Abstract
The abilities of monoclonal antibodies (MAbs) that bind to defined sequential epitopes on the dengue virus (DENV) nonstructural-1 (NS1) glycoproteins to cross-react with epitopes on the DENV envelope (E) glycoproteins were investigated. In this study, some of these MAbs cross-reacted with the DENV type 2 (DENV-2) E glycoprotein and with synthetic peptides representing X-ray crystallographically confirmed surface-exposed regions on this glycoprotein. MAb 1G5.3 cross-reacted with the flavivirus-conserved 101-WGNGCGLFG-109 fusion sequence, the 273-SSGNL-277 DENV-2 hinge region sequence, and the 156-GKHGKEIKIT-165 sequence of virulent DENV-2 strains. MAb 1G5.4-A1-C3 cross-reacted with the 67-NTTTESRCPT-76 and 156-GKHGKEIKIT-165 sequences of virulent DENV-2 strains, the 338-EIMDLDNRHV-347 sequence from a highly virulent DENV-2 (M2) strain, and two epitopes on a virulent DENV-3 strain (288-KMDKLELKG-296 and 323-RVEYRGEDAP-332), which all contained target ELK/KLE-type motifs (underlined). These MAbs showed reduced cross-reactions with the corresponding sequences from weakly pathogenic strains of all four DENV serotypes and had either no (MAb 1G5.4-A1-C3) or weak (MAb 1G5.3) neutralizing activity against them. MAb 1G5.3 more strongly neutralized DENV-2 strains with higher pathogenic capacities, while MAb 1G5.4-A1-C3 showed increasing neutralizing titers against the virulent DENV-3 strain and the moderately virulent and highly virulent (M2) DENV-2 strains. These cross-reactions with the E glycoprotein accord with the observation that MAb 1G5.3 caused dramatic and lethal antibody-enhanced replication (AER) of a DENV-2 strain in vivo. Together with in vivo AER studies of these DENV strains using MAb 1G5.4-A1-C3, these results may account for the increased pathogenic capacities of such strains, which is likely to have important implications for pathogenesis and vaccines.
The spread of dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) throughout the world has occurred through transportation of the more virulent viral strains from Southeast Asia, where DHF/DSS is the main cause of juvenile hospitalization (14). Strains of dengue virus type 2 (DENV-2) and DENV-3 are associated with most cases of DHF/DSS, but there are no reliable virulence marker sequences on pathogenic DENV strains. Nearly all DHF/DSS cases result from sequential infection with a virulent DENV strain of another serotype after the initial infection (14, 15). Patient antibodies bind to common epitopes on the heterologous virus, and instead of cross-neutralization, they can enhance the replication of DENV strains in target Fc receptor-bearing monocytes/macrophages, which has been hypothesized to account for DHF/DSS (15). The majority of evidence for antibody-enhanced replication (AER) of the DENVs comes from in vitro studies, but dramatic AER of a DENV-2 strain has also been demonstrated in vivo (10; see below).
Human immunoglobulin G (IgG) polyclonal antibodies (PAbs) generated against the DENV nonstructural-1 (NS1) glycoprotein could be detected only during the convalescent phase of primary DENV infections but were strongly identified during the acute phase of secondary DENV infections (37), suggesting that they may play a role in the pathogenesis of DHF/DSS. During DENV infections, human PAb responses were generated to multiple acidic (E or D)-aliphatic/aromatic (G, A, I, L or V/F, W, or Y)-basic (K or R) (ELK-type tri-amino acid) motifs present in either orientation (ELK/KLE-type motifs) on the DENV NS1 glycoproteins, and these responses were higher in DSS patients than in patients with mild disease (dengue fever [DF]) (7). Monoclonal antibody (MAb) 1G5.4-A1-C3 displayed the same reaction pattern as that for human DSS patient PAbs against multiple ELK/KLE-type epitopes on the DENV-2 NS1 glycoprotein, and therefore the cross-reaction of this MAb with other DENV proteins and human proteins is likely to be highly relevant in studies of DHF/DSS pathogenesis (7, 9). Three other MAbs generated to the DENV-2 NS1 protein also recognized short sequential amino acid sequences. MAb 1C6.3 reacted more specifically with multiple KELK-type motifs present in either orientation (KELK/KLEK-type motifs), MAb 3D1.4 recognized the LX1 (113-YSWKTWG-119) epitope, and MAb 1G5.3 recognized the 24C (301-TTASGKLIT-309) epitope (7, 9, 12).
Mouse PAbs and MAbs generated to the DENV-2 NS1 glycoprotein precipitated the DENV-2 NS1 glycoprotein, together with lower concentrations of the DENV-2 envelope (E) and premembrane (prM) glycoproteins (35), suggesting that common epitopes occur on these viral glycoproteins. This was further supported by the finding that PAbs, and some MAbs, raised to the DENV-2 NS1 glycoprotein could generate dramatic (>100,000-fold) and lethal AER of a DENV-2 strain in vivo (10).
Many epitope-reactive MAbs, defined by neutralizing DENV type or complex as well as by flavivirus subgroup and group, have been located within the E glycoprotein. These epitopes were identified by either the generation of escape mutations (13, 24, 25, 36), binding studies using recombinant protein fragments (26), reactions with recombinant constructs containing specific amino acid substitutions (4, 6, 17, 38, 39), or reactions with synthetic peptide sequences (1, 8, 18). From these studies, epitopes recognized by neutralizing MAbs were found to be located in each of three domains (domains I, II, and III) and could be confirmed to be surface exposed using the high-resolution X-ray crystal structure of the dimeric (prefusion) form of the DENV-2 (PR159S1 strain) E glycoprotein (27). Some of the sequences recognized by neutralizing MAbs and other surface-exposed sequences of the DENV-2 and DENV-3 E glycoproteins (27, 29) contained short (K)ELK/KLE(K)-type or SGK/KGS-type motifs, suggesting that MAbs 1G5.4-A1-C3, 1C6.3, and 1G5.3 are likely to cross-react with these motifs. Some DENV-2 strains which had greater human pathogenic capacities also contained additional (K)ELK/KLE(K)-type motifs or amino acid substitutions within some surface-exposed sequences on their E glycoproteins; such additional motifs may therefore affect the binding of these MAbs.
In this study, (i) six MAbs, including MAbs 1G5.4-A1-C3, 1C6.3, 3D1.4, and 1G5.3, which recognize sequential epitopes on the DENV-2 NS1 glycoprotein, were tested for the ability to cross-react with the E glycoprotein on purified DENV-2 particles; (ii) the cross-reactions of these MAbs were tested against an overlapping set of synthetic peptides sequentially spanning the entire sequence of domain III of the DENV-2 E glycoprotein and a panel of synthetic peptides representing sequential epitopes and other surface-exposed sequences on domains I, II, and III from the E glycoproteins of all four DENV serotypes and DENV-2 and DENV-3 strains with different human pathogenic capacities; and (iii) these MAbs were further tested against each DENV serotype and different DENV-2 and DENV-3 strains in plaque reduction neutralization tests. The results of this study may thus provide an explanation for the finding of weak cross-reactions of antibodies generated against the DENV-2 NS1 glycoprotein with the DENV-2 E glycoprotein (35) and their ability to cause dramatic AER of a DENV-2 strain in vivo (10) and may possibly locate the epitopes on the DENV-2 and DENV-3 E glycoproteins which may account for variations in their pathogenic capacities through increased AER potentials.
MATERIALS AND METHODS
Flavivirus growth.
The DENV strains used in this study were kindly supplied by J. G. Aaskov (DENV-2 strains NG-C [New Guinea 1944] and D-3V H-87 [Philippines 1956]), D. J. Gubler (DENV-3 strain SL-2783 [SL-91] [Sri Lanka 1991]), C. J. Leake (DENV-2 strain TR1751 [TR] [Trinidad 1953]), C. J. Lai (D-4V strain 814669 [Dominica 1981]), S. K. Lam (DENV-2 strain M725 [M2] [Malaysia 1987]), and R. E. Shope (DENV-1 strain WPAC74 [Nauru Island 1974]). The growth of the DENVs in mammalian fibroblast (Vero) cells was previously described (8, 9). These viruses were used to infect 70% confluent Vero cell monolayers maintained in medium 199 (M199; Sigma) containing 0.18% (wt/vol) NaHCO3, 3.5% (vol/vol) fetal bovine serum, and antibiotics (mammalian cell growth medium). After incubation at 37°C for 4 to 5 days, the supernatants were harvested, clarified by centrifugation, and stored at −80°C. For enzyme-linked immunosorbent assays (ELISAs) and plaque reduction neutralization tests (see below), these viruses were used to infect 70% confluent C6/36 (Aedes albopictus) cell monolayers maintained in Leibovitz (L-15) medium containing 10% (vol/vol) tryptose phosphate broth, 10% (vol/vol) fetal bovine serum, and antibiotics (Sigma) (insect cell growth medium) as described previously (10). After incubation at 28°C for 4 to 5 days, the supernatants were harvested, adjusted to pH 7.2 using 1 M Tris-HCl (pH 7.2), clarified by centrifugation at 250 × g, and stored at −80°C.
Virus purification.
The purification of DENV-2 particles was described previously (8). For this study, the DENV-2-infected supernatants from insect (C6/36) cells were slowly made to contain 7% (wt/vol) polyethylene glycol 8000 (Sigma) and 0.4% (wt/vol) NaCl by the addition of cold stock solutions, and the DENV-2 particles were precipitated overnight at 4°C. These supernatants were then centrifuged at 10,000 × g for 30 min at 4°C (JA10 rotor; Beckman), the supernatants were discarded, and the precipitated DENV-2 particles were resuspended in a minimal volume of 30 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, and 0.02% (wt/vol) NaN3 containing a panel of protease inhibitors (Sigma) (protein purification buffer [PPB]). The concentrated DENV-2 particles were then further purified by adding 0.5 ml of concentrated DENV-2 to a 20% (3.5 ml)/50% (1 ml) (wt/wt) discontinuous sucrose gradient prepared in 20 mM Tris-HCl, pH 7.4, containing 0.02% NaN3. These samples were then centrifuged in an SW55 swing-out rotor (Beckman) at 150,000 × g for 2 h at 4°C before 0.5-ml fractions were collected. The fractions were analyzed by immunoblot assay, and those containing the purified DENV-2 particles were pooled, diluted to 10% (wt/wt) sucrose, added to a 15-ml 10 to 50% (wt/wt) continuous sucrose gradient prepared with PPB in 17-ml ultracentrifuge tubes, and centrifuged in an AH627 swing-out rotor (Sorval, Dupont) at a mean gravitational force of 80,800 × g for 18 h at 4°C. Fractions (0.5 ml) were collected and analyzed by immunoblot assay, those containing the purified virus were pooled and diluted to 20 ml with PPB, and the DENV-2 particles were reprecipitated using 7% (wt/vol) polyethylene glycol 8000 and 0.4% (wt/vol) NaCl for 18 h at 4°C. These tubes were then centrifuged at 10,000 × g, the supernatants were discarded, the purified DENV-2 particles were resuspended in 1 ml of PPB, and aliquots were stored at −80°C. The protein concentration was then determined using serial sample dilutions in a microtiter plate-adapted bicinchoninic acid (BCA) protein assay (Pierce) (10 μl of sample/200 μl BCA reagent) using standard concentrations (0 to 1,000 μg/ml) of bovine serum albumin. After incubation at 37°C for 60 min, the optical densities at 570 nm were determined (MRX; Dynex), and the protein concentrations were established from a standard calibration curve.
Purification of DENV-2 NS1 glycoprotein.
Immunoaffinity purification of the native dimeric intracellular (iNS1) and extracellular (eNS1) forms of the DENV-2 NS1 glycoprotein from infected mammalian cell culture supernatants was described previously (11). To purify the DENV-2 eNS1 glycoprotein, clarified cell culture supernatants of DENV-2 (strain NG-C)-infected Vero cells were made to 30 mM Tris-HCl (pH 7.2) and 0.02% (wt/vol) NaN3 with protease inhibitors (PPB). These supernatants were slowly (1 ml/min) passed through an immunoaffinity column containing 12 mg of MAb 2A5.1 covalently coupled to 3.5 ml of cyanogen bromide-activated Sepharose 4B (Pharmacia). After being washed with PPB, the bound DENV-2 eNS1 protein was eluted using 20 mM diethylamine (DEA; pH 11.2) in PPB containing protease inhibitors, and 0.5-ml fractions were immediately neutralized using 0.1 ml of 1 M Tris-HCl (pH 7.2). To purify the DENV-2 iNS1 glycoprotein, DENV-2-infected mammalian cell monolayers were washed twice with PPB, scraped into PPB, made to 2% (vol/vol) Triton X-100 (Sigma), and emulsified overnight at 4°C. Detergent-insoluble material was pelleted by centrifugation at 20,000 × g (JA20 rotor; Beckman), and the supernatants were collected, diluted to 0.3 to 0.5% (vol/vol) Triton X-100 with PPB, and then slowly passed through the immunoaffinity column. The column was then washed with PPB, and the bound DENV-2 iNS1 glycoprotein was eluted and collected as described for the purification of DENV-2 eNS1 glycoprotein. The concentrations of DENV-2 eNS1 and iNS1 glycoproteins in each fraction were determined using a microtiter plate-adapted BCA protein assay (Pierce) (see above).
Preparation and purification of MAbs.
All animal experiments described were performed under a personal animal procedure license (PIL 70/6903) issued by the Home Office of the United Kingdom. The preparation and characterization of mouse MAbs against the DENV-2 E, prM, and NS1 glycoproteins were described previously (8, 11). Briefly, BALB/c mice were repeatedly immunized with either live DENV-2 (PR159 strain) or the immunoaffinity-purified DENV-2 (PR159 strain) NS1 glycoprotein, their splenocytes were fused with the SP2/0 Ag14 cell line, and specific MAb-secreting hybridomas were identified by ELISA and immunoblot assays. The hybridoma cell lines were cloned twice by limiting dilution, and their IgG subclasses were determined by either ELISA or radial immunodiffusion assays using mouse IgG subclass-specific sheep PAbs (Serotec, United Kingdom). High concentrations of each MAb were prepared in double-pristine primed BALB/c mice as described previously (11). The MAbs were further assessed for the ability to react with the E, prM (8, 10), or NS1 (9, 11) glycoproteins of other DENV serotypes and flaviviruses. The epitopes recognized by some of these MAbs were precisely located using panels of synthetic peptides from the DENV-2 E and NS1 glycoproteins (8, 9, 12). Of the MAbs used in this study, the neutralizing flavivirus group-reactive MAb 2C5.1 and DENV complex prM glycoprotein-reactive MAb 2A4.1 were of the IgG2a subclass (8); MAbs 1H7.4, 5H4.3, 3D1.4, 1G5.3, and 1C6.3 were of the IgG1 subclass (11); and MAb 1G5.4-A1-C3 was of the IgG2b subclass (9). MAbs of the IgG1 and IgG2b subclasses were diluted 1/20 in 0.1 M glycine-NaOH, pH 8.9, containing 3 M NaCl (binding buffer) and repeatedly passed through a 5.0-ml immunoaffinity column of Staphylococcus aureus protein A-Sepharose CL-4B (Sigma). After being washed with binding buffer, the MAbs were eluted using 0.1 M glycine-HCl, pH 5.5 (most IgG1 MAbs) or 4.5 (IgG2b and one IgG1 MAb [MAb 1C6.3]), and 1-ml fractions were immediately neutralized with 100 μl of 1 M Tris-HCl (pH 7.4). The MAb concentration in each fraction was determined using a microtiter plate-adapted BCA protein assay (see above), using standard concentrations of purified mouse IgG (Sigma). The MAb-containing fractions were pooled and concentrated to 1 to 10 mg/ml by reduced-pressure dialysis against PPB, and aliquots were stored at −80°C. MAbs 2C5.1 and 2A4.1, of the IgG2a subclass, were purified using the same steps but instead were loaded onto the protein A-Sepharose column with PPB, pH 7.8, and eluted with 0.1 M glycine-HCl, pH 3.0.
Immunoassays.
Indirect ELISAs and immunoblot assays using purified DENV-2 particles and the DENV-2 NS1 glycoprotein were described previously (7, 10). For ELISA, optimal concentrations of purified DENV-2 particles (1/250 dilution; 0.58 μg/ml) and DENV-2 eNS1 glycoprotein (2 to 3 μg/ml) were diluted in 0.16%/0.29% (wt/vol) sodium carbonate/bicarbonate (pH 9.8), loaded at 50 μl/well into ELISA plates (Immulon 2; Dynatech), and adsorbed overnight at 4°C. After being washed with phosphate-buffered saline (PBS) (pH 7.4), the plates were blocked for 2 h at 25°C with 1% (wt/vol) gelatin (Sigma) in PBS (100 μg/well). After being washed with PBS containing 0.05% Tween 20 (Sigma) (PBS/T), triplicate serial three- or fourfold dilutions of each MAb were prepared in PBS/T containing 0.25% (wt/vol) gelatin (PBS/T/G), with and without 10 mM DEA (Sigma; pH 11.0) (triplicate titrations with and without DEA), to gauge their avidity (33). After incubation at 25°C for 2 h, the contents of each well were aspirated. The plates were then washed with PBS/T, and the bound MAbs were detected by sequential steps using a 1/2,000 dilution of a horseradish peroxidase-labeled goat anti-mouse IgG (heavy and light chains; Jackson ImmunoResearch) prepared in PBS/T/G, washing with PBS/T, and incubation with 0.04% (wt/vol) o-phenylenediamine dihydrochloride (Sigma) and 0.003% (vol/vol) H2O2 in citrate-phosphate buffer (pH 5.0) (50 μl/well). After 10 min, the substrate reaction step was terminated by the addition of 2 M H2SO4 (25 μl/well), the absorbance values were determined at dual wavelengths of 492 and 620 nm (MRX; Dynex), and the reciprocal log10 50% end-point titers (1/log10t50) were determined for each MAb in the presence or absence of DEA. The avidity indexes (AIs) for each MAb against the DENV-2 particles and DENV-2 eNS1 glycoprotein were determined by the following formula: AI (%) = reciprocal anti-log10t50 with DEA/reciprocal anti-log10t50 without DEA × 100, as described previously (33) (or MAb concentration [ng/ml] at the reciprocal log10t50 without DEA/MAb concentration [ng/ml] at the reciprocal log10t50 with DEA × 100, which gave the same AI).
For immunoblot (Western blot) assays, 1,163-ng (8 μl), 292-ng (2.0 μl), and 73-ng (0.5 μl) samples of purified DENV-2 particles and 155-ng and 261-ng samples of iNS1 and eNS1, respectively, were heated at 100°C for 3 min in 2.0% (wt/vol) sodium dodecyl sulfate (SDS) and 33 mM phosphoric acid containing 0.71% (wt/vol) Trisma base (pH 6.8) (sample lysis buffer) with 0.01% (wt/vol) bromophenol blue and 10% (vol/vol) glycerol. These samples were subjected to discontinuous SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on 8 to 9% (wt/vol) resolving gels at 20 mA (Miniprotean II; Bio-Rad). The proteins were semidry electroblotted (Sartoblot II; Sartorius) at 160 mA/gel onto 0.2-μm-pore-size nitrocellulose membranes (Schleicher and Schuell, Germany) for 30 min. These immunoblot membranes were then air dried before being blocked using 2% (wt/vol) milk powder (Marvel; Cadbury's, United Kingdom) in PBS and then washed with PBS/T. MAb 1C6.3 or pools of PAbs, generated in high-responding congenic mouse strains (H-2s and H-2b) in response to repeated infections with 3.2 × 105 PFU of DENV-2 (TR1751 strain), were diluted to 1/200, while all of the other MAbs were diluted to 1/400, in PBS/T containing 2% milk powder (PBS/T/M) and incubated with the membranes at 25°C for 2 h. After being washed with PBS/T, the bound MAbs were detected by sequential steps with peroxidase-labeled secondary antibody (see above) and washing with PBS/T, 0.02% (wt/vol) 3,3′-diaminobenzidine tetrahydrochloride, and 0.06% (wt/vol) 4-chloro-1-naphthol (Sigma) containing 0.004% (vol/vol) H2O2 in PBS.
Plaque reduction neutralization tests were described previously (8). For these assays, serial twofold dilutions of each MAb, starting from 1/8 (or 1/2 for MAb 1H7.4), were prepared in mammalian cell growth medium (see above) (125 μl/well) in duplicate rows in two 48-well cell culture plates (Costar). Four serial 10-fold dilutions, starting from 1/100, of each DENV serotype/strain grown in C6/36 cells (each concentration in duplicate rows of two plates), prepared in mammalian cell growth medium (125 μl/well), were then added. Vero cells (2 × 105/250 μl of mammalian cell growth medium/well) were then added, and the plates were incubated at 37°C for 2 h, after which 500 μl/well of mammalian cell growth medium containing 1.5% (wt/vol) carboxymethyl cellulose (Sigma) was added. These plates were then incubated at 37°C with 5% CO2 for 7 days. The cells were then fixed by the addition of 8% (wt/vol) formaldehyde, incubated for 1 h, washed with H2O, stained with 0.1% (wt/vol) crystal violet-PBS for 1 h, washed with H2O, and then air dried. The average plaque reduction neutralization titer (PRNT) of each MAb which reduced the numbers of plaques by 50% was then determined.
Preparation and use of synthetic peptides.
The preparation and use of synthetic peptides on pins/gears (Chiron Mimetopes, United Kingdom) were described previously (7, 8, 9). Briefly, overlapping duplicate sets of 9-mer peptides sequentially moving two amino acid residues along the entire domain III sequence of the DENV-2 (NG-C strain) E protein (22) were prepared (8). Peptide sequences from domains I and II of different DENV-2 strains (22) were also prepared; these sequences had been identified either (i) by using short synthetic peptide sequences from the DENV-2 E glycoprotein (50-57, 127-134, 349-356 [1], 274-283 [8], and 278-297 [18]), (ii) by studying the neutralizing MAb escape mutations in the DENV-2 E glycoprotein (307-K to E [24], E-69-T to I, 71-E to D, 311-E to G, 112-S to G, 124-I to N [25], 169-S to P, 275-G to R, 386-K to N [36], and natural DENV-2 cell passage mutation 53-P to S [25]), (iii) by studying MAb reactions with recombinant DENV-2 particles containing the 106-G-to-Q or 107-L-to-K amino acid substitution in their E glycoproteins (4), or (iv) by being contained in DENV-2 E glycoprotein peptide sequences 35-55 and 352-368, which generated neutralizing PAbs in mice (32). Synthetic peptide sequences from other regions shown to be surface exposed by X-ray diffraction of the native dimeric form of the DENV-2 E glycoprotein (27) were also prepared. The DENV-2 strains were classified as either the Asian 1, Asian 2, American/Asian, Cosmopolitan, or American genotype, as described previously (40). Comparisons with corresponding sequences of DENV-3 (H87 and SL-91 strains) (20), using the X-ray crystallographic E glycoprotein structure of the DENV-3 H87 strain (29), were also performed. The structures of the E glycoproteins (DENV-2 structures, accession no. MMDB 23080, 25032, and 32273; DENV-3 structure, accession no. MMDB 32273) were obtained from the NCBI structural database (http://ncbi.nlm.nih.gov). Importantly, the original sequence of the American DENV-2 PR159S1 genotype candidate vaccine strain (NCBI accession number P12823) contained 71-D, 162-I, and 390-D in the E glycoprotein sequence (22), which are the markers of the American DENV-2 genotype strains. The DENV-2 PR159S1 strain used to prepare the high-resolution X-ray crystallographic structures (27, 28), but not the lower-resolution cryo-electron microscopic structure determination (43), contained the 71-D-to-E and 309-D-to-N substitutions present in the more virulent DENV-2 strains.
All of these synthetic peptides were prepared on 64-nM polypropylene gears by using activated 9-fluorenylmethoxy carbonyl-protected amino acid esters (Novabiochem, United Kingdom), the amino termini were acetylated, and the amino acid side chain protective groups were subsequently removed as described previously (7, 8, 9). These peptides were used in 96-well microtiter plates with 100-μl reagent volumes following each standard ELISA reaction step (see above), with the MAbs diluted to 100 times their ELISA titers (1/log10t50) against the DENV-2 eNS1 glycoprotein or DENV-2 particles (MAb 2C5.1). After each ELISA reaction, these peptides were recycled by immersion in disruption buffer (0.1% [wt/vol] SDS in 1% [wt/vol] NaHPO4-NaOH [pH 7.2]) at 58°C in an ultrasonic water bath for 20 min before being washed with hot water (58°C) and boiling methanol.
Sequence analysis of DENV E glycoproteins and secondary structural predictions of DENV-2 NS1 protein.
The amino acid sequence of the DENV-2 (strain NG-C) NS1 protein was analyzed using eight different computer algorithms (DPM, DSC, GOR4, HNNC, PHD, Predator, SIMPA96, and SOPM), and a consensus structural prediction of an alpha-helix, 310-helix, Pi-helix, β-bridge, extended-strand, β-turn, bend region, random coil, or ambiguous state was assigned to each amino acid, using the Pole Bio-Informatique Lyonnais database (http://pbil.univ-lyon1.fr). The E glycoprotein amino acid sequences of the different strains of each DENV serotype were obtained from the NCBI protein database (http://ncbi.nlm.nih.gov).
RESULTS
Purification of DENV-2 particles and DENV-2 NS1 protein.
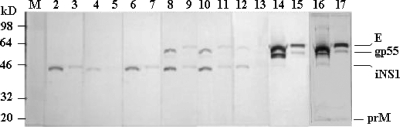
To test the cross-reactions of MAbs generated to the DENV-2 NS1 protein against the DENV-2 E and prM glycoproteins, DENV-2 particles were concentrated and sucrose gradient purified from infected insect (C6/36) cell culture supernatants. These purified DENV-2 particles contained high concentrations of the main envelope (E) glycoprotein and low concentrations of the truncated E (gp55), NS1 (gp46), and prM (gp20) glycoproteins (Fig. 1). In contrast, the immunoaffinity-purified preparations of the iNS1 (gp46) and eNS1 (gp 48-52) forms of the DENV-2 NS1 glycoproteins obtained from infected mammalian cells contained no contaminating E (gp60/55) or prM (gp20) glycoprotein.
FIG. 1.
Immunoblot analysis of purified DENV-2 particles and the DENV-2 NS1 glycoprotein. Three concentrations, 1,163 ng, 292 ng, and 73 ng, of purified DENV-2 particles from infected insect cell culture supernatants, in lanes 2, 3, and 4, respectively, and the immunoaffinity-purified iNS1 (155 ng) and eNS1 (262 ng) forms of the DENV-2 NS1 glycoprotein, in lanes 5 and 6, respectively, were subjected to SDS-PAGE and electroblotted onto nitrocellulose membranes, and the glycoproteins were detected using a mixture of MAbs generated against the DENV-2 E (MAb 2C5.1), NS1 (MAb 3D1.4), and prM (MAb 2A4.1) glycoproteins. The locations of each molecular weight marker in lane 1 and of the DENV-2 E (gp60), gp55 (truncated gp60), eNS1 (gp48-52), iNS1 (gp46), and prM (gp20) glycoproteins are shown.
Reaction of MAbs with purified DENV-2 particles and NS1 protein in ELISAs.
The abilities of six MAbs generated to the DENV-2 NS1 glycoprotein to cross-react with purified DENV-2 particles in an ELISA in the presence and absence of DEA were tested to gauge their relative avidities (33). In this study, all of the MAbs generated to the DENV-2 NS1 glycoprotein had moderately high to high ELISA titers against the homologous DENV-2 NS1 glycoprotein (1/log10t50, 4.52 to 6.12) (Table 1). DEA had a variable effect in reducing their ELISA titers against this protein. MAb 1H7.4, which bound to the DENV-2-specific LD2 epitope on the NS1 glycoprotein, showed the highest ELISA titer (1/log10t50 of 6.12 [3.14 ng/ml]) and AI (36.30%) against the homologous DENV-2 NS1 glycoprotein. MAb 5H4.3, which bound to the DENV-2 and -4 subcomplex 24A epitope on the NS1 glycoprotein, had a moderately high ELISA titer (1/log10t50 of 5.21 [17.14 ng/ml]) but a relatively high AI (34.67%) against this glycoprotein. MAbs 3D1.4 and 1G5.3, which bound to the DENV complex LX1 epitope and the DENV-2 and -4 subcomplex 24C epitope, respectively, had only moderately high ELISA titers and relatively low AIs (for MAb 3D1.4, 1/log10t50 of 5.88 [4.42 ng/ml] and AI of 13.81%; and for MAb 1G5.3, 1/log10t50 of 5.65 [5.75 ng/ml] and AI of 14.79%) against the DENV-2 NS1 glycoprotein. In contrast, MAbs 1G5.4-A1-C3 and 1C6.3, which bound to multiple ELK/KLE-type and KELK/KLEK-type epitope motifs, respectively, on the NS1 glycoproteins of all DENV serotypes, had relatively lower ELISA titers and AIs against the DENV-2 NS1 glycoprotein (for MAb 1G5.4-A1-C3, 1/log10t50 of 5.17 [15.95 ng/ml] and AI of 5.62%; and for MAb 1C6.3, 1/log10t50 of 4.52 [44.39 ng/ml] and AI of 4.47%). Despite MAbs 5H4.3 and 1G5.4-A1-C3 having similar ELISA titers (1/log10t50 of 5.21 and 5.17), which corresponded to their similar protein concentrations (17.14 and 15.85 ng/ml), MAb 5H4.3 had a much higher AI (34.67%) than did MAb 1G5.4-A1-C3 (5.62%). MAb concentrations at their ELISA titers (1/log10t50), which could be used to determine their Kd (dissociation constant) values (13), did not therefore correlate with their AIs. As expected, MAb 2C5.1, which bound to a flavivirus group epitope on the E glycoprotein, had the lowest ELISA titer (1/log10t50 of 2.28 [17,213.69 ng/ml]) and AI (2.88%) against the DENV-2 NS1 glycoprotein but had the highest ELISA titer (1/log10t50 of 5.46 [11.37 ng/ml]) and AI (11.21%) of all seven MAbs against purified DENV-2 particles. MAbs 1H7.4 and 5H4.3 had very low ELISA titers against DENV-2 particles, for which AIs could not be determined, while MAb 3D1.4 very weakly cross-reacted with DENV-2 particles (1/log10t50 of 2.02 [31,992.25 ng/ml]), with an AI of 6.31%, and therefore could only be observed to cross-react at very high MAb concentrations (>32.0 μg/ml). In contrast, MAbs 1G5.3 and 1G5.4-A1-C3 cross-reacted more strongly with DENV-2 particles, and both had similar ELISA titers and AIs (for MAb 1G5.3, 1/log10t50 of 3.43 [954.85 ng/ml] and AI of 2.34%; and for MAb 1G5.4-A1-C3, 1/log10t50 of 3.37 [1,006.73 ng/ml] and AI of 2.51%).
TABLE 1.
ELISA titers, concentrations, and AIs of MAbs against purified DENV-2 particles and the NS1 glycoprotein
| MAb | Concn (mg/ml) | Specificity (serotypes) | ELISA titer (concn [ng/ml])a
|
AI (%)b | |
|---|---|---|---|---|---|
| Without DEA | With DEA | ||||
| MAbs against DENV-2 NS1 glycoprotein | |||||
| 1H7.4 | 4.14 | NS1 (DENV-2) | 6.12 (3.14) | 5.68 (8.65) | 36.30 |
| 5H4.3 | 2.78 | NS1 (DENV-2 and -4) | 5.21 (17.14) | 4.75 (49.44) | 34.67 |
| 3D1.4 | 3.35 | NS1(DENV-1 to -4) | 5.88 (4.42) | 5.02 (31.99) | 13.82 |
| 1G5.3 | 2.57 | NS1 (DENV-2 and -4) | 5.65 (5.75) | 4.82 (38.90) | 14.78 |
| 1G5.4-A1-C3 | 2.36 | NS1 (DENV-1 to -4) | 5.17 (15.95) | 3.92 (283.73) | 5.62 |
| 1C6.3 | 1.47 | NS1 (DENV-1 to -4) | 4.52 (44.39) | 3.17 (993.84) | 4.47 |
| 2C5.1 | 3.28 | E (DENV-1 to -4) | 2.28 (17,213.69) | 0.74 (596,861.88) | 2.88 |
| MAbs against DENV-2 particles | |||||
| 1H7.4 | 4.14 | NS1 (DENV-2) | 0.62 (993,116.83) | ||
| 5H4.3 | 2.78 | NS1 (DENV-2 and -4) | 0.80 (440,600.31) | ||
| 3D1.4 | 3.35 | NS1 (DENV-1 to -4) | 2.02 (31,992.25) | 0.82 (507,043.02) | 6.31 |
| 1G5.3 | 2.57 | NS1 (DENV-2 and -4) | 3.43 (954.85) | 1.80 (40,731.76) | 2.34 |
| 1G5.4-A1-C3 | 2.36 | NS1 (DENV-1 to -4) | 3.37 (1,006.73) | 1.77 (40,078.55) | 2.51 |
| 1C6.3 | 1.47 | NS1 (DENV-1 to -4) | 2.51 (4,543.73) | 0.83 (217,428.93) | 2.09 |
| 2C5.1 | 3.28 | E (DENV-1 to -4) | 5.46 (11.37) | 4.51 (101.36) | 11.21 |
Average 1/log10t50 of MAbs in the presence or absence of DEA from triplicate assays, with the MAb concentration given in parentheses.
AI (%) = reciprocal anti-log10t50 with DEA/reciprocal anti-log10t50 without DEA × 100 = MAb concentration (ng/ml) at 1/log10t50 without DEA/MAb concentration (ng/ml) at 1/log10t50 with DEA × 100.
In conclusion, MAbs 1G5.3 and 1G5.4-A1-C3 showed moderate abilities to cross-react with DENV-2 particles, while MAb 1C6.3 and MAb 3D1.4 weakly and very weakly cross-reacted with DENV-2 particles, respectively.
Cross-reaction of MAbs generated to the DENV-2 NS1 glycoprotein with the DENV-2 E glycoprotein in immunoblot assays.
The abilities of the six MAbs generated to the DENV-2 NS1 glycoprotein to cross-react with the DENV-2 E glycoprotein were tested using purified DENV-2 particles in immunoblot assays. In this study, PAbs generated in mice in response to repeated live DENV-2 infections (7) strongly reacted with the full-length (gp60) and truncated (gp55) E glycoproteins but reacted only very weakly with the second envelope glycoprotein, prM (gp20), thereby confirming that only a low concentration of the prM glycoprotein was present on these purified DENV-2 particles (Fig. 2). MAbs 1H7.4 and 5H4.3, which were unreactive with DENV-2 particles in the ELISA, were, as expected, unreactive with the DENV-2 E (gp60 and 55) or prM glycoprotein, while they reacted with the low concentration of NS1 glycoprotein. MAbs 3D1.4 and 1C6.3 both had low ELISA titers against purified DENV-2 particles (Table 1), but only MAb 1C6.3 was observed to cross-react with the DENV-2 E glycoprotein in the immunoblot assay (Fig. 2). In contrast, MAbs 1G5.3 and 1G5.4-A1-C3, which showed higher ELISA titers against the DENV-2 particles, also showed much stronger cross-reactions with the DENV-2 E glycoprotein in immunoblot assays. MAb 2C5.1, which bound to a flavivirus group epitope on the E glycoprotein, as expected, strongly reacted with the E (gp60 and gp55) glycoprotein but also very weakly cross-reacted with the DENV-2 NS1 glycoprotein.
FIG. 2.
Cross-reactions of MAbs generated against the DENV-2 NS1 glycoprotein with the DENV-2 E glycoprotein. Two concentrations (1,163 ng [lanes 2, 4, 6, 8, 10, 12, 14, and 16] and 292 ng [lanes 3, 5, 7, 9, 11, 13, 15, and 17]) of purified DENV-2 particles were subjected to SDS-PAGE and electroblotted onto nitrocellulose membranes. Purified MAbs 1H7.4 (lanes 2 and 3), 5H4.3 (lanes 4 and 5), 3D1.4 (lanes 6 and 7), 1G5.3 (lanes 8 and 9), 1G5.4-A1-C3 (lanes 10 and 11), and 1C6.3 (lanes 12 and 13), generated against the DENV-2 NS1 glycoprotein, MAb 2C5.1 (lanes 14 and 15), generated against the DENV-2 E glycoprotein, and pooled mouse PAbs generated in response to repeated live DENV-2 infections (lanes 16 and 17) were diluted 1/200 (MAb 1C6.3 and PAbs) or 1/400 (all other MAbs), incubated with each membrane strip, and detected using an enzyme-labeled secondary antibody and substrate. The locations of each molecular size (kDa) marker and of the DENV-2 E (gp60), gp55 (truncated pg60), iNS1 (gp46), and prM (gp20) glycoproteins are shown.
Reactions of MAbs generated to the DENV-2 NS1 glycoprotein with synthetic peptide epitopes from the DENV-2 NS1 glycoprotein.
The binding of the six MAbs against epitopes represented by 9- or 11-amino-acid peptide sequences and the core (6 or 7 amino acid) LX1 and 24C epitope sequences was determined for subsequent comparison with their cross-reactions against peptide sequences from the DENV E glycoproteins (Table 2). In this study, MAb 1H7.4 and MAb 5H4.3 specifically bound to epitopes LD2 (25-33) and 24A (61-69) on the DENV-2 NS1 glycoprotein, respectively, which are cryptic or partially dependent upon conformation (12). In contrast, MAb 3D1.4, which specifically bound to the sequential LX1 epitope (113-121), more strongly reacted with the core LX1 epitope sequence (113-119) on the DENV NS1 glycoproteins but was unreactive with N′LX1 (109-117), which contained only part of the core LX1 epitope sequence. Thus, its reaction with the 113-119 peptide sequence was regarded as the maximum absorbance (2.63 = 100% [maximum absorbance] at a concentration of 442 ng/ml), against which its reaction with the 113-121 peptide sequence (A, 2.02; 76.8% of maximum absorbance) and its cross-reactions with surface-exposed sequences in the DENV E glycoproteins were compared. MAb 1G5.3 reacted most strongly with the full-length 24C epitope sequence (301-TTASGKLIT-309) (A, 3.88; 100.0% at 575 ng/ml) on the NS1 proteins of DENV-2 and DENV-4 but showed a slightly weaker reaction with the core six-amino-acid 24C epitope sequence, 302-307 (A, 3.01; 76.8% of maximum absorbance at 575 ng/ml). In contrast, MAb 1G5.4-A1-C3 recognized multiple ELK/KLE-type motifs (underlined) in the 24A epitope (61-TRLENLMWK-69), the N′LX1 epitope (109-TELKYSWKT-117), and the LX2/3 epitope (331-YGMEIRPLK-339) and also weakly reacted with the amino-terminal epitope (5-VVSWKNKELKC-15), the LD2 epitope (25-VHTWTEQYK-33), and the LX2/2 epitope (267-PWHLGKLEM-275). The maximum absorbance for this MAb was 1.14 (100.0%) at 1,595 ng/ml against the 109-117 peptide sequence, while the absorbance against the other peptides ranged from 57.8 to 75.4% of this maximum absorbance value. MAb 1C6.3, which was more reactive with KELK/KLEK-type motifs, more strongly identified these motifs (underlined) in the LX2/1 (208-TWKIEKASF-216), LX2/2 (267-PWHLGKLEM-275), and LX2/3 (331-YGMEIRPLK-339) epitopes but also weakly reacted with the amino-terminal (5-VVSWKNKELKC-15) and LD2 (25-VHTWTEQYK-33) epitopes. The maximum absorbance of this MAb was 1.22 (100.0%) at 4,439 ng/ml, and values of 58.2 to 83.6% of the maximal absorbance value were obtained against these other peptides.
TABLE 2.
Reactions of MAbs generated to the DENV-2 NS1 glycoprotein with sequential epitopes represented by synthetic peptides from the DENV-2 NS1 glycoprotein
| Peptide sequencea | Epitopeb | Average MAb ELISA absorbance (% of maximum absorbance)c
|
|||||
|---|---|---|---|---|---|---|---|
| 1H7.4 | 5H4.3 | 3D1.4 | 1G5.3 | 1G5.4-A1-C3 | 1C6.3 | ||
| 5-VVSWKNKELKC-15 | N terminus | 0.03 | 0.04 | 0.11 | 0.15 | 0.66 (57.8) | 0.71 (58.2) |
| 25-VHTWTEQYK-33 | LD2 | 2.14 | 0.21 | 0.09 | 0.25 | 0.80 (70.2) | 0.76 (62.3) |
| 61-TRLENLMWK-69 | 24A | 0.04 | 2.26 | 0.08 | 0.22 | 0.86 (75.4) | 0.66 |
| 109-TELKYSWKT-117 | N'LX1 | 0.05 | 0.05 | 0.22 | 0.05 | 1.14 (100.0) | 0.55 |
| 113-YSWKTWGKA-121 | LX1 | 0.06 | 0.02 | 2.02 (76.8) | 0.03 | 0.33 | 0.15 |
| 113-YSWKTWG-119 | LX1 core | 0.03 | 0.02 | 2.63 (100.0) | 0.03 | 0.21 | 0.09 |
| 208-TWKIEKASF-216 | LX2/1 | 0.03 | 0.02 | 0.06 | 0.28 | 0.45 | 1.22 (100.0) |
| 267-PWHLGKLEM-275 | LX2/2 | 0.03 | 0.02 | 0.07 | 0.30 | 0.52 | 1.02 (83.6) |
| 301-TTASGKLIT-309 | 24C | 0.03 | 0.03 | 0.10 | 3.88 (100.0) | 0.32 | 0.18 |
| 302-TASGKL-307 | 24C core | 0.03 | 0.02 | 0.05 | 3.01 (77.6) | 0.11 | 0.06 |
| 331-YGMEIRPLK-339 | LX2/3 | 0.05 | 0.05 | 0.07 | 0.06 | 0.85 (74.6) | 0.88 (72.1) |
Single-letter amino acid code for synthetic peptide sequences from the DENV-2 NS1 glycoprotein. The ELK/KLE-type and KELK/KLEK-type motifs are underlined.
Epitope on the DENV-2 NS1 glycoprotein with the full or core epitope sequences.
Average ELISA reactions of MAbs generated to the DENV-2 NS1 glycoprotein, diluted to 100 times their ELISA titer against the DENV-2 NS1 glycoprotein, and incubated with duplicate sets of each peptide sequence (standard deviation range of absorbance values, 0.00 to 0.02). Values above the absorbance cutoffs of 0.650 and 1.000 are shown in bold and underlined, respectively, and the percentages of maximum absorbance obtained against the 25-33, 61-69, 113-119, 301-309, 109-117, and 208-216 peptide sequences, using MAbs 1H7.4 (314 ng/ml), 5H4.3 (1,714 ng/ml), 3D1.4 (442 ng/ml), 1G5.3 (575 ng/ml), 1G5.4-A1-C3 (1,595 ng/ml), and 1C6.3 (4,439 ng/ml), respectively, are shown in parentheses.
Using a consensus from six different structural prediction algorithms, the LD2, LX1, 24C, LX2/2, and LX2/3 epitopes were predicted to adopt mixed extended-strand and random coil configurations, while the 24A and LX2/1 epitopes were predicted to occur in alpha-helical conformations.
Cross-reactions of MAbs generated to the DENV-2 NS1 glycoprotein with an overlapping set of synthetic peptides sequentially spanning domain III of the DENV-2 E glycoprotein.
Since domain III of the DENV E proteins contained epitopes defined by neutralizing DENV type or complex or flavivirus subgroup and bound by group-reactive MAbs (5, 26), the cross-reactions of the six MAbs generated to the DENV-2 NS1 glycoprotein were initially tested against a duplicate set of 47 overlapping synthetic peptides sequentially spanning this domain (amino acids 301 to 401) of DENV-2 (NG-C strain) (Fig. 3). In this study, only MAb 1G5.4-A1-C3 showed a relatively strong cross-reaction (A, 0.99; 86.8% of the maximal absorbance obtained against the DENV-2 NS1 109-119 peptide sequence) against one of these peptide sequences (peptide 10 [319-TIVIRVQYE-327]) (Fig. 3), which was surface exposed in the X-ray crystal structure of the native, dimeric DENV-2 E glycoprotein (27). MAb 1G5.3 and the control MAb (MAb 2C5.1 [C]) showed moderately strong and strong reactions against peptides 25 (349-GRLITVNPI-357) and 26 (351-LITVNPIVT-359), respectively, but these amino acid sequences were buried in the native dimeric (27) and trimeric (28, 43) DENV-2 E glycoprotein structures.
FIG. 3.
Cross-reactions of MAbs generated against the DENV-2 NS1 glycoprotein with overlapping synthetic peptides sequentially spanning the domain III sequence of the DENV-2 E glycoprotein. MAbs generated against either the DENV-2 NS1 (MAb 1H7.4, 5H4.3, 3D1.4, 1G5.3, 1G5.4-A1.C3, and 1C6.3) or E [control MAb 2C5.1 (C)] glycoprotein were diluted to 100 times their reciprocal log10t50 ELISA titer against the purified DENV-2 NS1 glycoprotein or DENV-2 particles (MAb 2C5.1, MAb 1H7.4 [314 ng/ml], 5H4.3 [1,714 ng/ml], 3D1.4 [442 ng/ml], MAb 1G5.3 [575 ng/ml], MAb 1G5.4-A1-C3 [1,595 ng/ml], MAb 1C6.3 [4,439 ng/ml], and MAb 2C5.1 [1,137 ng/ml]) and then incubated with duplicate sets of 47 overlapping synthetic peptides sequentially spanning the domain III (amino acids 301 to 401) sequence of the DENV-2 E glycoprotein. The average ELISA reaction (A492) against each pair of peptides is shown. Standard deviations ranged from 0.00 to 0.02.
Cross-reactions of MAbs generated to the DENV-2 NS1 glycoproteins with synthetic peptide sequences from surface-exposed regions of domains I, II, and III of the E glycoproteins of different DENV-2 genotypes and strains.
The reactions of the MAbs were tested against synthetic peptides representing surface-exposed sequences on the DENV-2 E glycoproteins of the mildly pathogenic (influenza-like symptom-associated) DENV-2 TR strain, the moderately pathogenic (DF-associated) DENV-2 NG-C strain, and the highly pathogenic (DSS-associated) DENV-2 M2 strain, of the American, Asian 2, and Asian 1 genotypes, respectively, as well as the corresponding E glycoprotein peptide sequences from highly pathogenic American/Asian and Cosmopolitan DENV-2 genotypes (Table 3). In this study, MAbs 1H7.4, 5H4.3, and MAb 3D1.4, which were unreactive with the DENV-2 E glycoprotein in the immunoblot assays, only very weakly cross-reacted with any of the 25 synthetic peptides which represented surface-exposed sequences on the DENV-2 E glycoproteins (Table 3). MAb 1C6.3, which weakly cross-reacted with the DENV-2 E glycoprotein, strongly cross-reacted only with the highly exposed 156-GKHGKEIKIT-165 peptide sequence that was present in all virulent DENV-2 strains and the corresponding 156-GKHGKEVKIT-165 sequence from mildly pathogenic American DENV-2 genotype strains, which contained the single 162-I-to-V substitution. These cross-reactions had absorbance values of 86.9% and 80.3% of the maximal absorbance obtained against the LX2/1 epitope of the DENV NS1 glycoprotein, respectively (Table 2). The cross-reactions of MAb 1C6.3 with the 156-165 peptide sequences on the DENV-2 E glycoprotein were therefore stronger than its reactions with the N-terminal, LD2, and LX2/3 epitopes on the DENV-2 NS1 glycoprotein (maximum absorbance values, 58.2 to 72.1%, respectively) (Table 2). In contrast, MAb 1G5.3, which showed a relatively strong cross-reaction with the DENV-2 E glycoprotein in the ELISA and immunoblot assay, showed a moderately strong cross-reaction with the 273-SSGNLLFTGH-282 domain I/II hinge sequence but more weakly cross-reacted with the short surface-exposed 273-SSGNL-277 sequence, the 97-VDRGWGNGC-105 and 101-WGNGCGLFG-109 sequences from the conserved flavivirus fusion sequence, and the 156-GKHGKEIKIT-165 sequences from virulent DENV-2 strains (e.g., DENV-2 strains NG-C and M2). The cross-reactions of MAb 1G5.3 with these surface-exposed sequences, however, had absorbance values of between 17.3 and 20.8% of the maximal absorbance obtained against the 24C epitope on the DENV-2 NS1 glycoprotein (Table 2). MAb 1G5.3 also showed a much weaker cross-reaction than MAb 1C6.3 with the corresponding 156-GKHGKEVKIT-165 sequence from the mildly pathogenic American DENV-2 genotype strains (e.g., DENV-2 TR) that contained the 162-I-to-V substitution (Table 3).
TABLE 3.
Cross-reactions of MAbs generated against the DENV-2 NS1 glycoprotein with synthetic peptides representing surface-exposed regions of DENV-2 E glycoproteins
| Peptide sequencea | DENV-2 genotype(s) (strains)b | Average MAb ELISA absorbance (% of maximum absorbance)c
|
|||||
|---|---|---|---|---|---|---|---|
| 1H7.4 | 5H4.3 | 3D1.4 | 1G5.3 | 1G5.4.A1.C3 | 1C6.3 | ||
| 44-ELIKTEAKQP-53 | Nearly all (TR, NG-C, M2) | 0.03 | 0.08 | 0.06 | 0.08 | 0.55 | 0.37 |
| 48-TEAKQPATLR-57 | Nearly all (TR, NG-C, M2) | 0.10 | 0.07 | 0.03 | 0.25 | 0.43 | 0.29 |
| 67-NTTTESRCPT-76 | As1, As2, Cos, Am/As (M2) | 0.04 | 0.04 | 0.05 | 0.15 | 0.92 (80.7) | 0.53 |
| 67-NTTTDSRCPT-76 | Am, some As2 (TR, NG-C) | 0.04 | 0.09 | 0.06 | 0.23 | 0.52 | 0.32 |
| 67-NTTTASRCPT-76 | Cos | 0.03 | 0.07 | 0.04 | 0.45 | 0.23 | 0.22 |
| 74-CPTQGEPSLN-83 | Nearly all (TR, NG-C, M2) | 0.04 | 0.05 | 0.03 | 0.03 | 0.06 | 0.05 |
| 74-CPTLGEPSLN-83 | A few As1, Am/As, Cos | 0.03 | 0.04 | 0.03 | 0.03 | 0.02 | 0.04 |
| 93-KHSMVDRGW-101 | All (TR, NG-C, M2) | 0.06 | 0.16 | 0.10 | 0.22 | 0.11 | 0.09 |
| 95-SMVDRGWGN-103 | All (TR, NG-C, M2) | 0.04 | 0.21 | 0.13 | 0.21 | 0.24 | 0.13 |
| 97-VDRGWGNGC-105 | All (TR, NG-C, M2) | 0.04 | 0.04 | 0.15 | 0.81 (20.8) | 0.48 | 0.25 |
| 99-RGWGNGCGL-107 | All (TR, NG-C, M2) | 0.04 | 0.06 | 0.07 | 0.38 | 0.24 | 0.21 |
| 101-WGNGCGLFG-109 | All (TR, NG-C, M2) | 0.02 | 0.11 | 0.13 | 0.67 (17.3) | 0.21 | 0.14 |
| 103-NGCGLFGKG-111 | All (TR, NG-C, M2) | 0.01 | 0.23 | 0.06 | 0.16 | 0.10 | 0.08 |
| 127-GKIVQPENLE-136 | Many (TR, M2) | 0.03 | 0.06 | 0.10 | 0.57 | 0.21 | 0.15 |
| 156-GKHGKEIKIT-165 | As1, As2, Cos, Am/As (NG-C, M2) | 0.03 | 0.02 | 0.14 | 0.67 (17.3) | 0.94 (82.5) | 1.06 (86.9) |
| 156-GKHGKEVKIT-165 | Am (TR) | 0.04 | 0.02 | 0.10 | 0.48 | 0.64 | 0.98 (80.3) |
| 273-SSGNLLFTGH-282 | All (TR, NG-C, M2) | 0.13 | 0.08 | 0.52 | 1.47 (37.9) | 0.16 | 0.08 |
| 273-SSGNL-277 | All (TR, NG-C, M2) | 0.03 | 0.03 | 0.05 | 0.69 (17.8) | 0.06 | 0.06 |
| 288-RMDKLQLKG-296 | All (TR, NG-C, M2) | 0.10 | 0.04 | 0.06 | 0.15 | 0.61 | 0.23 |
| 304-GKFKVVKEIA-313 | As1, As2, Cos, Am (TR, NG-C) | 0.04 | 0.05 | 0.09 | 0.18 | 0.22 | 0.36 |
| 304-GKFKIVKEIA-313 | Am/As, one Am | 0.05 | 0.04 | 0.10 | 0.14 | 0.23 | 0.32 |
| 304-GKFKVVEEIA-313 | A few As1 (M2) | 0.03 | 0.03 | 0.04 | 0.07 | 0.42 | 0.43 |
| 338-EIMDLEKRHV-347 | Nearly all (TR, NG-C) | 0.03 | 0.03 | 0.05 | 0.05 | 0.34 | 0.26 |
| 338-EIMDLDNRHV-347 | One As1 (M2) | 0.03 | 0.03 | 0.06 | 0.05 | 0.80 (70.2) | 0.28 |
| 385-GQLKLNWFKK-394 | Nearly all (TR, NG-C, M2) | 0.07 | 0.25 | 0.10 | 0.03 | 0.15 | 0.23 |
Amino acid sequences of synthetic peptides from DENV-2 E glycoproteins. Surface-exposed sequences are underlined, and amino acid substitutions in the different DENV-2 strains are shown in bold.
Sequences are present in the E glycoproteins of all, nearly all, many, a few, or one DENV-2 strain of the Asian 1 (As1), Asian 2 (As2), American/Asian (Am/As), American (Am), and Cosmopolitan (Cos) genotypes and present in the mildly pathogenic Trinidad 1751 (TR) (American), moderately pathogenic New Guinea-C (NG-C) (Asian 2), or highly pathogenic Malaysia M725 (M2) (Asian 1) DENV-2 strain (in parentheses).
Average ELISA absorbances (492 nm) of MAbs diluted to 100 times their ELISA titer (1/log10t50) obtained against the DENV-2 NS1 glycoprotein and incubated with each synthetic peptide in duplicate (standard deviation range, 0.00 to 0.02). Values above the absorbance cutoffs of 0.650 and 1.000 are shown in bold and underlined, respectively, and the percentages of maximum absorbance obtained against the 25-33, 61-69, 113-119, 301-309, 109-117, and 208-216 peptide sequences from the DENV NS1 glycoprotein, using MAb 1H7.4 (314 ng/ml), MAb 5H4.3 (1,714 ng/ml), MAb 3D1.4 (442 ng/ml), MAb 1G5.3 (575 ng/ml), MAb 1G5.4-A1-C3 (1,595 ng/ml), and MAb 1C6.3 (4,439 ng/ml), respectively (Table 2), are shown in parentheses.
MAb 1G5.4-A1-C3 cross-reacted strongly with the highly exposed 67-NTTTESRCPT-76 and 156-GKHGKEIKIT-165 sequences from virulent DENV-2 strains as well as moderately strongly with the 338-EIMDLDNRHV-347 sequence from the highly virulent DENV-2 M2 strain (22), which contained ELK/KLEK-type motifs (underlined). These cross-reactions had values of between 70.2 and 82.5% of the maximal absorbance obtained against the 109-117 peptide sequence of the DENV-2 NS1 glycoprotein (Table 2). The cross-reactions of MAb 1G5.4-A1-C3 with the 67-76 and 156-165 sequences from virulent DENV-2 strains (80.7 and 82.5% of the maximum absorbance, respectively) (Table 3) were therefore higher than those obtained against four of the five (N terminus and LD2, 24A, and LX2/3 epitopes) sequences it bound to on the DENV-2 NS1 glycoprotein (57.8 to 75.4% of the maximum absorbance) (Table 2). This MAb also showed reduced binding to the corresponding 67-NTTTDSRCPT-76 and 159-GKHGKEVKIT-168 sequences from DENV-2 strains associated with milder human disease and the 338-EIMDLEKRHV-347 sequence from other DENV-2 strains and genotypes (Table 3). The single replacement of 71-E by the shorter side chain of D (E side chain, -CH2-CH2-COOH; D side chain, -CH2-COOH) and the replacement of 365-I by the shorter side chain of V [I side chain, -CH(CH3)-CH2-CH3; V side chain, -CH(CH3)-CH3] therefore reduced the reaction of MAb 1G5.4-A1-C3 with these ELK/KLE-type motifs. This MAb also showed a much weaker cross-reaction with the corresponding 67-NTTTASRCPT-76 sequence from the Cosmopolitan DENV-2 genotype strains (22), in which the ELK/KLE-type motif was disrupted by the replacement of 71-E/D by the short aliphatic side chain (-CH3) of A. The replacement of 343-EK-344 by 343-DN-344, in which the large basic side chain of K (-CH2-CH2-CH2-NH2) was replaced by the short amine side chain of N (-CH2-CONH2), therefore created an additional ELK/KLE-type motif in the E glycoprotein (domain III) of the virulent (DSS-associated) DENV-2 M2 strain. MAb 1G5.4-A1-C3 was therefore likely to show different binding reactions with the E glycoproteins of the mildly (e.g., American genotype strain Trinidad TR1751 [TR]), moderately (e.g., Asian 2 genotype strain New Guinea-C [NG-C]), and highly (e.g., Asian 1 genotype strain Malaysia [M2]) pathogenic DENV-2 strains.
Cross-reactions with the corresponding E glycoprotein sequences from other DENV serotypes.
The corresponding surface-exposed peptide sequences were also prepared from the E glycoproteins of the D-1V Nauru Island (WPAC74) (genotype IV) strain, the prototype D-3V H87 (genotype I) strain, the virulent D-3V SL-91 (SL-2783) (genotype III) strain, and the D-4V (814669) Dominica (genotype II) strain. The cross-reactions of MAbs 1G5.3, 1G5.4-A1-C3, and 1C6.3 with these peptide sequences were then compared with those obtained against the corresponding sequences from mildly (TR), moderately (NG-C), and highly (M2) pathogenic DENV-2 strains (Table 4). In this study, MAb 1C6.3 strongly cross-reacted only with the 156-165 peptide sequence from the DENV-2 E glycoproteins (Table 3 and 4) and was only weakly reactive with corresponding peptide sequences from the E glycoproteins of the other three DENV serotypes or any other peptide sequences in this panel (Table 4). MAb 1G5.3 cross-reacted with the highly exposed 156-165 and 273-277 sequences on the DENV-2 E glycoprotein but not with the corresponding sequences from the other three DENV serotypes. This MAb, however, weakly cross-reacted with the 127-GK/NI/LVQP/Y/IENLE/K-136 sequences in domain II of the DENV-1, DENV-2, and DENV-4 E glycoproteins. This result was, however, probably due to its cross-reaction against the amino-terminal 127-GK/NI/LV-130 sequence, which is similar to that contained in the target 24C epitope on the DENV NS1 glycoprotein (302-TASGKLI/V-308) (Table 2), but the 127-129 sequence was not surface exposed in the native DENV-2 and DENV-3 E glycoproteins (27, 29). As shown among the different DENV-2 strains (Table 3), MAb 1G5.4-A1-C3 cross-reacted more weakly with the corresponding 67-NTTTDSRCPT-76 sequence from DENV-1 and DENV-3, which also contained the 71-E-to-D substitution (Table 4). This MAb was, as expected, unreactive with the corresponding sequence from DENV-4, which contained a 71-72-ES-to-AT substitution (71-NTTATRCPT-76), thereby eliminating the ELK/KLE-type motif. The cross-reaction of MAb 1G5.4-A1-C3 with the highly exposed 156-GKHGKEI/VKIT-165 sequence of DENV-2, which contained a KELK/KLEK-type motif, was also disrupted by amino acid substitutions in the corresponding sequences of the other three DENV serotypes. MAb 1G5.4-A1-C3 also showed a stronger cross-reaction with the corresponding 288-KMDKLELKG-296 sequence from domain I of nearly all DENV-3 strains, but not the prototype DENV-3 H87 strain, which contained ELK/KLE-type motifs (underlined), as well as with the highly exposed 323-RVEYRGEDAP-332 sequence from domain III of the virulent DENV-3 SL-91 (SL2783) strain, in which 323-K and 327-K of the DENV-3 H87 strain had been replaced by R residues (underlined) in these ELK/KLE-type motifs. These cross-reactions with the peptide sequences from the E glycoprotein of the DENV-3 SL-91 strain had absorbance values of 61.4% (288-296 sequence) and 58.8% (323-332 sequence) of the maximum absorbance obtained using this MAb against the 109-117 peptide sequence of the DENV-2 NS1 glycoprotein (Table 2). The 304-GSFKLEKEVA-313 sequence from domain III of the DENV-1 E glycoprotein uniquely contained a KELK/KLEK-type motif (underlined) (Table 4), but both MAb 1G5.4-A1-C3 and MAb 1C6.3 only weakly cross-reacted with this highly exposed sequence, probably due to the presence of the large-side-chained residues (F and E) located on either side of the core KLEK motif, as reported previously (9).
TABLE 4.
Cross-reactions of MAbs generated against the DENV-2 NS1 glycoprotein with the corresponding amino acid sequences of surface-exposed regions of the E glycoproteins of each DENV serotype and different DENV-2 and DENV-3 strains
| Serotype (strain)a | Amino acid sequenceb | Domain | Average MAb ELISA absorbance (% of maximum absorbance)c
|
||
|---|---|---|---|---|---|
| 1G5.3 | 1G5.4-A1-C3 | 1C6.3 | |||
| DENV-1 | 44-ELLKTEATNP-53 | I | 0.04 | 0.12 | 0.13 |
| DENV-2 | ELIKTEAKQP | I | 0.08 | 0.55 | 0.37 |
| DENV-3 | ELQKTEATQL | I | 0.03 | 0.13 | 0.09 |
| DENV-4 | ELTKTTAKEV | I | 0.12 | 0.22 | 0.11 |
| DENV-1/2 (TR, NG-C) | 67-NTTTDSRCPT-76 | II | 0.23 | 0.52 | 0.32 |
| DENV-2 (M2) | NTTTESRCPT | II | 0.15 | 0.92 (80.7) | 0.58 |
| DENV-3 | NITTDSRCPT | II | 0.23 | 0.53 | 0.32 |
| DENV-4 | NTTTATRCPT | II | 0.06 | 0.13 | 0.11 |
| DENV-1 | 127-GKIVQYENLK-136 | II | 0.48 | 0.47 | 0.52 |
| DENV-2 | GKIVQPENLE | II | 0.57 | 0.21 | 0.15 |
| DENV-3 (H87) | GKIVQHENLK | II | 0.35 | 0.18 | 0.22 |
| DENV-3 (SL-91) | GKVVQYENLK | II | 0.27 | 0.44 | 0.49 |
| DENV-4 | GNLVQIENLE | II | 0.55 | 0.53 | 0.47 |
| DENV-1 | 156-TEHGTIATIT-165 | I | 0.07 | 0.16 | 0.24 |
| DENV-2 | GKHGKEIKIT | I | 0.67 (17.3) | 0.94 (82.5) | 1.06 (86.9) |
| DENV-3 | ETQGVTAEIT | I | 0.06 | 0.28 | 0.31 |
| DENV-4 | SNHGVTAMIT | I | 0.11 | 0.14 | 0.24 |
| DENV-1 | 273-SGTTT-277 | I/II | 0.09 | 0.05 | 0.04 |
| DENV-2 | SSGNL | I/II | 0.69 (17.8) | 0.06 | 0.06 |
| DENV-3 | SGGTS | I/II | 0.06 | 0.04 | 0.03 |
| DENV-4 | GDGNH | I/II | 0.08 | 0.10 | 0.09 |
| DENV-1 | 288-KMDKLTLKG-296 | I | 0.03 | 0.21 | 0.22 |
| DENV-2 | RMDKLQLKG | I | 0.05 | 0.61 | 0.43 |
| DENV-3 (H87) | KMDKLKLKG | I | 0.05 | 0.36 | 0.28 |
| DENV-3 (SL-91) | KMDKLELKG | I | 0.04 | 0.70 (61.4) | 0.32 |
| DENV-4 | RMDKLRIKG | I | 0.03 | 0.45 | 0.40 |
| DENV-1 | 304-GSFKLEKEVA-313 | III | 0.12 | 0.38 | 0.43 |
| DENV-2 | GKFKVVKEIA | III | 0.04 | 0.22 | 0.36 |
| DENV-3 | NTFVLKKEVS | III | 0.03 | 0.23 | 0.22 |
| DENV-4 | GKFSIDKEMA | III | 0.04 | 0.15 | 0.13 |
| DENV-1 | 323-QVKYEGTDAP-332 | III | 0.05 | 0.39 | 0.10 |
| DENV-2 | RVQYEGDGSP | III | 0.03 | 0.37 | 0.10 |
| DENV-3 (H87) | KVEYKGEDAP | III | 0.10 | 0.48 | 0.24 |
| DENV-3 (SL-91) | RVEYRGEDAP | III | 0.09 | 0.67 (58.8) | 0.21 |
| DENV-4 | KVKYEGAGAP | III | 0.11 | 0.37 | 0.12 |
The mildly (TR), moderately (NG-C), and highly (M2) pathogenic DENV-2 strains and the moderately (H87) and highly (SL-91) pathogenic DENV-3 strains are indicated in parentheses.
Amino acid sequences of synthetic peptides containing the corresponding sequences from the E glycoprotein of each DENV serotype. Potential ELK/KLE-type and KELK/KLEK-type motifs are shown in bold, and surface-exposed sequences identified on the dimeric DENV-2 (PR159S1) and DENV-3 (H-87 strain) E glycoproteins (27, 29) are underlined.
Average ELISA absorbances (492 nm) of MAbs diluted to 100 times their ELISA titers (1/log10t50) obtained against the DENV-2 NS1 glycoprotein and incubated with each synthetic peptide in duplicate (standard deviation range, 0.00 to 0.02). Values above the absorbance cutoffs of 0.650 and 1.000 are shown in bold and underlined, respectively, and the percentages of maximum absorbance obtained against the 301-309, 109-117, and 208-216 peptide sequences of the DENV-2 NS1 glycoprotein, using MAb 1G5.3 (575 ng/ml), MAb 1G5.4-A1-C3 (1,595 ng/ml), and MAb 1C6.3 (4,439 ng/ml), respectively (Table 2), are shown in parentheses.
In conclusion, MAb 1C6.3 was expected to cross-react predominantly with the DENV-2 E glycoproteins due to its high 86.9% maximum absorbance value obtained against the 156-165 sequence, while MAb 1G5.3 was most likely to cross-react with the highly exposed 156-165 and 273-278 sequences on the DENV-2 E glycoprotein, against which 17.3 and 17.8% of the maximum absorbance value were obtained, as well as with the flavivirus-conserved 98-109 fusion sequence (17.3 and 20.8% of the maximum absorbance value). MAb 1G5.4-A1-C3 was likely to more strongly cross-react with the DENV-2 E glycoproteins of virulent DENV-2 strains, in particular the DSS-associated DENV-2 M2 strain, by obtaining very high (80.7, 82.5, and 70.2%) maximum absorbance values against the 67-76, 156-165, and 338-347 E glycoprotein sequences of these DENV-2 strains, respectively, as well as with the 288-296 and 323-332 sequences of the virulent DENV-3 SL-91 strain, for which 61.4 and 58.8% of the maximum absorbance values were obtained, respectively.
Plaque reduction neutralization test using MAbs generated against the DENV-2 NS1 glycoprotein.
The MAbs generated against the DENV-2 NS1 glycoprotein were tested for the ability to neutralize strains of different DENV-2 and DENV-3 genotypes, which had different human pathogenic capacities, as well as single DENV-1 and DENV-4 strains, to account for the different cross-reactions with the peptide sequences from their E glycoproteins (Tables 3 and 4). In this study, both MAb 1H7.4 and 5H4.3, which were unreactive with the DENV-2 E glycoprotein in either the ELISA or the immunoblot assay (Table 1; Figure 1), showed no neutralizing activity (PRNT of <1/2 and <1/8, respectively) against any of the strains of each DENV serotype tested (Table 5). MAb 3D1.4, which was very weakly cross-reactive with DENV-2 particles but was unreactive with the DENV-2 E and prM glycoproteins in the immunoblot assay, weakly neutralized (PRNT, 1/8) all three DENV-2 strains tested and had a slightly higher neutralizing titer (PRNT, 1/16) against the DENV-1 strain but did not neutralize the DENV-4 strain. MAb 1C6.3, which strongly cross-reacted only with the 156-165 peptide sequence from the E glycoproteins of DENV-2, only weakly neutralized (PRNT, 1/8) the moderately and highly virulent DENV-2 (NG-C and M2) strains and the virulent DENV-3 (SL-91) strain among this panel of seven different DENV strains. In contrast, MAb 1G5.3 showed the same neutralizing titer (PRNT, 1/16) against the DENV-1 strain, the DENV-2 TR strain, the DENV-3 H87 strain, and the DENV-4 strain (Table 5), probably through its cross-reaction with the conserved 97-109 fusion sequence (Table 3). This MAb, however, had a higher neutralizing titer (PRNT, 1/32) against the more pathogenic DENV-2 NG-C and M2 strains (Table 5), probably due to its stronger cross-reaction with the highly exposed 156-GKHGKEIKIT-165 sequence on the E glycoproteins of these more virulent DENV-2 strains (Table 3). MAb 1G5.4-A1-C3 showed more variable neutralizing titers against the different DENV serotypes and strains (Table 5). This MAb was unable to neutralize the DENV-2 TR strain, the DENV-3 H-87 strain, or the DENV-4 strain and weakly neutralized (PRNT, 1/8) the DENV-1 strain and the more virulent DENV-3 SL-91 strain but showed increasing neutralizing titers against the moderately (anti-NG-C strain PRNT, 1/16) and highly (anti-M2 strain PRNT, 1/32) pathogenic DENV-2 strains. These results were therefore probably due to this MAb's increased cross-reactions against the corresponding E glycoprotein sequences present on both the DENV-2 NG-C and M2 strains (156-GKGHGKEIKIT-165) or only on the DENV-2 M2 strain (67-NTTTESRCPT-76 and 338-EIMDLDNRHV-347), which contained amino acid substitutions (underlined) that increased the reaction of this MAb (Table 3). Similarly, the weak neutralization of the DENV-3 SL-91 strain was probably due to its stronger reaction with the ELK/KLE-type motifs in the 288-KMDKLELKG-296 sequence of domain I and the 323-RVEYRGEDAP-332 sequence of domain III of the E glycoprotein of this DENV-3 strain (Table 4). Both of these peptide sequences were highly exposed, with high atomic temperatures, on the native, dimeric DENV-3 E protein structure (29). As expected, the control MAb, 2C5.1, which bound to an epitope on all flavivirus E glycoproteins, very strongly neutralized (PRNT, >1/256) each of the DENV serotypes and strains (Table 5).
TABLE 5.
PRNTs of the six MAbs generated against the DENV-2 NS1 glycoprotein against each DENV serotype and different DENV-2 and DENV-3 strains
| MAb | PRNT againsta:
|
||||||
|---|---|---|---|---|---|---|---|
| DENV-1 strain NI | DENV-2
|
DENV-3
|
DENV-4 strain DOM | ||||
| TR | NG-C | M2 | H-87 | SL-91 | |||
| 1H7.4 | <1/2 | <1/2 | <1/2 | <1/2 | <1/2 | <1/2 | <1/2 |
| 5H4.3 | <1/8 | <1/8 | <1/8 | <1/8 | <1/8 | ||
| 3D1.4 | 1/16 | 1/8 | 1/8 | 1/8 | <1/8 | ||
| 1G5.3 | 1/16 | 1/16 | 1/32 | 1/32 | 1/16 | 1/16 | |
| 1G5.4-A1-C3 | 1/8 | <1/8 | 1/16 | 1/32 | <1/8 | 1/8 | <1/8 |
| 1C6.3 | <1/8 | <1/8 | 1/8 | 1/8 | <1/8 | 1/8 | <1/8 |
| 2C5.1(C) | >1/256 | >1/256 | >1/256 | >1/256 | >1/256 | >1/256 | >1/256 |
Average reciprocal dilutions of MAbs generated against the DENV-2 NS1 glycoprotein or the DENV-2 E glycoprotein [MAb 2C5.1(C)] from duplicate wells in two plates (quadruplicate), tested against DENV-1 (NI [Nauru Island strain]), DENV-2 (TR [Trinidad TR1751], NG-C [New Guinea-C], and M2 [Malaysia M725] strains), DENV-3 (H-87 and SL-91 [Sri Lanka 2783] strains) and DENV-4 (DOM [Dominica 814669 strain]), which reduced the plaques by 50%. Twofold serial dilutions were initiated at 1/2 for MAb 1H7.4 and at 1/8 for the other MAbs. Blank spaces indicate that the MAb was not tested.
DISCUSSION
The results from this study accounted for the earlier finding that both PAbs and one MAb (9A9) generated in mice to the DENV-2 NS1 glycoprotein precipitated low concentrations of the E glycoprotein (35). These antibodies were not shown to neutralize DENV-2, probably because the plaque reduction neutralization tests were performed at minimum serum dilutions of 1/20, rather than 1/8 (or 1/16), using DENV-2-infected suckling mouse brain homogenates (35), which contain high concentrations of the eNS1 glycoprotein. PAbs generated in monkeys to the NS1 glycoprotein of yellow fever virus could also precipitate the homologous E glycoprotein, and these PAbs from 2/5 animals neutralized the virus (PRNT, 1/32) (34). These results therefore strongly suggested that common epitopes also occur on the NS1 and E glycoproteins of this other flavivirus.
In this study, a relatively small set of MAbs were used, and these bound to epitopes on the DENV NS1 glycoprotein that could faithfully be represented by synthetic peptides, so their cross-reactions with common sequences within surface-exposed regions of the DENV E glycoprotein could easily be identified and tested. Since these synthetic peptides were covalently coupled to spacer molecules, all of their amino acid side chains, even in short amino acid sequences (e.g., 273-SSGNL-277), were exposed. This method therefore avoided the problems encountered when such short synthetic peptide sequences are adsorbed onto supports for solid-phase immunoassays (7). This method also more reliably allowed the relative MAb reaction intensities to simultaneously be compared between the peptide sequences from the DENV NS1 and E glycoproteins and between the corresponding sequences from the E glycoproteins of different DENV serotypes and strains. The high MAb ELISA reaction consistency using this methodology was demonstrated by the finding of very low standard deviations (A, 0.00 to 0.02) for MAb reactions against each pair of synthetic peptides, and this highlighted the uniformity of coating the 64-nM gears with synthetic peptides by using a very high excess of activated amino acid ester concentrations in the coupling reactions.
This study particularly concentrated on sequences which may account for variations in the pathogenic potentials of DENV-2 strains and, to a lesser extent, DENV-3 strains because DENV-2 strains show the greatest sequence variations (40) and also because these two DENV serotypes are those most commonly associated with DHF/DSS in the world. The findings that virulent strains of DENV-2 contain multiple short amino acid motifs in highly exposed regions of domains I, II, and III of the E glycoprotein and that amino acid substitutions may even increase these epitope numbers (e.g., the 338-347 sequence of the DENV-2 M2 strain) are consistent with the inability to find single virulence markers from E glycoprotein sequence analyses.
The ELK/KLE-type motifs, defined by MAb 1G5.4-A1-C3, were immunodominant in both mice and humans during live DENV infections, and DSS patients generated much stronger responses against these motifs than did DF patients (7). Interestingly, a MAb escape mutation on the DENV-2 E glycoprotein resulted in a loss of the glycosylation site through a 69-T-to-I mutation, together with a 71-E-to-D mutation (67-NTITDSRCPT-76) and three other mutations (25). This suggested that the highly exposed 67-NTTTESRCPT-76 sequence was at least part of the target epitope, which was also strongly identified by MAb 1G5.4-A1-C3. This 71-E marker was present in all highly virulent (DHF/DSS-associated) Asian 1, Asian 2, and Cosmopolitan strains, as well as the American/Asian DENV-2 strains imported into the Americas from Thailand, which subsequently underwent recombination (41) and displaced the weakly virulent American DENV-2 genotype strains (21). Interestingly, all virulent DENV-2 American/Asian genotype strains retained the highly exposed 71-E and 162-I (156-GKHGKEIKIT-165) markers during and after major DHF epidemics in Venezuela (41) and Cuba (31), suggesting that they are essential for DENV-2 viability/virulence. The DENV-2 strain responsible for the 1990 DHF/DSS epidemic in Brazil, however, contained both 162-I and a 160-K-to-E substitution (156-GKHGEEIKIT-165) (underlined) (22), which were retained in most other Brazilian (e.g., ROR1811, RGN53, MIG1269, BEL63650, and CEA2463) DENV-2 strains. This 160-E substitution is likely to further increase MAb 1G5.4-A1-C3 binding, while reducing MAb 1C6.3 binding, due to the replacement of the large K side chain (-CH2-CH2-CH2-NH2) by the shorter E side chain (-CH2-CH2-COOH), which may therefore act as an alternative virulence marker.
The increased ability of MAb 1G5.4-A1-C3 to neutralize the highly virulent (DSS-associated) DENV-2 M2 strain, probably due to the additional exposed ELK/KLE-type motif in domain III, is consistent with the observation that MAbs which bound to epitopes within this domain could more efficiently neutralize DENV-2 (5). Epitopes defined by neutralizing MAbs were located at either end of this domain (17, 24). However, with the exception of the virulent D-3V SL-91 strain, which contained replacements of K by the more antigenic R residue (7, 9) in the 323-332 sequence, MAb 1G5.4-A1-C3 only weakly cross-reacted with the ELK/KLE-type motifs in domain III. This was consistent with the weak ability and inability of this MAb to neutralize the weakly pathogenic DENV-1 and DENV-4 strains, respectively. The 383-EPG-385 sequence was shown to be essential for binding of the DENV-2 type-specific neutralizing MAb 3H5 (17) and was located immediately adjacent to the 385-GQLKLNWFKK-394 sequence, in which a 390-N-to-D substitution, present in nearly all American DENV-2 genotype strains (21), created an ELK/KLE-type motif (385-GQLKLDWFKK-394) (underlined). This substitution per se did not significantly reduce viral replication in the absence of antibodies (3) and was located very low on the side face of the DENV-2 E glycoprotein (27, 28, 43). Further studies are needed to assess whether MAb 1G5.4-A1-C3 can more effectively neutralize these weakly pathogenic strains of the American DENV-2 genotype than the DENV-2 TR strain used in this study, which contained a 390-N residue. Further studies are also needed to account for the ability of MAb 3D1.4 to neutralize strains of the DENV-1 and DENV-2 serotypes.
Interestingly, the original sequence of the American DENV-2 PR159S1 genotype candidate vaccine strain (NCBI accession number P12823) contained the American DENV-2 genotype 71-D, 162-I, and 390-D markers in the E glycoprotein (22). Yet, surprisingly, the DENV-2 PR159S1 strain used to prepare the high-resolution X-ray crystallographic structure (27, 28), but not the cryo-electron microscopic structure determination (43), contained the 71-D-to-E and 309-D-to-N substitutions present in the more virulent DENV-2 strains.
Sri Lankan (genotype III) DENV-3 strains isolated during DHF epidemics in the Americas (e.g., Cuba, Venezuela, Nicaragua, and Mexico) all contained the 291-KLELK-295 and 323-KLEYKGED-330 sequences, from domains I and III, respectively. The Venezuelan DENV-3 LARD6722 strain, however, contained a 291-KLELR-295 sequence (42), and several DENV-3 isolates from Brazil (e.g., the RGN576, GOI1100, D3BR/GO5/03, and D3BR/RP1/03 strains) contained the 323-KVEYRGED-330 sequence. Sequences containing these substitutions are expected to be identified more strongly by MAb 1G5.4-A1-C3, as shown by its stronger cross-reaction with the 323-RVEYRGEDAP-332 peptide sequence from the DENV-3 SL-91 strain.
Unlike MAb 1G5.4-A1-C3 (7, 9), MAb 1G5.3 was unreactive with its target SGK sequence, such as the DENV-conserved 393-K/RKGSS-397 sequence in domain III of the E glycoprotein, when this core tri-amino acid sequence was present in the reverse orientation. This MAb did, however, cross-react with the nonlinear β (“kl”) turn about 276-N (273-SSGNL-277), from the hinge region between domains I and II of DENV-2. A DENV-2 escape mutant generated with the flavivirus group-reactive neutralizing MAb 4G2 contained 169-S-to-P and 275-G-to-R (273-SSRNL-277) substitutions, which suggested that this short five-amino-acid surface-exposed sequence was at least part of the epitope bound by this MAb (36). MAb 4G2 was, however, unreactive with DENV-2 E glycoproteins containing a 106-G-to-Q or 107-L-to-K substitution (4) or other flavivirus glycoproteins with substitutions in 106-G or 107-L (6, 38, 39), strongly implicating the conserved fusion sequence as the target epitope for this MAb. MAb 1G5.3 also cross-reacted with the 97-105 and 101-109 peptide sequences, and its cross-reaction with the latter sequence, which contained both 106-G and 107-L, probably accounted for its ability to weakly neutralize all DENV serotypes. MAb 1G5.3 also had a higher neutralization titer against the more virulent DENV-2 strains, probably due to its stronger cross-reaction with the 156-165 sequence, which contained 162-I. Unlike MAb 1G5.4-A1-C3, however, the additional 160-K-to-E substitution in the 156-GKHGEEIKIT-165 sequence of many Brazilian American/Asian DENV-2 genotype strains is likely to disrupt its binding.
All neutralizing mouse MAbs diluted beyond their effective neutralizing concentrations could enhance the replication of DENV-2 via Fc receptor molecules on mouse macrophage cells in vitro (16). The relatively low neutralizing titers of MAbs 1G5.3 and 1G5.4-A1-C3 against these DENV-2 strains are likely to be reduced in the presence of high concentrations of the DENV-2 NS1 glycoprotein, as found in the plasmas of DHF/DSS patients (2, 23), together with the autoantigens identified by MAb 1G5.4-A1-C3 and PAbs generated against the DENV-2 NS1 glycoproteins (7, 9). This was confirmed by findings that the cross-reaction of MAb 1G5.3 with purified DENV-2 particles was reduced by the presence of high concentrations of the DENV-2 glycoprotein (10) and that MAb 1G5.3, as well as the PAbs generated to the DENV-2 NS1 glycoprotein, caused a lethal antibody-enhanced disease (AED) through the dramatic (>100,000-fold) AER of a DENV-2 strain in vivo (10). These results therefore strongly suggest that the DENV NS1 glycoprotein would be an unsuitable vaccine candidate, as originally proposed.
Since IgG antibodies generated against DENV NS1 glycoproteins were detected only during the acute phase of secondary DENV infections (37), the higher-titer antibody responses to ELK/KLE-type motifs found in DSS patients (7) may generate AER of virulent DENV strains, which has been implicated in DHF/DSS pathogenesis (15, 16). This hypothesis may therefore account for the finding that undiluted serum samples from patients who subsequently developed DHF/DSS generated the greatest AER of DENV-2 in vitro (19). The observation that virulent strains contained more highly antigenic ELK/KLE-type motifs or additional ELK/KLE-type motifs, as found in this study, may also account for the earlier finding that some (virulent) DENV-2 strains could be more effectively enhanced by DHF/DSS patients’ antibodies (30).
Histopathological and virological analyses, together with blocking studies, have been performed with the in vivo AER/AED model (unpublished data). MAbs 1G5.4-A1-C3 and 1C6.3 and a number of other MAbs which bind to common epitopes on the DENV E and NS1 glycoproteins are also being tested for the ability to generate AER/AED in vivo (10). This model will also be used to compare the reactions of PAbs generated to the E and NS1 glycoproteins of heterologous DENV serotypes to generate AER/AED of different DENV strains, in which antibody concentration and avidity, the relative concentrations of the E, (pr)M, and NS1 glycoproteins, and complement are likely to play important roles. The results from these studies are therefore likely to be important in understanding the pathogenesis of DHF/DSS and in the design of safe vaccines.
Acknowledgments
This work received financial support from the Sir Jules Thorn Charitable Trust and the Instituto Colombiano para el Desarrollo de la Ciencia y la Technologia Francisco Jose de Caldas (COLCIENCIAS) (no. 1215-04-14364).
I thank C. M. E. Romero-Vivas (Uninorte, Colombia) for helpful advice, M. A. Miles (LSH&TM, United Kingdom) for critically reviewing the manuscript, and two anonymous reviewers for their very valuable suggestions.
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Aaskov, J. G., H. M. Geysen, and T. J. Mason. 1989. Serologically defined linear epitopes in the envelope protein of dengue 2 (Jamaica strain 1409). Arch. Virol. 105:209-221. [DOI] [PubMed] [Google Scholar]
- 2.Aviruntun, P., N. Punyadee, S. Noisakran, C. Komoltri, S. Thiemmeca, K. Auethavornanan, A. Jairungsri, R. Kanlaya, N. Tangthawornchaikul, C. Puttanakitsakul, P. T. Yenchitsomanus, J. Mongkolsapaya, W. Kasinrerk, N. Sittisombut, M. Husmann, M. Blettner, S. Vasanawathana, S. Bhakdi, and P. Malasit. 2006. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis. 193:1078-1088. [DOI] [PubMed] [Google Scholar]
- 3.Cologna, R., and R. Rico-Hesse. 2003. American genotype structures decrease dengue virus output from human monocytes and dendritic cells. J. Virol. 77:3929-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crill, W. D., and G. J. Chang. 2004. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J. Virol. 78:13975-13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crill, W. D., and J. T. Roehrig. 2001. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 75:7769-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crill, W. D., N. B. Trainor, and G. J. Chang. 2007. A detailed mutagenesis study of flavivirus cross-reactive epitopes using West Nile virus-like particles. J. Gen. Virol. 79:853-859. [DOI] [PubMed] [Google Scholar]
- 7.Falconar, A. K. I. 2007. Antibody responses to immunodominant ELK-type motifs on the dengue virus nonstructural-1 glycoprotein during live dengue virus infections in mice and humans: implications for diagnosis, pathogenesis, and vaccine design. Clin. Vaccine Immunol. 15:493-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falconar, A. K. I. 1999. Identification of an epitope on the dengue virus membrane (M) protein defined by cross-reactive monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Arch. Virol. 144:2313-2330. [DOI] [PubMed] [Google Scholar]
- 9.Falconar, A. K. I. 1997. The dengue virus non-structural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesion proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch. Virol. 142:897-916. [DOI] [PubMed] [Google Scholar]
- 10.Falconar, A. K. I. 1999. The potential role of antigenic themes in dengue viral pathogenesis, p. 437-447. In S. G. Pandalai (ed.), Recent research developments in virology, vol. 1., part II. Transworld Research Network, Kerala, India. [Google Scholar]
- 11.Falconar, A. K. I., and P. R. Young. 1991. Production of dimer-specific and dengue group cross-reactive mouse monoclonal antibodies to the dengue 2 virus nonstructural glycoprotein. J. Gen. Virol. 72:961-965. [DOI] [PubMed] [Google Scholar]
- 12.Falconar, A. K. I., P. R. Young, and M. A. Miles. 1994. Precise location of sequential dengue virus subcomplex and complex B cell epitopes on the nonstructural-1 glycoprotein. Arch. Virol. 137:315-326. [DOI] [PubMed] [Google Scholar]
- 13.Goncalvez, A. P., R. H. Purcell, and C. J. Lai. 2004. Epitope determinants of a chimpanzee Fab antibody that efficiently cross-neutralizes dengue type 1 and type 2 viruses map to inside and in close proximity to fusion loop of the dengue type 2 virus envelope glycoprotein. J. Virol. 78:12919-12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halstead, S. B. 1997. Epidemiology of dengue and dengue hemorrhagic fever, p. 23-44. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, NY.
- 16.Halstead, S. B., C. N. Venkateshan, M. K. Gentry, and L. K. Larsen. 1984. Heterogeneity of infection enhancement of dengue 2 virus strains by monoclonal antibodies. J. Immunol. 132:1529-1532. [PubMed] [Google Scholar]
- 17.Hiramatsu, K., M. Tadano, R. Men, and C. J. Lai. 1996. Mutational analysis of a neutralising epitope on the dengue type 2 virus (DEN2) envelope protein: monoclonal antibody resistant DEN2/DEN4 chimeras exhibit reduced mouse neurovirulence. Virology 224:437-445. [DOI] [PubMed] [Google Scholar]
- 18.Kanai, R., K. Kar, K. Anthony, L. H. Gould, M. Ledizet, E. Fikrig, W. A. Marasco, R. A. Koski, and Y. Modis. 2006. Crystal structure of West Nile virus envelope glycoprotein reveals viral surface epitopes. J. Virol. 80:11000-11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kliks, S. C., A. Nisalak, W. E. Brandt, L. Wahl, and D. S. Burke. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 40:444-451. [DOI] [PubMed] [Google Scholar]
- 20.Lanciotti, R. S., J. G. Lewis, D. J. Gubler, and D. W. Trent. 1994. Molecular evolution and epidemiology of dengue-3 viruses. J. Gen. Virol. 75:65-75. [DOI] [PubMed] [Google Scholar]
- 21.Leitmeyer, K. C., D. W. Vaughn, D. M. Watts, R. Salas, I. Villalobos de Chalon, C. Ramos, and R. Rico-Hesse. 1999. Dengue virus structural differences that correlate with pathogenesis. J. Virol. 73:4738-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis, J. A., G.-J. Chang, R. S. Lanciotti, R. M. Kinney, L. W. Mayer, and D. W. Trent. 1993. Phylogenetic relationships of dengue-2 viruses. Virology 197:216-224. [DOI] [PubMed] [Google Scholar]
- 23.Libraty, D. H., P. R. Young, D. Pickering, T. P. Endy, S. Kalayanarooj, S. Green, D. W. Vaughn, A. Nisalat, F. A. Ennis, and A. L. Rothman. 2002. High circulating levels of the dengue virus non-structural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 186:1165-1168. [DOI] [PubMed] [Google Scholar]
- 24.Lin, B., C. R. Parish, J. M. Murray, and P. J. Wright. 1994. Localisation of a neutralising epitope on the envelope protein of dengue virus type 2. Virology 202:885-890. [DOI] [PubMed] [Google Scholar]
- 25.Lok, S. M., M. L. Ng, and J. Aaskov. 2001. Amino acid and phenotypic changes in dengue 2 virus associated with escape from neutralisation by IgM antibody. J. Med. Virol. 65:315-323. [DOI] [PubMed] [Google Scholar]
- 26.Megret, F., J. P. Hugnot, A. K. I. Falconar, M. K. Gentry, D. W. Morens, J. M. Murray, J. J. Schlesinger, P. J. Wright, P. R. Young, M. H. V. Van Regenmortel, and V. Deubel. 1992. Use of recombinant fusion proteins and monoclonal antibodies to define linear and discontinuous antigenic sites on the dengue virus envelope protein. Virology 187:408-491. [DOI] [PubMed] [Google Scholar]
- 27.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2003. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 100:6986-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313-319. [DOI] [PubMed] [Google Scholar]
- 29.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2005. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J. Virol. 79:1223-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morens, D. M., N. J. Marchette, M. C. Chu, and S. B. Halstead. 1998. Growth of dengue type 2 virus isolates in human blood leukocytes correlates with severe and mild dengue disease. Am. J. Trop. Med. Hyg. 45:644-651. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Roche, R., M. Alvarez, T. Gritsun, D. Rosario, S. Halstead, G. Kouri, E. A. Gould, and M. G. Guzman. 2005. Dengue virus type 2 in Cuba, 1997: conservation of E gene sequence in isolates obtained at different times during the epidemic. Arch. Virol. 150:415-425. [DOI] [PubMed] [Google Scholar]
- 32.Roehrig, J. T., A. J. Johnson, A. R. Hunt, R. A. Bolin, and M. C. Chu. 1990. Antibodies to dengue virus E-glycoprotein synthetic peptides identify antigenic conformation. Virology 177:668-675. [DOI] [PubMed] [Google Scholar]
- 33.Saito, A., Y. Hosaka, T. Nakagawa, S. Yamada, and K. Okuda. 1993. Relative avidity of serum immunoglobulin G antibody for the fimbria of Actinobacillus actinomycetemcomitans in patients with periodontitis. Infect. Immun. 61:332-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlesinger, J. J., M. W. Bradriss, C. B. Cropp, and T. P. Monath. 1986. Protection against yellow fever in monkeys by immunisation with yellow fever virus non-structural protein NS1. J. Virol. 60:1153-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlesinger, J. J., M. W. Bradriss, and E. E. Walsh. 1987. Protection of mice against dengue 2 virus encephalitis by immunisation with the dengue 2 virus non-structural glycoprotein NS1. J. Gen. Virol. 68:853-857. [DOI] [PubMed] [Google Scholar]
- 36.Serafin, I. L., and J. G. Aaskov. 2001. Identification of epitopes on the envelope (E) protein of dengue 2 and dengue 3 viruses using monoclonal antibodies. Arch. Virol. 146:2469-2479. [DOI] [PubMed] [Google Scholar]
- 37.Shu, P.-Y., L.-K. Chen, S.-F. Chang, Y.-Y. Yueh, I. Chow, L.-J. Chien, C. Chin, T. H. Lin, and J.-H. Huang. 2003. Comparison of capture immunoglobulin M (IgM) and IgG enzyme-linked immunosorbent assay (ELISA) and nonstructural protein NS1 serotype-specific IgG ELISA for differentiation of primary and secondary dengue virus infections. Clin. Diagn. Lab. Immunol. 10:622-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stiasny, K., S. Kiermayr, H. Holzmann, and F. X. Heinz. 2006. Cryptic properties of a cluster of dominant flavivirus cross-reactive antigenic sites. J. Virol. 80:9557-9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trainor, N. B., W. D. Crill, J. A. Robertson, and G. J. Chang. 2007. Mutation analysis of the fusion domain of St. Louis encephalitis virus envelope protein. Virology 360:398-406. [DOI] [PubMed] [Google Scholar]
- 40.Twiddy, S. S., J. J. Farrar, N. Vinh Chau, B. Wills, E. A. Gould, T. Gritsun, G. Lloyd, and E. C. Holmes. 2002. Phylogenetic relationships and different selection pressures among genotypes of dengue-2 viruses. Virology 298:63-72. [DOI] [PubMed] [Google Scholar]
- 41.Uzcategui, N. Y., D. Camacho, G. Comach, R. Cuello de Uzcategui, E. C. Holmes, and E. A. Gould. 2001. Molecular epidemiology of dengue type 2 in Venezuela: evidence for in situ virus evolution and recombination. J. Gen. Virol. 82:2945-2953. [DOI] [PubMed] [Google Scholar]
- 42.Uzcategui, N. Y., G. Comach, D. Camacho, M. Sacedo, M. Cabello de Quitana, M. Jiménez, G. Sierra, R. Cuello de Uzcategui, W. S. James, S. Turner, E. C. Holmes, and E. A. Gould. 1993. Molecular epidemiology of dengue virus type 3 in Venezuela. J. Gen. Virol. 84:1569-1575. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, W., P. R. Chipman, J. Corver, P. R. Johnson, Y. Zhang, S. Mukhopadhyay, T. S. Baker, J. H. Strauss, M. G. Rossman, and R. J. Kuhn. 2003. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus. Nat. Struct. Biol. 10:907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]