Abstract
Vaccines are needed against urinary tract infections (UTIs) in children, as episodes of pyelonephritis (PN) may cause renal scarring. Local immune mechanisms are regarded to confer protection, yet they have been poorly characterized for children. This study explores the local immune response in children by looking for newly activated pathogen-specific antibody-secreting cells (ASC), expected to appear transiently in the circulation as a response to UTI. Urinary tract-originating ASC specific to each patient's own pathogen or P fimbria were studied in 37 children with PN. The children were examined for recidivism and renal scarring in a 6-month follow-up study. Pathogen-specific ASC were found in 33/37 children, with the magnitude increasing with age. In contrast to the case for adults, with immunoglobulin A (IgA) dominance, in 18/33 cases IgM dominated the response, and this occurred more frequently in infants (63%) than in older children (30%). The most vigorous response was found to whole Escherichia coli bacteria (geometric mean, 63 ± 2,135 ASC/106 peripheral blood mononuclear cells [PBMC]), yet responses were found to P fimbriae (13 ± 33 ASC/106 PBMC), too. The response peaked at 1 to 2 weeks and was low/negligible 3 to 7 weeks after the beginning of symptoms. Recidivism was seen in seven patients, and renal scarring was seen in nine patients. In conclusion, a response of circulating ASC was found in children with UTIs, with the magnitude increasing with age. Since IgM is not present in urine, the IgM dominance of the response suggests that systemic immune mechanisms are more important in the immune defense in children than in adults. In 81% of patients, no recidivism was seen, suggesting a successful immune defense.
Urinary tract infection (UTI) represents one of the most common infections encountered in the practice of pediatrics today; UTIs comprise a severe problem, since renal scarring occurs in up to 20% of children with acute pyelonephritis (PN) (4, 11) and can result in an impairment of renal function. A vaccine against UTI is needed. Thorough knowledge of the immune mechanisms involved in protection would be beneficial in the development of effective vaccines.
UTIs are mostly of the ascending type, and therefore, the first defense line the pathogen encounters is the local immune system on the mucosa of the urinary tract, especially urinary antibodies. It has been shown that local antibodies can bind to the infecting bacteria and prevent their adherence to mucosal surfaces (29, 31, 33, 34) and the following infection. Accordingly, in animal experiments, local immunization eliciting local antibodies has been shown to protect against disease (36, 37). Recently, in adults, a vaginally administered vaccine has proven to be protective against reinfections in phase II trials (9, 35). If a urinary pathogen is able to cross the mucosal barrier, it must be cleared by the systemic immune mechanisms. It appears that an optimal defense state would involve both local and systemic immunity. The fact that 75% of previously infected women do not become reinfected (7) indicates that the immune mechanisms in patients experiencing their first episode of UTI may prove worth mimicking in developing vaccines against UTI. The view that local immunity is most effectively induced by local immunization (21) appears to apply to the genitourinary tract as well, as suggested by the protective efficacy of the vaginally administered vaccine against UTI (9, 35).
Knowledge on the human immune defense against UTI in children is sparse. Specific antibody responses to the infecting pathogen have been observed both in sera and in urines from both adults and children with UTIs (6, 17, 18, 20, 22, 25, 26, 28), with the levels being considerably lower in children than in adults. The local immune response to UTI has been investigated in adults by examining urinary tract-originating, newly recruited, pathogen-specific B cells in the peripheral blood. These cells are expected to be on their way back to the urinary mucosa to secrete antibodies locally (17, 18). These cells have proven to have unique homing potentials, as interpreted by their expression of homing receptors (Kantele et al., unpublished results). The present study was undertaken to look for circulating antibody-secreting cells (ASC) specific to urinary pathogens in children with UTIs. The results are expected to provide information on the immune response that will be useful in the development of vaccines against UTIs in children.
MATERIALS AND METHODS
The immune response to PN was studied in children by assessing peripheral blood ASC specific to the causative agent of the infection.
Patients.
Thirty-seven children (24 girls and 13 boys; aged 3 weeks to 15.4 years) suffering from PN caused by Escherichia coli participated in the study. None of the children had any known underlying diseases. One patient had a previous history of UTI. PN was diagnosed by the following criteria: typical clinical symptoms, rectum temperature over 38°C, C-reactive protein level of >40 mg/ml or blood leukocytosis of >10 × 109 cells/ml, leukocyturia (>10/μl), and a pathogenic bacterium isolated from urine (at least 105 CFU/ml) taken at the time of admission to the hospital. The urine samples for microbiological analysis were collected with help of a urine collection bag in four cases and by suprapubic aspiration in 33 cases. The E. coli strains of 23 patients were found to be P fimbriated (P+), as judged by a commercial P fimbria latex test carried out on the isolated E. coli (Orion Diagnostica, Helsinki, Finland).
Healthy laboratory personnel (five women, aged 18 to 32 years) volunteered as controls.
Renal ultrasonography (RUS) was performed on all 37 patients within 1 week of diagnosis. Voiding cystourethrography was performed on 30 patients approximately 1 month after UTI, and 99mTc-dimercaptosuccinic acid (DMSA) scanning was performed on 28 patients 6 to 18 months after the infection.
The study was approved by the ethics committees of the participating hospitals and institutions. Informed consent was obtained from parents of all patients. Human experimentation guidelines of the U.S. Department of Health and Human Services and those of the authors' institutions were followed in the conduct of clinical research.
Collection of blood samples.
Samples of blood were collected in the acute and in the convalescent phase of the disease. The difficulty of getting acute-phase samples at comparable stages of the disease (because the onset of symptoms varies and patients seek medical care after various times) was solved by taking two “acute-phase” samples. In order to catch the peak (or near the peak) of the ASC response, which according to our studies on oral vaccines (13, 14) is expected 7 days after antigen exposure, the sampling was carried out as follows. The acute-phase I specimens were collected 1 to 3 days after admission to the hospital, acute-phase II specimens were collected 1 week later, and convalescent-phase samples were collected 3 to 7 weeks after the onset of the disease. For patients not seeking medical care until 1 week after the onset of symptoms, only one acute-phase sample was taken. The definition “acute-phase sample” is later used for that acute-phase sample that yielded the larger number of ASC. Four healthy volunteers (all females, aged 23 to 38 years) belonging to the laboratory personnel served as controls. All blood samples were assayed by enzyme-linked immunospot (ELISPOT) assay immediately after they were drawn.
Preparation of antigen.
The following two types of antigen preparations were used: a preparation of the E. coli strain isolated from each patient's own urine and a preparation of isolated P fimbria.
Each patient's own E. coli strain was identified with conventional methods, grown on nutrient agar plates, suspended in phosphate-buffered saline (PBS; pH 7.4), and formalin killed (formaldehyde was added to a final concentration of 1%). The concentration of bacteria in the stock solution was estimated by the McFarland standard, and the bacteria were diluted to a final concentration of 108 bacteria/ml PBS.
The P fimbria isolate used in the ASC assay was obtained from Auli Pere (Department of General Microbiology, University of Helsinki). It had been isolated from strain IH 11086. This strain is of serotype O4:K12:H1 and was originally isolated from a patient with PN (24). The method of isolation of the P fimbria (19) and a detailed description of the IH 11086 strain (24) have been published elsewhere.
Isolation of mononuclear cells.
Mononuclear cells containing mainly lymphocytes were obtained by Ficoll-Paque (Pharmacia, Uppsala, Sweden) centrifugation of heparinized venous blood. The isolated cells were washed three times and suspended in culture medium to a concentration of 2 × 106 cells/ml as described earlier (12).
Assay of specific ASC.
Specific ASC were enumerated by ELISPOT assay (5, 12, 17, 18, 27). In this assay, isolated lymphocytes are allowed to secrete antibodies into microtiter plate wells previously coated with the antigen of interest. The secreted antibodies react with the antigen in the immediate vicinity of the secreting cell. These antibodies are visualized with enzyme-conjugated antisera followed by a substrate overlay in agarose; the latter immobilizes the decaying substrate and turns the area of antibody into a colored spot.
The plates (Maxisorp; Nunc, Copenhagen, Denmark) were coated by incubating a solution of whole bacteria prepared from each patient's own E. coli strain (see above) or a preparation of P fimbria at a concentration of 5 μg/ml PBS in the wells for 2 h at 37°C or overnight at 20°C. The next steps were described in detail earlier (12, 17, 18). Briefly, the nonspecific binding sites were blocked with 1% bovine serum albumin-PBS (30 min, 37°C), and then 105 cells in culture medium were incubated in each well (2 to 3 h, 37°C). The antibodies secreted during this time were detected with alkaline phosphatase-conjugated antisera (diluted 1:100 in 1% bovine serum albumin-PBS; Orion Diagnostica, Helsinki, Finland) followed by the substrate in 50°C agarose. For each patient, a total of 96 wells were studied, which means that 1.6 million cells were studied for each antigen-immunoglobulin (Ig) isotype combination.
Statistical methods.
The mean numbers of ASC are given as geometric means ± standard errors of the means (SEM). In order to be able to calculate the geometric means, zero values were replaced by the value 1. The significance of differences in the ASC responses was analyzed with Student's t test, using logarithmically transformed data. A person was regarded as a responder when having more than two specific ASC/106 cells. Nonresponders were included in all of the analyses as if they had one specific ASC/106 peripheral blood mononuclear cells (PBMC) in each Ig class or a total of three ASC/106 PBMC (IgA plus IgG plus IgM).
RESULTS
Patient demographics.
Twenty-four girls and 13 boys with acute PN participated. The mean age at the time of diagnosis was 25 (range, 1 to 185) months. The mean duration of fever before admission was 2.7 (range, 1 to 7) days, and the mean fever was 39.6°C (range, 39 to 40.4°C). The mean blood leukocytosis was 17.2 (range, 7.6 to 28.3) × 109 cells/ml, and the mean C-reactive protein concentration was 100 (range, 10 to 260) mg/liter.
Imaging studies and recidivism.
RUS was abnormal for 6 (16%) of the 37 patients. All abnormalities were mild to moderate hydronephrosis (posteroanterior diameter of >10 mm). Two of these patients had vesicoureteral reflux (VUR). None had obstructive uropathy. DMSA scans were abnormal for 9/28 (32%) patients, including 5 patients with VUR. Voiding cystourethrography was not performed on two patients with abnormal DMSA scans. Seven (19%) of the 37 patients had recidivist infections. Three of them had abnormal DMSA scans, one had VUR, and one had abnormal RUS.
ASC specific to each patient's own E. coli strain.
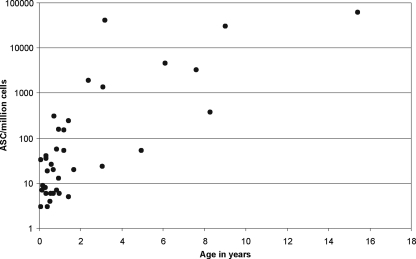
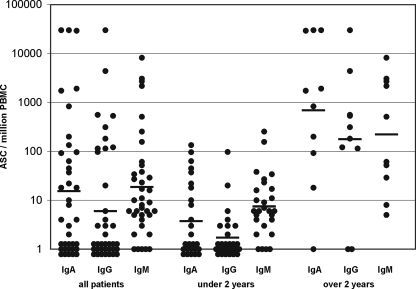
In the acute phase (less than 2 weeks after the estimated onset of the disease), pathogen-specific ASC were found in the blood of 33/37 children with PN. All four nonresponders were under 1 year old. The number of ASC was found to increase with increasing age (Fig. 1). The geometric mean (±SEM) of the ASC response was 63 ± 2,135 ASC/106 PBMC. In 18/33 (54.5%) cases, the response was dominated by IgM-secreting ASC, while IgA dominated in 15/33 (45.5%) cases and none of the patients had an IgG-dominated response. When the patients were divided into two groups according to age, the IgM dominance was found to be more frequent among children under 2 years (62.5%) than among those over 2 years (30%). For IgM-ASC, the geometric mean (±SEM) response of all patients was 19 ± 246 ASC/106 PBMC, that for IgA-ASC was 16 ± 1,348 ASC/106 PBMC, and that for IgG-ASC was 6 ± 815 ASC/106 PBMC (Fig. 2). Figure 2 shows separately the Ig isotype distributions for patients under and over 2 years.
FIG. 1.
Numbers of pathogen-specific ASC (the sum of ASC secreting antibodies of the IgA, IgG, and IgM isotypes) in the peripheral blood of 37 children with upper UTIs caused by Escherichia coli. The responses in infants were low and tended to increase with increasing age.
FIG. 2.
Ig class distribution of ASC responses in 37 children with UTIs. The distribution in all patients and separate presentations for children under and over 2 years of age are shown. The dominating Ig isotype was IgM.
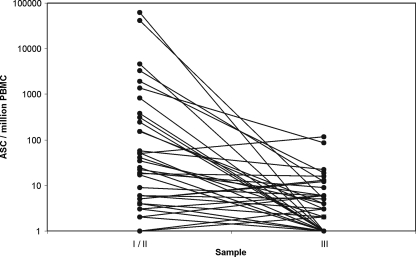
In the convalescent phase (3 to 7 weeks after the estimated onset of the disease), the numbers of ASC had decreased in most cases (Fig. 3), consistent with our previous study of adults (16, 17). For six patients, a slight increase was found in the convalescent phase: in two of the six patients, there was no response in the acute phase; in the rest, a response was seen in IgM only; and in all but one, the response was very low. This one patient had 51 ASC/106 PBMC (all IgM) in the acute phase and 115/106 PBMC, with IgA dominance, in the convalescent phase. This patient was the same, with five recidivist samples, in the follow-up.
FIG. 3.
Kinetics of pathogen-specific ASC response in children with upper UTIs caused by E. coli. The response declined to convalescent phase in most cases.
No ASC specific to urinary E. coli strains isolated from five PN patients were found in the blood of five healthy adult controls.
Since the present study was planned to explore the immune response to UTI in children of various ages, it revealed the increase in numbers of ASC with increasing age, but the number of patients of each age remained too low to allow a further statistical comparison between the numbers of ASC and the numbers of recidivist infections, signs of renal scarring, or presence of VUR. Of those with a response exceeding 10 ASC/106 PBMC, 5/24 had recidivist infection and 6/17 had signs of renal scarring. If the group with recidivism was compared to that with no recidivism, the geometric means of ASC responses did not differ from each other (58 versus 89 ASC/106 PBMC; P = 0.98), nor did those of the groups with signs of or no signs of renal scarring in DMSA scans (45 versus 208 ASC/106 PBMC; P = 0.19).
ASC specific to P fimbria.
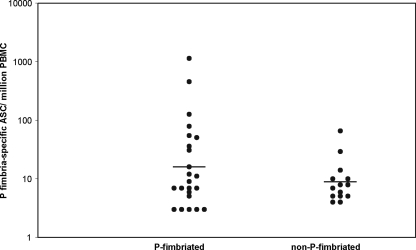
ASC specific to P fimbria were found in the blood of 18/23 patients with a P-fimbriated E. coli strain (P+) and in 12/14 patients with a non-P-fimbriated E. coli strain (P−) (Fig. 4). The geometric means (±SEM) of the P fimbria-specific ASC responses were 16 ± 52 and 9 ± 4 ASC/106 PBMC for the P+ and P− groups, respectively. For 13/18 P+ patients, a response was seen by more than one isotype: in most cases, the response was dominated by IgM; in 4/18 cases, it was dominated by IgA; and in 1 case, it was dominated by IgG. Among the P− patients, 11/12 patients had only an IgM response and 1/12 patients had only an IgA response.
FIG. 4.
P fimbria-specific ASC in children with UTIs caused by P-fimbriated or non-P-fimbriated E. coli.
DISCUSSION
We showed earlier that ASC specific to the pathogen can be found in the blood of adult patients for both lower and upper UTIs (18), and the present study was undertaken to look for a response in children. Since the mucosal immune response is believed to contribute to protection, it was of interest to look for signs of renal scarring during follow-up.
A response was found in 33/37 children with upper UTIs caused by E. coli. In our recent study, we found a response in 20/20 adult patients. The magnitude of the response in children (63 ± 2,135 ASC/106 PBMC) was found to be significantly lower than that observed in adults (1,643 ± 3,272 ASC/106 PBMC) (P < 0.001). The findings of nonresponding cases and lower magnitudes of responses than those of adults are suggested to be due to the less developed state of the immune system in children. This is supported by our notion that the magnitude of the response in children increased with increasing age. In fact, all of the nonresponding children were infants. A slow maturation of the urinary IgA system with age was also suggested by previous studies (30) showing lower amounts of total IgA in the urines of children than in those of adults. While these studies suggest a slower development of mucosal immune responses in the urinary tracts of children, previous studies by others have shown low systemic responsiveness in children, as measured by urinary IgG, serum antibodies (30), and interleukin-6 (2). The slow development of the immune system may account for the increased number of UTIs in infants compared to older children. Moreover, it certainly is a fact that needs to be considered in determining the timing of administration of possible future UTI vaccines for children.
The general pattern of the ASC response in children was found to be similar to that observed for adults: pathogen-specific ASC were observed in the acute phase of the disease, and a decline in their number was seen in the convalescent phase. However, the Ig isotype distribution of ASC in children was found to differ significantly from that for adults, even if both the previous study with adults and the present study with children examined the ASC response after the first episode of UTI. In adults, the dominating Ig class is IgA in most cases, and both the IgA- and IgG-ASC responses are more vigorous in magnitude than the IgM-ASC response (P < 0.001 for both) (18). In children, the responses were dominated by IgM in most cases. This difference in isotype distribution combined with the significantly lower responses observed in children accounts for the fact that the absolute numbers of IgM-ASC did not differ statistically between adults and children, even if a significant difference was found in the total response (IgA- plus IgG- plus IgM-ASC) (P < 0.003) as well as in IgA- and IgG-ASC responses (P < 0.003 and P < 0.002, respectively). In adults, the low IgM-ASC response appears to be a typical feature of the urinary tract (18), since the IgM-ASC response has been shown to be almost as prominent as the IgA-ASC response in adults when the antigen is administered via another mucosal surface, the gastrointestinal tract (13-15), even after booster immunization (16). Earlier studies have suggested that IgM might have only a minor role in the local immune system of the urinary tract (10), as IgM is rarely, if ever, present in the urine of healthy humans (8). Large macromolecules such as IgM do not usually pass the glomerular filter under normal conditions (32), and increased urine IgM is usually regarded as a predictor of impaired renal function (1). An increase in urinary IgM can be seen occasionally in UTI (28). The high IgM-ASC response could reflect a more systemic nature of the infection in children than in adults, and IgM-ASC could belong to the systemic part of the total ASC response, which is composed of local and systemic responses combined, as suggested previously (18).
We have shown recently for adults that an ASC response to P fimbria is elicited in UTI patients with a P+ E. coli strain, while those with a P− E. coli strain have no response (17). In the present study, 18/23 patients with a P+ E. coli strain had a P fimbria-specific ASC response. However, 12/14 patients with a P− E. coli strain also exhibited a response. In 11/12 cases, the latter was only an IgM response. Those with the highest responses tended to belong to the P+ group, yet most of the responses in both groups were low, with no statistical difference between the groups. The observation of a few significant P fimbria-specific ASC responses in P− patients can be explained partly by a possible failure to detect P fimbria with the latex test, as it is known that E. coli cells are subject to fimbrial phase variation in vivo in the urinary tract (23, 24): some strains have been shown to have a predominantly P fimbria-positive phase (e.g., 95%) in urine samples and a poor expression of P fimbriae (e.g., 1%) after growth on agar plates (23, 24). Unfortunately, the bacterial strains of the present study could not be tested for pap (3), the operon for P fimbria, as the strains were no longer available. Regarding the patients discussed above, the possibility exists that another, undetected P+ E. coli strain was simultaneously present in the urinary tract. A third explanation is a nonspecific stimulation of the immune system, as suggested by the increased numbers of Ig-secreting cells: in the course of a serious infection, the numbers of Ig-secreting cells far exceed the numbers of pathogen-specific ASC, as shown in our previous studies of adults with diarrhea (15) or PN (18). Polyclonal stimulation has been suggested as an explanation for the phenomenon (17, 18), and in fact, small numbers of ASC specific to an irrelevant antigen, trinitrophenyl, have also been found (18). In the present study, cells originally encountering a P-fimbriated E. coli strain in the normal microbial flora of the child's intestine could have been activated polyclonally.
Renal scarring may be associated with recurrences of PN more frequently in children than in adults. The present study revealed two major differences in the ASC response in children compared to that in adults, i.e., the lower magnitude of the response and the IgM dominance. Even if the data in the present study are not sufficient to allow such an analysis, in the future it would be interesting to determine if there exists a connection between a low ASC response or IgM dominance and renal scarring.
We conclude that the ASC assay can be used to measure the immune response to UTI in children. The response in children resembles that of adults, but it is lower in magnitude and the Ig isotype distribution of the response is different. IgM is the dominating Ig class and seems to have a more significant role than IgA in the defense against UTI in childhood.
Acknowledgments
This work was performed at the Division of Infectious Diseases, Helsinki University Central Hospital, at the Department of Pediatrics, Turku University Central Hospital of Turku, and at the Department of Medical Microbiology and Immunology, University of Turku.
This work was supported by a special Finnish governmental subsidy for health sciences research and by the Finnish Medical Association.
We do not have a commercial or other association that might pose a conflict of interest.
Footnotes
Published ahead of print on 9 January 2008.
REFERENCES
- 1.Bakoush, O., M. Segelmark, O. Torffvit, S. Ohlsson, and J. Tencer. 2006. Urine IgM excretion predicts outcome in ANCA-associated renal vasculitis. Nephrol. Dial. Transplant. 21:1263-1269. [DOI] [PubMed] [Google Scholar]
- 2.Benson, M., U. Jodal, W. Agace, M. Hellström, S. Mårild, S. Rosberg, M. Sjöström, B. Wettergren, S. Jönsson, and C. Svanborg. 1996. Interleukin (IL)-6 and IL-8 in children with febrile urinary tract infection and asymptomatic bacteriuria. J. Infect. Dis. 174:1080-1084. [DOI] [PubMed] [Google Scholar]
- 3.Blomfield, I. C. 2001. The regulation of pap and type 1 fimbriation in Escherichia coli. Adv. Microb. Physiol. 45:1-49. [DOI] [PubMed] [Google Scholar]
- 4.Broyer, M., C. Chantler, R. Donckerwolcke, J. H. Ehrich, G. Rizzoni, and K. Scharer. 1993. The pediatric registry of the European Dialysis and Transplant Association: 20 years' experience. Pediatr. Nephrol. 7:758-768. [DOI] [PubMed] [Google Scholar]
- 5.Czerkinsky, C. C., L. Å. Nilsson, H. Nygren, O. Ouchterlony, and A. Tarkowski. 1983. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 65:109-121. [DOI] [PubMed] [Google Scholar]
- 6.de Ree, J. M., and J. F. van den Bosch. 1987. Serological response to the P fimbriae of uropathogenic Escherichia coli in pyelonephritis. Infect. Immun. 55:2204-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foxman, B., B. Gillespie, J. Koopman, L. Zhang, K. Palin, P. Tallman, J. V. Marsh, S. Spear, J. D. Sobel, M. J. Marty, and C. F. Marrs. 2000. Risk factors for a second urinary tract infection among college women. Am. J. Epidemiol. 151:1194-1205. [DOI] [PubMed] [Google Scholar]
- 8.Holmgren, J., and J. W. Smith. 1975. Immunological aspects of urinary tract infections. Prog. Allergy 18:289-352. [PubMed] [Google Scholar]
- 9.Hopkins, W. J., J. Elkahwaji, L. M. Beierle, G. E. Leverson, and D. T. Uehling. 2007. Vaginal mucosal vaccine for recurrent urinary tract infections in women: results of a phase 2 clinical trial. J. Urol. 177:1349-1353. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins, W. J., D. T. Uehling, and E. Balish. 1987. Local and systemic antibody responses accompany spontaneous resolution of experimental cystitis in cynomolgus monkeys. Infect. Immun. 55:1951-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahnukainen, T., M. Chen, and G. Celsi. 2005. Mechanisms of renal damage owing to infection. Pediatr. Nephrol. 20:1043-1053. [DOI] [PubMed] [Google Scholar]
- 12.Kantele, A. 1990. Antibody secreting cells in the evaluation of the immunogenicity of an oral vaccine. Vaccine 8:321-326. [DOI] [PubMed] [Google Scholar]
- 13.Kantele, A. 1996. Peripheral blood antibody-secreting cells in the evaluation of the immune response to an oral vaccine. J. Biotechnol. 44:217-224. [DOI] [PubMed] [Google Scholar]
- 14.Kantele, A., H. Arvilommi, and I. Jokinen. 1986. Specific immunoglobulin-secreting human blood cells after peroral immunization against Salmonella typhi. J. Infect. Dis. 153:1126-1131. [DOI] [PubMed] [Google Scholar]
- 15.Kantele, A., H. Arvilommi, and R. Takala. 1988. Immune response to acute diarrhoea seen as circulating antibody secreting cells. J. Infect. Dis. 158:1011-1016. [DOI] [PubMed] [Google Scholar]
- 16.Kantele, A., and P. H. Mäkelä. 1991. Different profiles of the human immune response to primary and secondary immunization with Salmonella typhi Ty21a vaccine. Vaccine 9:423-427. [DOI] [PubMed] [Google Scholar]
- 17.Kantele, A., T. Möttönen, K. Ala-Kaila, and H. Arvilommi. 2003. P fimbria-specific B cell responses in patients with urinary tract infection. J. Infect. Dis. 188:1885-1891. [DOI] [PubMed] [Google Scholar]
- 18.Kantele, A., R. Papunen, E. Virtanen, T. Möttönen, L. Räsänen, K. Ala-Kaila, P. H. Mäkelä, and H. Arvilommi. 1994. Antibody secreting cells in acute urinary tract infection as indicators of local immune response. J. Infect. Dis. 169:1023-1028. [DOI] [PubMed] [Google Scholar]
- 19.Korhonen, T. K., E. L. Nurmiaho, H. Ranta, and C. S. Eden. 1980. New method for isolation of immunologically pure pili from Escherichia coli. Infect. Immun. 27:569-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenzie, H., and D. N. Young. 1987. Antibody to coliform antigens in urine samples from patients with symptoms of urinary tract infection. J. Clin. Pathol. 40:787-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mestecky, J. 1987. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J. Clin. Immunol. 7:265-276. [DOI] [PubMed] [Google Scholar]
- 22.Nicolle, L. E., J. Brunka, E. Ujack, and L. Bryan. 1989. Antibodies to major outer membrane proteins of Escherichia coli in urinary infection in the elderly. J. Infect. Dis. 160:627-633. [DOI] [PubMed] [Google Scholar]
- 23.Pere, A., B. Nowicki, H. Saxén, A. Siitonen, and T. K. Korhonen. 1987. Expression of P, type-1, and type 1c fimbriae of Escherichia coli in the urine of patients with acute urinary tract infection. J. Infect. Dis. 156:567-574. [DOI] [PubMed] [Google Scholar]
- 24.Pere, A., V. Väisänen-Rehn, M. Rehn, J. Tenhunen, and T. K. Korhonen. 1986. Analysis of P fimbriae on Escherichia coli O2, O4, and O6 strains by immunoprecipitation. Infect. Immun. 51:618-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratner, J. J., V. L. Thomas, B. A. Sanford, and M. Forland. 1981. Bacteria-specific antibody in the urine of patients with acute pyelonephritis and cystitis. J. Infect. Dis. 143:404-412. [DOI] [PubMed] [Google Scholar]
- 26.Salit, I. E., J. Hanley, L. Clubb, and S. Fanning. 1988. The human antibody response to uropathogenic Escherichia coli: a review. Can. J. Microbiol. 34:312-318. [DOI] [PubMed] [Google Scholar]
- 27.Sedgwick, J. D., and P. G. Holt. 1983. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 57:301-309. [DOI] [PubMed] [Google Scholar]
- 28.Sohl-Åkerlund, Å., B. Ahlstedt, L. Å. Hanson, and U. Jodal. 1979. Antibody responses in urine and serum against E. coli O-antigen in childhood urinary tract infection. Acta Pathol. Microbiol. Scand. C 87:29-36. [PubMed] [Google Scholar]
- 29.Svanborg-Edén, C., B. Andersson, L. Hagberg, L. Å. Hanson, H. Leffler, G. Maqnusson, G. Noori, J. Dahmén, and T. Söderström. 1983. Receptor analogues and anti-pili antibodies as inhibitors of bacterial attachment in vivo and in vitro. Ann. N. Y. Acad. Sci. 409:580-592. [DOI] [PubMed] [Google Scholar]
- 30.Svanborg-Edén, C., R. Kulhavy, S. Mårild, S. J. Prince, and J. Mestecky. 1985. Urinary immunoglobulins in healthy individuals and children with acute pyelonephritis. Scand. J. Immunol. 21:305-313. [DOI] [PubMed] [Google Scholar]
- 31.Svanborg-Edén, C., and A.-M. Svennerholm. 1978. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect. Immun. 22:790-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tencer, J., I.-M. Frick, B. W. Öquist, P. Alm, and B. Rippe. 1998. Size-selectivity of the glomerular barrier to high molecular weight proteins: upper size limitations of shunt pathways. Kidney Int. 53:709-715. [DOI] [PubMed] [Google Scholar]
- 33.Thumbikat, P., C. Waltenbaugh, A. Schaeffer, and D. J. Klumpp. 2006. Antigen-specific responses accelerate bacterial clearance in the bladder. J. Immunol. 176:3080-3086. [DOI] [PubMed] [Google Scholar]
- 34.Trinchieri, A. L., D. Braceschi, D. Tiranti, S. Dell'Aqua, A. Mandressi, and E. Pisani. 1990. Secretory immunoglobulin A and inhibitory activity of bacterial adherence to epithelial cells in urine from patients with urinary tract infections. Urol. Res. 18:305-308. [DOI] [PubMed] [Google Scholar]
- 35.Uehling, D. T., W. J. Hopkins, E. Balish, Y. Xing, and D. M. Heisey. 1997. Vaginal mucosal immunization for recurrent urinary tract infection: phase II clinical trial. J. Urol. 157:2049-2052. [PubMed] [Google Scholar]
- 36.Uehling, D. T., W. J. Hopkins, J. Jensen, and E. Balish. 1987. Vaginal immunization against induced cystitis in monkeys. J. Urol. 137:327-329. [DOI] [PubMed] [Google Scholar]
- 37.Uehling, D. T., L. J. James, W. J. Hopkins, and E. Balish. 1991. Immunization against urinary tract infection with a multivalent vaginal vaccine. J. Urol. 146:223-226. [DOI] [PubMed] [Google Scholar]