Abstract
Anaplasma phagocytophilum causes human granulocytic anaplasmosis by inducing immunopathologic responses. Its immunodominant Msp2 protein is encoded by a family of >100 paralogs. Msp2 (msp2) expression modulates in the absence of immune pressure, and prolonged in vitro passage modulates in vivo virulence. Because programmed MSP2 expression occurs in Anaplasma marginale, we hypothesized a similar event in A. phagocytophilum in vivo, with specific Msp2 expression triggering immunopathologic injury or clinical manifestations of disease. We examined msp2 transcripts in 11 B6 mice and 6 horses inoculated with low- or high-passage A. phagocytophilum Webster strain. Blood was sequentially obtained through 3 weeks postinfection for msp2 reverse transcription-PCR. Horses were additionally assessed for clinical manifestations, seroconversion, complete blood count, blood chemistry, and cytokine gene transcription. In both species, there was no consistent emergence of msp2 transcripts, and all 22 msp2 variants were detected in both passage groups. Clinical severity was much higher for high-passage-infected than for low-passage-infected horses, preceded by higher levels of blood gamma interferon transcription on day 7. Antibody was first detected on day 7, and all horses seroconverted by day 22, with a trend toward lower antibody titers in low-passage-infected animals. Leukocyte and platelet counts were similar between experimental groups except on day 13, when low-passage-infected animals had more profound thrombocytopenia. These findings corroborate studies with mice, where msp2 diversity did not explain differences in hepatic histopathology, but differ from the paradigm of low-passage A. phagocytophilum causing more significant clinical illness. Alteration in transcription of msp2 has no bearing on clinical disease in horses, suggesting the existence of a separate proinflammatory component differentially expressed with changing in vitro passage.
Human granulocytic anaplasmosis (HGA) is a tick-borne disease caused by Anaplasma phagocytophilum. A. phagocytophilum strains from different geographic regions vary in their antigenic content (1, 11). Antigenic variability results chiefly from differential expression of immunodominant surface proteins encoded by a multigene family (msp2, p44) characterized by conserved sequences flanking a hypervariable region (HVR) which is predicted to contain B-cell and T-cell epitopes (8, 24). The related MSP2 antigenic variation of Anaplasma marginale leads to persistent, relapsing infection in ruminants, a feature not present in mammals or in humans who develop febrile infection with A. phagocytophilum. For A. phagocytophilum, Msp2 may have a role as an adhesin to bind to mammalian cells (19).
A. phagocytophilum msp2 transcription and expression change with increasing lengths of in vitro propagation (24). Horses experimentally infected by A. phagocytophilum passaged in vitro develop clinical manifestations either typical of natural virulent disease when infected with low-passage bacteria or with significantly diminished clinical signs and laboratory features when infected with high-passage bacteria (20). In contrast, the murine model of HGA is imperfect since infection does not cause clinical signs in mice, yet the development of histopathologic lesions, such as inflammatory liver lesions that mimic those in infected humans and horses, indicates that this model is an important tool for investigating A. phagocytophilum pathogenesis (4, 7, 14). Moreover, histopathologic lesions in mice are directly linked to the production of gamma interferon (IFN-γ) and not to bacterial load (15), and Msp2 is the major immunological target of the host immune response (1, 13). In this study comparing two different animal models, we investigated whether there is a specific pattern of programmed Msp2 expression and whether that pattern differs between high- and low-passage infection in the mouse and horse models of HGA by analyzing transcript diversity and kinetics. In addition, whether programmed Msp2 expression correlates with clinical disease in the equine model of HGA was examined.
MATERIALS AND METHODS
Cultivation of A. phagocytophilum in vitro.
A. phagocytophilum was maintained in RPMI 1640 medium supplemented with 5% fetal bovine serum and 2 mM l-glutamine until approximately 90% of the cells contained morulae. Cultures were coordinated so that low-passage (passage 7 to 8) and high-passage (passage 17 to 22) materials were available for inoculation. On the day of inoculation, infected and uninfected HL-60 cells were centrifuged (200 × g, 10 min) to concentrate the cells, and then the cell pellet was resuspended in serum-free RPMI 1640 medium.
Horses and clinical assessment.
Six horses were selected for infection with A. phagocytophilum. All horses were screened at baseline and verified to be seronegative for A. phagocytophilum and to lack A. phagocytophilum DNA in blood by quantitative PCR. Horses were infected intravenously with 1 × 106 A. phagocytophilum Webster strain-infected HL-60 cells. Three horses were infected with low-passage inoculum (<10 in vitro passages) and three with high-passage inoculum (20 to 26 in vitro passages). Blood was obtained from the horses on days 0, 2, 5, 7, 9, 13, 14, 15, 16, 20, and 22 by jugular vein venipuncture. Physical examination, complete blood count and serum chemistry, and body temperature were measured at these intervals to characterize clinical responses to infection. Clinical parameters that were examined and scored included ataxia, ranging from mild lack of coordination to recumbency (grade 1 to 5); lethargy, ranging from slightly quiet and less responsive to obtunded (grade 1 to 4); limb swelling, ranging from slight congestion above the fetlock to edema above and below the carpus and hock (grade 1 to 4); reluctance to move, ranging from slow but walks with leading to no walking even with leading (grade 1 to 4); and presence of petechiae (number observed on oral mucosa).
Mice.
Eleven C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were maintained in a barrier facility and received food and water ad libitum. Baseline blood samples were taken from each mouse on day 0. Mice (four in each group) were then inoculated intraperitoneally with 1 × 106 HL-60 cells highly infected with low-passage (passage 5) or high-passage (passage 26) A. phagocytophilum. Three mice served as controls and received 1 × 106 uninfected HL-60 cells. Survival blood sampling was then performed on days 2, 7, 10, 14, and 21 by facial vein puncture, except for on day 21, when samples were acquired by terminal cardiac puncture. Mice were evaluated for clinical signs such as ruffled fur, reduced activity, failure to eat or drink, a hunched posture, and lack of interaction with cage mates.
Serology.
Antibody responses were detected in serum samples of horses as has been previously described (20). Briefly, A. phagocytophilum antibodies were detected by an indirect immunofluorescent-antibody assay using cultured A. phagocytophilum as the antigen, and the cutoff titer for a positive serological response was ≥25. Antibody responses in mice were not determined since the small serial samples taken were used exclusively for RNA extraction, with little serum left for adequate serological measurements.
RNA preparation and RT-PCR for determination of bacterial load and equine cytokine transcript quantitation.
Total cellular RNA was isolated using the QIAamp RNA blood minikit (Qiagen Inc., Valencia, CA) from whole blood of low- and high-passage A. phagocytophilum-infected and mock-infected (uninfected HL-60 cell-inoculated) mice on days 2, 7, 14, and 21 postinfection and from horses on days 0, 2, 5, 7, 9, 13, 14, 15, 16, 20, and 22. Blood was either used immediately or placed in RNAlater per the manufacturer's recommendations (Ambion, Austin, TX). For horses, RNA was used directly in quantitative reverse transcriptase 5′ nuclease assays for analysis of equine cytokine mRNA and for 22 A. phagocytophilum msp2 variants (8, 24), with the primers, probes, and GenBank accession numbers listed in Table 1. Transcripts for msp2 were quantitated using a standard curve determined from plasmids for each of the 22 variants examined. Equine cytokine transcripts analyzed included interleukin-6 (IL-6), IL-8, IL-10, IL-12B (IL-12 p40 gene), IL-18, IFN-γ, CCL5 (RANTES gene), and SLC11A1 (nRAMP gene) (21, 22). For mice, reverse transcription-PCR (RT-PCR) was performed with Superscript one-step RT-PCR and Platinum Taq (Invitrogen) with 30 ng RNA according to the instructions of the manufacturer. Broad-range primers for amplification of all msp2 cDNAs were used as previously described (8, 24). The reactants were subjected to electrophoresis in 1% agarose gels to confirm the presence of a 550-bp amplicon as visualized by ethidium bromide staining.
TABLE 1.
PCR primer and TaqMan probe sequences for determination of msp2 HVRs by real-time RT-PCR
| msp2 variant | Primer sequences (5′→3′) | Product length (bp) | Probe sequence (5′→3′) | Accession no. |
|---|---|---|---|---|
| RWMSP11 | TCCACTATCGATGGGAAGGTTTG | 84 | TGACAAAGAAGGGAAACGATACTCATGCG | AF443403 |
| CGTCTTATCTGCATATACTCCATGACTT | ||||
| WMSP9 | CATCCTAGTATTGATGGCAAGGTTTG | 106 | CACTAGTAACTATGGAGCGTATGCTGCGACAA | AF443412 |
| CATCGCTCGTTTTGCTAGCA | ||||
| WMSP4 | TTCCGATATTGATGGGAAGATTTG | 107 | CTGCGGATAATTTTGGGAAGTATGGCG | AF443407 |
| TGGTCGTACTATCCGTAGCTATATCAG | ||||
| WMSP6 | CCGAGATTGATGGGAAGATTTG | 86 | CGGAAGGCTGGTGACAGTAGCGGCA | AF443411 |
| ATCCGTTTCTTCCCCATACTTG | ||||
| WMSP16 | CTACGATTGGGGACAAGGTTTG | 95 | CGATACGGCCAAAAAAGATCATTATGCAAA | AF443402 |
| TGCACTTCCCTTGTCAGTAGTCTT | ||||
| WMSP19 | CCTACCATTGATGGGAAGGTTTG | 95 | TGGACATAGTACACCAACGACGTTAACTGCC | AF443404 |
| ACGTCTGACTCTACAGCGTACTTACC | ||||
| WMSP5 | GCTCCTAAGATCGATAAGCAAGTTTG | 104 | AGCGGAACAAGATATGCTAAGTACCTCGAAGAAG | AF443409 |
| CCAGCATTGCTAGACGTTCCA | ||||
| WMSP10 | ATCCTAATATCGATGGGAAGGTTTG | 94 | AGCACGAACTACGCGAAATATGTAGAGGTAACG | AF443400 |
| CCATCGTTGCTTCCGCTTT | ||||
| RWMSP25 | CCTGAAATTGATGGGAAGGTTTG | 135 | CGGTAGGTACAGCGCAGGGAAAGGAG | AF443397 |
| GCTTTTATTCAGCCCACTACACTGT | ||||
| WMSP2 | GCCATCGATGGGAAGGTTTG | 71 | ACTGGTAGCCATGCTGACCTAGCGCC | AF443405 |
| AACTTTTTCCCCGCATTCGT | ||||
| WMSP45 | CACTCTAATATCGATGGGAAGGTTTG | 84 | AGGAGGGAAAAGCATGGGAGTCAAGGT | AF443408 |
| ATCACACGAACCTGCTTTGGT | ||||
| WMSP15 | CCTACCATCGATGGTAAGGTTTGT | 77 | TGGCGGAAAAGGCTCAGGGCA | AF443401 |
| GCAAACGTCGATGGGCTACT | ||||
| RWMSP57 | CATTCCGGTATTGATAAGAAGGTTTG | 121 | AGATAATGGCTCACTGGCCGACTATACGG | AF443398 |
| GAGCCGTCTTATTCGTCTGTGA | ||||
| WMSP3 | TTTCTCATCCTAGTATTGATAAGAAGGTTTG | 86 | TCATGCGAAAGGAGGAGCGGATAATACG | AF443406 |
| AGCGTTACCCCGTATTTTTCC | ||||
| WMSP52 | ATCCCGGTATTGATAAGAAGGTTTG | 87 | CGGGAGCGACGGCGGGAGTAATATA | AF443410 |
| TCGGGTCAGCCGCATAAG | ||||
| WMSP1 | ATCCTAATATCGATGGGAAGGTTTG | 94 | CACACGAGTGCGGATAGCTACGGTGTG | AF443399 |
| CTCGCCTGGCCTGTTAACTC | ||||
| WMSP17 | TCCCGGTATTGATAAGAAGGTTTG | 85 | ACGAAAGCCCAAAGTTCTGGAAAGTATGGTAA | AF443396 |
| CTTCGTACCGGTTTTATCAGCAT | ||||
| WMSP13 | TTCCGAGATTGGTAAGAAGGTTTG | 92 | CGACCCCGAGCACGTTTGGAACATAT | AF443413 |
| AGCCCACAGGTGTTGAATCAG | ||||
| RW17 | ATTCCGAGATTGGTAAGAAGGTTTG | 85 | TGACGAAGAATTACGACAGTGGGAGCAA | AF412831 |
| CCATTCGATTCTGTCCCGTACT | ||||
| RW1 | CACCCCGATATTAATAAGAAGGTTTG | 102 | CGAGGAAGGACAGTGGTGGTACTAGATATGCG | AY253530 |
| TTCAGGGTTGCTGCTCTTATTAGTC | ||||
| RW23 | CCGGTATTGGTAAGAAGGTGTGTG | 84 | AGCGGAAGGATGGTGATACTACGAACAGG | AF135262 |
| CCGCACCAACTATATACTTTGCAA | ||||
| RW20 | CGGCATTGGTAAGAAGGTTTGT | 125 | CAATTTATATGCCGTTTATGCTGAGAGGACGG | AY064524 |
| CAGCACACACAGCCACTTTTC |
Cloning and sequencing of RT-PCR products from mice.
RT-PCR products were cloned into the pGEM-T Easy vector (Promega, Madison, WI), followed by transformation into Escherichia coli DH5α, and then plated onto LB agar containing 50 μg/ml ampicillin. Plasmids were purified (Wizard SV 96 plasmid DNA purification system; Promega) and assessed for insert size after EcoRI digestion, and when an insert of the appropriate size insert (∼550 bp) was detected, 10 to 20 from each cloning reaction were sequenced to compare at least 10 msp2 transcripts from each mouse at each interval. All sequences were aligned with A. phagocytophilum Webster strain msp2 references (18, 24) using ClustalX (25). The numbers of clones that aligned with each reference msp2 or into a new clade were identified to document the diversity of msp2 transcription that emerged as infection progressed in vivo with reference to low- or high-passage bacteria.
Statistical analysis.
Chi-square analysis was conducted to compare clinical data from low- and high-passage-infected horses and the expected distribution of clinical signs. All other statistical analyses comparing continuous data between both low- and high-passage groups were analyzed using Student's t test, with significance set at a P value of <0.05.
RESULTS
Equine studies.
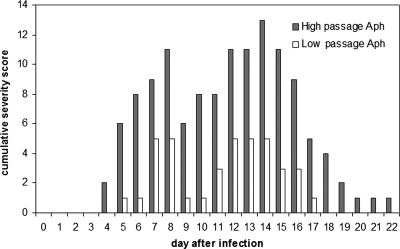
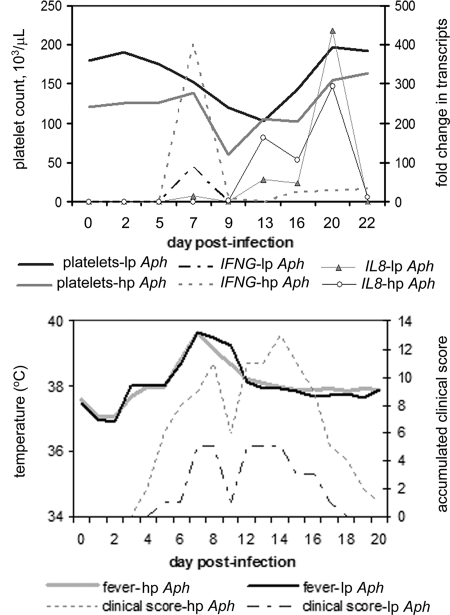
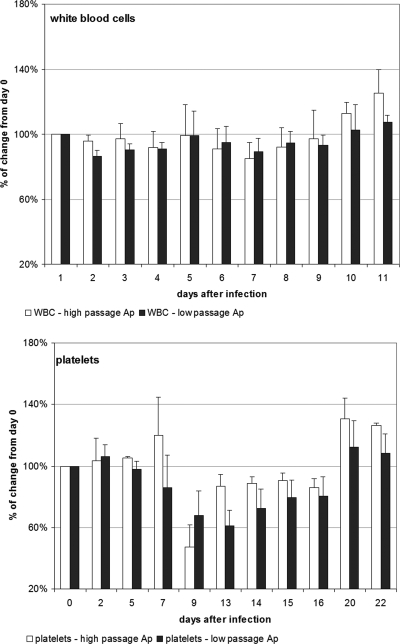
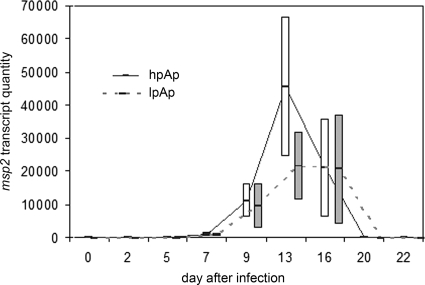
A. phagocytophilum morulae were detected in equine neutrophils at between days 13 and 16 postinfection, a result which was similar for both low- and high-passage-infected groups. Body temperatures started to increase at day 6 after infection and peaked between days 7 and 11. At day 15 body temperatures returned to baseline values. There were no differences noted in fever between the two infected groups. Antibody was first detected on day 7 for most horses in the low- and high-passage groups, and all seroconverted by day 22, with antibody titers reaching between 400 and 600 (Table 2). There was also a trend (P = 0.1, two-sided Student's t test) toward a lower antibody titer in low-passage-inoculated horses (geometric mean of 400, versus 1,600 in high-passage-inoculated horses). Accumulated clinical scores reached a peak of 5 for low-passage-inoculated horses, compared to 13 in high-passage-inoculated horses (Fig. 1). Clinical signs were most prominent between days 5 and 18, with high-passage-inoculated horses still exhibiting clinical signs through day 22 (Fig. 1). There was also a bimodal pattern exhibited by both groups, in which the first peak of clinical manifestations occurred around day 7 to 9 and the second peak occurred during days 13 to 16 (Fig. 2). In contrast to our prior work, the more severe clinical manifestations (P < 0.001, χ2 test) were observed in high-passage A. phagocytophilum-infected animals. Both clinical manifestations and thrombocytopenia, noted to begin predominantly around day 9 (Fig. 2), were preceded by elevated blood IFN-γ transcript levels on day 7, which were significantly higher in high-passage-infected horses than in low-passage-infected horses (P = 0.045, Student's t test) (Fig. 2). The elevations in IFN-γ transcript levels on day 7 also corresponded to the initiation of fever in these animals (Fig. 2). In concert with previous observations (20), when results were normalized to starting platelet counts, low-passage-infected horses were found to develop a more profound thrombocytopenia than high-passage-infected animals, but only on day 13 (P < 0.05, Student's t test) (Fig. 3). There were also increasing levels of IL-8 transcripts in high- and low-passage A. phagocytophilum-infected animals at between 2 and 3 weeks postinfection, rising to the highest levels at day 20, although levels did not differ between these groups (Fig. 2).
TABLE 2.
Equine A. phagocytophilum antibody titers determined by indirect immunofluorescent-antibody test
| Group | Titers (geometric mean titer) on the following day postinfection:
|
||
|---|---|---|---|
| 0 | 7 | 22 | |
| Low-passage infection | <25, <25, <25 (<25) | <25, 25, 100 (31) | 1,600, 400, 400 (635) |
| High-passage infection | <25, <25, <25 (<25) | <25, 25, 400 (50) | 1,600, 1,600, 1,600 (1,600) |
FIG. 1.
Cumulative severity scores for both low- and high-passage-infected horses, with three animals represented in each group. Clinical parameters that were examined and included in the severity score were ataxia (grade 1 to 5), lethargy (grade 1 to 4), limb swelling (grade 1 to 4), reluctance to move (grade 1 to 4), and presence of petechiae (number observed on oral mucosa). Aph, A. phagocytophilum.
FIG. 2.
(Top) Curves for low- and high-passage-infected horses, with fold change in IFN-γ and IL-8 transcription plotted with platelet counts to demonstrate that thrombocytopenia is preceded by increasing IFN-γ but not IL-8 transcription. lp Aph and hp Aph, low- and high-passage A. phagocytophilum infection, respectively. (Bottom) Curves for low- and high-passage-infected horses, with fever plotted against accumulated clinical scores. Note the increase in clinical scores and fever at days 7 to 10. A second peak in clinical score severity occurred between days 12 and 16, which was unrelated to fever but coincided with the presence of morulae between days 13 and 16 followed by a peak in IL-8 transcription (see Results).
FIG. 3.
When results were normalized to starting platelet counts, low-passage-infected horses developed a more profound thrombocytopenia than high-passage-infected animals, but only on day 13 (P < 0.05, Student's t test). No significant differences were noted in the white blood cell (WBC) count change between low- and high-passage-infected horses throughout the entire experimental period. Ap, A. phagocytophilum. Error bars indicate standard errors of the means.
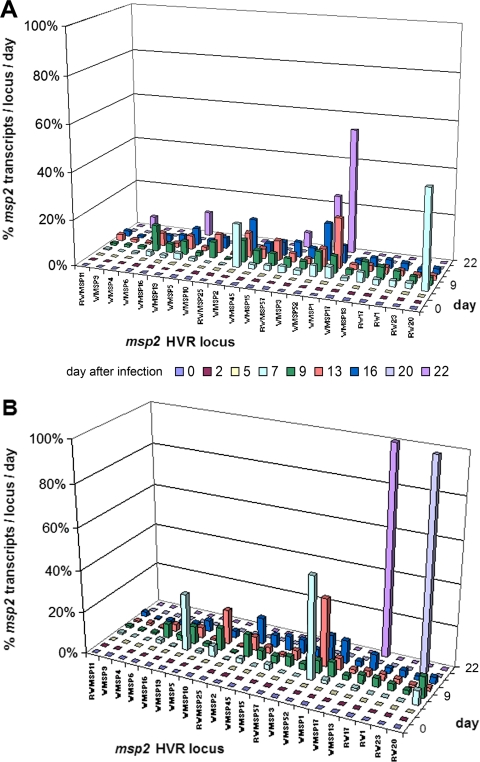
Multiple HVR transcripts were detected in each infected horse at each time interval. Between days 7 and 16, when the majority of msp2 transcripts were detected, the diversity was very broad (Fig. 4A and B). The total number of transcripts detected, as a reflection of bacterial load, was not different between low-passage- and high-passage-infected horses (P > 0.05) (Fig. 5). The differences in clinical findings could not be attributed to bacterial load or msp2 transcript diversity, which varied considerably among all six low- and high-passage-inoculated horses; however, when assessed by group, there appeared to be a relationship between increased bacterial numbers and the second clinical score peak observed in both groups.
FIG. 4.
Diversity of msp2 gene transcription of 22 variants in the peripheral blood of horses infected with high-passage (A) or low-passage (B) A. phagocytophilum. Transcription became evident by day 7 in both groups, peaked between days 13 and 16, and was mostly resolved by day 22. Of the 22 msp2 variants examined, all were detected in both groups of horses, and the patterns of transcription appeared to be similar.
FIG. 5.
Mean quantities of msp2 transcripts in low- and high-passage-infected horses through day 22 after infection. Note the similarity in emergence of transcripts between days 7 and 20, with a greater number of transcripts in high-passage- than in low-passage-infected horses. This reflects the disproportionate emergence of a few msp2 transcripts in high-passage- compared to low-passage-infected animals at day 13. Bars represent standard errors of the means.
Analysis of msp2 transcripts in mice.
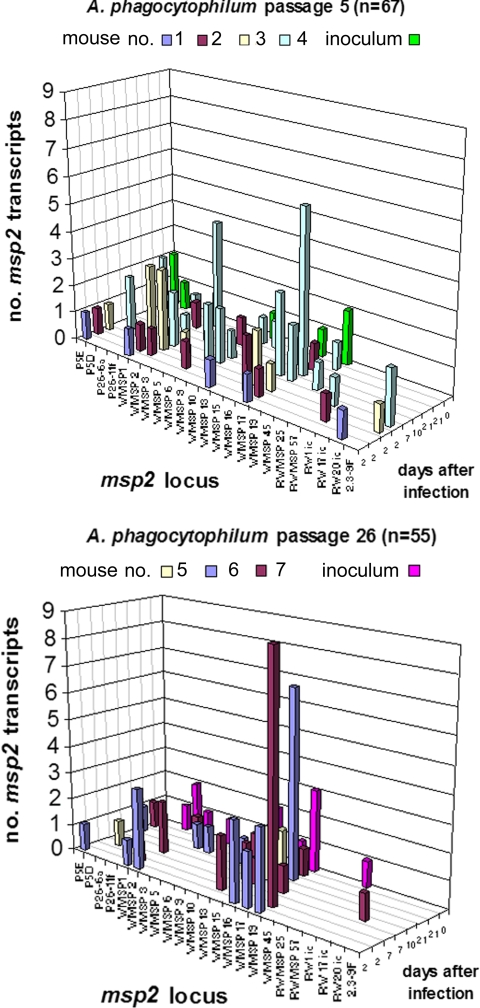
From among the msp2 HVR amplicons after reverse transcription, 67 plasmid clones were generated from the low-passage A. phagocytophilum-infected mice and 8 clones from the low-passage inoculum prior to infection; 55 plasmid clones were similarly derived from high-passage A. phagocytophilum-infected mice and 12 from the high-passage inoculum prior to infection (Fig. 6). Four unique transcripts emerged from the initial inocula, including two from each passage. After infection in mice, msp2 transcripts were detected on days 2 through 21. As shown in Fig. 6, the most frequent transcripts were WMSP1 (low passage), WMSP2 (low passage), WMSP16 (low and high passages), WMSP17 (low passage), and WMSP19 (high passage), each comprising more than 10% of the total number of transcript clones. In contrast, in horses WMSP1 (high passage), WMSP2 (low and high passages), WMSP10 (low passage), and WMSP52 (low and high passage) comprised more than 10% of the total copy number detected. No reproducible pattern of msp2 transcription was found among mice, among inocula, or over time. Among mice in both the low- and high-passage groups, a total of 29 unique msp2 transcripts were detected. Each mouse had multiple (one to eight) msp2 transcripts representing heterogeneous populations of A. phagocytophilum. Some variants dominated the population (>50%) in individual animals, although these variants did not emerge with any pattern and occurred at different times. In the inocula, only two variants were shared between low and high passage, and the transcript repertoire present in both inocula was not reproduced in mice over the 21-day study.
FIG. 6.
msp2 transcript diversity found in mice inoculated with passage 5 and passage 26 Webster strain A. phagocytophilum. Note the lack of programmed transcript emergence within and among mice over time. Each mark for “days after infection” represents a different single mouse at that specific time point.
DISCUSSION
In both the mouse and equine models of HGA, A. phagocytophilum msp2 transcription displays a high degree of diversity that emerges within 2 days in mice and by day 7 in horses and lasts through 21 to 22 days. The rapidity of variant emergence and lack of consistent variant emergence between and within animals suggest that msp2 gene recombination is not driven by adaptive or innate immunity and is instead a random and inherent property of the organism. The expression of a large repertoire of variants of A. phagocytophilum msp2 was also noted to occur within ticks and among various strains of A. phagocytophilum (3, 12), and this diversity also occurs at different time points of infection (2) and between mammalian and tick host cells (26). The benefit of diverse variants is the generation of heterogeneous bacterial populations, which ensure survival of the bacteria in the bloodstreams of various mammalian hosts until they are acquired in the tick blood meal. Additional diversity in ticks suggests a role in that host as well.
A. phagocytophilum msp2 transcription displayed a high degree of diversity in both experimental groups of horses, and this diversity in transcription of msp2 had no bearing on clinical disease in horses. This observation suggests the existence of a separate proinflammatory component differentially expressed with changing in vitro passage (10). These findings corroborate studies in mice, in which msp2 diversity did not explain differences in T-cell responses or differences in hepatic histopathology, a disease correlate (9). This discrepancy between msp2 expression and clinical disease in A. phagocytophilum differs from the case for its relative A. marginale, in which its MSP2 has properties, such as segmental gene conversion, that contribute to specific CD4+ T-cell epitopes resulting in strong IFN-γ responses, clinical disease, and clearing of infection by macrophage activation (6).
Although no model is ideal for HGA investigation, given the clinical and pathological similarities to human disease, the distribution and kinetics of infection, and knowledge of the effects of the transmission vector and route, the horse provides many desirable attributes not attained with other models. It is clear that hypotheses generated from other models greatly benefit by validation in the horse. For example, we previously examined and demonstrated that IFN-γ plays a critical role in induction of inflammatory histopathology (15, 16). Although we did not use a histopathological correlate in horses, we demonstrated that IFN-γ expression in infected horses parallels that observed in the murine model and potentially plays a role in clinical manifestations and perhaps hematologic abnormalities such as platelet number and function (5). In this study, increased IFN-γ expression on day 7 corresponded with fever and clinical disease severity and immediately preceded thrombocytopenia in high-passage-infected horses and to a lesser extent in low-passage-infected horses (Fig. 2), as previously observed in mice (15, 16); a similar phenotype of increased cytokine expression and clinical severity observed has also been observed in a canine model of HGA (unpublished data). Increased IL-8 expression has not been shown to correlate with disease severity in HGA, although IL-8 plays a role in A. phagocytophilum infection and propagation (23) and ultimately HGA pathogenesis.
In both the murine and equine models, we also clearly demonstrated that there is a lack of a specific and reproducible program for A. phagocytophilum msp2 expression in animals inoculated with both low- and high-passage bacteria. The unique feature of this comparative study is that each animal was followed from inoculation for 21 to 22 days, when bacteremia or clinical signs subsided. Over this interval, msp2 variant emergence was unique for each individual and each species studied. Although we previously demonstrated a lack of programmed msp2 expression in mice inoculated with low- and high-passage organisms (9), the prior study design allowed for evaluation of transcript diversity only at various intervals in individual cohorts of mice per interval. The advantage of the current approach is the ability to sequentially evaluate transcript emergence within individual animals over time.
The evidence presented in this paper refutes our original hypothesis and supports the concept that changing Msp2 expression has little or no impact on clinical disease outcome and that passage in vitro, associated with Msp2 expression changes, is not followed by a specific repertoire of msp2 transcripts during infection. This is in contrast to the case for A. marginale, among other Anaplasmataceae, which establishes persistent infections and likely relies on changing pfam01617 surface antigen 2 expression to generate antigenic diversity and immune evasion (17). We propose to continue studies to elucidate the antigenic triggers that influence proinflammatory responses and that ultimately influence clinical outcomes and histopathologic severity. Once these are better understood, the goal of developing appropriate treatment regimens for patients and animals with granulocytic anaplasmosis will be more easily addressed.
Acknowledgments
We thank Dennis J. Grab for his assistance in preparing the manuscript.
This study was funded by NIAID grant R01AI41213 (to J.S.D.).
Footnotes
Published ahead of print on 19 December 2007.
REFERENCES
- 1.Asanovich, K. M., J. S. Bakken, J. E. Madigan, M. Aguero-Rosenfeld, G. P. Wormser, and J. S. Dumler. 1997. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J. Infect. Dis. 176:1029-1034. [DOI] [PubMed] [Google Scholar]
- 2.Barbet, A. F., A. M. Lundgren, A. R. Alleman, S. Stuen, A. Bjoersdorff, R. N. Brown, N. L. Drazenovich, and J. E. Foley. 2006. Structure of the expression site reveals global diversity in MSP2 (P44) variants in Anaplasma phagocytophilum. Infect. Immun. 74:6429-6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbet, A. F., P. F. Meeus, M. Belanger, M. V. Bowie, J. Yi, A. M. Lundgren, A. R. Alleman, S. J. Wong, F. K. Chu, U. G. Munderloh, and S. D. Jauron. 2003. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect. Immun. 71:1706-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borjesson, D. L., and S. W. Barthold. 2002. The mouse as a model for investigation of human granulocytic ehrlichiosis: current knowledge and future directions. Comp. Med. 52:403-413. [PubMed] [Google Scholar]
- 5.Borjesson, D. L., J. L. Brazzell, and R. Feferman. 2005. Platelet dysfunction after association with Anaplasma phagocytophilum in vitro. Ann. N.Y. Acad. Sci. 1063:413-415. [DOI] [PubMed] [Google Scholar]
- 6.Brown, W. C., K. A. Brayton, C. M. Styer, and G. H. Palmer. 2003. The hypervariable region of Anaplasma marginale major surface protein 2 (MSP2) contains multiple immunodominant CD4+ T lymphocyte epitopes that elicit variant-specific proliferative and IFN-gamma responses in MSP2 vaccinates. J. Immunol. 170:3790-3798. [DOI] [PubMed] [Google Scholar]
- 7.Bunnell, J. E., E. R. Trigiani, S. R. Srinivas, and J. S. Dumler. 1999. Development and distribution of pathologic lesions are related to immune status and tissue deposition of human granulocytic ehrlichiosis agent-infected cells in a murine model system. J. Infect. Dis. 180:546-550. [DOI] [PubMed] [Google Scholar]
- 8.Caspersen, K., J. H. Park, S. Patil, and J. S. Dumler. 2002. Genetic variability and stability of Anaplasma phagocytophila msp2 (p44). Infect. Immun. 70:1230-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi, K. S., D. G. Scorpio, N. C. Barat, and J. S. Dumler. 2007. Msp2 variation in Anaplasma phagocytophilum in vivo does not stimulate T cell immune responses or interferon-gamma production. FEMS Immunol. Med. Microbiol. 49:374-386. [DOI] [PubMed] [Google Scholar]
- 10.Choi, K. S., and J. S. Dumler. 2007. A mitogenic component in polar lipid-enriched Aanaplasma phagocytophilum membranes. Clin. Vaccine Immunol. 14:1260-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumler, J. S., K. M. Asanovich, J. S. Bakken, P. Richter, R. Kimsey, and J. E. Madigan. 1995. Serologic cross-reactions among Ehrlichia equi, Ehrlichia phagocytophila, and human granulocytic Ehrlichia. J. Clin. Microbiol. 33:1098-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felek, S., S. Telford III, R. C. Falco, and Y. Rikihisa. 2004. Sequence analysis of p44 homologs expressed by Anaplasma phagocytophilum in infected ticks feeding on naive hosts and in mice infected by tick attachment. Infect. Immun. 72:659-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ijdo, J. W., Y. Zhang, E. Hodzic, L. A. Magnarelli, M. L. Wilson, S. R. Telford III, S. W. Barthold, and E. Fikrig. 1997. The early humoral response in human granulocytic ehrlichiosis. J. Infect. Dis. 176:687-692. [DOI] [PubMed] [Google Scholar]
- 14.Lepidi, H., J. E. Bunnell, M. E. Martin, J. E. Madigan, S. Stuen, and J. S. Dumler. 2000. Comparative pathology, and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am. J. Trop. Med. Hyg. 62:29-37. [DOI] [PubMed] [Google Scholar]
- 15.Martin, M. E., J. E. Bunnell, and J. S. Dumler. 2000. Pathology, immunohistology, and cytokine responses in early phases of human granulocytic ehrlichiosis in a murine model. J. Infect. Dis. 181:374-378. [DOI] [PubMed] [Google Scholar]
- 16.Martin, M. E., K. Caspersen, and J. S. Dumler. 2001. Immunopathology and ehrlichial propagation are regulated by interferon-gamma and interleukin-10 in a murine model of human granulocytic ehrlichiosis. Am. J. Pathol. 158:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noh, S. M., K. A. Brayton, D. P. Knowles, J. T. Agnes, M. J. Dark, W. C. Brown, T. V. Baszler, and G. H. Palmer. 2006. Differential expression and sequence conservation of the Anaplasma marginale msp2 gene superfamily outer membrane proteins. Infect. Immun. 74:3471-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 19.Park, J., K. S. Choi, and J. S. Dumler. 2003. Major surface protein 2 of Anaplasma phagocytophilum facilitates adherence to granulocytes. Infect. Immun. 71:4018-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pusterla, N., J. E. Madigan, K. M. Asanovich, J. S. Chae, E. Derock, C. M. Leutenegger, J. B. Pusterla, H. Lutz, and J. S. Dumler. 2000. Experimental inoculation with human granulocytic Ehrlichia agent derived from high- and low-passage cell culture in horses. J. Clin. Microbiol. 38:1276-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pusterla, N., W. D. Wilson, P. A. Conrad, B. C. Barr, G. L. Ferraro, B. M. Daft, and C. M. Leutenegger. 2006. Cytokine gene signatures in neural tissue of horses with equine protozoal myeloencephalitis or equine herpes type 1 myeloencephalopathy. Vet. Rec. 159:341-346. [DOI] [PubMed] [Google Scholar]
- 22.Pusterla, N., W. D. Wilson, P. A. Conrad, S. Mapes, and C. M. Leutenegger. 2006. Comparative analysis of cytokine gene expression in cerebrospinal fluid of horses without neurologic signs or with selected neurologic disorders. Am. J. Vet. Res. 67:1433-1437. [DOI] [PubMed] [Google Scholar]
- 23.Scorpio, D. G., M. Akkoyunlu, E. Fikrig, and J. S. Dumler. 2004. CXCR2 blockade influences Anaplasma phagocytophilum propagation but not histopathology in the mouse model of human granulocytic anaplasmosis. Clin. Diagn. Lab. Immunol. 11:963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scorpio, D. G., K. Caspersen, H. Ogata, J. Park, and J. S. Dumler. 2004. Restricted changes in major surface protein-2 (msp2) transcription after prolonged in vitro passage of Anaplasma phagocytophilum. BMC Microbiol. 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, X., Z. Cheng, C. Zhang, T. Kikuchi, and Y. Rikihisa. 2007. Anaplasma phagocytophilum p44 mRNA expression is differentially regulated in mammalian and tick host cells: involvement of the DNA binding protein ApxR. J. Bacteriol. 189:8651-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]