluated with Pearson's correlation coefficient test and Student's t test.
All values are given as medians, and all viral load values are log10 values unless otherwise specified. P values of <0.05 were considered significant.
RESULTS
Characteristics of patients.
Twenty-four HAART-treated asymptomatic HIV-1-infected patients with CD4+ T-cell counts of ≥350/mm3 and plasma HIV RNA levels of <1.7 log10 copies/ml were enrolled in the study. They had received a median of four lines of ART (interquartile range [IQR], one to seven lines) when they stopped taking HAART. During LIPTHERA I, 8 patients (33.5%) received two nucleoside reverse transcriptase inhibitors (NRTIs) plus one protease inhibitor (PI) and 16 patients (66.5%) received two NRTIs plus one nonnucleoside reverse transcriptase inhibitor.
The median baseline and nadir CD4+ T-cell counts were 629/mm3 (IQR, 397 to 1,132) and 313/mm3 (IQR, 1 to 481), respectively. The nadir CD4+ cell counts were stratified as follows: 200 to 350 cells/mm3, 13 patients (54.2%); >350 cells/mm3, 7 patients (29.2%); and <200 cells/mm3, 4 patients (16.6%). The median total time on ART was 37.2 months (range, 20.4 to 63.6 months).
All patients received the full vaccination schedule of three injections. Three patients were withdrawn from the study at W24 because their viral loads exceeded 1.7 log10 copies/ml (Tables 1 and 2).
TABLE 1.
Baseline characteristics of patients
| Parameter | Value |
|---|---|
| No. of patients | 24 |
| Median (range) age (yr) | 35.8 (28.3-55.1) |
| Gender (no. [%]) | |
| Male | 18 (75) |
| Female | 6 (25) |
| CDC status (%) | |
| A | 54.2 |
| B | 33.3 |
| C | 12.5 |
| Median (range) time for primary ARV (yr) | 3.5 (1.9-8.7) |
| Median (range) time of HIV serology at time of first | |
| ARV (yr) | 3.4 (0-10.2) |
| No. (%) of patients receiving HAART during the trial | |
| Two NRTIs + PI | 8 (33.5) |
| Two NRTIs + nonnucleoside RT inhibitor | 16 (66.5) |
| Median (range) CD4 cell count before immunization | |
| (cells/mm3) | 629 (397-1132) |
| Median (range) nadir CD4 cell count (cells/mm3) | 313 (1-481) |
| No. (%) of patients with median plasma HIV RNA of | |
| <1.7 log10 copies/ml | 24 (100) |
| Median (range) antiviral therapy duration (wk) | 3.1 (1.7-5.3) |
| Median (range) CD4 cell count at treatment interruption | |
| (cells/mm3) | 722 (383-1029) |
| No. (%) of patients with median plasma HIV RNA of | |
| <1.7 log10 copies/ml at treatment interruption | 21 (87.5) |
TABLE 2.
Virological and immunological parameters at treatment interruption and follow-up
| Parameter | Value |
|---|---|
| No. of patients with HIV RNA load of <1.7 log10 | |
| copies/ml at wk 24/total no. of patients (%) | 21/24 (87.5) |
| No. of patients who stopped HAART at wk 24/total | |
| no. of patients | 21/24 |
| Median (range) peak HIV RNA load (log10copies/ml) | 4.26 (3-5) |
| Median (range) time to peak HIV RNA load (wk) | 4 (2-6) |
| No. of patients who resumed therapy/total no. of patients | 13/21 |
| Median (range) delay in resuming therapy (wk) | 60 (9.2-68.4) |
| Median (range) CD4 cell count at time of restarting | |
| therapy (cells/mm3) | 551 (156-778) |
| Median (range) HIV RNA load at time of resuming | |
| therapy (log10copies/ml) | 4.8 (2.9-5.7) |
| Median (range) time to achieve undetectable pVL after | |
| resuming HAART (wk) | 8 (4-16) |
| Median (range) CD4 cell count at W96 in patients not | |
| resuming treatment (cells/mm3) | 412 (299-832) |
| Median (range) HIV RNA load at W96 in patients not | |
| resuming treatment (log10 copies/ml) | 4 (1.7-4.6) |
Tolerability and safety.
No serious adverse events occurred during the vaccination period. Pain and induration at the injection site were the most common adverse events (n = 10). Eight patients reported asthenia and four reported fever after vaccination.
After HAART interruption (LIPTHERA II), eight, five, and four patients had asthenia, monomeric herpes zoster reactivation, and an HIV seroconversion-like syndrome, respectively. The latter syndrome occurred a median of 4 weeks (range, 1 to 6 weeks) after HAART interruption, and all four patients concerned resumed HAART without reaching the pVL and CD4+ T-cell count criteria (pVL of <4.47 log10 copies/ml and CD4+ count of >250 cells/mm3). All symptoms resolved within a median of 2 weeks (range, 1 to 4 weeks) after resuming HAART.
Anti-HIV immunological responses and CD4+ T-cell proliferative responses.
Twenty-three of the 24 patients had interpretable T-cell responses to at least one of the six HIV-1 large peptides.
At baseline (W0), 30% of the patients (7/23 patients) responded to at least one of the six peptides, compared to 74% of patients (17/23 patients) after the three vaccine injections. These responses were detected at least twice during the study in 11/23 patients (48%). New T-cell responses to at least one large peptide were detected after vaccination in 16/23 patients (70%), and these responses were detected on at least two occasions in 5/23 (22%) patients.
CD4+ T-cell responses were directed mainly to the Gag 2 peptide, as described in detail elsewhere (51).
Lymphoproliferative responses to HIV-1 peptides, either before or after immunization, did not significantly influence the probability of remaining off HAART (data not shown).
CD8+ T-cell responses.
Six (26%) of 23 assessable patients had CD8+ T-cell responses to HIV-1 epitopes at baseline, and 13/23 patients (57%) had new responses after vaccination. These new responses were detected on at least two occasions in 8/23 patients (35%). Among the 13 patients with new CD8+ T-cell responses, 4, 4, 2, and 3 patients had new responses to one, two, three, and four peptides, respectively. Most of the CD8+ T-cell epitopes recognized in vaccinated patients were located in the G2, N1, and N2 peptides, as in healthy volunteers (21).
Outcome after HAART interruption.
The dynamics of HIV replication were evaluated for 96 weeks in 21 patients. The median time to pVL rebound after HAART interruption was 4 weeks (IQR, 2 to 6 weeks), with a median peak pVL of 4.26 (IQR, 3 to 5) log10 copies/ml. Forty-eight weeks after HAART interruption, 9/21 patients (41%) were still off therapy, all with pVLs of <4.47 log10 copies/ml (<4 log10 copies/ml in 4 patients). Seven of these nine patients had new CD4+ or CD8+ T-cell responses after vaccination.
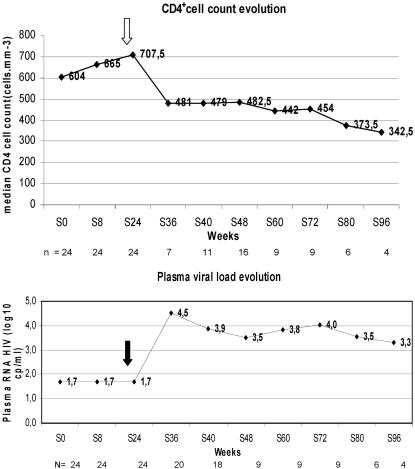
Thirteen of the 21 patients resumed HAART after a median interval of 60 weeks (IQR, 9.2 to 68.4 weeks), with a median pVL of 4.8 (2.9 to 5.7) log10 copies/ml and a median CD4+ T-cell count of 551/mm3 (range, 156 to 778/mm3). Eight patients were still off HAART at 96 weeks, with a median pVL of 4 (IQR, 1.7 to 4.6) log10 copies/ml and a median CD4+ T-cell count of 412 (IQR, 299 to 832)/mm3. Interestingly, one of these eight patients had an undetectable pVL (<50 copies/ml) at W96 (Table 2; Fig. 1).
FIG. 1.
(Top) Median CD4 T-cell counts before and after HAART interruption (indicated by an arrow). (Bottom) Median pVLs before and after HAART interruption (indicated by an arrow).
Impact of nadir CD4+ T-cell count on outcome after HAART interruption.
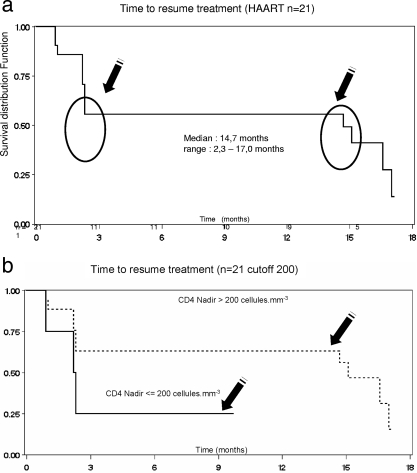
The kinetics of HAART resumption was biphasic. Almost half of the patients resumed HAART between W8 and W12, and the rest resumed HAART after W63 (64 to 68 weeks).
Eight patients were still off therapy at 96 weeks. The median time to HAART resumption in the remaining 13 patients was 60 weeks (IQR, 9.2 to 68.4 weeks) (Fig. 2a). The median baseline CD4+ T-cell counts in these 8 and 13 patients were 617 (IQR, 397 to 832)/mm3 and 648 (IQR, 447 to 1,132)/mm3, respectively (Table 1; Fig. 1).
FIG. 2.
(a) Kaplan-Meier curve of the time to HAART resumption. At W60, 62% of the patients remained off therapy. One subset of patients resumed therapy early, after between 9 and 12 weeks, while a second subset resumed therapy after between 48 and 56 weeks. (b) Kaplan-Meier curve of the time to HAART resumption. Responses are shown according to the CD4 T-cell nadir (>200 versus ≤200 cells/mm3).
Thirteen patients resumed HAART, with a median CD4+ T-cell count of 551 (156 to 778)/mm3. The median count at treatment interruption for patients who remained off therapy was 412 (IQR, 299 to 832)/mm3 at W96. Only 4 of the 21 assessable patients had a nadir CD4+ T-cell count above 350 cells/mm3, and 5 of the 8 patients who remained off therapy at 96 weeks had a CD4+ T-cell nadir of between 200 and 250/mm3.
Kaplan-Meier analysis showed a trend toward a relation between a CD4+ T-cell nadir of 200/mm3 and the risk of HAART resumption (Fig. 2b).
The nadir CD4+ cell count correlated with the CD4+ T-cell count slope during treatment interruption (r = 0.72; P = 0.007).
DISCUSSION
This study shows that HIV-1-specific T-cell responses can be induced by lipopeptide vaccination of chronically infected patients on HAART. The efficacy of the lipopeptide vaccine used in this study was first tested in HIV-seronegative volunteers in an open phase I study called VAC 04 (21, 38), in which T-cell proliferative responses were detected in 25/26 subjects (92%) and CD8+ T-cell responses were detected in 19/22 subjects (86%).
Structured treatment interruption has been explored as a means of offsetting the complexity and toxicity of antiretroviral treatment, and also the development of viral resistance (6, 23, 34, 46). Previous reports suggest that CD4+ T-cell counts are predictive of the outcome after treatment discontinuation (29, 36, 44, 45), even if HAART interruption consistently leads to a fall in the CD4+ T-cell count and to pVL rebound (10, 28, 30, 49).
In the present study, new CD4+ T-cell proliferative responses to at least one HIV-1 large peptide were detected after vaccination in 16/23 patients (70%), and new CD8+ T-cell responses were detected in 13/23 patients (57%). Ninety percent of the 23 immunized patients developed CD4+ and/or CD8+ responses to at least one HIV-1 peptide.
Functional alterations and impaired maturation of HIV-1-specific CD4+ and CD8+ T cells have been shown to correlate with disease progression. The rationale of therapeutic immunization for HIV infection is based on studies suggesting that cytotoxic T lymphocytes are involved in controlling HIV-1 replication and also that CD4+ T-cell proliferative responses (essentially to p24) correlate with better control of HIV-1 viremia. The reversible cell-mediated immune deficits associated with chronic HIV-1 infection have important implications for immunotherapy (35). In this study, the aim of therapeutic immunization was to induce new HIV-1-specific CD4+ T cells and also to induce new HIV-1-specific CD8+ T cells in patients with chronic infection, as is the case in long-term nonprogressors (40, 51).
Levy et al. (30) randomized HIV-1-infected patients to continue with their antiretroviral regimen, either alone or combined with four injections of the ALVAC-HIV (vCP1433) and Lipo-6T vaccines, followed by three cycles of IL-2. Proliferative responses to p24 antigen remained stable in 47% of patients (15/34 patients) in the vaccine-IL-2 group, compared with 24% (8/33 patients) of controls (P = 0.049). After stopping HAART, 24% of the patients in the vaccine-IL-2 group lowered their viral set point, compared to 5% of controls (P = 0.027), suggesting that immunization before HAART cessation might contribute to controlling viral replication. In contrast, no significant difference in the time before resuming therapy was found between the two groups (median, 54 versus 69.5 days; P = 0.11). In the Vacciter study, 79% of patients resumed therapy after a median of 6.3 weeks (IQR, 5.9 to 7.1 weeks) (25). Harrer et al. reported that therapeutic vaccination with recombinant HIV-1 nef-expressing modified vaccinia virus Ankara gave similar results: 8/14 patients resumed HAART after a median treatment interruption of 15 weeks (range, 4 to 37 weeks) (25). Molina et al. examined whether intermittent IL-2 therapy could defer antiretroviral (ARV) therapy in ARV-naive HIV-1-infected patients. The patients were randomized to receive IL-2 (n = 66) or no treatment (n = 64). ARV therapy was deferred by a median of 48 weeks in the IL-2 arm (P = 0.02), and at W96, 35% of subjects in the IL-2 arm versus 59% of patients in the control group had experienced treatment failure, defined as a CD4 cell count of <300/mm3, ARV initiation, or the occurrence of an AIDS-defining event or death. Moreover, treatment failure was significantly more rapid in the control arm. In naive patients with a baseline CD4 cell count between 300 and 500/mm3, IL-2 administration substantially deferred the initiation of ARV without affecting HIV replication (32). In the TILT pilot study, involving a total of 86 ARV-experienced patients, the use of IL-2 before ARV interruption prolonged the time off ARV. Ninety-six weeks after ARV interruption, 66% of control patients had resumed ARV therapy, compared with only 34% of IL-2-treated patients (1). The different trials of candidate vaccines with and without IL-2 are not comparable, however, because of different inclusion and exclusion criteria and different schedules of vaccination and follow-up. It is nonetheless noteworthy that in the LIPTHERA trial, treatment resumption was necessary after a median of 60 weeks (range, 9.2 to 68.4 weeks) for only 62% of patients. In the case control study by Molina et al., IL-2 deferred ARV therapy for 48 weeks. The LIPTHERA trial provides proof of concept for treatment-experienced patients that therapeutic vaccination can defer the resumption of ARV treatment. In a retrospective cohort study involving 105 patients, 57% of patients had not resumed therapy a median of 114 weeks after immunization (43). In a recent study (ACTG A5086) of STI in 167 HIV-infected patients with preserved immune function, Skiest et al. found that only 46/167 patients (27.5%) resumed HAART at week 96 (43), but it is noteworthy that the baseline CD4+ cell count was higher than that for the LIPTHERA study population (833/mm3 [668 to 989] versus 629/mm3 [132 to 397]). The best predictor of the CD4+ T-cell decline and of the risk of resuming ARV therapy was the nadir CD4+ T-cell count, as in other studies (27, 29, 32, 46, 50). Treatment interruption in patients with a pre-ARV CD4+ cell count nadir above 200/mm3 was not associated with adverse events.
In conclusion, this study shows that a significant percentage of chronically HIV-1-infected patients can remain off antiretroviral drug therapy for long periods after vaccination with a mixture of six HIV lipopeptides.
Acknowledgments
We thank Jean Paul Viard and David Young for critical reviews of the manuscript and Thu-Huyen Nguyen and Eka Chakvetadze for clinical management. We also thank Elisette Maréchal-da-Silva and the patients who participated in the study.
Footnotes
Published ahead of print on 9 January 2008.
REFERENCES
- 1.Angus, B., F. Lampe, G. Tambussi, C. Katlama, M. Youle, I. Williams, B. Clotet, M. Fisher, F. Post, A. Babiker, and TILT Trial Steering Committee. 2007. The TILT trial—a pilot trial of ART interruption with and without the use of IL-2, abstr. 477. 14th Conf. Retrovir. Opportun. Infect., Los Angeles, CA, 25 to 28 February 2007.
- 2.Autran, B., P. Debre, B. Walker, and C. Katlama. 2003. Therapeutic vaccines against HIV need international partnerships. Nat. Rev. Immunol. 3:503-508. [DOI] [PubMed] [Google Scholar]
- 3.BenMohamed, L., S. L. Wechsler, and A. B. Nesburn. 2002. Lipopeptide vaccines—yesterday, today and tomorrow. Lancet Infect. Dis. 2:425-431. [DOI] [PubMed] [Google Scholar]
- 4.BHIVA Writing Committee on Behalf of the BHIVA Executive Committee. 2001. British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy. HIV Med. 2:276-313. [DOI] [PubMed] [Google Scholar]
- 5.Boschi, A., T. Carmine, P. Ortolani, G. Moscatelli, G. Morigi, and M. Arlotti. 2004. CD4+ cell-count-guided treatment interruptions in chronic HIV-infected patients with good response to highly active antiretroviral therapy. AIDS 18:2381-2389. [PubMed] [Google Scholar]
- 6.Carcelain, G., R. Tubiana, A. Samri, V. Calvez, C. Delaugerre, H. Agut, C. Katlama, and B. Autran. 2001. Transient mobilization of human immunodeficiency virus (HIV)-specific CD4 T helper cells fails to control virus rebounds during intermittent antiretroviral therapy in chronic HIV type 1 infection. J. Virol. 75:234-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardiello, P., E. Hassink, J. Ananworanich, P. Srasuebkul, T. Samor, A. Mahanontharit, K. Ruxrungtham, B. Hirschel, J. Lange, P. Phanuphak, and D. A. Cooper. 2005. A prospective, randomized trial of structured treatment interruption for patients with chronic HIV type 1 infection. Clin. Infect. Dis. 40:594-600. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter, C. J., D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, S. M. Hammer, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, P. G. Yeni, and P. A. Volberding. 2000. Antiretroviral therapy in adults. Updated recommendations of the International AIDS Society—USA Panel. JAMA 283:381-390. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter, C. J., M. A. Fischl, S. M. Hammer, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, R. T. Schooley, M. A. Thompson, S. Vella, P. G. Yeni, and P. A. Volberding. 1998. Antiretroviral therapy for HIV infections in 1998. Updated recommendations of the International AIDS Society—USA Panel. JAMA 280:78-86. [DOI] [PubMed] [Google Scholar]
- 10.Chun, T. W., R. T. Davey, Jr., M. Ostrowsky, J. Shawn Justement, D. Engel, J. I. Mullins, and A. S. Fauci. 2000. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat. Med. 6:757-761. [DOI] [PubMed] [Google Scholar]
- 11.Daniel, N., B. Charmeteau, S. Grabar, G. Pialoux, D. Salmon, N. Bonilla, M. Dupuis, C. Troadec, W. Rozenbaum, H. Gahéry-Ségard, J. G. Guillet, and M. Andrieu. 2004. Use of well-defined HIV-derived epitopes to evaluate CD4(+) and CD8(+) T cell responses in patients with chronic HIV-1 infection treated with HAART. AIDS Res. Hum. Retrovir. 20:827-835. [DOI] [PubMed] [Google Scholar]
- 12.Delaugerre, C., M. A. Valantin, M. Moroux, M. Bonmarchand, G. Carcelain, C. Duvivier, R. Tubiana, A. Simon, F. Bricaire, H. Agut, B. Autran, C. Katlama, and V. Calvez. 2001. Re-occurrence of HIV-1 drug mutations after treatment re-initiation following interruption in patients with multiple treatment failure. AIDS 15:2189-2191. [DOI] [PubMed] [Google Scholar]
- 13.DHHS Panel on Clinical Practices for Treatment of HIV Infection. 1 December 2007, posting date. Guidelines for use of antiretroviral agents in HIV-infected adults and adolescents. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 14.Dorrell, L. 2006. Therapeutic immunization for the control of HIV-1: where are we now? Int. J. STD AIDS 17:436-441. [DOI] [PubMed] [Google Scholar]
- 15.Dybul, M., T. W. Chun, D. Yoder, B. Hidalgo, M. Belson, K. Hertogs, B. Larder, R. L. Dewar, C. H. Fox, C. W. Hallawan, J. S. Justement, S. A. Migueles, J. A. Metcalf, R. T. Davey, M. Daucher, P. Pandya, M. Baseler, D. J. Ward, and A. S. Fauci. 2001. Short-cycle structured intermittent treatment of chronic HIV infection with highly active antiretroviral therapy: effects on virologic, immunologic, and toxicity parameters. Proc. Natl. Acad. Sci. USA 98:15161-15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dybul, M. 2002. Structured treatment interruption: approaches and risks. Curr. Infect. Dis. 4:175-180. [DOI] [PubMed] [Google Scholar]
- 17.Esparza, J., and S. Osmanov. 2003. HIV vaccines: a global perspective. Curr. Mol. Med. 3:183-193. [DOI] [PubMed] [Google Scholar]
- 18.Fagard, C., A. Oxenius, H. Günthard, F. Garcia, M. Le Braz, G. Mestre, M. Battegay, H. Furrer, P. Vernazza, E. Bernasconi, A. Telenti, R. Weber, D. Leduc, S. Yerly, D. Price, S. J. Dawson, T. Klimtait, T. V. Pereneger, A. McLean, B. Clotet, J. M. Gatell, L. Perrin, M. Plana, R. Phillips, and B. Hirschel. 2003. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch. Intern. Med. 163:1220-1226. [DOI] [PubMed] [Google Scholar]
- 19.Fischer, M., R. Hafner, C. Schneider, A. Trkola, B. Joos, H. Joller, B. Hirschel, R. Weber, and H. F. Günthard. 2003. HIV RNA in plasma rebounds within days during structured treatment interruptions. AIDS 17:195-199. [DOI] [PubMed] [Google Scholar]
- 20.Forum for Collaborative HIV Research. 2002. Immune-based therapies: a review of clinical endpoints used in trials of selected immunologic agents. HIV Clin. Trials 3:58-88. [DOI] [PubMed] [Google Scholar]
- 21.Gahery, H., N. Daniel, B. Charmeteau, L. Ourth, A. Jackson, M. Andrieu, J. Choppin, D. Salmon, G. Pialoux, and J. G. Guillet. 2006. New CD4+ and CD8+ T cell responses induced in chronically HIV-1 infected patients after immunization with an HIV type 1 lipopeptide vaccine. AIDS Res. Hum. Retrovir. 22:684-694. [DOI] [PubMed] [Google Scholar]
- 22.Gahery-Segard, H., G. Pialoux, S. Figueiredo, C. Igéa, M. Surenaud, J. Gaston, H. Gras-Masse, J. P. Levy, and J. G. Guillet. 2003. Long-term specific immune responses induced in humans by a human immunodeficiency virus type 1 lipopeptide vaccine: characterization of CD8+-T-cell epitopes recognized. J. Virol. 77:11220-11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallant, J. E. 2002. Current status of antiretroviral treatment interruption and intermittent therapy strategies. MedGenMed 4:19. [PubMed] [Google Scholar]
- 24.Harari, A., F. Petipierre, F. Vallelian, and G. Pantaleo. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103:966-972. [DOI] [PubMed] [Google Scholar]
- 25.Harrer, E., M. Bäuerle, B. Ferstl, P. Chaplin, B. Petzold, L. Mateo, A. Handley, M. Tzatzaris, J. Vollmar, S. Bergmann, M. Rittmaier, K. Eismann, S. Müller, J. R. Kalden, B. Spriewald, D. Willbold, and T. Harrer. 2005. Therapeutic vaccination of HIV-1-infected patients on HAART with a recombinant HIV-1 nef-expressing MVA: safety, immunogenicity and influence on viral load during treatment interruption. Antivir. Ther. 10:285-300. [PubMed] [Google Scholar]
- 26.Harrigan, P. R., M. Whaley, and J. S. Montaner. 1999. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. AIDS 13:F59-F62. [DOI] [PubMed] [Google Scholar]
- 27.Hoggs, R. S., B. Yip, K. J. Chan, E. Wood, K. J. Craib, M. V. O'Shaugnessy, and J. S. Montaner. 2001. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA 286:2568-2577. [DOI] [PubMed] [Google Scholar]
- 28.International Study Group on CD4-Monitored Treatment Interruptions. 2005. CD4 cell-monitored treatment interruption in patients with a CD4 cell count >500 × 106 cells/l. AIDS 19:287-294. [PubMed] [Google Scholar]
- 29.Lange, C. G., M. M. Lederman, K. Medvik, R. Asaad, M. Wild, R. Kalayjian, and H. Valdez. 2003. Nadir CD4+T-cell count and numbers of CD28+ CD4+-T cells predict functional responses to immunization in chronic HIV-1 infection. AIDS 17:2015-2023. [DOI] [PubMed] [Google Scholar]
- 30.Levy, Y., H. Gahery-Segard, C. Durier, A. S. Lascaux, C. Goujard, V. Meiffredy, C. Rouzioux, R. El Habib, M. Beumont-Mauviel, J. G. Guillet, J. F. Delfraissy, and J. P. Aboulker. 2005. Immunological and virological efficacy of a therapeutic immunization combined with interleukin-2 in chronically HIV-1-infected patients. AIDS 19:279-286. [PubMed] [Google Scholar]
- 31.Lichterfeld, M., D. E. Kaufmann, X. G. Yu, S. K. Mui, M. M. Addo, M. N. Johnston, D. Cohen, G. K. Robbins, E. Pae, G. Alter, A. Wurcel, D. Stone, E. S. Rosenberg, B. D. Walker, and M. Altfed. 2004. Loss of HIV-1-specific CD8+T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+T cells. J. Exp. Med. 200:701-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molina, J. M., Y. Levy, S. Hamonic, I. Fournier, M. Bentata, G. Beck-Wirth, I. Madeleine, F. Jeanblanc, D. Sereni, F. Simon, and J. Aboulker. 2007. Intermittent interleukin-2 therapy to defer antiretroviral therapy in patients with human immunodeficiency virus infection, abstr. H-718. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother.
- 33.Molinier-Frenkel, V., H. Gahery-Segard, M. Mehtali, C. Le Boulaire, S. Ribault, P. Boulanger, T. Tursz, J. G. Guillet, and F. Farace. 2000. Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J. Virol. 74:7678-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montaner, J. S., M. Harris, and R. Hogg. 2005. Structured treatment interruptions: a risky business. Clin. Infect. Dis. 40:601-603. [DOI] [PubMed] [Google Scholar]
- 35.Pallela, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, and S. D. Holmberg. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 36.Papasavvas, E., J. R. Kostman, K. Mounzer, R. M. Grant, R. Gross, C. Gallo, L. Azzoni, A. Foulkes, B. Thiel, M. Pistilli, A. Mackiewicz, J. Shull, and S. J. Montaner. 2004. Randomized, controlled trial of therapy interruption in chronic HIV-1 infection. PLoS Med. 1:218-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasquier, C., N. Millot, R. Njouom, K. Sandress, M. Cazabat, J. Puel, and J. Izopet. 2001. HIV-1 subtyping using phylogenetic analysis of pol gene sequences. J. Virol. Methods 94:45-54. [DOI] [PubMed] [Google Scholar]
- 38.Pialoux, G., H. Gahery-Segard, S. Sermet, H. Poncelet, S. Fournier, L. Gérard, A. Tartar, H. Gras-Masse, J. P. Levy, and J. G. Guillet. 2001. Lipopeptides induce cell-mediated anti-HIV immune responses in seronegative volunteers. AIDS 15:1239-1249. [DOI] [PubMed] [Google Scholar]
- 39.Ratto-Kim, S., L. D. Loomis-Price, N. Aronson, J. Grimes, C. Hill, C. Williams, R. El Habib, D. L. Birx, and J. H. Kim. 2003. Comparison between env-specific T-cell epitopic responses in HIV-1-uninfected adults immunized with combination of ALVAC-HIV (vCP205) plus or minus rgp 160 MN/LAI-2 and HIV-1-infected adults. J. Acquir. Immune Defic. Syndr. 32:1-17. [DOI] [PubMed] [Google Scholar]
- 40.Robbins, G. K., M. M. Addo, H. Troung, A. Rathod, K. Habeeb, B. Davis, H. Heller, N. Basgoz, R. D. Walker, and E. S. Rosenberg. 2003. Augmentation of HIV-1-specific T helper cell responses in chronic HIV-1 infection by therapeutic immunization. AIDS 17:1121-1126. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz, L., J. Martinez-Picado, J. Romeu, R. Paredes, M. K. Zayat, S. Marfil, E. Negredo, G. Sirera, C. Tural, and B. Clotet. 2000. Structured treatment interruption in chronically HIV-1-infected patients after long-term viral suppression. AIDS 14:397-403. [DOI] [PubMed] [Google Scholar]
- 42.Skiest, D. J., P. Morrow, B. Allen, J. McKinsey, C. Crosby, B. Foster, and R. D. Hardy. 2004. It is safe to stop antiretroviral therapy in patients with preantiretroviral CD4 cell counts >250 cells/μl. J. Acquir. Immune Defic. Syndr. 37:1351-1357. [DOI] [PubMed] [Google Scholar]
- 43.Skiest, D. J., Z. Su, D. V. Havlir, K. R. Robertson, R. W. Croombs, P. Cain, T. Peterson, A. Krambrink, N. Jahed, D. McMahon, and D. M. Margolis. 2007. Interruption of antiretroviral treatment in HIV-infected patients with preserved immune function is associated with a low rate of clinical progression: a prospective study by AIDS clinical trials group 5170. J. Infect. Dis. 195:1426-1436. [DOI] [PubMed] [Google Scholar]
- 44.Tarwater, P. M., M. Parish, and J. E. Gallant. 2003. Prolonged treatment interruption after immunologic response to highly active antiretroviral therapy. Clin. Infect. Dis. 37:1541-1548. [DOI] [PubMed] [Google Scholar]
- 45.Tebas, P., K. Henry, K. Mondy, S. Deeks, H. Valdez, C. Cohen, and W. G. Powderly. 2002. Effect of prolonged discontinuation of successful antiretroviral therapy on CD4+ T cell decline in human immunodeficiency virus-infected patients: implications for intermittent therapeutic strategies. J. Infect. Dis. 186:851-854. [DOI] [PubMed] [Google Scholar]
- 46.Toulson, A. R., P. R. Harrigan, B. Yip, M. Harris, R. S. Hogg, W. Dong, K. V. Heath, and J. S. Montaner. 2004. Evaluation of outcomes following structured interruptions (STI) among patients with prior nadir CD4 cell counts >200 cells/mm3. Can. J. Infect. Dis. 15:S29A. [DOI] [PubMed] [Google Scholar]
- 47.Tubiana, R., G. Carcelain, M. Vray, K. Gourlain, C. Dalban, A. Chermak, C. Rabian, D. Vittecoq, A. Simon, E. Bouvet, R. El Habib, D. Costagliola, V. Calvez, B. Autran, and C. Katlama. 2005. Therapeutic immunization with a human immunodeficiency virus (HIV) type-recombinant canarypox vaccine in chronically HIV-infected patients: the Vacciter study (ANRS 094). Vaccine 23:4292-4301. [DOI] [PubMed] [Google Scholar]
- 48.Reference deleted.
- 49.Wong, J. K., M. Hezareh, H. F. Günthard, D. V. Havlir, C. C. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV-1 despite prolonged suppression of plasma viremia. Science 278:1291-1295. [DOI] [PubMed] [Google Scholar]
- 50.Yeni, P. G., S. M. Hammer, C. C. Carpenter, D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Voderbing. 2002. Antiretroviral treatment for adult HIV infection in 2002. Updated recommendations of the International AIDS Society—USA Panel. JAMA 288:222-235. [DOI] [PubMed] [Google Scholar]
- 51.Younes, S. A., B. Yassine-Diabb, A. R. Dumont, M. R. Boulassel, Z. Grossman, J. P. Routy, and R. P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin-2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]