Abstract
We have previously shown that DNA immunization with PspA (pneumococcal surface protein A) DNA is able to elicit protection comparable to that elicited by immunization with PspA protein (with alum as adjuvant), even though the antibody levels elicited by DNA immunization are lower than those elicited by immunization with the protein. This work aims at characterizing the ability of sera to bind to the pneumococcal surface and to mediate complement deposition, using BALB/c wild-type and interleukin-4 knockout mice. We observed that higher anti-PspA levels correlated with intense antibody binding to the pneumococcal surface, while elevated complement deposition was observed with sera that presented balanced immunoglobulin G1 (IgG1)/IgG2a ratios, such as those from DNA-immunized mice. Furthermore, we demonstrated that gamma interferon and tumor necrosis factor alpha were strongly induced after intraperitoneal pneumococcal challenge only in mice immunized with the DNA vaccine. We therefore postulate that although both DNA and recombinant protein immunizations are able to protect mice against intraperitoneal pneumococcal challenge, an optimized response would be achieved by using a DNA vaccine and other strategies capable of inducing balanced Th1/Th2 responses.
Diseases caused by Streptococcus pneumoniae, such as pneumonia, meningitis, and bacteremia, are major public health problems. The need to achieve broader serotype coverage at a lower cost has thus stimulated attempts to develop a vaccine based on conserved protein antigens of pneumococci.
Pneumococcal surface protein A (PspA) is a promising candidate for the development of a cost-effective protein-based vaccine. PspA is an exposed virulence factor present in all pneumococcal strains. It is a highly immunogenic antigen and affects host-pathogen interactions by inhibiting complement activation by the classic and alternative pathways (23). Many groups have shown that recombinant PspA (rPspA) can elicit an antibody response that protects against lethal challenge in different animal models (26), and a recent human trial showed an increase in specific anti-PspA immunoglobulin G (IgG) levels after immunization with rPspA (20). Furthermore, sera from humans immunized with PspA were able to passively protect mice against challenge with various pneumococcal strains (4). The precise characterization of the optimal anti-PspA antibody response has not been addressed, though.
Although immunity to pneumococcal diseases has long been assumed to depend mainly on the humoral immune response, Malley and collaborators have recently shown antibody-independent immunity mediated by CD4+ T cells in a murine model of pneumococcal colonization (16). There is also accumulating evidence of the importance of T cells in resistance to pneumococcal disease in humans. It is well established that human immunodeficiency virus-infected and splenectomized patients are at high risk for developing severe pneumococcal infections (7, 22). Activated T lymphocytes with a Th1 cytokine profile were shown to be highly engaged in the in vivo immune response to pneumococci in humans (11). Interleukin-12 (IL-12) deficiency has also been correlated with increased susceptibility to pneumococcal infections, presumably due to a deficient T-cell response (10). In mice, the development of disease and the host response to the pneumococcal challenge have been studied with different strains of wild-type (WT) and knockout (KO) animals (13, 14, 18, 25, 27), but all of these studies were performed with nonimmunized mice only and reported that resolution of infection in the naïve host is mediated primarily through phagocytosis and intracellular killing by neutrophils and macrophages, which are recruited to the site of infection in response to proinflammatory cytokine and interleukin release by T lymphocytes. In accordance with this, it has been suggested that gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) produced by NK and T cells have a crucial involvement not only in the natural host defense but also in acquired resistance against S. pneumoniae (12, 24). Furthermore, events which mediate the early innate release of pro- and anti-inflammatory cytokines in response to this pathogen may have a profound influence on the quality and intensity of the subsequent adaptive immune response.
Our previous results showed that protective Th1 immune responses against pneumococcal infection in a mouse model can be achieved successfully by DNA vaccines using vectors expressing PspA (8, 17). The PspA fragment used in this study comprises the N-terminal region through the proline-rich region without the signal sequence from a clade 3 (family 2) PspA and is expressed in a secreted form by a DNA vaccine vector (pSec-pspA3NS). A previous study by our group showed that immunization of mice with the same fragment as a recombinant protein was able to induce anti-PspA antibodies that increased C3 deposition only on the pneumococci with PspA from the same family (family 2). On the other hand, PspA hybrids containing fused portions of both families (families 1 and 2) were able to increase C3 deposition on pneumococci bearing PspA fragments from both families (5).
The present study aims at characterizing the induction and functionality of the antibodies induced by immunization against S. pneumoniae, using PspA as a DNA vaccine and a recombinant protein in wild-type BALB/c mice. IL-4 KO mice were also used in order to alter the polarization of the immune response. Furthermore, mice immunized with DNA vaccine and recombinant protein were also analyzed in terms of cytokine release in response to an intraperitoneal pneumococcal challenge.
MATERIALS AND METHODS
Immunization of mice.
Five- to 7-week-old female BALB/c WT or IL-4 KO mice from ICB-USP (Universidade de São Paulo, São Paulo, Brazil) were supplied with food and water ad libitum. Animal experimental protocols were approved by the ethics committee of Instituto Butantan (São Paulo, Brazil). Groups of four to six animals were lightly anesthetized and inoculated intramuscularly (i.m.) with 50 μl of 10 μM cardiotoxin (Laxotan, Valence, France) into each tibialis anterior muscle 5 days before starting immunization with the DNA vaccine (6). A vector (pSec-pspA3NS) expressing a secreted fragment of PspA3 containing the N-terminal region through the proline-rich region (8) was used in this work. Immunization was performed i.m. with 50 μg of DNA or subcutaneously with 5 μg of rPspA3NS, using 50 μg of Al(OH)3 as adjuvant, on days 1 and 21. Alum-immunized mice or animals injected with the empty vector (pSec2a) were used as controls.
Detection of anti-PspA-specific antibodies through ELISA.
Mice were bled individually from the retroorbital plexus 2 weeks after the last immunization for detection of antibodies through enzyme-linked immunosorbent assay (ELISA) in plates coated with rPspA3NS. The assay was performed using goat anti-mouse IgG, anti-mouse IgG1, anti-mouse IgG2a, and anti-goat IgG conjugated with horseradish peroxidase (Southern Biotech). Standard curves were generated using mouse IgG, IgG1, and IgG2a.
Bacterial strains and growth conditions.
S. pneumoniae strains P-30 (serotype 6B; PspA clade 3), from Universidade Federal de Goiás (Goiânia, Brazil), and St 679/99 (serotype 6B; PspA clade 3), from Instituto Adolfo Lutz (São Paulo, Brazil), were cultured, aliquoted, and stored at −80°C in Todd-Hewitt 0.5% yeast extract containing 20% glycerol. To estimate the challenge dose and viability of bacterial stocks, groups of nonimmune mice were challenged with serial dilutions of the standard inoculum.
i.p. challenge.
Immunized mice were challenged by intraperitoneal (i.p.) injection of 500 CFU of St 679/99 in 0.5 ml of saline 2 weeks after the final immunization. Animals were then observed for 1 week, and inactive, sick animals were euthanized.
Antibody binding and complement deposition.
Antibody binding and complement deposition assays were performed as previously described (23). Briefly, S. pneumoniae P-30 frozen stocks were plated on blood agar overnight, grown in Todd-Hewitt 0.5% yeast extract to an optical density at 600 nm of 0.4 to 0.5 (∼108 CFU/ml), and harvested by centrifugation. Bacteria were washed and resuspended in phosphate-buffered saline (PBS) and then incubated with 25% pooled sera for 30 min at 37°C. For the antibody binding assay, bacteria were then washed with PBS and resuspended in fluorescein isothiocyanate-conjugated goat anti-mouse IgG (MP Biomedicals). For complement deposition, after another wash with PBS, bacteria were resuspended in gelatin-Veronal buffer (Sigma) and incubated for 30 min at 37°C with 10% normal mouse serum as a source of complement. The bacteria were then washed, resuspended in fluorescein isothiocyanate-conjugated goat anti-mouse complement C3 (MP Biomedicals), and incubated for 30 min at 4°C in the dark. Finally, in both assays, bacteria were washed twice with PBS, resuspended in 1% formaldehyde, and analyzed using FACSCalibur flow cytometry (BD Biosciences).
ELISPOT assay for detection of IFN-γ-, IL-4-, or TNF-producing spleen cells.
Pooled spleen cells for each group were obtained from immunized BALB/c mice 2 weeks after the final immunization (before challenge or 16 h after i.p. challenge with St 679/99), and cytokine-secreting cells were detected using IFN-γ, IL-4, and TNF enzyme-linked immunospot (ELISPOT) sets (BD Biosciences). Splenocytes were incubated for 20 h in the presence of rPspA3NS (5 μg/ml). Preparation of spleen cells was performed as described previously (21), and the medium was supplemented with polymyxin B (10 μg/ml) to avoid nonspecific stimulation from lipopolysaccharide present in rPspA. An antibiotic-antimycotic solution (Gibco) containing penicillin G sodium (100 units/ml), streptomycin sulfate (100 μg/ml), and amphotericin B (250 ng/ml) was added to the medium to eliminate live pneumococci from spleen cells obtained from infected mice. Mean numbers of spot-forming cells (SFCs) were calculated from triplicate wells.
ELISA detection of IFN-γ or TNF-α in the supernatants of spleen cell cultures.
Cytokines in the supernatants of spleen cell cultures stimulated for 72 h with rPspA3NS (5 μg/ml) were assayed by sandwich ELISA (Peprotech).
Statistical analysis.
Differences in anti-PspA antibody concentrations between groups were analyzed by Student's t test, and those in the overall survival rate were analyzed by the Fisher exact test (P ≤ 0.05).
RESULTS
Humoral immune response elicited by DNA vaccine or recombinant protein.
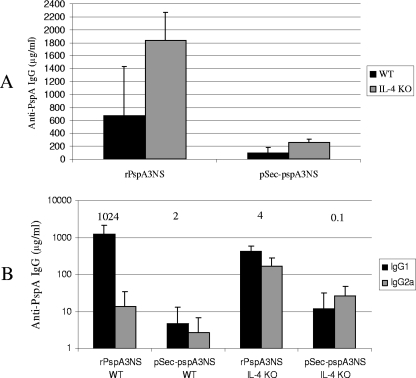
Groups of BALB/c WT and IL-4 KO mice were immunized i.m. with the DNA vaccine (pSec-pspA3NS) or subcutaneously with recombinant protein, using alum as an adjuvant (rPspA3NS). As shown in Fig. 1A, total IgG antibody levels were higher in mice immunized with rPspA3NS than in those immunized with the DNA vaccine, with a statistical difference for IL-4 KO animals (P = 0.02). The levels of antibody to PspA elicited by both protein and DNA immunization were higher in the IL-4 KO mice than in the WT mice, with a statistical difference for mice immunized with the recombinant protein (P = 0.01). When the IgG isotypes of the anti-PspA antibodies elicited in the WT and IL-4 KO mice by the protein and DNA immunizations were determined, it was observed that only WT mice immunized with recombinant protein showed a marked predominance of IgG1 antibodies (Fig. 1B). Balanced amounts of IgG1 and IgG2a antibodies were observed for the other immunized groups.
FIG. 1.
Induction of anti-PspA antibodies in sera from BALB/c WT and IL-4 KO mice immunized with recombinant protein or DNA vaccine. Total anti-PspA IgG (A) or anti-PspA IgG1 and IgG2a (B) antibodies were detected through ELISA in plates coated with rPspA3NS, using sera obtained from individual mice (six animals per group) 2 weeks after the last immunization. Numbers above columns are mean IgG1/IgG2a titer ratios.
Antibody binding and complement deposition.
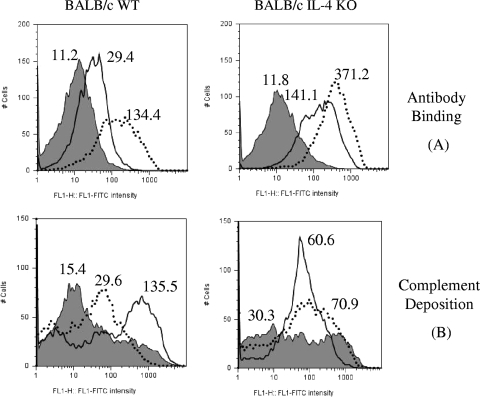
In order to investigate whether anti-PspA antibodies detected by ELISA would be able to bind to the surfaces of intact pneumococci (Fab region adequacy) and to mediate C3 deposition (Fc region activity), sera from mice immunized with pSec-pspA3NS or rPspA3NS were incubated with pneumococcal isolate P-30, and the amounts of bound antibody and complement C3 were determined (Fig. 2). Sera from alum-immunized mice (control group) are represented by the gray area in each graph. Sera from WT and IL-4 KO mice immunized with rPspA3NS (dotted lines) showed higher binding capacities than did sera from pSec-pspA3NS-immunized mice (solid lines) (Fig. 2A), indicating a strict correlation between total anti-PspA IgG concentration and antibody binding to the bacterial surface. However, when the ability of the immune sera to mediate C3 deposition onto the pneumococcal surface was analyzed (Fig. 2B), sera from WT animals injected with pSec-pspA3NS demonstrated a more pronounced increase in C3 deposition than did those from rPspA3NS-immunized mice, despite a lower binding capacity. Furthermore, sera from IL-4 KO mice immunized with both recombinant protein and DNA vaccines showed similar abilities to mediate complement deposition. A direct comparison between the data on complement deposition obtained for WT and IL-4 KO mice would not be reliable, since background C3 deposition mediated by the sera of control animals differed significantly between the two strains of animals.
FIG. 2.
Binding of anti-PspA antibodies and complement deposition on the pneumococcal surface. Pooled sera from BALB/c WT and IL-4 KO mice immunized with a recombinant protein or DNA vaccine were tested for the abilities to bind to the pneumococcal surface (A) and to mediate C3 deposition (B). S. pneumoniae strain P-30 was incubated with 25% serum from mice immunized with DNA vaccine vector pSec-pspA3NS (solid lines)-, recombinant PspA3NS (dotted lines)-, or alum-immunized animals (gray areas). The median fluorescence of the bacteria is shown for each sample. Results are representative of two independent experiments.
Cytokine secretion elicited by DNA vaccine or recombinant protein.
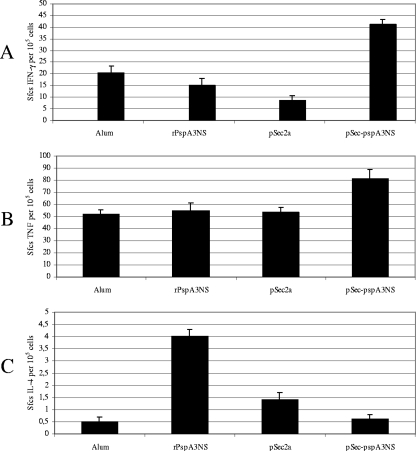
In order to investigate the role of cellular immune responses, we next immunized BALB/c WT mice with pSec-pspA3NS or rPspA3NS and, 2 weeks after the last immunization, isolated splenocytes 16 h after challenge with S. pneumoniae St 679/99. In the ELISPOT assay, the number of SFCs after in vitro stimulation with rPspA was determined, and results are shown in Fig. 3. After pneumococcal challenge, we observed a more pronounced increase in SFCs secreting IFN-γ in the pSec-pspA3NS-immunized group than in the control groups (alum and pSec2a) and the rPspA3NS-immunized group (Fig. 3A); very similar results were obtained for TNF secretion for the same immunized groups of mice (Fig. 3B). In contrast to the results with IFN-γ, a fourfold increase in IL-4 SFCs was observed in rPspA3NS-immunized mice compared with the number after pSec-pspA3NS immunization (Fig. 3C).
FIG. 3.
Cytokine responses in spleen cells induced by DNA vaccine and recombinant protein after i.p. challenge with S. pneumoniae. Splenocytes isolated from immunized BALB/c WT mice 16 h after i.p. challenge with pneumococci were incubated for 20 h with PspA (5 μg/ml) or medium only as a control (nonstimulated) in ELISPOT plates coated with antibodies against the indicated cytokines. The numbers of SFCs secreting IFN-γ (A), TNF (Β), and IL-4 (C) were detected through ELISPOT. The average number of spots in triplicate wells was calculated by subtracting the number in nonstimulated wells and considered to be the number of SFCs/105 cultured splenocytes. Results are representative of two independent experiments.
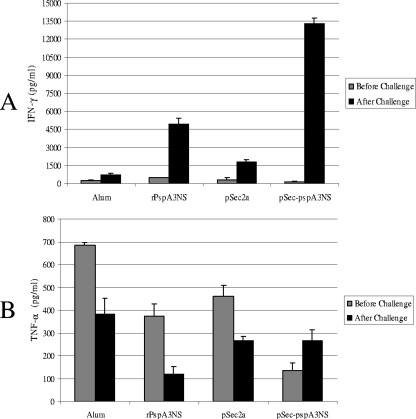
The presence of IFN-γ in the supernatant of splenocytes was also determined by ELISA before and after pneumococcal challenge (Fig. 4). Cells obtained from mice immunized with pSec-pspA3NS and restimulated with PspA in vitro showed a considerable improvement in IFN-γ secretion after challenge, which was more pronounced than that for immunization with rPspA3NS, corroborating the ELISPOT data. Secretion of IFN-γ was not detected in any of the groups before the challenge (Fig. 4A). We also looked for TNF-α, and interestingly, we observed that all groups showed larger amounts of this cytokine before than after the challenge, except for the pSec-pspA3NS-immunized group, which showed a trend toward an enhancement of TNF-α secretion after challenge with pneumococci (Fig. 4B).
FIG. 4.
Cytokine responses in spleen cells induced by DNA vaccine and recombinant protein. Splenocytes isolated from immunized BALB/c WT mice before or 16 h after i.p. challenge with pneumococci were incubated for 72 h with PspA (5 μg/ml) or medium only as a control (nonstimulated). IFN-γ (A) and TNF-α (B) levels in the supernatants were detected through sandwich ELISA. Results are representative of two independent experiments.
Protection elicited by DNA vaccine or recombinant protein.
Immunized BALB/c mice were then challenged i.p. with St 679/99, and survival was analyzed (Table 1). Both formulations with PspA elicited statistically significant protection in WT mice at similar levels, which is in accordance with previous results published by our group. IL-4 KO mice also showed enhancement of survival with both the recombinant protein and DNA vaccines, but rPspA protection had borderline significance. Both strategies thus showed a capacity to elicit protection against pneumococcal challenge.
TABLE 1.
Survival of BALB/c mice after challenge with S. pneumoniae 679/99
| Vaccine | WT mice
|
IL-4 KO mice
|
||||
|---|---|---|---|---|---|---|
| No. of survivors/ total no. of mice | % Survival | P value | No. of survivors/ total no. of mice | % Survival | P value | |
| Alum | 0/5 | 0 | 2/5 | 40 | ||
| rPspA3NS | 4/5 | 80 | 0.02a | 5/5 | 100 | 0.08 |
| pSec2a | 0/5 | 0 | 0/5 | 0 | ||
| pSec-pspA3NS | 5/5 | 100 | 0.004b | 4/4 | 100 | 0.007b |
Statistically different from animals injected with alum (P ≤ 0.05).
Statistically different from animals injected with pSec2a (P ≤ 0.05).
DISCUSSION
Genetic immunization with vectors expressing PspA has previously been shown to be protective against pneumococcal challenge (3, 8, 17, 19). Our previous results showed that this immunization elicited a more balanced anti-PspA IgG1/IgG2a response that protected mice against i.p. pneumococcal challenge at levels similar to those for recombinant protein immunization, despite significantly lower total anti-PspA IgG production (8). In this work, we show that recombinant protein immunization elicited higher levels of anti-PspA antibodies than did DNA immunization in WT and IL-4 KO mice. We also observed an increment in total anti-PspA IgG in IL-4 KO mice immunized with either rPspA3NS or pSec-pspA3NS compared to WT animals. Higher antibody responses to PspA have previously been observed in IL-4 KO mice than in WT mice, and this is assumed to be due to the lack of suppression of the IgG response by IL-4 (14). In our present studies, we also analyzed the IgG1/IgG2a ratio, and only WT mice immunized with recombinant protein showed a marked predominance of IgG1 (IgG1/IgG2a ratio = 1,024), while balanced IgG1/IgG2a ratios were observed for the other groups (ratio of 4 for pSec-pspA3NS-immunized WT mice, 2 for rPspA3NS-immunized IL-4 KO mice, and 0.1 for pSec-pspA3NS-immunized IL-4 KO mice).
We evaluated the ability of antibodies elicited in sera from mice immunized with rPspA and pSec-pspA3NS to bind to the pneumococcal surface and to mediate complement deposition. In the case of both WT and IL-4 KO mice, antibody to PspA in sera from mice immunized with rPspA showed much more binding to pneumococci than did antibody in sera from mice immunized with pSec-pspA3NS. These results confirm the functionality of the antibodies detected by ELISA and indicate a direct correlation between larger amounts of total anti-PspA IgG and an increase in antibody binding to the surfaces of intact pneumococci. On the other hand, an increase in antibody binding to pneumococci did not show a correlation with an enhancement of complement deposition. A more accurate correlate of complement deposition seemed to be the IgG1/IgG2a ratio: a more balanced response elicited by pSec-pspA3NS (ratio = 4) correlated with a much more robust deposition of complement than that for the Th2-biased response elicited by rPspA3NS (ratio = 1,024) in WT mice. This hypothesis is further reinforced by the data obtained with IL-4 KO mice, with balanced responses (ratio of 2 for rPspA3NS and 0.1 for pSec-pspA3NS) mediating similar depositions of complement.
Since IgG2a is the isotype with the greatest capacity to mediate complement deposition onto the pneumococcal surface (1), it could be reasonable to correlate a higher total IgG2a concentration with an increase in complement deposition. We propose, though, that large amounts of IgG1 elicited by the recombinant protein could block the binding of anti-PspA IgG2a antibodies, thus compromising complement deposition. This competition between anti-PspA IgG1 and IgG2a binding to the pneumococcal surface might be the reason why a balanced IgG1/IgG2a response might be beneficial in mediating complement deposition. The ability of Ig from one isotype to prevent the effector activity by another isotype has been described previously for Neisseria meningitidis (9).
Although passive protection experiments have shown that antibodies against PspA are sufficient to induce protection against fatal infection in mice (4), the increasing evidence for the role of cellular immune responses in pneumococcal disease prompted us to further characterize the profiles of cytokines induced in animals immunized with DNA vaccine and recombinant protein after challenge with S. pneumoniae. Splenocytes from mice immunized with the DNA vaccine showed secretion of IFN-γ and TNF-α, whereas animals injected with recombinant protein showed induction of IL-4 when restimulated in vitro with rPspA. These results corroborate the data on the IgG isotype ratio induced by immunization, with recombinant protein inducing a Th2-biased response with a high IgG1/IgG2a ratio and secretion of IL-4. In contrast, immunization with the DNA vaccine showed a trend toward a Th1 response, with a balanced IgG1/IgG2a ratio and secretion of IFN-γ and TNF-α. The role of IFN-γ in the resolution of pneumococcal infection is well described for different mouse models (24, 27), but the role of TNF-α is controversial.
A previous study reported that after intranasal pneumococcal infection, TNF-α receptor KO mice exhibit elevated bacteremia and progress more rapidly to a fatal outcome (12). Although studies showed that TNF-α may be dispensable in pulmonary responses, it appears to be a necessary beneficial regulator of systemic organ protection in pneumococcal disease (15). The role of TNF-α may be a double-edged sword, since TNF-α is important in the early stages of infection but its levels need to be controlled later to avoid an exacerbated inflammatory response (12).
A recent study reported that Toll-like receptor 9 (TLR9)-deficient mice, but not TLR1-, TLR2-, TLR4-, or TLR6-deficient mice, are more susceptible to respiratory bacterial infection caused by pneumococci and that pneumococcal infection triggers a TLR9- and MyD88-dependent activation of phagocytic activity from resident macrophages, leading to an early clearance of bacteria from the lower respiratory tract (2). It is known that TLR9 induces an inflammatory response by recognition of unmethylated CpG present in prokaryotic DNA. We thus postulate that DNA vaccines could induce an immune response similar to the one elicited by bacterial infection, which would be much more suitable than the response elicited by immunization with recombinant protein with alum as an adjuvant in terms of the profile of both humoral and cellular responses. Although it is clear from our data that immunizations with both recombinant protein and DNA vaccines elicit immune responses which are sufficient for protection against lethal challenge with S. pneumoniae, immunization with DNA vaccines showed a response that is qualitatively superior, with the induction of a balanced IgG1/IgG2a anti-PspA antibody response that correlated with a more efficient mediation of complement deposition as well as a specific cellular immune response characterized by the secretion of proinflammatory cytokines. We thus propose that a vaccine based on PspA should be administered with adjuvants that induce a more balanced Th1/Th2 response, such as unmethylated CpG oligodeoxynucleotides.
Acknowledgments
We thank Jorge Mário da Costa Ferreira, Jr., for help with fluorescence-activated cell sorter analysis.
This work was supported by CAPES, CNPq, FAPESP, Fundação Butantan, and the Millenium Institute-Gene Therapy Network (MCT-CNPq).
Footnotes
Published ahead of print on 9 January 2008.
REFERENCES
- 1.Abbas, A. K., A. H. Lichtman, and J. S. Pober. 1997. Cellular and molecular immunology, 3rd ed. W. B. Saunders Company, Philadelphia, PA.
- 2.Albiger, B., S. Dahlberg, A. Sandgren, F. Wartha, K. Beiter, H. Katsuragi, S. Akira, S. Normark, and B. Henriques-Normark. 2007. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell. Microbiol. 9:633-644. [DOI] [PubMed] [Google Scholar]
- 3.Bosarge, J. R., J. M. Watt, D. O. McDaniel, E. Swiatlo, and L. S. McDaniel. 2001. Genetic immunization with the region encoding the alpha-helical domain of PspA elicits protective immunity against Streptococcus pneumoniae. Infect. Immun. 69:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briles, D. E., S. K. Hollingshead, J. King, A. Swift, P. A. Braun, M. K. Park, L. M. Ferguson, M. H. Nahm, and G. S. Nabors. 2000. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694-1701. [DOI] [PubMed] [Google Scholar]
- 5.Darrieux, M., E. N. Miyaji, D. M. Ferreira, L. M. Lopes, A. P. Lopes, B. Ren, D. E. Briles, S. K. Hollingshead, and L. C. Leite. 2007. Fusion proteins containing family 1 and family 2 PspA fragments elicit protection against Streptococcus pneumoniae that correlates with antibody-mediated enhancement of complement deposition. Infect. Immun. 75:5930-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, H. L., M. L. Michel, M. Mancini, M. Schleef, and R. G. Whalen. 1994. Direct gene transfer in skeletal muscle: plasmid DNA-based immunization against the hepatitis B virus surface antigen. Vaccine 12:1503-1509. [DOI] [PubMed] [Google Scholar]
- 7.Ejstrud, P., B. Kristensen, J. B. Hansen, K. M. Madsen, H. C. Schonheyder, and H. T. Sorensen. 2000. Risk and patterns of bacteraemia after splenectomy: a population-based study. Scand. J. Infect. Dis. 32:521-525. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira, D. M., E. N. Miyaji, M. L. Oliveira, M. Darrieux, A. P. Areas, P. L. Ho, and L. C. Leite. 2006. DNA vaccines expressing pneumococcal surface protein A (PspA) elicit protection levels comparable to recombinant protein. J. Med. Microbiol. 55:375-378. [DOI] [PubMed] [Google Scholar]
- 9.Griffiss, J. M., and M. A. Bertram. 1977. Immunoepidemiology of meningococcal disease in military recruits. II. Blocking of serum bactericidal activity by circulating IgA early in the course of invasive disease. J. Infect. Dis. 136:733-739. [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi, S., N. K. Day, R. P. Nelson, Jr., P. Emmanuel, J. E. Duplantier, C. S. Christodoulou, and R. A. Good. 1998. Interleukin 12 deficiency associated with recurrent infections. Proc. Natl. Acad. Sci. USA 95:13125-13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemp, K., H. Bruunsgaard, P. Skinhoj, and B. Klarlund Pedersen. 2002. Pneumococcal infections in humans are associated with increased apoptosis and trafficking of type 1 cytokine-producing T cells. Infect. Immun. 70:5019-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerr, A. R., J. J. Irvine, J. J. Search, N. A. Gingles, A. Kadioglu, P. W. Andrew, W. L. McPheat, C. G. Booth, and T. J. Mitchell. 2002. Role of inflammatory mediators in resistance and susceptibility to pneumococcal infection. Infect. Immun. 70:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan, A. Q., Q. Chen, Z. Q. Wu, J. C. Paton, and C. M. Snapper. 2005. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on Toll-like receptor 2. Infect. Immun. 73:298-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan, A. Q., Y. Shen, Z. Q. Wu, T. A. Wynn, and C. M. Snapper. 2002. Endogenous pro- and anti-inflammatory cytokines differentially regulate an in vivo humoral response to Streptococcus pneumoniae. Infect. Immun. 70:749-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirby, A. C., J. G. Raynes, and P. M. Kaye. 2005. The role played by tumor necrosis factor during localized and systemic infection with Streptococcus pneumoniae. J. Infect. Dis. 191:1538-1547. [DOI] [PubMed] [Google Scholar]
- 16.Malley, R., K. Trzcinski, A. Srivastava, C. M. Thompson, P. W. Anderson, and M. Lipsitch. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. USA 102:4848-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyaji, E. N., D. M. Ferreira, A. P. Lopes, M. C. Brandileone, W. O. Dias, and L. C. Leite. 2002. Analysis of serum cross-reactivity and cross-protection elicited by immunization with DNA vaccines against Streptococcus pneumoniae expressing PspA fragments from different clades. Infect. Immun. 70:5086-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizrachi-Nebenzahl, Y., S. Lifshitz, R. Teitelbaum, S. Novick, A. Levi, D. Benharroch, E. Ling, and R. Dagan. 2003. Differential activation of the immune system by virulent Streptococcus pneumoniae strains determines recovery or death of the host. Clin. Exp. Immunol. 134:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore, Q. C., J. R. Bosarge, L. R. Quin, and L. S. McDaniel. 2006. Enhanced protective immunity against pneumococcal infection with PspA DNA and protein. Vaccine 24:5755-5761. [DOI] [PubMed] [Google Scholar]
- 20.Nabors, G. S., P. A. Braun, D. J. Herrmann, M. L. Heise, D. J. Pyle, S. Gravenstein, M. Schilling, L. M. Ferguson, S. K. Hollingshead, D. E. Briles, and R. S. Becker. 2000. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18:1743-1754. [DOI] [PubMed] [Google Scholar]
- 21.Nascimento, I. P., W. O. Dias, R. P. Mazzantini, E. N. Miyaji, M. Gamberini, W. Quintilio, V. C. Gebara, D. F. Cardoso, P. L. Ho, I. Raw, N. Winter, B. Gicquel, R. Rappuoli, and L. C. Leite. 2000. Recombinant Mycobacterium bovis BCG expressing pertussis toxin subunit S1 induces protection against an intracerebral challenge with live Bordetella pertussis in mice. Infect. Immun. 68:4877-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicoletti, C., M. C. Brandileone, M. L. Guerra, and A. S. Levin. 2007. Prevalence, serotypes, and risk factors for pneumococcal carriage among HIV-infected adults. Diagn. Microbiol. Infect. Dis. 57:259-265. [DOI] [PubMed] [Google Scholar]
- 23.Ren, B., A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2004. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect. Immun. 72:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubins, J. B., and C. Pomeroy. 1997. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect. Immun. 65:2975-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun, K., S. L. Salmon, S. A. Lotz, and D. W. Metzger. 2007. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect. Immun. 75:1196-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai, S. S. 2006. Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies. Crit. Rev. Microbiol. 32:139-153. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto, N., K. Kawakami, Y. Kinjo, K. Miyagi, T. Kinjo, K. Uezu, C. Nakasone, M. Nakamatsu, and A. Saito. 2004. Essential role for the p40 subunit of interleukin-12 in neutrophil-mediated early host defense against pulmonary infection with Streptococcus pneumoniae: involvement of interferon-gamma. Microbes Infect. 6:1241-1249. [DOI] [PubMed] [Google Scholar]