Abstract
Due to the ubiquitous nature of porcine circovirus type 2 (PCV2) in the pig population and the increasing use of PCV2 vaccines in breeding herds, the majority of dams have been exposed to field PCV2 or PCV2 vaccines, resulting in piglets with varied levels of passively acquired PCV2 maternal antibodies. The objective of the current research was to investigate the influence of passively acquired anti-PCV2 antibodies on PCV2 vaccine efficacy. Sixty 26-day-old pigs were divided into four groups: vaccinated pigs with no maternal PCV2 antibodies at the time of vaccination (VAC-NEG; n = 9), vaccinated pigs with maternal PCV2 antibodies at the time of vaccination (VAC-POS; n = 21), nonvaccinated pigs with no maternal antibodies at the time of challenge (NVAC-CNEG; n = 15), and nonvaccinated pigs with maternal antibodies at the time of challenge (NVAC-CPOS; n = 15). Vaccinations and challenges were performed on trial days 0 and 28, respectively, according to group designation. The pigs were monitored for clinical signs of disease daily and weighed weekly, and blood was collected weekly. All pigs were necropsied on trial day 49, and tissues were evaluated for macroscopic and microscopic lesions. Serum was evaluated using PCV2 immunoglobulin G (IgG) and PCV2 IgM enzyme-linked immunosorbent assays, quantitative PCV2 PCR, and a serum PCV2 neutralizing antibody test. In comparison to NVAC-CPOS pigs, VAC-POS animals had significantly (P < 0.01) less severe microscopic PCV2-associated lymphoid lesions and significantly (P < 0.04) reduced PCV2 genomic copies in serum following PCV2 challenge. These results indicate that vaccination with Suvaxyn PCV2 One Dose reduces viremia and prevents microscopic lesions associated with PCV2 in the presence of maternal antibodies.
Porcine circovirus type 2 (PCV2) is a small, nonenveloped, single-stranded DNA virus that is ubiquitous in the swine population (1). PCV2 continues to have a major economic impact on the global swine industry. It has been associated with systemic infection (1), respiratory disease (7, 8), enteritis (9), reproductive failure (10, 13, 23), and porcine dermatitis and nephropathy syndrome (20, 22). In order to combat the growing problems associated with PCV2-associated disease, several vaccines were introduced into the U.S. market in 2006. Initial reports on the efficacy of these products indicated that vaccination is an effective tool to reduce PCV2-associated losses in production systems (15).
Due to the combination of vaccine usage and the ubiquitous nature of PCV2, the majority of females in breeding herds have been exposed to field PCV2 or PCV2 vaccines, and their piglets have varied levels of passively acquired PCV2 antibodies. In a previous report, sows from six U.S. breeding herds were tested for the presence of PCV2 antibodies, and 50 to 80% of dams from the herds had sample-to-positive (S/P) ratios greater then 0.6, 15 to 42% had S/P ratios between 0.2 and 0.6, and 0 to 8% were seronegative (18). This study also indicated that maternal antibodies decay over a wide window of time (2 to 15 weeks of age) in growing pigs within a population, depending on the initial concentration of maternal antibodies (18).
Although maternal antibodies against PCV2 are present at various levels in the swine population, their ability to completely prevent infection has not been proven. In a study by McKeown et al., 12-day-old commercial pigs with various levels of maternal antibodies were infected with a PCV2 infectious clone (11). Results indicated that high levels of maternal antibodies provided some protection (reduced peak viremia levels) but did not completely prevent infection. In animals with low levels of maternal antibodies, protection was not conferred (11). These results agreed with a study by Ostanello et al. which suggested that while maternal antibody levels were related to the development of PCV2-associated disease, they were unable to prevent clinically silent PCV2 infections (19).
While vaccines have been shown to be effective when used on pigs with no or low levels of passively acquired PCV2 antibodies, the effect of high levels of maternal antibodies on PCV2 vaccination remains unknown. Veterinary practitioners have raised concerns that pigs with detectable levels of passively acquired PCV2 antibodies may not develop a protective immune response to PCV2 vaccines. The study described in this report was designed to investigate the influence of passively acquired PCV2 antibodies on PCV2 vaccine efficacy, using one of the commercial PCV2 vaccines available in the United States. The hypothesis of the study was that vaccination in the presence of maternal antibody would result in protection (reduced PCV2 genomic copies and reduced PCV2-associated microscopic lesions) of pigs following challenge with PCV2.
MATERIALS AND METHODS
Experimental design.
Sixty 26- to 28-day-old, PCV2 PCR-negative piglets with various levels of passively acquired PCV2 antibody levels, as determined with a PCV2 open reading frame 2-based enzyme-linked immunosorbent assay (ELISA) (12), were blocked by PCV2 antibody level and randomly divided into four groups: vaccinated pigs with negative PCV2 ELISA S/P ratios at the time of vaccination (VAC-NEG; n = 9), vaccinated pigs with positive PCV2 ELISA S/P ratios at the time of vaccination (VAC-POS; n = 21), nonvaccinated pigs with negative PCV2 ELISA S/P ratios at the time of challenge (NVAC-CNEG; n = 15), and nonvaccinated pigs with positive PCV2 ELISA S/P ratios at the time of challenge (NVAC-CPOS; n = 15). Vaccinations and challenges were performed on trial days 0 and 28, respectively, according to group designation (Table 1). The pigs were monitored for clinical signs of disease daily and weighed and bled weekly. On trial day 49 (21 days after PCV2 challenge [DPC]), all pigs were necropsied and the tissues were evaluated for macroscopic and microscopic lesions. The experimental protocol was approved by the Iowa State University Institutional Animal Care and Use Committee.
TABLE 1.
Study design summary
| Group | No. of animals | Vaccinationa | ELISA status, day 0 (S/P ratio)b | Challengec | ELISA status, day 28 (S/P ratio)b |
|---|---|---|---|---|---|
| VAC-NEG | 9 | Yes | Negative (9/9) | Yes | Positive (6/9) |
| VAC-POS | 21 | Yes | Positive (21/21) | Yes | Positive (17/21) |
| NVAC-CNEG | 15 | No | Positive (12/15) | Yes | Negative (15/15) |
| NVAC-CPOS | 15 | No | Positive (15/15) | Yes | Positive (15/15) |
Animals were vaccinated on trial day zero with Suvaxyn PCV2 One Dose (Fort Dodge Animal Health, Inc., Fort Dodge, IA).
An S/P ratio of less then 0.3 was considered negative; pigs with an S/P ratio of ≥0.3 were considered positive.
Inoculation with PCV2 on trial day 28.
Animals and housing.
Colostrum-fed, cross-bred, specific-pathogen-free pigs were purchased from a herd that is routinely tested for major swine pathogens and known to be free of porcine reproductive and respiratory syndrome virus, swine influenza virus, and Mycoplasma hyopneumoniae. The farm was positive for PCV2 as determined by serology on sows; however, PCV2-associated disease was not present in the source farm or the offspring from this farm. The pigs were weaned at 2 weeks of age and transported to the Livestock Infectious Disease Isolation Facility at Iowa State University, Ames. The pigs were bled on arrival to confirm PCV2 PCR-negative status. On the day of delivery, the pigs were randomly assigned to one of two rooms, each containing six 2.5- by 3.6-m raised wire decks equipped with one nipple drinker and one self-feeder.
Vaccination.
On trial day zero, nine pigs with PCV2 ELISA S/P ratios below 0.3 (VAC-NEG) and 21 pigs with S/P ratios equal to or higher than 0.3 (VAC-POS) received 2 ml of a commercially available killed PCV1-2 chimeric vaccine (Suvaxyn PCV2 One Dose; Fort Dodge Animal Health, Inc., Fort Dodge, IA). The injection was given intramuscularly into the right neck according to the manufacturer's instructions.
Inoculation.
Each pig received 4 ml (1 ml intramuscularly and 3 ml intranasally) of PCV2 inoculum. The inoculum used was the fifth passage of PCV2 isolate 40895 (2, 3) at an approximate concentration of 104.7 50% tissue culture infective doses.
Serology.
Blood samples were collected upon arrival of the pigs at the research facility and weekly thereafter until necropsy. The serum was tested by the PCV2 open reading frame 2-based immunoglobulin G (IgG) ELISA (12). Samples were considered positive if the calculated S/P ratio was 0.3 or greater. A cutoff of 0.3 was used based on Iowa State University Laboratory protocols and was previously determined (unpublished data) to provide optimal sensitivity and specificity for the assay.
Samples were also tested by the Ingezim PCV2 ELISA IgM assay (Ingenasa, Madrid, Spain). The ELISA cutoff value was determined by multiplying by 0.4 the average optical density at 450 nm of the IgM-positive control wells. A fluorescence focus neutralization assay was done on trial day 0, 24, and 28 serum samples in order to determine the presence of neutralizing antibodies (NAs) against PCV2 according to the standard Iowa State University Veterinary Diagnostic Laboratory operating protocol. PCV2 isolate ISU-98-15237 was used in this assay.
Clinical evaluation.
Following PCV2 inoculation, the pigs were monitored daily and scored for severity of clinical respiratory disease, using scores ranging from 0 (normal) to 6 (severe dyspnea and abdominal breathing) (6). In addition, pigs were evaluated daily for clinical signs, including sneezing, ranging from 0 (no sneezing) to 3 (severe persistent sneezing), and jaundice. Rectal temperatures, wasting, and behavioral changes such as lethargy were recorded daily. The pigs were weighed on the day of vaccination and weekly thereafter until necropsy.
PCV2 DNA quantification.
DNA extraction from serum samples collected on trial days 0, 7, 14, 21, 28, 35, 42, and 48 was performed using the QIAamp DNA Mini kit (Qiagen, Valencia, CA). DNA extracts were used for quantification of PCV2 genomic DNA copy numbers by real-time PCR as described previously (17).
Necropsy.
All pigs were humanely euthanized by phenobarbital overdose and necropsied on trial day 49 (21 DPC). The total extent of macroscopic lung lesions (ranging from 0 to 100%) was estimated and scored. Additionally, the sizes of lymph nodes, ranging from 0 (normal) to 3 (four times the normal size), were estimated and recorded (14). Lungs were insufflated with fixative as previously described (6). Sections of lymph nodes (superficial inguinal, mediastinal, tracheobronchial, and mesenteric), tonsil, thymus, ileum, kidney, colon, spleen, and liver were collected at necropsy, fixed in 10% neutral-buffered formalin, and routinely processed for histological examination.
Histopathology.
Microscopic lesions were evaluated by a pathologist blinded to the group designation of animal tissues. Sections were scored for the presence and severity of interstitial pneumonia, ranging from 0 (normal) to 6 (severe diffuse) (6). Sections of heart, liver, kidney, ileum, and colon were evaluated for the presence of lymphohistiocytic inflammation and scored from 0 (none) to 3 (severe). Lymphoid tissues, including lymph nodes (tracheobronchiolar, mesenteric, mediastinal, superficial inguinal, and external iliac), tonsil, and spleen, were evaluated for the presence of lymphoid depletion, ranging from 0 (normal) to 3 (severe), and histiocytic inflammation and replacement of follicles, ranging from 0 (normal) to 3 (severe) (16). The overall microscopic lymphoid lesion score, which accounts for lymphoid depletion, histiocytic inflammation, and PCV2 antigen present in lymphoid tissues, was calculated as previously described (16) and ranged from 0 (normal) to 9 (severe).
Immunohistochemistry.
Immunohistochemistry (IHC) for detection of PCV2-specific antigen was performed on selected formalin-fixed and paraffin-embedded sections of lymph nodes (superficial inguinal, mediastinal, tracheobronchial, and mesenteric), tonsil, spleen, and thymus using a rabbit polyclonal antiserum (21). PCV2 antigen scoring was done by a pathologist blinded to animal group designation. Scores ranged from 0 (no signal) to 3 (more than 50% of lymphoid follicles contained cells with PCV2 antigen staining) (16). The mean group score was determined for each tissue and compared among groups.
Statistical analysis.
Summary statistics were calculated for all groups to assess the overall quality of the data, including normality. Continuous data collected over time were analyzed using multivariate analysis of variance (ANOVA). If a multivariate ANOVA was significant (P < 0.05), a nonparametric Kruskal-Wallis one-way ANOVA was done at each time point. If significant (P < 0.05) differences were seen at a time point, pairwise Wilcoxon tests were used to assess differences between groups (data were nonparametric). In order to summarize and simplify the clinical observations, a response feature analysis and a chi-square test were used. The clinical scores for each pig were reduced to one weekly mean score, and the resulting values were subjected to statistical analysis. Daily rectal temperature data were analyzed with response feature analysis on average weekly temperature. Nonrepeated measures of necropsy and histopathology data were assessed using a nonparametric Kruskal-Wallis one-way ANOVA. If this nonparametric ANOVA test was significant (P < 0.05), then pairwise Wilcoxon tests were used to assess differences between groups.
RESULTS
Clinical presentation.
None of the pigs developed clinical disease during the duration of the experiment. None of the pigs developed fever, and the average daily rectal temperatures were not significantly (P = 0.57) different among the groups. Similarly, the average daily weight gain did not significantly (P = 0.76) differ among the groups.
Macroscopic lesions.
No remarkable gross lesions were observed. Individual vaccinated and nonvaccinated pigs had mild lung lesions characterized by failure of the lungs to collapse and small, focal, cranioventral consolidated areas of pneumonia. There was no significant (P > 0.05) difference between the groups for the mean size of lymph nodes.
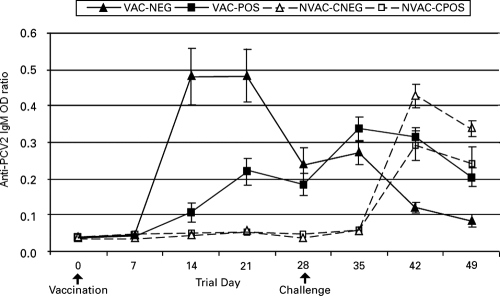
Anti-PCV2 IgM antibody levels.
The group mean anti-PCV2 IgM antibody levels are summarized in Fig. 1. The VAC-POS group had significantly (P < 0.01) higher IgM antibody levels from trial days 21 to 35 than the NVAC-CPOS group. Assessment of the IgM antibody levels between the VAC-NEG and NVAC-CNEG groups indicated that the VAC-NEG group had a significantly (P < 0.01) higher IgM response than the NVAC-CNEG group from days 14 to 35. On trial days 42 and 49 (7 and 14 DPC), these groups remained significantly (P < 0.01) different, but the IgM response was higher in the NVAC-CNEG group. In order to assess the effect of maternal antibody on IgM response, the VAC-POS and VAC-NEG groups were compared. On trial days 14 and 21, VAC-NEG pigs had significantly (P < 0.01) higher IgM responses.
FIG. 1.
Group mean optical density (OD) ratios and standard errors for anti-PCV2 IgM antibody responses. Trial day 0 = vaccination; trial day 28 = PCV2 challenge; trial day 49 = necropsy.
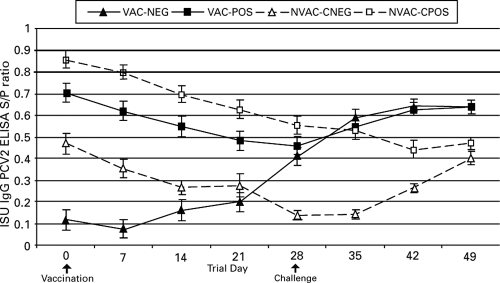
Anti-PCV2 IgG antibody levels.
The mean group anti-PCV2 IgG serum levels for trial day zero (0 DPV) to trial day 48 (21 DPC) are summarized in Fig. 2. To assess the effect of maternal antibody on vaccination, the IgG levels in VAC-POS and VAC-NEG animals were addressed on trial days 0, 7, 14, and 21. VAC-POS pigs had significantly (P < 0.01) higher IgG levels than VAC-NEG pigs from trial days 0 to 21. Following challenge on day 28, no significant differences in IgG levels were noted between the two groups. In nonvaccinated animals, the IgG levels following challenge were significantly (P < 0.01) higher in NVAC-CPOS pigs compared to NVAC-CNEG pigs from days 28 to 42. To address vaccine efficacy, VAC-POS animals were next compared to NVAC-CPOS pigs. Results indicated that VAC-POS animals had significantly (P < 0.01) higher IgG levels at trial days 42 and 49 (14 and 21 DPC) in comparison to NVAC-CPOS animals. Additionally, VAC-NEG animals had significantly (P < 0.01) higher IgG levels on trial days 28, 35, 42, and 49 in comparison to NVAC-CNEG pigs.
FIG. 2.
Group mean S/P ratios and standard errors for anti-PCV2 IgG antibody responses on different trial days. Trial day 0 = vaccination; trial day 28 = PCV2 challenge; trial day 49 = necropsy.
Neutralizing antibodies.
The group mean NA titers and standard errors are presented in Table 2. In order to assess maternal antibody effects on neutralizing antibody formation, VAC-POS pigs were compared to VAC-NEG pigs. As expected, VAC-POS pigs had significantly higher NA levels on days 0 and 14 (P < 0.01 for both days) compared to VAC-NEG animals. The data from the nonvaccinated animals were similar; NVAC-CPOS animals had significantly higher NA titers on trial days 0 (P < 0.01), 14 (P = 0.03), and 28 (P < 0.01) compared to NVAC-CNEG pigs.
TABLE 2.
Log-transformed PCV2 neutralizing antibody titers
| Group | Log-transformed group mean (SE) PCV2 NA titer on trial daya:
|
||
|---|---|---|---|
| 0 | 14 | 28 | |
| VAC-NEG | 1.36 (0.08)A | 1.78 (0.13)A | 2.85 (0.24)A |
| VAC-POS | 2.51 (0.10)B | 2.44 (0.10)B | 2.41 (0.10)B |
| NVAC-CNEG | 1.95 (0.06)C | 1.74 (0.07)A,C | 1.58 (0.14)C |
| NVAC-CPOS | 2.71 (0.10)B | 1.99 (0.18)A,D | 2.45 (0.12)A,B |
Significantly different (P < 0.05) group means within each column are connected by different superscript letters (A, B, C, and D).
In order to assess the effects of vaccination, VAC-POS animals were compared to NVAC-CPOS animals. Results of this comparison indicated that these two groups were significantly (P < 0.04) different on day 14, with higher levels in the VAC-POS pigs. Comparison of VAC-NEG to NVAC-CNEG animals indicated significantly higher NA levels in NVAC-CNEG on day 0 (P < 0.01) which were reversed on day 28, at which point the VAC-NEG levels were significantly (P = 0.01) higher.
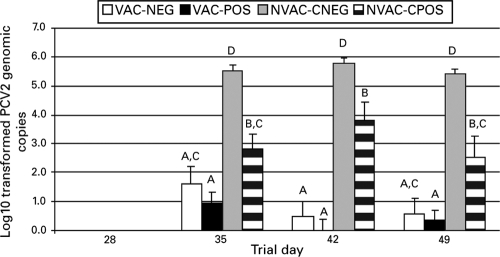
Incidence and amount of PCV2 DNA in serum.
PCV2 DNA was not detected in any of the serum samples on trial days 0, 7, 14, 21, or 28. The number of PCV2 genomic copies in serum was significantly reduced in VAC-POS pigs in comparison to NVAC-CPOS pigs on day 35 (7 DPC) (P = 0.04), on day 42 (14 DPC) (P < 0.01), and on day 49 (21 DPC) (P = 0.01) (Fig. 3). Additionally, VAC-NEG pigs had significantly (P < 0.01) fewer PCV2 genome copies compared to NVAC-CNEG pigs on trial days 35, 42, and 49 (Fig. 3).
FIG. 3.
Log-transformed group means for PCV2 DNA copies per ml of serum. Trial day 28 = PCV2 challenge; trial day 49 = necropsy. Different letters correspond to significant (P < 0.05) differences between groups for that trial day.
Microscopic lesions and IHC staining.
Mean scores and standard errors for lesions in lungs and lymphoid tissue sections are summarized in Table 3. Specific lymphoid tissue lesions (lymphoid depletion and histiocytic replacement) and IHC scores are summarized in Table 4. Comparison of microscopic lesions between VAC-POS and VAC-NEG groups revealed no significant (P = 0.28 for interstitial pneumonia; P = 0.93 for overall lymphoid lesions) differences. Comparison of microscopic interstitial pneumonia scores revealed significantly (P = 0.02) less severe interstitial pneumonia in NVAC-CPOS animals and NVAC-CNEG pigs. Additionally, a significant reduction in microscopic lung lesions (P = 0.01) and overall lymphoid lesions (P < 0.01) was noted between VAC-NEG and NVAC-CNEG animals, and there was a significant (P < 0.01) reduction in overall lymphoid lesions in VAC-POS pigs compared to NVAC-CPOS pigs.
TABLE 3.
Microscopic lesion scores for lung (interstitial pneumonia) and lymphoid tissues (overall lymphoid severity)
| Group | Mean (SE) scorea
|
|
|---|---|---|
| Interstitial pneumoniab | Overall lymphoid severityc | |
| VAC-NEG | 0.11 (0.11)A | 0.48 (0.18)A |
| VAC-POS | 0.38 (0.15)A | 0.69 (0.22)A |
| NVAC-CNEG | 0.87 (0.19)B | 3.76 (0.58)B |
| NVAC-CPOS | 0.27 (0.15)A | 2.77 (0.51)B |
Significantly different (P < 0.05) group means within each column are indicated by different superscript letters (A or B).
Possible scores for interstitial pneumonia ranged from 0 (normal) to 6 (severe diffuse interstitial pneumonia).
The mean cumulative value from seven lymphoid tissues (tonsil, spleen, and five lymph nodes) is reported (possible range, 0 to 9) and includes scores for lymphoid depletion (possible range, 0 to 3), inflammation (possible range, 0 to 3), and the amount of PCV2 (possible range, 0 to 3). Possible cumulative scores for overall severity included normal (score of 0), mild (score range, 1 to 3), moderate (range, 4 to 6), and severe (range, 7 to 9).
TABLE 4.
PCV2 immunohistochemistry, lymphoid depletion, and histiocytic replacement scores in selected lymphoid tissues
| Tissue and test | Mean (SE) scoreb for vaccination group
|
|||
|---|---|---|---|---|
| VAC-NEG | VAC-POS | NVAC-CNEG | NVAC-CPOS | |
| Lymph nodes | ||||
| IHCa | 0.00 (0.00)A | 0.19 (0.15)A | 1.67 (0.27)B | 1.00 (0.22)B |
| LDc | 0.44 (0.17)A | 0.43 (0.13)A | 1.73 (0.28)B | 1.33 (0.27)B |
| HRc | 0.11 (0.11)A | 0.29 (0.12)A | 1.67 (0.25)B | 1.13 (0.24)B |
| Tonsil | ||||
| PCV2 IHCa | 0.00 (0.00)A | 0.10 (0.10)A | 0.60 (0.19)B | 0.53 (0.17)A,B |
| LDc | 0.11 (0.11)A,B | 0.00 (0.00)B | 0.40 (0.16)A | 0.27 (0.15)A |
| HRc | 0.00 (0.00)A | 0.05 (0.05)A | 0.53 (0.22)A | 0.27 (0.15)A |
| Spleen | ||||
| PCV2 IHCa | 0.00 (0.00)A,B | 0.10 (0.10)B | 0.47 (0.13)C | 0.33 (0.13)A,C |
| LDc | 0.11 (0.11)A | 0.14 (0.08)A | 0.80 (0.17)B | 0.87 (0.19)B |
| HRc | 0.11 (0.11)A,B | 0.14 (0.10)B | 0.80 (0.17)C | 0.60 (0.21)A,C |
Scores ranged from 0 (no signal) to 3 (more than 50% of the lymphoid follicles contained cells with PCV2 antigen staining).
Significantly different (P < 0.05) group means within each row are connected by different superscript letters (A, B, or C).
Scores ranged from 0 (normal) to 3 (severe). LD, lymphoid depletion; HR, histocytic replacement.
DISCUSSION
In order to determine whether passively acquired, maternally derived PCV2 antibodies interfere with the efficacy of vaccination against PCV2, colostrum-fed specific-pathogen-free pigs originating from a PCV2-seropositive population were used in this study. This ensured that the study population contained pigs with and without maternal antibodies at the time of vaccination and inoculation.
The study design included four groups. Due to the logistical difficulties of finding sufficient numbers of PCV2-seronegative animals, a vaccinated, nonchallenged group and a nonvaccinated, nonchallenged group were not included in the study. While this is less than ideal, previous publications have provided evidence that PCV2 vaccination (with the live chimeric PCV1-2 vaccine) is efficacious in the pig model using PCV2-negative pigs and is not associated with microscopic lesions (4, 5). Evidence of naturally acquired PCV2 infection was lacking in the animals studied in this trial, and PCV2 DNA was not detected in any of the serum samples prior to trial day 35 (7 DPC). Microscopic lesions and PCV2 real-time PCR data from the nonvaccinated groups are consistent with PCV2 infection following the challenge on trial day 28.
Overall, in comparison to NVAC-CPOS pigs, VAC-POS animals had significantly (P < 0.01) lower microscopic lymphoid tissue lesion scores and significantly (P < 0.05) fewer PCV2 genomic copies following PCV2 challenge. These findings confirm previous studies, which have indicated that vaccination prevents microscopic PCV2-associated lesions and reduces PCV2 viremia (4, 5). Additionally, the anti-PCV2 IgG levels in the VAC-POS animals increased between trial days 28 (challenge) and 35, while IgG levels in NVAC-CPOS animals declined (Fig. 2). These results are consistent with the challenge acting as a booster to the previous vaccination in the VAC-POS animals. There were no significant differences in macroscopic lung lesions between VAC-POS and NVAC-CPOS animals. This is likely due to the use of the single PCV2 challenge model, in which clinical signs and lung lesions are rarely seen. In contrast, significantly more severe microscopic lesions were observed in VAC-POS animals compared to NVAC-CPOS animals.
After PCV2 challenge, there were no significant differences in severity of PCV2-associated microscopic lesions, anti-PCV2 IgG levels following challenge, levels of PCV2 viremia, or amounts of PCV2 antigen in tissue sections between VAC-NEG and VAC-POS pigs. These results suggest that the vaccine is equally effective in reducing PCV2-associated lesions and viremia regardless of the level of passively acquired antibodies at the time of vaccination. The only significant differences between VAC-POS and VAC-NEG animals included the anti-PCV2 IgM levels and the NA titers. Specifically, the IgM response in VAC-POS animals was significantly lower and delayed by 1 week compared to VAC-NEG animals. One explanation for the IgM response is that in animals which were ELISA positive at vaccination, maternal antibody inhibited a strong IgM response. The NA titers in this trial indicated that vaccination induced an NA response in the VAC-NEG group. In the VAC-POS group, we were unable to determine if the NA response was consistent with vaccination or due to passive maternal antibody (which likely contains NA). Therefore, the ability of the vaccine to induce an NA response in the presence of maternal antibodies warrants further investigation.
In contrast to the vaccinated animals, there were significant differences between the NVAC-CNEG and NVAC-CPOS pigs following challenge. As expected, the NVAC-CNEG animals had more severe microscopic lung lesions and higher levels of PCV2 viremia than the NVAC-CPOS animals. Additionally, initial differences in anti-PCV2 IgG levels and differences in NA titers were most likely due to the presence of passively acquired maternal NA. Following challenge, IgG levels in the NVAC-CNEG pigs increased, in contrast to the NVAC-CPOS group. This was most likely due to maternal IgG inhibition in animals which had received passive immunization.
PCV2 infection typically occurs at the mid- to late nursery phase or early in the finisher phase of production in North American swine production systems and, thus, vaccination should occur several weeks prior to this stage of production. The results from this study indicate that the PCV2 vaccine used in this study is effective in reducing viremia and microscopic lesions consistent with PCV2 infection, even when used in pigs with passively acquired antibodies at the time of vaccination. This suggests that practitioners can recommend the use of this PCV2 vaccine on pigs at an early age, such as around 26 days old, and well ahead of typical PCV2 exposure.
Acknowledgments
We thank Paul Thomas and Matt Boogerd for assistance with animal work, John Johnson and staff at the Iowa State University Veterinary Diagnostic Laboratory for assistance with serology, and Fort Dodge Animal Health, Inc., for providing the inoculum.
Footnotes
Published ahead of print on 19 December 2007.
REFERENCES
- 1.Allan, G. M., and J. A. Ellis. 2000. Porcine circoviruses: a review. J. Vet. Diagn. Investig. 12:3-14. [DOI] [PubMed] [Google Scholar]
- 2.Fenaux, M., P. G. Halbur, M. Gill, T. E. Toth, and X. J. Meng. 2000. Genetic characterization of type 2 porcine circovirus (PCV-2) from pigs with postweaning multisystemic wasting syndrome in different geographic regions of North America and development of a differential PCR-restriction fragment length polymorphism assay to detect and differentiate between infections with PCV-1 and PCV-2. J. Clin. Microbiol. 38:2494-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenaux, M., P. G. Halbur, G. Haqshenas, R. Royer, P. Thomas, P. Nawagitgul, M. Gill, T. E. Toth, and X. J. Meng. 2002. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: characterization of clinical disease, virus distribution, and pathologic lesions. J. Virol. 76:541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenaux, M., T. Opriessnig, P. G. Halbur, F. Elvinger, and X. J. Meng. 2004. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J. Virol. 78:6297-6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenaux, M., T. Opriessnig, P. G. Halbur, and X. J. Meng. 2003. Immunogenicity and pathogenicity of chimeric infectious DNA clones of pathogenic porcine circovirus type 2 (PCV2) and nonpathogenic PCV1 in weanling pigs. J. Virol. 77:11232-11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halbur, P. G., P. S. Paul, M. L. Frey, J. Landgraf, K. Eernisse, X. J. Meng, M. A. Lum, J. J. Andrews, and J. A. Rathje. 1995. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 32:648-660. [DOI] [PubMed] [Google Scholar]
- 7.Harms, P. A., P. G. Halbur, and S. D. Sorden. 2002. Three cases of porcine respiratory disease complex associated with porcine circovirus type 2 infection. J. Swine Health Prod. 10:27-30. [Google Scholar]
- 8.Kim, J., H. K. Chung, and C. Chae. 2003. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet. J. 166:251-256. [DOI] [PubMed] [Google Scholar]
- 9.Kim, J., Y. Ha, K. Jung, C. Choi, and C. Chae. 2004. Enteritis associated with porcine circovirus 2 in pigs. Can. J. Vet. Res. 68:218-221. [PMC free article] [PubMed] [Google Scholar]
- 10.Ladekjær-Mikkelsen, A. S., J. Nielsen, T. Storgaard, A. Bøtner, G. Allan, and F. McNeilly. 2001. Transplacental infection with PCV-2 associated with reproductive failure in a gilt. Vet. Rec. 148:759-760. [PubMed] [Google Scholar]
- 11.McKeown, N. E., T. Opriessnig, P. Thomas, D. K. Guenette, F. Elvinger, M. Fenaux, P. G. Halbur, and X. J. Meng. 2005. Effects of porcine circovirus type 2 (PCV2) maternal antibodies on experimental infection of piglets with PCV2. Clin. Diagn. Lab. Immunol. 12:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nawagitgul, P., P. A. Harms, I. Morozov, B. J. Thacker, S. D. Sorden, C. Lekcharoensuk, and P. S. Paul. 2002. Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based enzyme-linked immunosorbent assays for detection of antibodies to PCV. Clin. Diagn. Lab. Immunol. 9:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor, B., H. Gauvreau, K. West, J. Bogdan, M. Ayroud, E. G. Clark, C. Konoby, G. Allan, and J. A. Ellis. 2001. Multiple porcine circovirus 2-associated abortions and reproductive failure in a multisite swine production unit. Can. Vet. J. 42:551-553. [PMC free article] [PubMed] [Google Scholar]
- 14.Opriessnig, T., M. Fenaux, P. Thomas, M. J. Hoogland, M. F. Rothschild, X. J. Meng, and P. G. Halbur. 2006. Evidence of breed-dependent differences in susceptibility to porcine circovirus type-2-associated disease and lesions. Vet. Pathol. 43:281-293. [DOI] [PubMed] [Google Scholar]
- 15.Opriessnig, T., X. J. Meng, and P. G. Halbur. 2007. Porcine circovirus type 2-associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet. Diagn. Investig. 19:591-615. [DOI] [PubMed] [Google Scholar]
- 16.Opriessnig, T., E. L. Thacker, S. Yu, M. Fenaux, X. J. Meng, and P. G. Halbur. 2004. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet. Pathol. 41:624-640. [DOI] [PubMed] [Google Scholar]
- 17.Opriessnig, T., S. Yu, J. M. Gallup, R. B. Evans, M. Fenaux, F. Pallares, E. L. Thacker, C. W. Brockus, M. R. Ackermann, P. Thomas, X. J. Meng, and P. G. Halbur. 2003. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Vet. Pathol. 40:521-529. [DOI] [PubMed] [Google Scholar]
- 18.Opriessnig, T., S. Yu, E. L. Thacker, and P. G. Halbur. 2004. Derivation of porcine circovirus type 2-negative pigs from positive breeding herds. J. Swine Health Prod. 12:186-191. [Google Scholar]
- 19.Ostanello, F., A. Caprioli, F. A. Di, M. Battilani, G. Sala, G. Sarli, L. Mandrioli, F. McNeilly, G. M. Allan, and S. Prosperi. 2005. Experimental infection of 3-week-old conventional colostrum-fed pigs with porcine circovirus type 2 and porcine parvovirus. Vet. Microbiol. 108:179-186. [DOI] [PubMed] [Google Scholar]
- 20.Rosell, C., J. Segalés, J. A. Ramos-Vara, J. M. Folch, G. M. Rodríguez-Arrioja, C. O. Duran, M. Balasch, J. Plana-Durán, and M. Domingo. 2000. Identification of porcine circovirus in tissues of pigs with porcine dermatitis and nephropathy syndrome. Vet. Rec. 146:40-43. [DOI] [PubMed] [Google Scholar]
- 21.Sorden, S. D., P. A. Harms, P. Nawagitgul, D. Cavanaugh, and P. S. Paul. 1999. Development of a polyclonal-antibody-based immunohistochemical method for the detection of type 2 porcine circovirus in formalin-fixed, paraffin-embedded tissue. J. Vet. Diagn. Investig. 11:528-530. [DOI] [PubMed] [Google Scholar]
- 22.Wellenberg, G. J., N. Stockhofe-Zurwieden, M. F. De Jong, W. J. Boersma, and A. R. Elbers. 2004. Excessive porcine circovirus type 2 antibody titres may trigger the development of porcine dermatitis and nephropathy syndrome: a case-control study. Vet. Microbiol. 99:203-214. [DOI] [PubMed] [Google Scholar]
- 23.West, K. H., J. M. Bystrom, C. Wojnarowicz, N. Shantz, M. Jacobson, G. M. Allan, D. M. Haines, E. G. Clark, S. Krakowka, F. McNeilly, C. Konoby, K. Martin, and J. A. Ellis. 1999. Myocarditis and abortion associated with intrauterine infection of sows with porcine circovirus 2. J. Vet. Diagn. Investig. 11:530-532. [DOI] [PubMed] [Google Scholar]