Abstract
The 53-kDa proteins in larval excretory and secretory (E-S) products were expressed from five Trichinella species (T. spiralis, T. britovi, T. nativa, T. pseudospiralis, and T. papuae), using the Escherichia coli expression system, and the antibody responses to the 53-kDa recombinant proteins in mice infected with Trichinella spp. were analyzed by Western blotting. The 53-kDa protein is conserved among the five Trichinella species, with >60% similarity in amino acid sequences. The 53-kDa recombinant proteins of T. spiralis and T. pseudospiralis reacted to sera from mice infected with T. spiralis and T. pseudospiralis at 8 days postinfection (p.i.), respectively. An antibody against the 53-kDa recombinant protein of T. spiralis recognized the 53-kDa protein in the crude extracts from adult worms and 30-day p.i. muscle larvae and E-S products from muscle larvae of T. spiralis but did not recognize any proteins from T. pseudospiralis. The sera from the mice infected with T. spiralis strongly reacted with the 53-kDa recombinant protein of T. spiralis but did not react with the 53-kDa recombinant proteins of T. britovi, T. nativa, T. pseudospiralis, and T. papuae. Similarly, the sera from mice infected with T. britovi, T. nativa, T. pseudospiralis, or T. papuae strongly reacted with the 53-kDa recombinant proteins of T. britovi, T. nativa, T. pseudospiralis, or T. papuae, respectively. These results showed that the 53-kDa recombinant proteins provide early and species-specific antibody responses in mice infected with Trichinella spp.
Nematodes of the genus Trichinella infect a broad range of mammals, birds, and reptiles and cause significant food-borne illness in humans (5).
In domestic animals and wildlife, the meat digestion and microscopic inspection method is considered to be the most useful method for detecting these parasites, but it is somewhat cumbersome to perform (8). In human trichinellosis, most clinical symptoms and biological signs are nonspecific, and so immunological techniques for the detection of antibody against Trichinella antigens are important for making a diagnosis of trichinellosis (1). Many techniques have been adapted for detecting antibodies against Trichinella antigens, such as indirect immunofluorescence, Western blotting, and an enzyme-linked immunosorbent assay (ELISA) (6, 14, 24). Crude antigens and excretory and secretory (E-S) antigens from muscle larvae are widely used for ELISAs and Western blotting, but these antigens may give rise to cross-reactivity to other antigenically related parasites (3). An ELISA using purified tyvelose-containing antigen, which is secreted from muscle larvae of Trichinella spp., is sensitive and specific for immunodiagnosis of trichinellosis, but it is not useful for making an early diagnosis (during the intestinal and migratory phases of the infection) (7).
The 53-kDa glycoprotein secreted from T. spiralis is a candidate immunodiagnostic antigen for trichinellosis, because this protein is present in much greater amounts in the E-S products (25), and the homologue of the 53-kDa glycoprotein of T. spiralis is present in E-S products of other species in the genus Trichinella (15, 16, 22). The use of the 53-kDa recombinant protein for detection of antibodies against Trichinella antigens has already been described (9, 25).
The humoral immune response to Trichinella spp. has been studied in different host species, and the studies may be used to identify useful antigens for the diagnosis of or protection from Trichinella infection (4, 12, 19). In the present study, each of the 53-kDa proteins from T. spiralis, T. britovi, T. nativa, T. pseudospiralis, and T. papuae was produced using the Escherichia coli expression system, and the humoral immune response and the antigenic recognition of the recombinant proteins were analyzed in mice infected with different Trichinella species.
MATERIALS AND METHODS
Parasites and material sampling.
Five Trichinella species (T. spiralis, T. britovi, T. nativa, T. pseudospiralis, and T. papuae) were used in this study. The detailed data (code, original hosts, locality, and country of origin) of these species are shown in Table 1. The code is that used by the Trichinella Reference Centre in Rome.
TABLE 1.
Codes, original hosts, and geographical origins of five Trichinella species
| Species | Isolate code | Host origin | Geographic region |
|---|---|---|---|
| T. spiralis | ISS413 | Wild bore | Poland |
| T. britovi | ISS119 | Red fox | Italy |
| T. nativa | ISS410 | Polar bear | Arctic |
| T. pseudospiralis | ISS13 | Raccoon | Russia (Caucasus) |
| T. papuae | ISS572 | Wild pig | Papua New Guinea |
Muscle-stage larvae of Trichinella spp. from mice at 15 days and 30 days postinfection (p.i.) were isolated by pepsin-HCl digestion (11). Adult worms of Trichinella spp. were isolated from infected mouse intestines at 6 days p.i. Newborn larvae of Trichinella spp. were isolated from female adult worms according to the methods of Takada and Tada (18). Crude saline extracts of parasites or E-S products from 30-day p.i. muscle larvae of Trichinella spp. were prepared by conventional methods (21, 22).
Infection sera and antisera.
Infection sera were obtained from BALB/c mice infected with 300 larvae of T. spiralis and T. pseudospiralis at 8, 13, 18, 23, 30, 50, 90, and 120 days p.i., and they were obtained from BALB/c mice infected with 300 larvae of T. britovi, T. nativa, and T. papuae at 30 days p.i. Polyclonal antibodies against the recombinant 53-kDa proteins of T. spiralis and T. pseudospiralis were produced in BALB/c mice injected intradermally with approximately 100 μg of the recombinant protein and complete Freund's adjuvant. This was followed by four booster injections of 100 μg of the recombinant protein mixed with incomplete Freund's adjuvant at 2-week intervals.
Preparation of Trichinella cDNA.
Total RNA was isolated from 30-day p.i. muscle larvae using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Reverse transcription was performed using SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions. In brief, the 20-μl reaction volume consisted of 3 μg of the sample RNA, 1 μl of 0.5 μg/μl oligo(dT)12-18, 1 μl 10 mM deoxynucleotide triphosphate mix, 4 μl First-Strand buffer (Invitrogen), 1 μl 0.1 M dithiothreitol, 1 μl RNase inhibitor, and 1 μl SuperScript III reverse transcriptase. The reaction mixture was incubated at 50°C for 60 min and then inactivated by heating at 70°C for 15 min.
Amplification of genes of Trichinella 53-kDa proteins by PCR and DNA sequencing.
The genes encoding the full-length 53-kDa proteins of T. nativa and T. papuae were amplified by PCR from 30-day p.i. muscle larva cDNA using oligonucleotide primers with BamHI and EcoRI restriction enzyme sites added (underlined in the following sequences). The primers for amplification of the genes were designed from the reported nucleotide sequence of the T. spiralis 53-kDa protein (GenBank accession number U25127) as follows: 5′-CGG GAT CCC GAT GTT CAG CAT CAC ATT AAA and 5′-CGG AAT TCC GGA CAG ATT GCT TAA TGA AGC. The PCR mixture (total volume of 100 μl) consisted of 10 μl of 100 ng of cDNA, 10 μl of 10× PCR buffer, 4 μl of 2.5 mM each deoxynucleotide triphosphate, 1.5 U of Taq polymerase, and 10 μl of a 10 μM concentration of each primer. DNA was amplified for 35 cycles (each cycle consisted of 30 s of denaturation at 94°C, 30 s of annealing at 52°C, and 60 s of extension at 72°C). The purified PCR products were digested with BamHI and EcoRI, and the resulting fragments were cloned into a pGEM-3Zf (+) vector (Promega, Madison, WI). The nucleotide sequences were determined using ABI PRISM BigDye primer cycle sequencing kits (Applied Biosystems Japan, Tokyo, Japan) and an ABI PRISM 3100 genetic analyzer (Applied Biosystems Japan). The DNA sequences were assembled and analyzed using the DNASIS software (Hitachi Software Engineering, Tokyo, Japan). The BLAST network service was used to search the DNA and protein databases at the National Center for Biotechnology Information.
Expression and purification of the recombinant protein.
The genes encoding pro-proteins of the 53-kDa proteins of T. spiralis, T. britovi, T. nativa, T. pseudospiralis, and T. papuae were amplified by PCR from 30-day p.i. muscle larva cDNA using oligonucleotides primers with BamHI and EcoRI restriction enzyme sites added to assist the cloning into the pTrcHis expression vector (Invitrogen). The primers for the amplification of the 53-kDa proteins are shown in Table 2.
TABLE 2.
Primer pairs used to amplify genes encoding pro-proteins of the 53-kDa proteins of Trichinella spp.
| Target | Sequencea |
|---|---|
| T. spiralis | CGCGGATCCGCGGTCTACAGACAATGAGAATGTTG |
| CGGAATTCCGTTAGAACAACAACTGTAGTTCTG | |
| T. britovi | CGCGGATCCGCGGTCTACAGACAATGAGAATGCTG |
| CGGAATTCCGTTAGAACAACAACTGTAGTTCTG | |
| T. nativa | CGCGGATCCGCGGTCTACAGACAATGAGAATGCTG |
| CGGAATTCCGTTAGAACAACAACTGTAGTTCTG | |
| T. pseudospiralis | CGCGGATCCGCGGTCTTCAGACACTGATAAGGTTG |
| CGGAATTCCGTTAGAACAACAACTGTAGTTCTG | |
| T. papuae | CGCGGATCCGCGGTCTACAGACATTGAGAAGGTTG |
| CGGAATTCCGTTAGAACAACAACTGTAGTTCTG |
Underlined portions indicate the BamHI or EcoRI restriction enzyme site.
The PCR products were digested with BamHI and EcoRI and cloned into the pTrcHis vector. The recombinant plasmids were transformed into an E. coli DH5α strain, and the expression of polyhistidine-containing recombinant proteins was induced by adding isopropyl β-d-thiogalactopyranoside at a final concentration of 1 mM at 37°C for 3 h. The induced cells were harvested and disrupted by sonication in 20 mM Tris-HCl buffer (pH 8.0). Recombinant proteins expressed as inclusion bodies were solubilized completely with 6 M urea in 20 mM Tris-HCl buffer (pH 8.0) and then purified with a His trap kit (GE Healthcare Bio-Sciences) for affinity purification of the histidine-tagged proteins according to the manufacturer's instructions. For the refolding of recombinant proteins, the urea was removed from the sample protein solutions by stepwise dilution with 20 mM Tris-HCl buffer (pH 8.0) in a His trap chelating column (2). The recombinant proteins were eluted with 500 mM imidazole, and then imidazole was removed from the sample with a PD-10 column (GE Healthcare Bio-Sciences, Piscataway, NJ) and analyzed with sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) to assess its purity.
Western blot analysis.
Test samples included crude extracts from adult worms, newborn larvae, 15-day p.i. muscle larvae, 30-day p.i. muscle larvae, E-S products from 30-day p.i. muscle larvae, and the recombinant proteins. Twenty μg of crude extract, E-S products, or 0.2 μg of recombinant protein was separated by 12% SDS-PAGE, electrotransferred to nitrocellulose sheets, and immunostained with antibodies against the recombinant proteins (1:100 dilution) or sera from mice infected with Trichinella spp. (1:100 dilution). Goat anti-mouse antibody-alkaline phosphatase conjugate (1:15,000 dilution; Sigma Chemical Co., St. Louis, MO) was used as the second antibody, and the alkaline phosphatase was developed in 5-bromo-4-chloro-3-indolyl-p-toluidine salt and nitroblue tetrazolium.
Nucleotide sequence accession numbers.
The nucleotide sequence data for the genes encoding 53-kDa proteins of T. nativa and T. papuae have been deposited in the GenBank, EMBL, and DDBJ databases under accession numbers DQ399908 and DQ399909, respectively.
RESULTS
Sequence analysis of the 53-kDa protein of five Trichinella species.
The amino acid sequences of the 53-kDa proteins of T. spiralis, T. britovi, and T. pseudospiralis have been previously reported under accession numbers AAA97512, CAD86782, and AAK29415, respectively. The genes encoding the 53-kDa proteins of T. nativa and T. papuae were sequenced in this study, and the amino acid sequences were deduced.
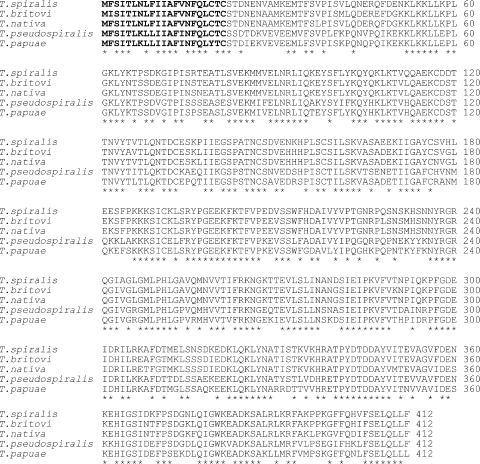
All genes encoding the 53-kDa proteins of five Trichinella species were 1,239 bp in length and encoded a protein of 412 amino acid residues. Weight matrix calculations based on the algorithm determined by von Heijne (20) allow predictions of the most likely cleavage site for the N-terminal signal sequence. For all five Trichinella species, the possible signal sequence cleavage sites were predicted after cysteine 21 (Fig. 1). Alignment of the amino acid sequences of the 53-kDa proteins among the five Trichinella species is shown in Fig. 1. The 53-kDa proteins seem to comprise the conserved and nonconserved regions. For example, the N-terminal region (positions 22 to 51) of the pro-protein is nonconservative (27% identical), and the C-terminal region (positions 377 to 412) is conservative (61% identical). The pairwise amino acid sequence comparisons for the five species are shown in Table 3. The 53-kDa protein of T. spiralis (encapsulated species) showed 90.7% and 89.5% sequence similarities to those of T. britovi and T. nativa (encapsulated species), respectively, but showed low sequence similarities (66.6% and 68.8%) to those of T. pseudospiralis and T. papuae (nonencapsulated species). The 53-kDa protein of T. britovi is 95.7% identical to that of T. nativa, whereas the 53-kDa protein of T. pseudospiralis is 78.7% identical to that of T. papuae but has low sequence similarity to those of encapsulated species (from 62.4% to 66.6%).
FIG. 1.
Deduced amino acid sequences of the 53-kDa proteins of T. spiralis (GenBank accession number AAA97512), T. britovi (GenBank accession number CAD86782), T. nativa (GenBank accession number DQ399908), T. pseudospiralis (GenBank accession number AAK29415), and T. papuae (GenBank accession number DQ399909). Amino acid residues conserved in all five sequences are indicated by asterisks. The putative signal peptides are indicated in boldface. The numbers along the margin designate the positions of amino acid residues.
TABLE 3.
Similarity of amino acid sequences among five Trichinella species
| Species | Identity (%) with:
|
||||
|---|---|---|---|---|---|
| T. spiralis | T. britovi | T. nativa | T. pseudospiralis | T. papuae | |
| T. spiralis | 100 | ||||
| T. britovi | 90.7 | 100 | |||
| T. nativa | 89.5 | 95.7 | 100 | ||
| T. pseudospiralis | 66.6 | 62.7 | 62.4 | 100 | |
| T. papuae | 68.8 | 65.9 | 65.3 | 78.7 | 100 |
Western blot analysis with antibody against the recombinant 53-kDa protein.
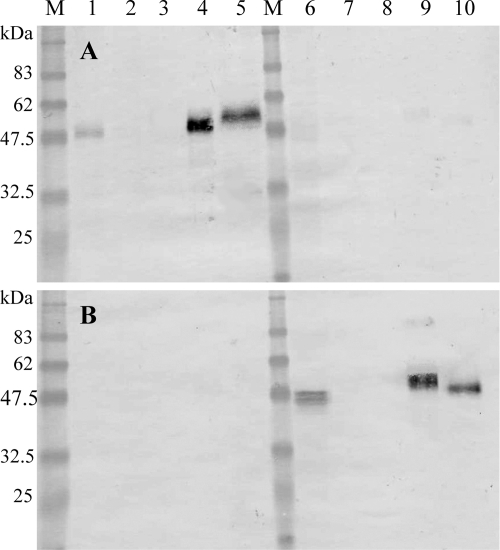
Figure 2A shows that the antibody against the 53-kDa recombinant protein of T. spiralis immunostained proteins that migrated at approximately 47 to 50 kDa in the crude extracts from adult worms of T. spiralis (lane 1), approximately 48 to 55 kDa in the crude extracts from 30-day p.i. muscle larvae of T. spiralis (lane 4), and approximately 53 to 58 kDa in the E-S products from 30-day p.i. muscle larvae of T. spiralis (lane 5), but the antibody against the 53-kDa recombinant protein of T. spiralis did not immunostain any proteins in the crude extracts or in the E-S products of T. pseudospiralis (lanes 6 to 10).
FIG. 2.
Western blot analysis of crude extracts from T. spiralis adult worms (lane 1), T. spiralis newborn larvae (lane 2), T. spiralis 15-day p.i. muscle larvae (lane 3), T. spiralis 30-day p.i. muscle larvae (lane 4), E-S products from T. spiralis muscle larvae (lane 5), crude extracts from T. pseudospiralis adult worms (lane 6), T. pseudospiralis newborn larvae (lane 7), T. pseudospiralis 15-day p.i. muscle larvae (lane 8), T. pseudospiralis 30-day p.i. muscle larvae (lane 9), and E-S products from T. pseudospiralis muscle larvae (lane 10) immunostained with antibody against the T. spiralis 53-kDa recombinant protein (A) or immunostained with antibody against the T. pseudospiralis 53-kDa recombinant protein (B). M, molecular mass standards; sizes in kDa are shown on the left side.
Figure 2B shows that the antibody against the 53-kDa recombinant protein of T. pseudospiralis immunostained proteins that migrated at approximately 44 to 47 kDa in the crude extracts from adult worms of T. pseudospiralis (lane 6), approximately 48 to 55 kDa in the crude extracts from 30-day p.i. muscle larvae of T. pseudospiralis (lane 9), and approximately 47 to 50 kDa in the E-S products from 30-day p.i. muscle larvae of T. pseudospiralis (lane 10), but the antibody against the 53-kDa recombinant protein of T. pseudospiralis did not immunostain any proteins in crude extracts or in E-S products of T. spiralis (lane 1 to 5).
Western blot analysis of the recombinant 53-kDa proteins with sera from infected mice.
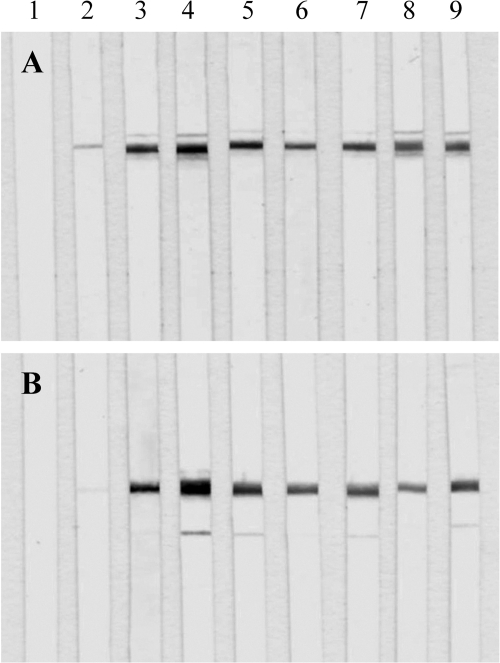
The 53-kDa recombinant protein of T. spiralis was positively immunostained with sera from mice infected with T. spiralis at 8, 13, 18, 23, 30, 50, 90, and 120 days p.i. (Fig. 3A, lanes 2 to 9, respectively), but it was not immunostained with serum from uninfected mice (Fig. 3A, lane 1).
FIG. 3.
(A) Western blot analysis of the T. spiralis 53-kDa recombinant protein immunostained with sera from noninfected mice (lane 1) or sera from mice infected with T. spiralis at 8 days p.i. (lane 2), 13 days p.i. (lane 3), 18 days p.i. (lane 4), 23 days p.i. (lane 5), 30 days p.i. (lane 6), 50 days p.i. (lane 7), 90 days p.i. (lane 8), and 120 days p.i. (lane 9). (B) Western blot analysis of the T. pseudospiralis 53-kDa recombinant protein immunostained with sera from noninfected mice (lane 1) or sera from mice infected with T. pseudospiralis at 8 days p.i. (lane 2), 13 days p.i. (lane 3), 18 days p.i. (lane 4), 23 days p.i. (lane 5), 30 days p.i. (lane 6), 50 days p.i. (lane 7), 90 days p.i. (lane 8), and 120 days p.i. (lane 9).
The 53-kDa recombinant protein of T. pseudospiralis was positively immunostained with sera from mice infected with T. pseudospiralis at 8, 13, 18, 23, 30, 50, 90, and 120 days p.i. (Fig. 3B, lanes 2 to 9, respectively), but it was not immunostained with sera from uninfected mice (Fig. 3B, lane 1).
Cross-reactivity analysis of the recombinant 53-kDa proteins by Western blotting.
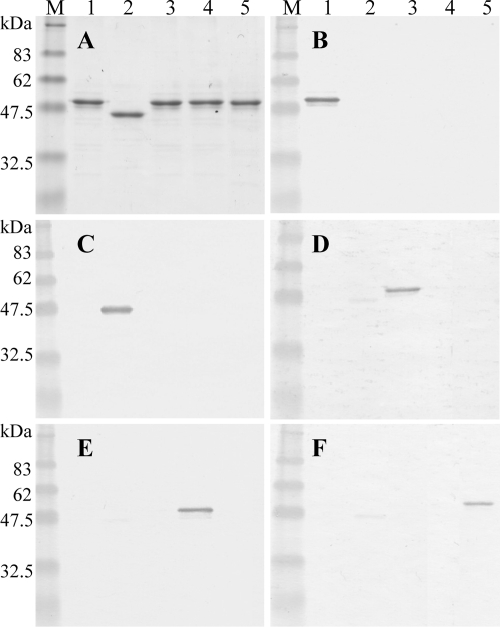
The 53-kDa recombinant proteins of Trichinella spp. could be purified to a single band level by SDS-PAGE using a His trap kit and elution with 500 mM imidazole (Fig. 4A). The 53-kDa recombinant proteins of T. spiralis, T. nativa, T. papuae, and T. britovi migrated at approximately 48 to 50 kDa (Fig. 4A, lanes 1, 3, 4, and 5, respectively), but the 53-kDa recombinant protein of T. pseudospiralis migrated at approximately 45 to 47 kDa (Fig. 4A, lane 2), whereas the estimated molecular masses of the 53-kDa proteins of T. spiralis, T. pseudospiralis, T. nativa, T. papuae, and T. britovi are 46,451 Da, 46,791 Da, 46,288 Da, 46,887 Da, and 46,244 Da, respectively. This discrepancy in the molecular masses of the recombinant proteins between T. pseudospiralis and the other species may be due to differences in posttranslation modifications after protein synthesis in E. coli.
FIG. 4.
(A) SDS-PAGE analysis of the purified 53-kDa recombinant proteins of T. spiralis (lane 1), T. pseudospiralis (lane 2), T. nativa (lane 3), T. papuae (lane 4), and T. britovi (lane 5). (B to F) Cross-reactivity analysis by Western blotting results with the 53-kDa recombinant proteins of T. spiralis (lane 1), T. pseudospiralis (lane 2), T. nativa (lane 3), T. papuae (lane 4), and T. britovi (lane 5) immunostained with sera from mice infected with T. spiralis (B), T. pseudospiralis (C), T. nativa (D), T. papuae (E), or T. britovi (F). M, molecular mass standards; sizes in kDa are shown on the left side.
The sera from mice infected with T. spiralis strongly reacted with the 53-kDa recombinant protein of T. spiralis (Fig. 4B, lane 1) but did not react with the 53-kDa recombinant proteins of T. pseudospiralis, T. nativa, T. papuae, and T. britovi (Fig. 4B, lanes 2 to 5, respectively). Similarly, the sera from mice infected with T. pseudospiralis, T. nativa, T. papuae, or T. britovi strongly reacted with the 53-kDa recombinant proteins of T. pseudospiralis, T. nativa, T. papuae, or T. britovi, respectively (Fig. 4C to F). The 53-kDa recombinant protein of T. pseudospiralis weakly cross-reacted to the sera from mice infected with T. nativa (Fig. 4D, lane 2), with T. papuae (Fig. 4E, lane 2), and with T. britovi (Fig. 4F, lane 2).
DISCUSSION
To date, two clades in the genus Trichinella have been identified: the encapsulated clade and the nonencapsulated clade (26). Muscle larvae in encapsulated species develop a thick collagen capsule, and the nonencapsulated species develop only a very thin collagen capsule (23). The current study showed that the amino acid sequence of the 53-kDa protein of T. spiralis (an encapsulated species) was highly identical to those of T. britovi and T. nativa (encapsulated species), but it showed a low sequence similarity to those of T. pseudospiralis and T. papuae (nonencapsulated species). On Western blotting, the 53-kDa recombinant proteins of T. spiralis, T. britovi, T. nativa, T. pseudospiralis, and T. papuae reacted almost exclusively to sera from mice infected with T. spiralis, T. britovi, T. nativa, T. pseudospiralis, or T. papuae, respectively. In addition, the antibody against the 53-kDa recombinant protein of T. spiralis only recognized the 53-kDa protein of T. spiralis. These data showed that the level of sequence similarities among the 53-kDa proteins is not consistent with the intensity of antibody responses to the 53-kDa recombinant proteins in mice infected with Trichinella spp.
The native 53-kDa protein in E-S products of T. spiralis shows a marked heterogeneity in glycosylation (15, 16) and has multiple protein isoforms (10). The different molecular masses of the native 53-kDa proteins from the different developmental stages (Fig. 2) may be due to variable glycosylation after protein synthesis. In addition, the variations in the molecular masses of the 53-kDa proteins between T. spiralis and T. pseudospiralis may be due to small differences in the amino acid sequences leading to a change in the number of glycosylation sites (15).
The native 53-kDa glycoprotein of T. spiralis is strongly recognized by sera from BALB/c mice infected with T. spiralis but weakly recognized by sera from BALB/c mice infected with T. britovi (15), suggesting that the 53-kDa glycoprotein of T. spiralis bears species-specific epitopes. The epitope, which is only present in T. spiralis, is a 47-amino-acid sequence containing two α-helix regions flanked by random coils (13). The present results showed that the 53-kDa recombinant proteins of T. britovi, T. nativa, T. pseudospiralis, and T. papuae also bear species-specific epitopes and induce an immunological reaction during infection. The species-specific antigenicity of the 53-kDa proteins is only due to protein epitopes, because the recombinant proteins, which are unglycosylated proteins, only contain protein epitopes.
The 53-kDa glycoprotein is one of the E-S products and is highly concentrated in the E-S products, and so this protein may be a candidate immunodiagnostic antigen for trichinellosis (25). However, in contrast to the case with BALB/c mouse infection, the sera from Swiss CD-1 mice infected with T. britovi strongly recognized the 53-kDa protein of T. spiralis (15). These results show that the species-specific epitopes present on the 53-kDa protein are differently recognized in different mouse strains, and this may be due to differences in the infective capabilities of T. spiralis in the hosts (15). In this study, the antibody response to the 53-kDa recombinant protein was tested only in sera from BALB/c mice. Therefore, further experimentation is needed to confirm that the 53-kDa recombinant proteins can be used for species-specific antigens in making an immunodiagnosis in different hosts (e.g., humans, domestic animals, or wildlife).
Although an early immunodiagnosis of trichinellosis during the intestinal or migratory phase of infection is essential to limit infection with Trichinella spp., to date there are no available tests to achieve this purpose (7). Western blotting showed that the 53-kDa protein could be expressed not only by muscle larvae but also by adult worms, and the antibody response to the recombinant 53-kDa protein occurred from 8 days p.i. These results suggested that the 53-kDa protein secreted by adult worms induces an early antibody response during Trichinella infection.
Eight species (i.e., T. spiralis, T. britovi, T. nativa, T. murrelli, T. nelsoni, T. pseudospiralis, T. papuae, and T. zimbabwensis) and three genotypes (i.e., Trichinella T6, T8, and T9) have all been identified in the genus Trichinella, to date (26). Only five species in the genus Trichinella were observed for species-specific antibody responses to the 53-kDa recombinant proteins. However, the present results are applicable for other Trichinella species for immunodiagnosis of trichinellosis, because the recombinant 53-kDa proteins of the other Trichinella species showed similar antibody responses against five species in our preliminary data.
Western blotting is a sensitive and specific immunodiagnostic method which is generally used for confirmation of antibody detection (17). This study demonstrated that Western blotting using the 53-kDa recombinant protein is useful for species identification and early immunodiagnosis of trichinellosis and could provide more reliable results for routine diagnosis. Therefore, the use of recombinant antigens may provide a new source of diagnostic reagents and more reliable results when making a diagnosis of trichinellosis.
Footnotes
Published ahead of print on 9 January 2008.
REFERENCES
- 1.Capó, V., and D. D. Despommier. 1996. Clinical aspects of infection with Trichinella spp. Clin. Microbiol. Rev. 9:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colangeli, R., A. Heijbel, A. M. Williams, C. Manca, J. Chan, K. Lyashchenko, and M. L. Gennaro. 1998. Three-step purification of lipopolysaccharide-free, polyhistidine-tagged recombinant antigens of Mycobacterium tuberculosis. J. Chromatogr. B 714:223-235. [DOI] [PubMed] [Google Scholar]
- 3.de la Rosa, J. L., P. Alcántara, and D. Correa. 1995. Investigation of cross-reactions against Trichinella spiralis antigens by enzyme-linked immunosorbent assay and enzyme-linked immunoelectrotransfer blot assay in patients with various diseases. Clin. Diagn. Lab. Immunol. 2:122-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denkers, E. Y., D. L. Wassom, C. J. Krco, and C. E. Hayes. 1990. The mouse antibody response to Trichinella spiralis defines a single, immunodominant epitope shared by multiple antigens. J. Immunol. 144:3152-3159. [PubMed] [Google Scholar]
- 5.Dupouy-Camet, J. 2000. Trichinellosis: a worldwide zoonosis. Vet. Parasitol. 93:191-200. [DOI] [PubMed] [Google Scholar]
- 6.Dupouy-Camet, J., W. Kociecka, F. Bruschi, F. Bolás-Fernández, and E. Pozio. 2002. Opinion on the diagnosis and treatment of human trichinellosis. Expert Opin. Pharmacother. 3:1117-1130. [DOI] [PubMed] [Google Scholar]
- 7.Escalante, M., F. Romarís, M. Rodríguez, E. Rodríguez, J. Leiro, M. T. Gárate, and F. M. Ubeira. 2003. Evaluation of Trichinella spiralis larva group 1 antigens for serodiagnosis of human trichinellosis. J. Clin. Microbiol. 42:4060-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamble, H. R., A. S. Bessonov, K. Cuperlovic, A. A. Gajadhar, F. van Knapen, K. Noeckler, H. Schenone, and X. Zhu. 2000. International Commission on Trichinellosis: recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet. Parasitol. 93:393-408. [DOI] [PubMed] [Google Scholar]
- 9.Jung, D., J. P. Teifke, A. Karger, K. Michael, S. Venz, W. Wittmann, K. Kindermann, K. Nöckler, and E. Mundt. 2007. Evaluation of baculovirus-derived recombinant 53-kDa protein of Trichinella spiralis for detection of Trichinella-specific antibodies in domestic pigs by ELISA. Parasite Res. 100:429-437. [DOI] [PubMed] [Google Scholar]
- 10.Nagano, I., Z. Wu, T. Boonmars, and Y. Takahashi. 2004. Molecular cloning and characterisation of two kinds of proteins in excretory-secretory products of Trichinella pseudospiralis. Int. J. Parasitol. 34:491-500. [DOI] [PubMed] [Google Scholar]
- 11.Nagano, I., Z. Wu, and Y. Takahashi. 2006. Molecular cloning and characterization of an Rcd1-like protein in excretory-secretory products of Trichinella pseudospiralis. Parasitology 133:785-792. [DOI] [PubMed] [Google Scholar]
- 12.Parkhouse, R. M., and L. L. Harrison. 1989. Antigens of parasitic helminths in diagnosis, protection and pathology. Parasitology 99:5-19. [DOI] [PubMed] [Google Scholar]
- 13.Perteguer, M. J., E. Rodríguez, F. Romarís, M. Escalante, P. Bonay, F. M. Ubeira, and M. T. Gárate. 2004. Minor interspecies variations in the sequence of the gp53 TSL-1 antigen of Trichinella define species-specific immunodominant epitopes. Mol. Immunol. 41:421-433. [DOI] [PubMed] [Google Scholar]
- 14.Robert, F., B. Weil, N. Kassis, and J. Dupouy-Camet. 1996. Investigation of immunofluorescence cross-reactions against Trichinella spiralis by Western blot (immunoblot) analysis. Clin. Diagn. Lab. Immunol. 3:575-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romarís, F., M. A. Dea-Ayuela, F. Bolás, A. R. Martinez-Fernandez, M. L. Sanmartin, and F. M. Ubeira. 2003. Heterogeneity and immunogenicity of the Trichinella TSL-1 antigen gp53. Parasite Immunol. 25:297-305. [DOI] [PubMed] [Google Scholar]
- 16.Romarís, F., M. Escalante, S. Lorenzo, P. Bonay, T. Gárate, J. Leiro, and F. M. Ubeira. 2002. Monoclonal antibodies raised in Btk(xid) mice reveal new antigenic relationships and molecular interactions among gp53 and other Trichinella glycoproteins. Mol. Biochem. Parasitol. 125:174-183. [DOI] [PubMed] [Google Scholar]
- 17.Sofronic-Milosavljevic, L., N. Ilic, M. Djordjevic, M. Savic, A. Gruden-Movsesijan, K. Cuperlovic, and K. D. Murrell. 2005. Anti-Trichinella antibodies detected in chronically infected horses by IFA and Western blot, but not by ELISA. Vet. Parasitol. 132:107-111. [DOI] [PubMed] [Google Scholar]
- 18.Takada, N., and T. Tada. 1988. Collection of newborn larvae of Trichinella spiralis in vitro. Jpn. J. Parasitol. 37:251-253. [Google Scholar]
- 19.Takahashi, Y. 1997. Antigens of Trichinella spiralis. Parasitol. Today 13:104-106. [DOI] [PubMed] [Google Scholar]
- 20.Von Heijne, G. 1986. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 14:4683-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakelin, D., P. K. Goyal, M. S. Dehlawi, and J. Hermanek. 1994. Immune responses to Trichinella spiralis and T. pseudospiralis in mice. Immunology 81:475-479. [PMC free article] [PubMed] [Google Scholar]
- 22.Wu, Z., I. Nagano, and Y. Takahashi. 1998. Differences and similarities between Trichinella spiralis and T. pseudospiralis in morphology of stichocyte granules, peptide maps of excretory and secretory (E-S) products and messenger RNA of stichosomal glycoproteins. Parasitology 116:61-66. [DOI] [PubMed] [Google Scholar]
- 23.Xu, D., Z. Wu, I. Nagano, and Y. Takahashi. 1997. A muscle larva of Trichinella pseudospiralis is intracellular, but does not form a typical cyst wall. Parasitol. Int. 46:1-5. [Google Scholar]
- 24.Yera, H., S. Andiva, C. Perret, D. Limonne, P. Boireau, and J. Dupouy-Camet. 2003. Development and evaluation of a Western blot kit for diagnosis of human trichinellosis. Clin. Diagn. Lab. Immunol. 10:793-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarlenga, D. S., and H. R. Gamble. 1990. Molecular cloning and expression of an immunodominant 53-kDa excretory-secretory antigen from Trichinella spiralis muscle larvae. Mol. Biochem. Parasitol. 42:165-174. [DOI] [PubMed] [Google Scholar]
- 26.Zarlenga, D. S., B. M. Rosenthal, G. La Rosa, E. Pozio, and E. P. Hoberg. 2006. Post-Miocene expansion, colonization, and host switching drove speciation among extant nematodes of the archaic genus Trichinella. Proc. Natl. Acad. Sci. USA 103:7354-7359. [DOI] [PMC free article] [PubMed] [Google Scholar]