Abstract
The use of anti-tumor necrosis factor (TNF) agents as a treatment for chronic inflammatory conditions has been shown to be associated with an increased risk of developing tuberculosis. We studied the effect of the anti-TNF antibody infliximab on antimycobacterial immunity in 26 patients with rheumatoid arthritis or ankylosing spondylitis by use of an in vitro whole-blood model employing a reporter mycobacterium. Blood samples taken before and 30 min and 7 days after a 2-hour infliximab infusion were compared in terms of their abilities both to suppress luminescence of Mycobacterium bovis bacillus Calmette-Guérin lux and to secrete chemokines and cytokines 24 and 96 h after infection. No immediate effect of infliximab on mycobacterial luminescence was detected using this bioassay, irrespective of whether patients were receiving their first (n = 14) or maintenance (n = 12) doses of infliximab. Moreover, no effect on mycobacterial luminescence was detected when blood was taken 7 days after infliximab treatment (n = 7). By contrast, there was a significant reduction in the chemokines implicated in cellular trafficking, namely, interleukin-8, macrophage-inhibitory protein-1α (MIP-1α), MIP-1β (24 h and 96 h), and monocyte chemoattractant protein-1 (MCP-1) (24 h) following BCG lux strain infection in the 30-minute post-infliximab-infusion blood samples (P < 0.05). This effect was sustained by MIP-1β and MCP-1 (24 h; P < 0.05) at 7 days after infusion. Our results suggest that the development of tuberculosis in infliximab-treated patients is not directly related to the mycobactericidal effects of TNF but may be due to inhibition of TNF-dependent chemokine gradients disrupting cellular migration necessary to maintain the integrity of the granuloma.
Anti-tumor necrosis factor (TNF) agents are increasingly used to treat a range of chronic inflammatory conditions including rheumatoid arthritis (RA), ankylosing spondylitis (AS), and Crohn's disease. One of the currently approved and widely used agents is infliximab, a chimeric monoclonal antibody (75% human, 25% mouse protein) which targets both soluble and membrane-bound forms of TNF by binding with high avidity and specificity (14). Thus, infliximab neutralizes the effect of this cytokine and its ability to induce other downstream biological mediators associated with chronic inflammatory diseases.
However, anti-TNF therapy is also associated with an increased risk of developing opportunistic infections, particularly tuberculosis (TB) (7, 15, 18, 20, 35). This risk appears to be greater with infliximab than with other similar agents (8, 35, 36, 38), although the mechanisms remain unclear (10). TNF has pleiotropic functions in the host response to TB infection, including the promotion of orderly granuloma formation and containment of disease and the induction of macrophage apoptosis as well as the stimulation and release of other cytokines, chemokines, and adhesion molecules. However, TNF is also believed to contribute to the necrosis characteristic of TB (16, 37).
Infliximab-associated TB tends to occur early, often within the first 3 months of therapy (20), and in most instances appears to arise from the reactivation of latent TB infection (19). Furthermore, disease is often extrapulmonary, disseminated, and associated with poor granuloma formation (9, 14, 21, 23). A number of experimental studies, both in vitro and in animals, indicate the crucial role of TNF in granuloma formation (2, 12, 30). In addition to a possible direct mycobactericidal effect, TNF is also essential for the early expression of chemokines and recruitment of leukocytes (particularly macrophages) to the site of infection (1, 2, 28). Poor granuloma formation in TB following anti-TNF treatment may therefore be a mechanism for infliximab-associated TB, although data from humans are limited compared to those from animal models.
One of the recently developed models to study immune responses to mycobacteria in humans is the Mycobacterium bovis bacillus Calmette-Guérin (BCG) lux whole-blood assay (17). This in vitro technique evaluates the metabolic activity (measured as luminescence) of a recombinant reporter strain of mycobacteria when exposed to whole blood (17, 31, 32). In addition, soluble mediators (chemokines and cytokines) secreted into culture supernatant can be measured simultaneously. Differences in the degrees of suppression of mycobacterial luminescence have been demonstrated in this model, which relates well to the underlying sensitization to mycobacterial antigens in healthy individuals and to clinical phenotype.
We employed this whole-blood assay and chemokine and cytokine measurement to study antimycobacterial immunity in patients receiving infliximab. Our observations provide a mechanistic insight into how infliximab may predispose individuals to tuberculous infection.
MATERIALS AND METHODS
Human subjects.
Ethical permission for this study was granted by the Harrow Local Research Ethics Committee (ethics submission no. 3126). Twenty-six adult patients aged between 32 and 81 years (mean, 60 years) with either RA (24/26) or AS (2/26) were recruited from the Rheumatology Clinic at Northwick Park Hospital, Harrow, United Kingdom. Patient information regarding purified protein derivative Heaf grade skin status (Heaf grade 0, 16 patients; Heaf grade 1 to 4, 10 patients) and evidence of BCG scar/history of BCG vaccination (BCG, 8; no BCG, 11; undetermined, 7) was collected. Only one patient had a known past history of previously treated TB. Infliximab treatment was administered to RA and AS patients (3 and 5 mg/kg of body weight, respectively) at 0, 2, and 6 weeks (loading regimen) and every 8 weeks thereafter (maintenance therapy). All doses were given as a 2-h infusion, following standard protocols. Each patient served as their own internal control to avoid confounding factors such as the use of other drugs and disease severity.
Peripheral blood (10 ml) was taken from each patient prior to starting the infusion of infliximab (pre-anti-TNF), and a second blood sample was taken approximately 30 min after completion of the infusion (post-anti-TNF), i.e., 2.5 h after the start of the infusion. Seven of the 26 patients had an additional blood sample taken 7 days later (7 days post-anti-TNF). Whole-blood samples were collected in sodium heparin Vacutainers (BD Biosciences) and diluted with equal volumes of RPMI 1640 (Sigma) containing l-glutamine and sodium bicarbonate supplemented with 12 mM HEPES (Sigma).
Reporter BCG.
Mycobacterium bovis BCG, Montreal strain, obtained from Young's group, Imperial College London, was transformed with the reporter plasmid construct pSMT1 as previously described in detail elsewhere (31). This plasmid carries the lux strain AB genes from Vibrio harveyi under the control of a mycobacterial strong constitutive promoter (hsp 60). M. bovis BCG lux was grown to mid-log phase in Middlebrook 7H9 broth (Difco, Detroit, MI) containing 0.2% glycerol, 0.05% Tween 80, 10% albumin-dextrose-catalase (Difco, Detroit, MI) enrichment, and 50 μg/ml hygromycin (Roche). Aliquots were prepared in 15% glycerol and stored at −80°C. The number of CFU per milliliter was determined by serial dilutions on 7H11 agar (Difco, Detroit, MI) containing 0.5% glycerol, 10% oleic acid-albumin-dextrose-catalase (Difco, Detroit, MI) enrichment, and 50 μg/ml hygromycin. Prior to each assay, a vial of BCG lux was thawed and inoculated into 15 ml of 7H9 broth and incubated at 37°C for 48 h in a shaking incubator (150 rpm). Mycobacteria were then diluted in phosphate-buffered saline (Sigma) to obtain a luminescence reading of 1 × 107 relative light units (RLU)/ml. Luminescence was determined by preparing serial 10-fold dilutions in phosphate-buffered saline (total volume, 1 ml) and measuring for a period of 20 s in a luminometer (Berthold Autolumat LB953) following injection of 0.1 ml of substrate (1% n-decyl aldehyde; Sigma). Luminescence readings are expressed as RLU. Throughout these experiments, approximately 3 RLU corresponded to 1 CFU.
BCG lux whole-blood assays.
The BCG lux whole-blood assay has been described in detail elsewhere (17). In brief, BCG lux was inoculated in diluted whole blood (in triplicate) prepared as described above to give a final inoculum of 3 × 105 CFU/ml (1 × 106 RLU/ml) (multiplicity of infection, 1 CFU per monocyte). Luminescence was measured at the time of inoculation (T0) and at 24 h and 96 h following incubation at 37°C. Mycobacterial metabolic activity (measured as luminescence) was expressed as a luminescence ratio (LR) calculated as follows: (RLU of BCG at T24)/(RLU of BCG at T0) and (RLU of BCG at T96)/(RLU of BCG at T0) for the 24-h and 96-h time points, respectively. All assays were performed double blinded.
Cytokine and chemokine assays.
Supernatants were aspirated from BCG lux-infected whole-blood samples at 0 h, 24 h, and 96 h and from uninfected blood samples at 0 h (uninfected chemokine/cytokine controls). Enzyme-linked immunosorbent assays (ELISA) were performed using the commercially available DuoSet ELISA development systems (R&D Systems, Minneapolis, MN). The sensitivity of the assays was 5 pg/ml (interleukin-6 [IL-6]), 31 pg/ml (IL-10), 17 pg/ml (gamma interferon [IFN-γ]), and 96 pg/ml (IL-8). Assays for IFN-inducible protein of 10 kDa (IP-10), macrophage-inhibitory protein-1α (MIP-1α), MIP-1β, monocyte chemoattractant protein-1 (MCP-1), RANTES (regulated on activation normal T-cell expressed and secreted), and IL-12p40 were determined using Beadlyte Luminex multiplex assays (Upstate Ltd., Charlottesville, VA). The sensitivities of the assays were 2 pg/ml, 3 pg/ml, 8 pg/ml, 30 pg/ml, 4 pg/ml, and 3 pg/ml, respectively. All assays were performed following the manufacturer's recommendations.
Statistical analysis.
All data were tested for normal Gaussian distribution but were not found to be normally distributed. Therefore, the Wilcoxon signed-rank test was used to analyze nonparametric data. Statistical significance was assumed when the P value was <0.05. All data quoted are median and interquartile range (IQR).
RESULTS
Mycobacterial luminescence before and immediately after infliximab infusion.
To determine whether treatment of RA or AS patients with infliximab leads to increased growth of BCG lux, we simultaneously inoculated whole blood from 26 patients taken before and approximately 30 min after infliximab infusion (irrespective of infliximab infusion number) with 105 CFU/ml BCG lux mycobacteria, and the LRs were compared.
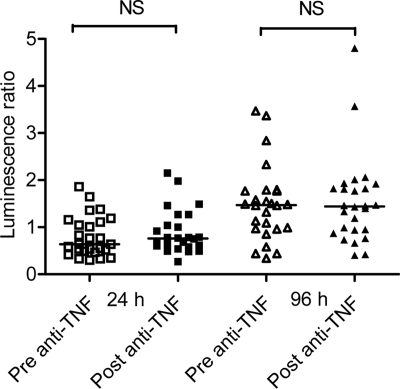
There was no significant difference in the luminescence of BCG lux in pre- and post-infliximab-infusion blood samples after 24 h of incubation (pre-infliximab median, 0.64 [IQR, 0.3 to 1.86]; post-infliximab median, 0.76 [IQR, 0.27 to 2.15]; P = 0.43) and 96 h of incubation (pre-infliximab median, 1.47 [IQR, 0.3 to 3.47]; post-infliximab median, 1.44 [IQR, 0.4 to 4.8]; P = 0.68) (Fig. 1).
FIG. 1.
BCG lux luminescence in whole blood taken before (pre-anti-TNF) and 30 min after (post-anti-TNF) infliximab infusion from 26 adult patients with RA (24/26) or AS (2/26). The metabolic activity of BCG lux was determined as an LR at 24 and 96 h following infection in both the pre- and post-anti-TNF blood samples according to the following formula: LR = RLU of BCG lux at 96 h (or 24 h)/RLU of BCG lux at 0 h. Horizontal lines show median values for each group. NS, not significant using the Wilcoxon signed-rank test.
We postulated that mycobacterial luminescence pre- and postinfusion might vary according to the number of infliximab infusions received. We therefore stratified the data by consideration of the 14/26 pairs of samples that were taken before and after the first dose of infliximab and the 12/26 that were taken during maintenance therapy (2nd to 10th infliximab doses). No significant impairment in antimycobacterial activity was detected at 96 h for either of the sample groups (for first infliximab infusion at 96 h, pre-infliximab median, 1.16 [IQR, 0.34 to 3.47], post-infliximab median, 1.45 [IQR, 0.40 to 2.0] [P = 0.326]; for maintenance therapy at 96 h, pre-infliximab median, 1.57 [IQR, 0.96 to 3.37], post-infliximab median, 1.39 [IQR, 0.76 to 4.80] [P = 0.677]) (data not shown). LRs at 24 h and 96 h after infliximab infusion were also stratified according to purified protein derivative skin test results (Heaf grade 0 and Heaf grade 1 to 4), the presence/absence of BCG scar/history of BCG vaccination, and underlying disease (RA/AS). No significant differences were noted (data not shown). The addition of various concentrations of infliximab or an anti-TNF antibody (R & D Systems) (18) added in vitro to whole blood from “healthy adults” did not have any direct effect on BCG lux luminescence in our whole-blood assay (data not shown).
Chemokine and cytokine production before and immediately after infliximab infusion.
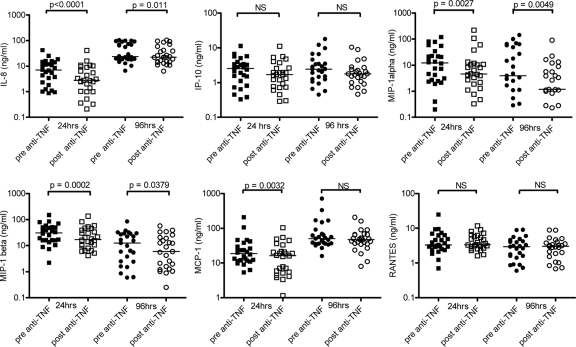
Supernatants harvested from BCG lux-infected cultures at 24 and 96 h and from uninfected chemokine/cytokine control cultures at 0 h were analyzed for secretion of chemokines and cytokines, which are TNF dependent and/or play a significant role in antimycobacterial immunity (IL-10 and IFN-γ production were analyzed only for the 96-h supernatants). TNF was not assayed because of the confounding presence of infliximab, which may have interfered with ELISA results. There was a significant reduction in median IL-8 (24 h, 2.5-fold [P < 0.0001]; 96 h, 1.28-fold [P = 0.011]), MIP-1α (24 h, 2.7-fold [P = 0.0027]; 96 h, 3.3-fold [P = 0.0049]), and MIP-1β (24 h, 1.8-fold [P = 0.0002]; 96 h, 2.1-fold [P = 0.0379]) secretion at both 24 h and 96 h for the BCG lux-infected post-infliximab-infusion blood samples (Fig. 2). A significant reduction was seen in MCP-1 production in the post-infliximab-infusion sample at the 24-h time point only (24 h, 1.15-fold [P = 0.0032]). There was no significant difference in IP-10 or RANTES chemokine production (Fig. 2) or in IL-6, IL-10, IL-12p40, and IFN-γ cytokine secretion for either 24- or 96-h pre- and postinfusion samples (data not shown).
FIG. 2.
Chemokine (IL-8, IP-10, MIP-1α, MIP-1β, MCP-1, RANTES) production in BCG lux-infected whole blood taken before (pre-anti-TNF) and 30 min after (post-anti-TNF) infliximab infusion from 26 adult patients with RA or AS was measured. Pre- and post-infliximab blood samples inoculated in triplicate were incubated for 24 and 96 h. Supernatants were harvested at each of these time-points and analyzed by ELISA or Luminex multiplex assays. Horizontal lines represent the median for the group. NS, not significant using the Wilcoxon signed-rank test.
To ensure that the levels of IL-8, MIP-1α, MIP-1β, and MCP-1 production and the significant differences observed at 24 and/or 96 h pre- and postinfusion were not already present at baseline (0 h) and hence were due to the effect of infection with BCG lux and the presence of infliximab, we measured these chemokines in the uninfected chemokine/cytokine control supernatants at 0 h. There was no difference in IL-8 (pre-infliximab median, 0 pg/ml, post-infliximab median, 0 pg/ml [IQR, 0 to 214.8]; P = 0.156) or MIP-1α (pre-infliximab median, 7.72 pg/ml, post-infliximab median, 6.08 pg/ml [IQR, 4.80 to 43.54]; P = 0.06) production between pre- and postinfusion blood samples. Furthermore, we observed increased levels of chemokine production at both subsequent time points (24 and 96 h), clearly demonstrating that these chemokines were induced by the infecting BCG lux organism (Fig. 2).
By contrast, we noted a statistically significant decrease in MIP-1β and MCP-1 production at 0 h in the post-infliximab blood samples (MIP-1β, 1.55-fold [P = 0.0016]; MCP-1, 1.23-fold [P = 0.0123]). However, production of these chemokines was extremely low at 0 h in both pre- and post-infliximab samples (MIP-1β pre-infliximab median, 32 pg/ml; MIP-1β post-infliximab median, 21 pg/ml; MCP-1 pre-infliximab median, 103 pg/ml; MCP-1 post-infliximab median, 84 pg/ml) compared to the significantly increased levels in response to BCG lux infection at 24 h (MIP-1β pre-infliximab median, 31,071 pg/ml; MIP-1β post-infliximab median, 17,241 pg/ml; MCP-1 pre-infliximab median, 18,783 pg/ml; MCP-1 post-infliximab median, 16,287 pg/ml) and 96 h (MIP-1β pre-infliximab median, 12,638 pg/ml; MIP-1β post-infliximab median, 5,958 pg/ml; MCP-1 pre-infliximab median, 50,418 pg/ml; MCP-1 post-infliximab median, 48,203 pg/ml) (Fig. 2), where subsequently differences in chemokine production occurred between the pre- and postinfusion samples due to the presence of infliximab.
Mycobacterial luminescence and cytokine/chemokine production before and 7 days after infliximab infusion.
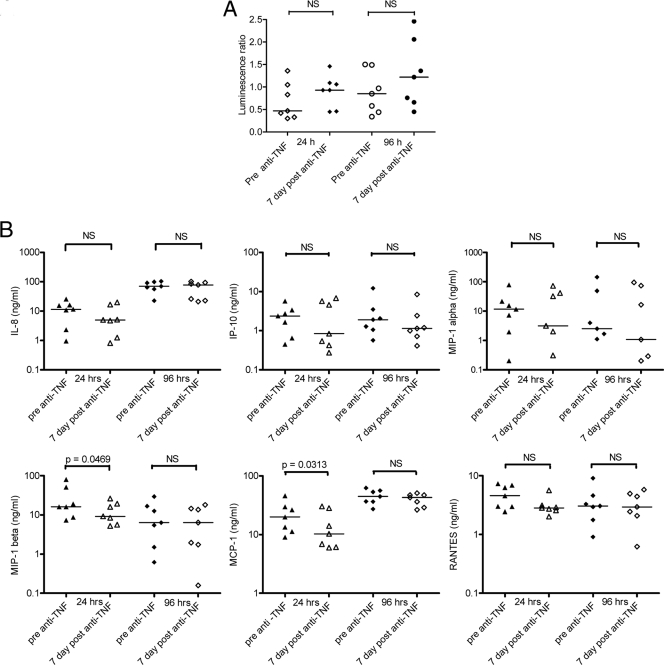
Although the BCG lux-infected whole-blood cultures were incubated for 96 h, the actual blood sample containing infliximab was taken 30 min after the infusion. This sample and assay schedule may not have allowed adequate time for infliximab to exert its potential immunosuppressive action. We therefore extended our analysis in a subgroup of seven patients receiving their first dose of infliximab and repeated the assay at 7 days after infliximab infusion, the half-life of infliximab being approximately 8.5 days. For this subset of donors, there was again no significant difference in BCG lux luminescence between the pre- and 7-day-post-infliximab samples at 24 h and 96 h (24-h pre-infliximab median, 0.47 [IQR, 0.3 to 1.36]; 7-day post-infliximab median, 0.93 [IQR, 0.45 to 1.46] [P = 0.109]; 96-h pre-infliximab median, 0.85 [IQR, 0.34 to 1.5]; 7-day post-infliximab median, 1.22 [IQR, 0.45 to 2.46] [P = 0.156]) (Fig. 3A). However, we did observe a significant reduction in both MIP-1β (24 h, 1.75-fold [P = 0.0469]) and MCP-1 (1.9-fold [P = 0.032]) in the 7-day-post-infliximab-infusion blood samples 24 h after infection (Fig. 3B). This effect was not sustained for either chemokine at 96 h. Although there were no other statistically significant reductions in chemokine or cytokine production, a trend in reduced secretion was noted for all other chemokines at the 24-h time point (Fig. 3B). No differences in chemokine/cytokine levels between the pre- and 7-day-post-infliximab-infusion blood samples were detected in the uninfected chemokine/cytokine controls at 0 h (data not shown).
FIG. 3.
(A) BCG lux luminescence in whole blood taken from seven patients before and 7 days after receiving their first infliximab infusion. Luminescence was measured after 24-h and 96-h incubation. Each point represents the median of triplicate determinations. Horizontal lines represent median values for the group. NS, not significant using the Wilcoxon signed-rank test. (B) Chemokine (IL-8, IP-10, MIP-1α, MIP-1β, MCP-1, RANTES) production in BCG lux-infected whole blood of seven patients receiving their first infliximab infusion was measured. Whole blood was taken before (pre anti-TNF) and 7 days after (7 day post anti-TNF) infliximab infusion. Infected pre- and 7-day-post-infliximab blood samples were incubated for 24 and 96 h. Supernatants were harvested at each of these time points and analyzed by ELISA or Luminex multiplex assays. Horizontal lines represent median values for the group. NS, not significant using the Wilcoxon signed-rank test.
DISCUSSION
TNF appears to play a central role in the host immune response to Mycobacterium tuberculosis, being involved in both pathogenesis and protection (12). We set out to study the effects of the anti-TNF drug infliximab on RA/AS patient antimycobacterial immunity by use of an in vitro whole-blood assay employing recombinant reporter mycobacteria (BCG lux whole-blood assay).
Evidence from both animal models and humans shows that anti-TNF treatment, in addition to its valuable therapeutic ability to reduce the severity of inflammatory disease, is associated with increased susceptibility to opportunistic infection, particularly TB (19) and other atypical mycobacterial infections (6, 24, 26). Rapid and lethal reactivation of TB infection has been reported for TNF-deficient mice with high bacterial loads in the lungs, spleen, and liver (5). Similarly, TNF receptor 1 (p55 receptor) knockout (TNFR1−/−) mice and mice treated with neutralizing TNF antibody have been shown to be extremely susceptible to M. tuberculosis infection; loss of granuloma structure was a striking observation (13). These findings, emphasizing the important role of TNF in antimycobacterial immunity, have also been confirmed by numerous other studies summarized in the reviews by Ehlers (9) and Tufariello et al. (34).
Our initial hypothesis was that the administration of the anti-TNF agent infliximab would lead to increased mycobacterial luminescence in the BCG lux whole-blood assay as an expression of decreased antimycobacterial immune responses. However, this was not found to be the case. There was no difference in mycobacterial luminescence in whole-blood assays performed before and 30 min after infliximab infusion. This was irrespective of whether patients were receiving their first or subsequent maintenance doses of infliximab. Although samples were taken relatively soon after a 2-h infliximab infusion, there was a considerable subsequent time period in vitro (96 h) in which the effects of the anti-TNF antibody could manifest. Moreover, as anti-TNF may take some time to modify overall immunological responses in vivo, and since the drug has a half-life of approximately 8.5 days (25), an additional blood sample taken 7 days after infliximab infusion also demonstrated no differences in mycobacterial luminescence in blood taken before and 7 days after infliximab infusion. These results are similar to studies by Saliu et al. (29) and Kampmann et al. (17), in which neither infliximab nor an anti-TNF antibody (R & D Systems) added to whole blood in vitro had any effect on the control of M. tuberculosis or BCG lux growth, respectively. For practical reasons, we used the BCG lux organism in this whole-blood model rather than M. tuberculosis. It is possible that different immunological and molecular mechanisms involved in the restriction of the growth of the pathogenic strains exist.
In addition to measuring antimycobacterial activity using the BCG lux whole-blood assay, we analyzed the changes in a number of pivotal chemokines and cytokines involved in antimycobacterial immunity. Furthermore the expression of many of these biological mediators is evident in rheumatoid joints (4, 11). Saliu et al. (29) showed that the addition of infliximab in vitro suppressed IFN-γ and IL-10 production in whole blood infected with M. tuberculosis culture filtrate. In addition, infliximab reduced the proportion of TB-responsive CD4 cells by 70%. In contrast, a recent report demonstrated that short-term infliximab treatment (2 to 14 weeks) of RA patients did not affect the production of IFN-γ and other proinflammatory (IL-1β, IL-6) and immunosuppressive (IL-10) cytokines when whole-blood cultures were stimulated with heat-killed M. tuberculosis in vitro, although RA patients already had a reduced capacity to release IFN-γ compared to healthy controls (27). Our observations correlate with these findings in that no differences were observed in IFN-γ, IL-6, IL-10, or IL-12p40 production after infliximab infusion. However, we found a highly significant reduction at both 24 h and 96 h postinfection in the levels of chemokines IL-8 (CXCL8), MIP-1α (CCL3), MIP-1β (CCL4), and MCP-1 (CCL2) (96 h only) in the culture supernatants of blood taken 30 min after infliximab infusion. Similarly, macrophage production of C-X-C and C-C chemokines is reduced in response to M. tuberculosis infection by an anti-TNF antibody added in vitro (2, 3), delayed chemokine induction has been shown for TNF-deficient mice (28), and a significant reduction in serum IL-8, MCP-1, and RANTES occurred in patients with active RA after an initial infusion of infliximab (22).
However, when we examined a small subset of seven patients whose blood was taken 7 days after infliximab infusion, the reduction was sustained only for MIP-1β and MCP-1 (24 h), although a trend of reduction in all chemokine secretion was observed after infliximab infusion. This might be because the effect of anti-TNF on the production of certain chemokine production occurs early in infection or is short-lived; alternatively, it may be that this analysis was underpowered to detect these effects. The expression of chemokines appears to be partially controlled by TNF production (33). Algood et al. (2) demonstrated that chemokine production was not completely abrogated in bone marrow-derived murine macrophages following neutralization of TNF or in TNFR1−/− macrophages. Infection of TNF−/− mice with M. tuberculosis also resulted in an initial 2-week delay in the induction of chemokine mRNA, but thereafter chemokine expression developed independently of TNF (28), suggesting that other pathways or molecules may also be involved in the stimulation of chemokines. This may explain why the effect of infliximab on cytokine secretion in this assay was not sustained in the 7-day blood sample.
C-C chemokines including MIP-1α, MIP-1β, MCP-1, and RANTES and the C-X-C chemokines IL-8 and IP-10, produced by various cell types, including neutrophils, monocytes/macrophages, and lymphocytes in response to mycobacterial infection, play a potent role in the orchestrated cellular recruitment and trafficking of inflammatory cells (reviewed by Algood et al. [1]) vital for granuloma formation and thus the containment of mycobacteria (30).
In summary, our data suggest that the development of TB in infliximab-treated patients is not directly related to the mycobactericidal effects of TNF but may be due to inhibition of TNF-dependent chemokine gradients disrupting cellular migration necessary to maintain the integrity of the granuloma. This implies that granulomas are dynamic and active structures with considerable turnover, even in latent TB infections.
Acknowledgments
We thank all the patients and staff at Northwick Park Hospital who were involved in this study.
This work was supported by the Wellcome Trust (reference no. 060079, 064261 and GR077273MA).
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Algood, H. M., J. Chan, and J. L. Flynn. 2003. Chemokines and tuberculosis. Cytokine Growth Factor Rev. 14:467-477. [DOI] [PubMed] [Google Scholar]
- 2.Algood, H. M., P. L. Lin, and J. L. Flynn. 2005. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin. Infect. Dis. 41(Suppl. 3):S189-S193. [DOI] [PubMed] [Google Scholar]
- 3.Algood, H. M., P. L. Lin, D. Yankura, A. Jones, J. Chan, and J. L. Flynn. 2004. TNF influences chemokine expression of macrophages in vitro and that of CD11b+ cells in vivo during Mycobacterium tuberculosis infection. J. Immunol. 172:6846-6857. [DOI] [PubMed] [Google Scholar]
- 4.Andreakos, E. T., B. M. Foxwell, F. M. Brennan, R. N. Maini, and M. Feldmann. 2002. Cytokines and anti-cytokine biologicals in autoimmunity: present and future. Cytokine Growth Factor Rev. 13:299-313. [DOI] [PubMed] [Google Scholar]
- 5.Botha, T., and B. Ryffel. 2003. Reactivation of latent tuberculosis infection in TNF-deficient mice. J. Immunol. 171:3110-3118. [DOI] [PubMed] [Google Scholar]
- 6.Boulman, N., M. Rozenbaum, G. Slobodin, and I. Rosner. 2006. Mycobacterium fortuitum infection complicating infliximab therapy in rheumatoid arthritis. Clin. Exp. Rheumatol. 24:723. [PubMed] [Google Scholar]
- 7.Brassard, P., A. Kezouh, and S. Suissa. 2006. Antirheumatic drugs and the risk of tuberculosis. Clin. Infect. Dis. 43:717-722. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello, C. A. 2005. Differences between anti-tumor necrosis factor-a monoclonal antibodies and soluble TNF receptors in host defense impairment. J. Rheumatol. Suppl. 74:40-47. [PubMed] [Google Scholar]
- 9.Ehlers, S. 2003. Role of tumour necrosis factor (TNF) in host defence against tuberculosis: implications for immunotherapies targeting TNF. Ann. Rheum. Dis. 62(Suppl. 2):ii37-ii42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlers, S. 2005. Why does tumor necrosis factor targeted therapy reactivate tuberculosis? J. Rheumatol. Suppl. 74:35-39. [PubMed] [Google Scholar]
- 11.Feldmann, M., and R. N. Maini. 1999. The role of cytokines in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 38(Suppl. 2):3-7. [PubMed] [Google Scholar]
- 12.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 13.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 14.Gardam, M. A., E. C. Keystone, R. Menzies, S. Manners, E. Skamene, R. Long, and D. C. Vinh. 2003. Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect. Dis. 3:148-155. [DOI] [PubMed] [Google Scholar]
- 15.Giles, J. T., and J. M. Bathon. 2004. Serious infections associated with anticytokine therapies in the rheumatic diseases. J. Intensive Care Med. 19:320-334. [DOI] [PubMed] [Google Scholar]
- 16.Islam, N., A. R. Kanost, L. Teixeira, J. Johnson, R. Hejal, H. Aung, R. J. Wilkinson, C. S. Hirsch, and Z. Toossi. 2004. Role of cellular activation and tumor necrosis factor-alpha in the early expression of Mycobacterium tuberculosis 85B mRNA in human alveolar macrophages. J. Infect. Dis. 190:341-351. [DOI] [PubMed] [Google Scholar]
- 17.Kampmann, B., P. O. Gaora, V. A. Snewin, M. P. Gares, D. B. Young, and M. Levin. 2000. Evaluation of human antimycobacterial immunity using recombinant reporter mycobacteria. J. Infect. Dis. 182:895-901. [DOI] [PubMed] [Google Scholar]
- 18.Keane, J. 2005. TNF-blocking agents and tuberculosis: new drugs illuminate an old topic. Rheumatology (Oxford) 44:714-720. [DOI] [PubMed] [Google Scholar]
- 19.Keane, J. 2004. Tumor necrosis factor blockers and reactivation of latent tuberculosis. Clin. Infect. Dis. 39:300-302. [DOI] [PubMed] [Google Scholar]
- 20.Keane, J., S. Gershon, R. P. Wise, E. Mirabile-Levens, J. Kasznica, W. D. Schwieterman, J. N. Siegel, and M. M. Braun. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098-1104. [DOI] [PubMed] [Google Scholar]
- 21.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56:731-740. [DOI] [PubMed] [Google Scholar]
- 22.Klimiuk, P. A., S. Sierakowski, I. Domyslawska, and J. Chwiecko. 2006. Regulation of serum chemokines following infliximab therapy in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 24:529-533. [PubMed] [Google Scholar]
- 23.Mohan, V. P., C. A. Scanga, K. Yu, H. M. Scott, K. E. Tanaka, E. Tsang, M. M. Tsai, J. L. Flynn, and J. Chan. 2001. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect. Immun. 69:1847-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mufti, A. H., B. W. Toye, R. R. McKendry, and J. B. Angel. 2005. Mycobacterium abscessus infection after use of tumor necrosis factor alpha inhibitor therapy: case report and review of infectious complications associated with tumor necrosis factor alpha inhibitor use. Diagn. Microbiol. Infect. Dis. 53:233-238. [DOI] [PubMed] [Google Scholar]
- 25.Nestorov, I., M. I. Sadek, E. Sada, Z. Toossi, S. K. Schwander, E. A. Rich, C. Ameixa, and J. S. Friedland. 2005. Clinical pharmacokinetics of TNF antagonists: how do they differ? Semin. Arthritis Rheum. 34(Suppl 1):12-18. [DOI] [PubMed] [Google Scholar]
- 26.Okubo, H., M. Iwamoto, T. Yoshio, H. Okazaki, T. Kato, M. Bandoh, and S. Minota. 2005. Rapidly aggravated Mycobacterium avium infection in a patient with rheumatoid arthritis treated with infliximab. Mod. Rheumatol. 15:62-64. [DOI] [PubMed] [Google Scholar]
- 27.Popa, C., M. G. Netea, P. Barrera, T. R. Radstake, P. L. van Riel, B. J. Kullberg, and J. W. Van der Meer. 2005. Cytokine production of stimulated whole blood cultures in rheumatoid arthritis patients receiving short-term infliximab therapy. Cytokine 30:72-77. [DOI] [PubMed] [Google Scholar]
- 28.Roach, D. R., A. G. Bean, C. Demangel, M. P. France, H. Briscoe, and W. J. Britton. 2002. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 168:4620-4627. [DOI] [PubMed] [Google Scholar]
- 29.Saliu, O. Y., C. Sofer, D. S. Stein, S. K. Schwander, and R. S. Wallis. 2006. Tumor-necrosis-factor blockers: differential effects on mycobacterial immunity. J. Infect. Dis. 194:486-492. [DOI] [PubMed] [Google Scholar]
- 30.Saunders, B. M., and A. M. Cooper. 2000. Restraining mycobacteria: role of granulomas in mycobacterial infections. Immunol. Cell Biol. 78:334-341. [DOI] [PubMed] [Google Scholar]
- 31.Snewin, V. A., M. P. Gares, P. O. Gaora, Z. Hasan, I. N. Brown, and D. B. Young. 1999. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect. Immun. 67:4586-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tena, G. N., D. B. Young, B. Eley, H. Henderson, M. P. Nicol, M. Levin, and B. Kampmann. 2003. Failure to control growth of mycobacteria in blood from children infected with human immunodeficiency virus and its relationship to T cell function. J. Infect. Dis. 187:1544-1551. [DOI] [PubMed] [Google Scholar]
- 33.Tessier, P. A., P. H. Naccache, I. Clark-Lewis, R. P. Gladue, K. S. Neote, and S. R. McColl. 1997. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J. Immunol. 159:3595-3602. [PubMed] [Google Scholar]
- 34.Tufariello, J. M., J. Chan, and J. L. Flynn. 2003. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect. Dis. 3:578-590. [DOI] [PubMed] [Google Scholar]
- 35.Wallis, R. S., M. S. Broder, J. Y. Wong, M. E. Hanson, and D. O. Beenhouwer. 2004. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin. Infect. Dis. 38:1261-1265. [DOI] [PubMed] [Google Scholar]
- 36.Wallis, R. S., and S. Ehlers. 2005. Tumor necrosis factor and granuloma biology: explaining the differential infection risk of etanercept and infliximab. Semin. Arthritis Rheum. 34:34-38. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson, R. J., L. E. DesJardin, N. Islam, B. M. Gibson, R. A. Kanost, K. A. Wilkinson, D. Poelman, K. D. Eisenach, and Z. Toossi. 2001. An increase in expression of a Mycobacterium tuberculosis mycolyl transferase gene (fbpB) occurs early after infection of human monocytes. Mol. Microbiol. 39:813-821. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe, F., K. Michaud, J. Anderson, and K. Urbansky. 2004. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum. 50:372-379. [DOI] [PubMed] [Google Scholar]