Abstract
Dendritic cells are the only antigen-presenting cells that can present exogenous antigens to both helper and cytolytic T cells and prime Th1-type or Th2-type cellular immune responses. Given their unique immune functions, dendritic cells are considered attractive “live adjuvants” for vaccination and immunotherapy against cancer and infectious diseases. The present study was carried out to assess whether the reinjection of autologous monocyte-derived dendritic cells loaded with an aldithriol-2-inactivated primary isolate of feline immune deficiency virus (FIV) was able to elicit protective immune responses against the homologous virus in naive cats. Vaccine efficacy was assessed by monitoring immune responses and, finally, by challenge with the homologous virus of vaccinated, mock-vaccinated, and healthy cats. The outcome of challenge was followed by measuring cellular and antibody responses and viral and proviral loads and quantitating FIV by isolation and a count of CD4+/CD8+ T cells in blood. Vaccinated animals exhibited clearly evident FIV-specific peripheral blood mononuclear cell proliferation and antibody titers in response to immunization; however, they became infected with the challenge virus at rates comparable to those of control animals.
Dendritic cells (DCs) are the only antigen-presenting cells that are able to present exogenous antigens to both helper and cytolytic T cells, prime naive T cells, and skew the immune response toward Th1 type or Th2 type. In order to do so, DCs must undergo maturation after the uptake of antigen, a process that can be started by several stimuli, such as inflammatory cytokines and microbial products like lipopolysaccharide (LPS) (20, 23). Once mature, DCs can reach draining lymph nodes, where they present antigen and express the necessary molecules to start an immune response. Nowadays, DCs can easily be generated in culture by differentiating monocytes from peripheral blood in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). Therefore, given their unique immune functions, DCs are considered attractive “live adjuvants” for vaccination and immunotherapy against cancer and infectious diseases (25).
Although culture conditions for the generation of monocyte-derived DCs (MDCs) have not been standardized so far, a general consensus on several points has been reached to generate MDCs for clinical use in humans (2). MDCs should be grown in the absence of xenogeneic proteins, such as those present in fetal calf serum (FCS) or others. MDCs should be mature to exert their functions once reinjected in vivo, since immature DCs are tolerogenic (23). Most studies using MDCs as vehicles and adjuvants to induce an immune response against viruses have been carried out with already infected patients or animals (11, 12). Lu and colleagues have reported that autologous MDCs pulsed with aldithriol-2 (AT2)-inactivated whole human immunodeficiency virus 1 (HIV-1) are promising means to treat chronic HIV-1 infection (12). Unfortunately, very few studies have addressed the ability of DCs to induce adaptive immunity against subsequent viral infection in healthy subjects (10, 21) and, to our knowledge, only one of these has been performed to attempt the prevention of lentiviral infection (9). In the latter study, autologous MDCs loaded with simian immunodeficiency virus peptides from Tat, Rev, and Env induced a distinct cellular immune response in monkeys but failed to control challenge inoculation with the homologous virus.
Feline immunodeficiency virus (FIV) is a nonprimate lentivirus that has long been studied as a model for HIV (22). The infection it establishes in domestic cats resembles human AIDS, causing progressive immune deficiency; therefore, it is considered a model to test possible vaccination strategies against HIV-1. Vaccines consisting of inactivated virus and/or inactivated virus-infected cells have been shown to provide some protection against homologous and, sometimes, against heterologous challenges as well (7, 14, 16, 19, 27). However, the protective effects exerted have shown strong limitations or have proved difficult to reproduce (1, 5, 15). Thus, improved immunogens are actively searched for.
We have recently described a protocol to generate feline MDCs in the absence of xenogeneic protein for the very purpose of using them as cell substrate to vaccinate cats against FIV (3). The present study was carried out to assess whether MDCs loaded with AT2-inactivated FIV (FIV-MDCs) are able to elicit protective immune responses against the homologous virus in naive cats. This immunization strategy elicited clearly evident FIV-specific peripheral blood mononuclear cell (PBMC) proliferation and antibody, yet the cats showed no evidence of increased resistance to challenge with the homologous virus.
MATERIALS AND METHODS
Media and reagents.
The cell culture medium used was RPMI 1640 supplemented with 3% feline plasma, unless otherwise stated, 2 mM l-glutamine, 1% nonessential amino acids, and 50 μg/ml gentamicin. Recombinant feline IL-4 and recombinant feline GM-CSF were purchased from R&D Systems (Minneapolis, MN).
Animals, cells, and culture conditions.
Specific-pathogen-free female cats were bought from IFFA Credo (L'Arbresle, France) after they had been vaccinated against rabies, and they were kept in our climate-controlled animal facility under conditions required by the European Community Law. The cats were enrolled for this study when they were 2 years old. Heparinized jugular venous blood was obtained under light anesthesia. The IL-2-dependent MBM cell line has previously been described (13). In the present study, these cells were grown in feline plasma obtained by heparin, as pools from healthy donors from our animal facility, instead of in FCS.
Preparation of AT2-inactivated virus.
The Pisa-M2 isolate of FIV (FIV-M2) was selected for the study because it has been passed a limited number of times in tissue cultures and presents growth and neutralization features typical of wild-type lentiviruses (14). The virus used to load MDCs was produced in MBM cells growing in 3% cat plasma and inactivated by incubation with AT2 at a final concentration of 300 μM at 4°C for 2 h, as described previously (5). The virus thus treated was then concentrated and purified on a sucrose gradient for 3 h at 15,000 × g. A single stock (AT2-FIV) was prepared, characterized, and used throughout the study: it contained 800 μg/ml protein.
Preparation and analysis of FIV-MDCs.
PBMCs were obtained by gradient centrifugation over a Ficoll-Paque layer for 30 min at 550 × g. MDCs were generated from a maximum of 35 ml of blood at any one time, as described previously (3). Cells isolated were washed in apyrogenic saline, counted, and resuspended in medium at 3 × 106 cells/ml. Three-milliliter aliquots were distributed in six-well plates, and then autologous plasma was added. After 24 h, adhering cells were washed delicately twice with warm medium and then 1 ml of medium containing 3% autologous plasma, recombinant feline IL-4 (10 ng/ml), and recombinant feline GM-CSF, (50 ng/ml) was added. Every other day, fresh IL-4 and GM-CSF were added in 100 μl of medium. After 5 days of culture, MDCs were incubated with 20 μg/ml of AT2-FIV in a volume of 200 μl at 37°C in 5% CO2 for 2 h and then the volume was brought to 1 ml and fresh cytokines were added. The same day, LPS from Escherichia coli O127:B8 (Sigma, St. Louis, MO) was used at 10 ng/ml to stimulate the maturation of antigen-loaded and unloaded (mock) MDCs.
Flow cytometric analysis.
Live-cell staining was carried out in 50 μl of RPMI, 0.2% bovine serum albumin, and 0.1% NaN3 on ice for 30 min. The monoclonal antibodies (MAbs) used were anti-major histocompatibility complex class II (MHC-II) (42.3; Peter F. Moore, University of California, Davis) and anti-feline B7.1 (B7.1.66), a kind gift from Wayne Tompkins, North Carolina State University (24); the negative control for all immunoglobulin G1 isotype MAbs was L8D8 (3). A fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G antiserum (Sigma) was used as a secondary antibody where needed. Cells were fixed in phosphate-buffered saline paraformaldehyde for 20 min on ice.
MLR.
A mixed leukocyte reaction (MLR) was performed as described previously (3). Briefly, FIV-MDCs were prepared as described above and compared for their capacities to induce alloreactivity with MDCs induced or not induced to mature with 50 ng/ml LPS for 24 h. Stimulating MDCs (1 ×103 or 3 ×103) were added to 105 allogeneic PBMCs in 96-well plates in triplicate (stimulator-to-responder ratio of 1:100 or 1:30, respectively) and cultured for 4 days in medium. Human serum (10%) was added to decrease background incorporation according to standard protocols (14). We assessed proliferation by adding 1 μCi/well [methyl-3H]thymidine (Amersham Biosciences, Milan, Italy) 18 h before harvesting the cells and counting them on a β-plate counter (Wallace PerkinElmer, Milan, Italy). Results are expressed as counts per minute.
Immunization and challenge protocol and schedule.
For immunization, FIV-MDCs were reinjected into the corresponding cats 48 h after the addition of LPS. The cells were counted, and the viability assessed ranged between 74 and 87% in the different inocula. All cells obtained were resuspended in a final volume of 1 ml of saline and injected subcutaneously. To this aim, two skin sites located on each thigh, close to the popliteal lymph nodes, were pretreated with Aldara cream (Laboratoires 3M Santé, Pontoise, France). This cream contains 5% imiquimod, and we applied it at the site of the inoculum 20 min before cells were injected, after shaving the site. A single-use packet containing 0.25 g of cream was used for two cats. MDCs were generated, loaded, and reinjected three times at 1-month intervals and once again 2 months before challenge. Two months after the last FIV-MDC inoculum, cats were challenged intravenously with 10 50% cat infectious doses (CID50) of FIV-M2 obtained from plasma pooled from three infected cats and titrated as described previously (14). Infection was monitored for virus load, antibody titers, cellular FIV-M2-specific responses, and CD4+/CD8+ T-cell counts, as described below.
PBMC proliferation assay.
To assess specific cellular immunity elicited by vaccination, cats were bled regularly after vaccination and 1.5 × 105 PBMCs were cultured in quadruplicate in 96-well plates in 200 μl medium containing 10% human serum and incubated with either 1 μg/well of purified sonicated FIV-M2 or 0.5 μg/ml concanavalin A or no stimulus. After 4 days, 1 μCi of [3H]thymidine (Amersham Biosciences) was added and, 18 h later, plates were harvested and counted. Results are reported as stimulation index (S.I.), the ratio of radioactivity incorporated by test PBMCs in the presence versus that incorporated in the absence of antigen; it was considered positive when the value was >5.
Assays for antibodies.
Total FIV-M2 binding antibodies were measured by enzyme-linked immunosorbent assay (ELISA) as previously described (14). The titers were expressed as reciprocals of the highest serum dilutions that gave optical density readings at least threefold higher than the average values obtained with 10 control FIV-negative sera plus three times the standard deviation. Neutralizing antibodies were measured after prior adsorption of sera from vaccinated cats with MBM cells (5). The assay was carried out against 10 50% tissue culture infectious doses of FIV-M2 by using MBM cells as indicators and a 50% inhibition of p25 production as the readout.
Western blot analysis was carried out after separation of whole-purified FIV-M2 on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, as previously described (5). Sera of cats were diluted 1:50.
Viral load.
To determine plasma viremia, viral RNA was extracted from plasma by using the QIAamp viral RNA kit (Qiagen, Milan, Italy), reverse transcribed, and amplified by reverse transcription TaqMan PCR (TM-PCR). To determine proviral load in PBMCs, genomic DNA was extracted from the PBMCs using the QIAamp DNA blood mini kit (Qiagen). Proviral DNA was quantified from 0.4 μg of genomic DNA by TM-PCR under the same conditions used for cDNA amplification, except that the reaction mixture volume was 25 μl. Reverse transcription and amplification conditions and evaluation of the sensitivity of the assay (200 copies/ml plasma and 100 copies/μg of genomic DNA, respectively) have previously been described (18).
Infectious units were assessed for PBMCs: 106 cells were serially diluted 10-fold and cocultured with 5 × 105 MBM cells in RPMI 1640 medium containing 10% FCS, 10 U/ml recombinant human IL-2 (Roche Diagnostics, Monza, Italy), and 5 μg/ml concanavalin A (Sigma). Culture supernatants were monitored biweekly for p25 production by ELISA for up to 5 weeks to obtain infectious units/106 PBMCs, as described previously (14).
Determination of CD4+ and CD8+ T-cell numbers in whole peripheral blood from enrolled cats.
Quantitative determination of CD4+ and CD8+ T cells in peripheral blood was assessed as follows. Thirty microliters of heparinized peripheral blood was incubated on ice with 3 μl of anti-feline CD4- or CD8-phycoerythrin (clone vpg34 or vpg9, respectively; AbD Serotec, Raleigh, NC) for 30 min. Red blood cells were lysed, and leukocytes were fixed by the addition of 30 μl of OptiLyse (Beckman Coulter, Milan, Italy), incubation for 10 min at room temperature, and then the addition of 300 μl of distilled water for 10 min. After lysis, 30 μl of FlowCount microspheres containing 1,014 microspheres/μl (Beckman Coulter) was added. Samples were analyzed by flow cytometry by acquiring a number of microspheres equivalent to 1 μl.
RESULTS
Generation and characterization of FIV-MDCs.
We have previously described the generation of feline MDCs in conditions established to avoid any contact of the cells with foreign protein, implying the use of autologous plasma and feline cytokines. We have also shown that the only maturation stimulus tested leading to full feline MDC maturation in the cat was LPS and that the most suitable surface markers to follow maturation were MHC-II and B7.1 expression levels, while the best functional assay was considered to be MLR (2, 3).
For the present study, preliminary experiments were carried out with the cats enrolled for the vaccination trial to evaluate the numbers and state of MDCs that could be obtained after treatment with AT2-FIV and maturation by LPS. A maximum of 35 ml of blood was drawn from single cats, and 50 × 106 to 150 × 106 PBMCs were typically recovered from such a volume. In our hands, between 0.5 to 3% PBMCs differentiated into MDCs. After loading with AT2-FIV and maturation with LPS, the numbers of MDCs obtained were in the range of 0.1 to 3.5 × 106 per bleeding, a yield that is very close to the one we had in previous experiments (4). This ruled out the possibility of freezing PBMCs/MDCs for future injections and analyzing the state of MDCs each time they were generated from experimental cats. Indeed, either procedure would have led to a loss of the MDCs incompatible with the experiment planned.
MDCs were loaded with AT2-FIV for 2 h on day 5 of culture. Given that there was no way to assess what AT2-FIV dose is appropriate to optimally load MDCs for the purposes of the present study, pulsing was carried out with 20 μg/ml of AT2-FIV, an arbitrarily chosen dose that is approximately four times higher than the one used to load PBMCs in proliferation experiments in our protocols.
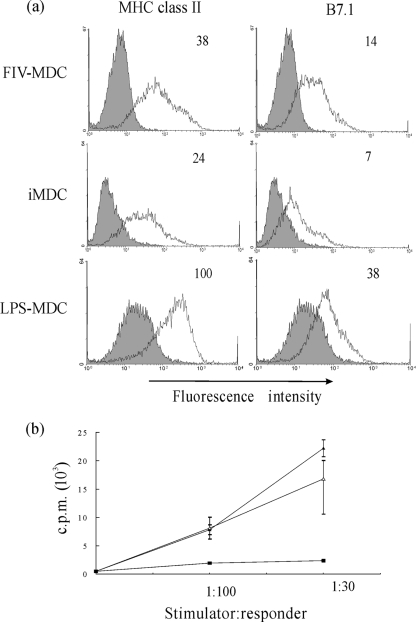
MDCs were induced to mature by exposing them to 10 ng/ml of LPS for 48 h immediately after loading with the FIV antigen. In previous experiments (3), the dose of 50 ng/ml had been used to obtain DC maturation, but we noticed that quite a number of MDCs tended to die after treatment with this LPS dose, while 10 ng/ml still brought along maturation without visible cytotoxicity (data not shown). MDCs loaded with AT2-FIV and matured with 10 ng/ml LPS were then compared (for MHC-II and B7.1 expression levels) to immature MDCs and to MDCs matured with the dose of 50 ng/ml previously used and for the ability to activate MLR. FIV-MDCs exhibited high expression levels of MHC-II and B7.1 compared to those exhibited by immature MDCs (Fig. 1a). The ability of MDCs to turn on alloreactivity was also upregulated (Fig. 1b). Although surface marker expression was lower on FIV-MDCs than on MDCs matured with 50 ng/ml of LPS, MLR induction was generally comparable, if not higher.
FIG. 1.
Maturation state of MDCs used for vaccination. (a) Histogram plots of AT2-FIV-loaded MDCs matured with 10 ng/ml of LPS (FIV-MDC), immature MDCs (iMDC), and MDCs matured with 50 ng/ml of LPS (LPS-MDC). All cells were stained with MAb anti-MHC-II or anti-B7.1 (open histograms) or with the respective isotype-matched negative controls (filled histograms). Numbers at the top right corner of each panel represent the geometric mean of fluorescence intensity of positive cells. (b) MLR by FIV-MDCs (▴), iMDCs (▪), and LPS-MDCs (▵). MDCs were incubated with 105 allogeneic PBMCs at the indicated stimulator-to-responder ratios for 4 days, and then [methyl-3H]thymidine was added to the wells for another 18 h. Results are expressed as the means ± standard errors of triplicates. Data are representative of four experiments. c.p.m., counts per minute.
Vaccination experiment.
Based on the above findings, this experiment was performed by (i) generating fresh MDCs before each inoculation time, (ii) setting up the MDC cultures 7 days before the inoculation took place, and (iii) preparing FIV-MDCs by loading with 20 μg/ml of AT2-FIV at day 5 of culture, followed by 10 ng/ml LPS for two additional days. A total of 16 cats were enrolled in the experiment and randomly divided into three groups. One group of eight received autologous FIV-MDCs generated and matured as described above (vaccine group). A second group of three cats received autologous MDCs that had been treated in a manner the same as that for the group of eight cats, except that they had been exposed to medium instead of FIV (mock group). The final group of five cats was left untreated until challenge (naive group). MDCs were reinjected subcutaneously into two sites pretreated with the Toll-like receptor 7 agonist imiquimod in an attempt to increase the chances of obtaining satisfactory migration of MDCs to the draining lymph nodes (2, 17). This procedure was repeated three times at 1-month intervals and once again after an additional interval of 3 months (boost). Table 1 shows the numbers of MDCs injected into each cat at each dose. As can be seen, these numbers differed greatly from one cat to another and from one preparation to the next for the same cat, varying from a minimum of 0.2 × 106 to up to 3.9 × 106 cells per inoculum. No adverse reactions were noticed in the cats, except for local bland inflammation and swelling of regional lymph nodes for some of the cats. Eight weeks following the last immunizing dose, no virus could be found in the PBMCs of any vaccinated cat either by isolation in MBM cells or by TM-PCR, thus confirming parallel tissue culture findings showing that AT2-FIV contained no detectable residual FIV infectivity (data not shown).
TABLE 1.
Numbers of MDCs inoculated per immunization into each experimental cat
| Cat ID | DCs inoculated (106) at indicated intervala
|
|||
|---|---|---|---|---|
| 1st | 2nd | 3rd | Boost | |
| Vaccine group | ||||
| EB | 0.2 | 1.5 | 0.6 | 0.2 |
| EC | 0.6 | 2.5 | 1.1 | 1.2 |
| EV | 3.1 | 0.7 | 0.6 | 0.6 |
| FC | 0.3 | 0.3 | 0.2 | 1 |
| GR | 2.6 | 1.1 | 1.9 | 0.6 |
| GT | 1.6 | 0.6 | 3.8 | 3.5 |
| GV | 0.3 | 0.7 | 2.9 | 1.9 |
| GX | 0.4 | 0.8 | 1.5 | 1.2 |
| Mock group | ||||
| FD | 2.6 | 0.5 | 1.2 | 1.5 |
| GA | 1.6 | 0.7 | 3.9 | 1.5 |
| GB | 0.2 | 1.3 | 2 | 1.5 |
MDCs were cultured and treated as described. The day of immunization, those obtained from each cat were harvested, counted, and reinjected in the cats. Numbers represent total live cells, as evaluated by trypan blue exclusion. ID, identification code.
Immune responses elicited by FIV-MDCs.
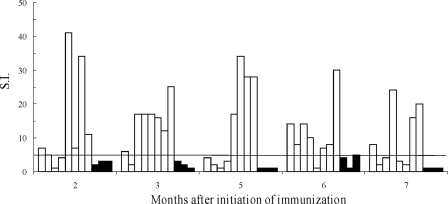
The ability of FIV-MDCs to elicit specific T-cell responses was investigated by examining the proliferative response of PBMCs to FIV at the time of each immunization and at the time of challenge. Figure 2 shows that overall, the vaccine group exhibited much higher S.I. values than the mock group did, thus indicating that FIV-MDCs were effective at inducing a measurable specific T-cell response. Indeed, as many as 27 out of the total 40 determinations that were performed with the PBMCs of vaccinated cats prior to challenge gave S.I. values of >5, whereas none of the 15 determinations that were performed with the PBMCs of the mock group did. In addition, four cats exhibited peak S.I. values of ≥30, which had been a rare finding in our previous FIV vaccination experiments. However, although there was a tendency toward greater S.I. values at late times of immunization, peak proliferation was observed at different sampling time points in different vaccines. In fact, only four animals had a clearly positive S.I. at the time of challenge (Table 2).
FIG. 2.
FIV-M2-specific cell proliferation by PBMCs after the start of immunization. PBMCs from cats of the vaccine group (white columns) or the mock group (black columns) were tested for FIV-M2-specific proliferation at different months after the initiation of immunization. Proliferation is expressed as S.I., which is calculated as the ratio of radioactivity incorporated in the presence and in the absence of antigen. The time of challenge corresponds to month 7; month 5 corresponds to the time of the booster.
TABLE 2.
Summary of the state of cats at the time of challengea
| Cat ID | Total no. of MDCs inoculated (106) | Anti-FIV ELISA antibody titer | Anti-FIV immunoblot reactivity for:
|
PBMC proliferation (S.I.)b | CD4+ T cells/μl of blood | CD8+ T cells/μl of blood | ||
|---|---|---|---|---|---|---|---|---|
| gp120 | p25 | p17 | ||||||
| Vaccine group | ||||||||
| EB | 2.5 | 400 | − | + | − | 8 | 647 | 583 |
| EC | 5.4 | 100 | + | ++ | ++ | 2 | 1,430 | 2,178 |
| EV | 5 | 1,600 | + | ++ | + | 4 | 1,105 | 856 |
| FC | 1.8 | <100 | ND | ND | ND | 24 | 1,550 | 1,275 |
| GR | 6.2 | 400 | + | ++ | − | 3 | 1,638 | 814 |
| GT | 9.5 | 800 | + | ++ | − | 2 | 1,643 | 1,714 |
| GV | 5.8 | 800 | + | ++ | + | 16 | 845 | 540 |
| GX | 3.9 | 1,600 | + | + | − | 20 | 1,638 | 814 |
| Mock group | ||||||||
| FD | 5.8 | <100 | − | − | − | 1 | 1,462 | 1,145 |
| GA | 7.7 | <100 | − | − | − | 1 | 2,471 | 1,346 |
| GB | 5.0 | <100 | − | − | − | 1 | 2,190 | 1,154 |
| Naive group | ||||||||
| GS | 0 | <100 | ND | ND | ND | 1 | 1,143 | 1,001 |
| GU | 0 | <100 | ND | ND | ND | 1 | 1,521 | 1,026 |
| GY | 0 | <100 | ND | ND | ND | 1 | 1,112 | 466 |
| GZ | 0 | <100 | ND | ND | ND | 2 | 1,266 | 471 |
| HB | 0 | <100 | ND | ND | ND | 1 | 1,235 | 847 |
ID, identification code; −, no reactivity; +, reactive; ++, strongly reactive; ND, not done.
S.I., stimulation index.
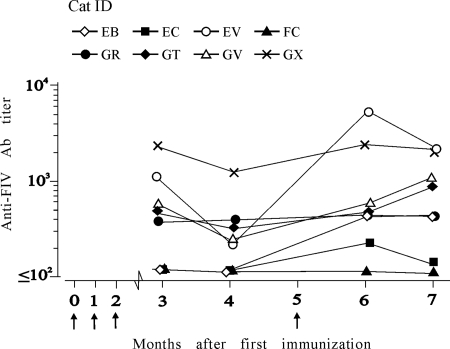
Antibody responses were monitored by ELISA using whole purified and sonicated FIV-M2 as an antigen. Figure 3 depicts the results obtained with sera of animals of the vaccine group. Five out of eight cats responded well, since they were already antibody positive at titers that ranged between 200 and 1,600 at 3 months after the first immunizing dose, tended to decline slightly at the subsequent sampling, and underwent a generally moderate increase after the boost. The cat identified as FC (cat FC), which had received the lowest total dose of FIV-MDCs (1.8 × 106), showed no detectable antibody titer throughout the prechallenge observation period. Cat EB, which had received the second lowest number of FIV-MDCs (2.5 × 106), and cat EC, which had received an average dose (5.4 × 106), responded very weakly and only after receiving the boost. Thus, by the time of challenge, four vaccinees had substantial levels of FIV antibody, two had moderate levels of FIV antibody, and two had no FIV antibody or very little. In Western blot, the sera showed the strongest reactivity against the capsid protein p25, while reactivity to gp120 was modest or undetectable. Variable reactivity was found to protein p17. All sera tested negative in neutralization assays performed as described in Materials and Methods. As expected, mock-vaccinated and naive cats were uniformly antibody negative (Table 2).
FIG. 3.
FIV-M2-binding antibodies (Ab) in sera during the course of immunization. Total FIV-M2 antibodies were measured by ELISA against whole purified and sonicated FIV-M2 at the times indicated. Titers were expressed as reciprocals of the highest serum dilutions that gave optical density readings at least threefold higher than the average values obtained with 10 FIV-negative sera plus three times the standard deviation. The times of immunization are shown by arrows.
Outcome of challenge.
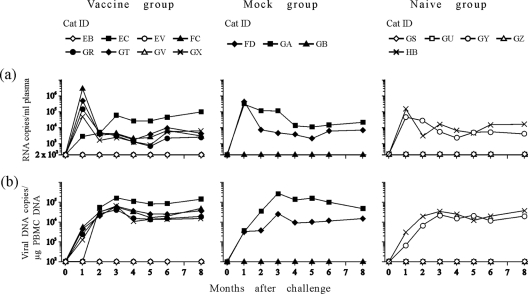
Two months after the immunization cycle was completed, all study cats were challenged intravenously with 10 CID50 of ex vivo FIV-M2 (14). The take of challenge virus was then monitored by evaluating both plasma viral loads and proviral loads in the PBMCs by TM-PCR at monthly intervals for 8 months. As shown by Fig. 4, only two of five naive controls and two of three mock-vaccinated cats became infected after challenge, possibly indicating that the virus titer had declined since it had been determined. Yet, in the vaccinees, there were no indications that FIV-MDCs had conferred any degree of resistance to FIV. Five out of eight animals of this group became infected, and these presented plasma viremia, proviral loads, and infectious units in the PBMCs (not shown) that were similar in size and kinetics to the controls that became infected, with no apparent correlation with the FIV immune state exhibited before or at the time of challenge. The infectious loads in PBMCs, measured by quantitative virus isolation in MBM cells at 3, 4, and 5 months from challenge, qualitatively confirmed the data obtained by TM-PCR (data not shown).
FIG. 4.
FIV-M2 plasma viremia (a) and proviral loads (b) in PBMCs determined by TM-PCR. Cats GV, EV (vaccinated group), GB (mock), and GZ, GU, and GS (controls) turned out to not be infected after challenge.
FIV-specific immune responses were also monitored throughout the postchallenge observation period. PBMCs of vaccinees generated S.I. values of >5 in 5 of 40 determinations, with no significant differences between those who became infected and those who did not (3 out of 25 versus 2 out of 15, respectively), thus possibly suggesting that the cell-mediated response elicited by FIV-MDCs tended to persist only shortly following challenge and was not recalled appreciably by infection. Control animals that became infected did not mount measurable proliferative responses at the times tested after challenge. On the other hand, while mock and naive animals that became infected underwent the expected clearly evident FIV antibody response, the vaccinees that became infected showed only a marginal increase relative to prechallenge (data not shown).
Circulating CD4+ T cells were monitored starting 4 months before challenge. Prior to challenge, all cats, which were approximately 2 years old at the beginning of our study, had stable CD4+ T-cell percentages and, after challenge, the cats that became infected showed the expected drops in CD4+ T-cell percentages regardless of whether they had received FIV-MDCs, while the others had stable values. CD8+ T cells underwent no appreciable changes before and after challenge (data not shown).
DISCUSSION
Current consensus is that a successful vaccine against HIV must stimulate polyfunctional CD8+ as well as CD4+ T cells capable of secreting IL-2 and other cytokines, whereas neutralizing antibody is envisioned as an important but difficult goal (6). DCs can elicit both CD4+ and CD8+ T cells, thanks to unique abilities as antigen-presenting cells (26), and virus-loaded DC immunotherapy has given encouraging results in the control of viral infections in infected individuals of different species (11, 12). On the other hand, very few data are available on the ability of DCs to prime protective immunity against viruses in naive individuals as yet. To begin to shed light on this issue, we tested an MDC-based vaccine in the FIV-cat animal model. Many issues must be considered in the design of such an experiment: to name but two, the kind of antigen to load MDCs with and their maturation stage. FIV protective antigens are not well known; in addition, the cat is not a syngeneic animal model. Therefore, protective peptides may differ from one individual to another. For this reason, we loaded MDCs with whole AT2-inactivated FIV (5). We then used the same virus isolate to challenge vaccinated cats. Although the feline DC maturation stage is not as straightforward to define as that for human DCs, where well-defined markers allow us to distinguish between mature and immature cells, correlates of feline MDC maturation, such as MHC-II and B7.1 expression levels and the ability to induce MLR, have been described previously (3). In the present study, after MDCs were loaded with antigen, the maturation stimulus used in vitro was LPS for all MDCs, because previous work has shown LPS is the only stimulus known to induce the ability of MDCs to start MLR in the feline model. After such treatment, MDCs did express higher levels of MHC-II and B7.1 and induced MLR; however, to maximize MDC migration to local lymph nodes, the sites of inocula were pretreated with imiquimod. This substance has been shown to be an agonist of Toll-like receptor 7 that is used to create local subcutaneous environments rich with inflammatory cytokines and that has been reported to induce the maturation of DCs and facilitate their homing to draining lymph nodes in other animal species (17).
Because MDCs were generated from PBMCs, there was a natural limit to the number of cells that could be obtained. Since freezing decreases the number of viable cells available, we chose to generate fresh MDCs every time they were needed for immunization rather than using prestored cells. This procedure yielded numbers of MDCs varying widely from one cat to another and from one injection to the next, as already reported in previous works (3, 4); therefore, cats did not receive the same numbers of MDCs. However, we did not notice any correlation between responses to FIV and the number of MDCs received by each cat. Nevertheless, the fact that a cat that had not produced any antibodies or cell proliferation against FIV had received only a total of less than 2 × 106 MDCs suggested that the numbers of MDCs injected may not have been much above the minimum required for an efficient immunization.
The cell-mediated responses generated by FIV-MDCs were measured by lymphoproliferation from 2 months after the first inoculum (after two injections of MDCs). For most cats, lymphoproliferation was found to be much stronger than detected after vaccination with FIV-infected MBM cells fixed with paraformaldehyde or in infected cats in this and previous studies of ours (14). Antibody titers were measured from 3 months after the first inoculum. A rise in titer was detected for most cats after the boost (2 months before challenge). However, these antibodies were not neutralizing as determined in a lymphoid cell-based assay. Of note, in previous experiments (14), cats immunized with whole uninfected MBM cells (where the virus used throughout the present study was produced) failed to generate immune responses that could be detected with the same assays used here, excluding the possibility that reactivity to cell components incorporated in the virions could contribute to the above findings.
After a total of four DC injections, cats were challenged with 10 CID50 of the homologous virus injected intravenously. Such a low dose was used in an effort to maximize the likelihood of demonstrating a protective effect and to mimic natural FIV infection that presumably takes place in the cat by inoculum of low doses of virus (8). As a matter of fact, the infecting dose turned out to be even lower than intended and not all control cats were infected. Nonetheless, vaccinated cats turned viremic at a similar, if not higher, rate and, with kinetics similar to those of controls, either mock vaccinated or not. As expected, coincidently with the sharp rise in viral load observed 1 month after challenge, a marked decline in the percentage of blood CD4+ T cells was detected in cats that became infected, demonstrating that the immunization protocol used was unable to prevent not only infection but also the consequent reduction in CD4+ T lymphocyte numbers (data not shown).
The results of the present study do not seem to support the hope that FIV-loaded MDCs can elicit protective immunity better than other forms of antigen; however, some important questions remain open. MDC maturation, for instance, was induced by adding a substance such as LPS, which had been found to be an appropriate stimulus to mature feline DCs, as judged by MLR in previous studies (3), and maturation was also confirmed in the present experiment. In addition, local treatment of the site of MDC injection with imiquimod was also performed to make sure maturation was at its maximum level. However, the possibility still exists that the MDCs injected were not at the right maturation stage to start an efficient protective response in cats. Next, although we did not notice a correlation between the number of MDCs injected and the levels of either cell-mediated or humoral immunity achieved, we cannot exclude the possibility that the numbers of MDCs injected might not have been sufficient. Lastly, we were unable to assess the antigen-loaded state of FIV-MDCs (perhaps there was too little or too much antigen), since this assessment would have required the use of antigen-specific T-cell stimulation assays, which is impossible with nonsyngeneic animals that are antigen naive at the time of inoculation (2).
Acknowledgments
This work was supported by grants from the Ministero della Salute-Istituto Superiore di Sanità “Programma per l'AIDS” Rome, Italy.
We thank Wayne Tompkins for his generous gift of anti-B7.1 antibody.
Footnotes
Published ahead of print on 23 January 2008.
REFERENCES
- 1.Dunham, S. 2006. Lessons from the cat: development of vaccines against lentiviruses. Vet. Immunol. Immunopathol. 112:67-77. [DOI] [PubMed] [Google Scholar]
- 2.Figdor, C. G., I. J. de Vries, W. J. Lesterhuis, and C. J. Melief. 2004. Dendritic cell immunotherapy: mapping the way. Nat. Med. 10:475-480. [DOI] [PubMed] [Google Scholar]
- 3.Freer, G., D. Matteucci, P. Mazzetti, L. Bozzacco, and M. Bendinelli. 2005. Generation of feline dendritic cells derived from peripheral blood monocytes for in vivo use. Clin. Diagn. Lab. Immunol. 12:1202-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freer, G., D. Matteucci, P. Mazzetti, F. Tarabella, V. Catalucci, and M. Bendinelli. 2007. Effects of feline immunodeficiency virus on feline monocyte-derived dendritic cells infected by spinoculation. J. Gen. Virol. 88:2574-2582. [DOI] [PubMed] [Google Scholar]
- 5.Giannecchini, S., P. Isola, O. Sichi, D. Matteucci, M. Pistello, L. Zaccaro, D. Del Mauro, and M. Bendinelli. 2002. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: failure to protect and possible enhancement of challenge infection by four cell-based vaccines prepared with autologous lymphoblasts. J. Virol. 76:6882-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heeney, J. L., and S. A. Plotkin. 2006. Immunological correlates of protection from HIV infection and disease. Nat. Immunol. 7:1281-1283. [DOI] [PubMed] [Google Scholar]
- 7.Hosie, M. J., R. Osborne, J. K. Yamamoto, J. C. Neil, and O. Jarrett. 1995. Protection against homologous but not heterologous challenge induced by inactivated feline immunodeficiency virus vaccines. J. Virol. 69:1253-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosie, M. J., and J. A. Beatty. 2007. Vaccine protection against feline immunodeficiency virus: setting the challenge. Aust. Vet. J. 85:5-12. [DOI] [PubMed] [Google Scholar]
- 9.Klase, Z., M. J. Donio, A. Blauvelt, P. A. Marx, K. T. Jeang, and S. M. Smith. 2006. A peptide-loaded dendritic cell based cytotoxic T-lymphocyte (CTL) vaccination strategy using peptides that span SIV Tat, Rev, and Env overlapping reading frames. Retrovirology 3:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, W., D. K. Krishnadas, J. Li, D. L. Tyrrell, and B. Agrawal. 2006. Induction of primary human T cell responses against hepatitis C virus-derived antigens NS3 or core by autologous dendritic cells expressing hepatitis C virus antigens: potential for vaccine and immunotherapy. J. Immunol. 176:6065-6075. [DOI] [PubMed] [Google Scholar]
- 11.Lu, W., X. Wu, Y. Lu, W. Guo, and J. M. Andrieu. 2003. Therapeutic dendritic-cell vaccine for simian AIDS. Nat. Med. 9:27-32. [DOI] [PubMed] [Google Scholar]
- 12.Lu, W., L. C. Arraes, W. T. Ferreira, and J. M. Andrieu. 2004. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 10:1359-1365. [DOI] [PubMed] [Google Scholar]
- 13.Matteucci, D., P. Mazzetti, F. Baldinotti, L. Zaccaro, and M. Bendinelli. 1995. The feline lymphoid cell line MBM and its use for feline immunodeficiency virus isolation and quantitation. Vet. Immunol. Immunopathol. 46:71-82. [DOI] [PubMed] [Google Scholar]
- 14.Matteucci, D., M. Pistello, P. Mazzetti, S. Giannecchini, D. Del Mauro, L. Zaccaro, P. Bandecchi, F. Tozzini, and M. Bendinelli. 1996. Vaccination protects against in vivo-grown feline immunodeficiency virus even in the absence of detectable neutralizing antibodies. J. Virol. 70:617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matteucci, D., M. Pistello, P. Mazzetti, S. Giannecchini, D. Del Mauro, I. Lonetti, L. Zaccaro, C. Pollera, S. Specter, and M. Bendinelli. 1997. Studies of AIDS vaccination using an ex vivo feline immunodeficiency virus model: protection conferred by a fixed-cell vaccine against cell-free and cell-associated challenge differs in duration and is not easily boosted. J. Virol. 71:8368-8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matteucci, D., A. Poli, P. Mazzetti, S. Sozzi, F. Bonci, P. Isola, L. Zaccaro, S. Giannecchini, M. Calandrella, M. Pistello, S. Specter, and M. Bendinelli. 2000. Immunogenicity of an anti-clade B feline immunodeficiency fixed-cell virus vaccine in field cats. J. Virol. 74:10911-10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair, S., C. McLaughlin, A. Weizer, Z. Su, D. Boczkowski, J. Dannull, J. Vieweg, and E. Gilboa. 2003. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J. Immunol. 1:6275-6282. [DOI] [PubMed] [Google Scholar]
- 18.Pistello, M., M. Moscardini, P. Mazzetti, S. Macchi, F. Bonci, P. Isola, G. Freer, D. Matteucci, S. Specter, and M. Bendinelli. 2002. Development of feline immunodeficiency virus ORF-A (tat) mutants: in vitro and in vivo characterization. Virology 298:84-95. [DOI] [PubMed] [Google Scholar]
- 19.Pu, J., J. Coleman, E. Coisman, T. Sato, M. Tanabe, J. Arai, and J. K. Yamamoto. 2005. Dual-subtype FIV vaccine (Fel-O-Vax FIV) protection against a heterologous subtype B FIV isolate. J. Feline Med. Surg. 7:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallusto, F., and A. Lanzavecchia. 2002. The instructive role of dendritic cells on T-cell responses. Arthritis Res. 4:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schon, E., A. M. Harandi, I. Nordstrom, J. Holmgren, and K. Eriksson. 2001. Dendritic cell vaccination protects mice against lethality caused by genital herpes simplex virus type 2 infection. J. Reprod. Immunol. 50:87-104. [DOI] [PubMed] [Google Scholar]
- 22.Sparger, E. 2006. FIV as a model for HIV: an overview, p. 149-199. In H. Friedman, S. Specter, and M. Bendinelli (ed.), Infectious agents and pathogenesis: in vivo models of HIV disease and control. Springer, New York, NY.
- 23.Steinman, R. M., and J. Banchereau. 2007. Taking dendritic cells into medicine. Nature 449:419-426. [DOI] [PubMed] [Google Scholar]
- 24.Tompkins, M. B., M. E. Bull., J. L. Dow, J. M. Ball, E. W. Collisson, B. J. Winslow, A. P. Phadke, T. W. Vahlenkamp, and W. A. Tompkins. 2002. Feline immunodeficiency virus infection is characterized by B7+CTLA4+ T cell apoptosis. J. Infect. Dis. 185:1077-1093. [DOI] [PubMed] [Google Scholar]
- 25.Tüttenberg, A., E. Schmitt, J. Knop, and H. Jonuleit. 2007. Dendritic cell-based immunotherapy of malignant melanoma: success and limitations. J. Dtsch. Dermatol. Ges. 5:190-196. [DOI] [PubMed] [Google Scholar]
- 26.Villadangos, J. A., W. R. Heath, and F. R. Carbone. 2007. Outside looking in: the inner workings of the crosspresentation pathway within dendritic cells. Trends Immunol. 28:45-47. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto, J. K., T. Okuda, C. D. Ackley, H. Louie, E. Pembroke, H. Zochlinski, R. J. Munn, and M. B. Gardner. 1991. Experimental vaccine protection against feline immunodeficiency virus. AIDS Res. Hum. Retrovir. 7:911-922. [DOI] [PubMed] [Google Scholar]