Abstract
During the spring in 2005 and 2006, 39,095 northward-migrating land birds were captured at 12 bird observatories in eastern Canada to investigate the role of migratory birds in northward range expansion of Lyme borreliosis, human granulocytic anaplasmosis, and their tick vector, Ixodes scapularis. The prevalence of birds carrying I. scapularis ticks (mostly nymphs) was 0.35% (95% confidence interval [CI] = 0.30 to 0.42), but a nested study by experienced observers suggested a more realistic infestation prevalence of 2.2% (95% CI = 1.18 to 3.73). The mean infestation intensity was 1.66 per bird. Overall, 15.4% of I. scapularis nymphs (95% CI = 10.7 to 20.9) were PCR positive for Borrelia burgdorferi, but only 8% (95% CI = 3.8 to 15.1) were positive when excluding nymphs collected at Long Point, Ontario, where B. burgdorferi is endemic. A wide range of ospC and rrs-rrl intergenic spacer alleles of B. burgdorferi were identified in infected ticks, including those associated with disseminated Lyme disease and alleles that are rare in the northeastern United States. Overall, 0.4% (95% CI = 0.03 to 0.41) of I. scapularis nymphs were PCR positive for Anaplasma phagocytophilum. We estimate that migratory birds disperse 50 million to 175 million I. scapularis ticks across Canada each spring, implicating migratory birds as possibly significant in I. scapularis range expansion in Canada. However, infrequent larvae and the low infection prevalence in ticks carried by the birds raise questions as to how B. burgdorferi and A. phagocytophilum become endemic in any tick populations established by bird-transported ticks.
Ixodes scapularis is the tick vector of a number of medically important zoonoses in northeastern North America, including Lyme borreliosis and human granulocytic anaplasmosis (HGA), caused by Borrelia burgdorferi sensu stricto and Anaplasma phagocytophilum, respectively (56). A number of field studies have shown that migratory passerines are hosts for I. scapularis in North America (2, 5, 37, 46, 49, 53, 54, 61) and that these birds carry nymphal I. scapularis northward into and through Canada during spring migration (25, 35, 50). It has been hypothesized that migratory birds are dispersing I. scapularis over some considerable distance in Canada, where they could pose a public health risk for two reasons. First, such adventitious ticks appear capable of surviving through the molt to quest and attach to humans and domesticated animals (and possibly infect them with tick-borne pathogens) even in areas that are currently thought too cold to sustain reproducing populations of I. scapularis (38). Second, bird-borne ticks introduced into new locations could be instrumental in expanding the northern range of reproducing I. scapularis populations (32, 39), particularly if warming due to climate change enhances that possibility (40). Up to 1997, the only established population of I. scapularis known in Canada was that at Long Point, Ontario (4, 30). However, the number of known I. scapularis populations in Canada has risen from 1 to 13 (38; L. R. Lindsay, unpublished data) in the last decade, a period that may have witnessed the first effects of global warming (41, 48). Most of the Canadian populations of I. scapularis are geographically isolated from one another and occur on southeast-facing shores of the Great Lakes (in Ontario), Lake of the Woods (in Manitoba), and the coast of Nova Scotia. Such locations suggest that I. scapularis range expansion in Canada is less likely to be due to the local spread of ticks by terrestrial hosts than to the migratory birds that frequently make landfall at these locations on their northward migration (33). Even so, that migratory birds alone are responsible for the distribution of “adventitious” ticks elsewhere in Canada (39) remains a hypothesis.
Migratory birds are increasingly considered important in the global dispersal of zoonotic pathogens (21). Migratory birds have been implicated as important in dispersing B. burgdorferi sensu lato because (i) some migratory bird species are efficient reservoirs of some genotypes of B. burgdorferi sensu lato in Europe (19) and of B. burgdorferi sensu stricto (from here on referred to simply as B. burgdorferi) in North America (2, 46, 47), (ii) the stress of migration is thought to render some species possible “super-spreaders” (18), and (iii) the birds transport the tick vectors as well as the pathogens (11). While it is likely that migratory birds do introduce infected ticks into Canada (35, 50), how important bird-borne I. scapularis ticks are in the establishment of endemic transmission cycles of I. scapularis-borne pathogens remains to be investigated.
In this study, we aimed to quantify the role of northward-migrating passerines in spring as hosts transporting I. scapularis and to investigate the extent to which bird-borne ticks could explain the occurrence of “adventitious” ticks observed by passive surveillance and the establishment of new I. scapularis populations in Canada. We also investigated the role of migratory birds as a route of introduction of different genotypes of the Lyme disease agent, B. burgdorferi, which could have consequences for clinical and serological diagnosis of Lyme disease in Canada (60, 62), and the introduction of A. phagocytophilum, the agent of HGA.
MATERIALS AND METHODS
Estimation of I. scapularis infestations of migratory passerines.
Twelve bird observatories in the Canadian Migration Monitoring Network (1 in Nova Scotia, 1 in Quebec, and 10 in Ontario [http://www.bsc-eoc.org/national/cmmn.html]) participated in the study (Table 1; Fig. 1). At each bird observatory, staff members were asked to examine the heads of as many captured birds as possible for the presence of ticks, using a binocular head loupe, during the spring northward migration from the start of capture in March to the end of June in the years 2005 and 2006. Our study was confined to this period because birds captured after June are likely to be resident rather than migrating and birds captured after mid-July are increasingly likely to be birds migrating south (for data on individual bird species, see reference 43). Staff members were asked to remove all ticks observed by using fine forceps, and the ticks were placed in sterile tubes and dispatched to the National Microbiology Laboratory, Winnipeg, for identification using appropriate taxonomic keys (10, 23) and for molecular assays (see below).
TABLE 1.
Numbers of birds of each species that carried ticks and numbers of I. scapularis ticks they carried that were infected with B. burgdorferi
| Species | No. of birds carrying ticks of any species/no. examined (%) | No. (%) of I. scapularis-infested birds | No. of infected I. scapularis nymphs/no. tested | No. of infected I. scapularis larvae/no. tested | No. of individuals carrying infected ticks (no. that carried >1 infected tick) |
|---|---|---|---|---|---|
| Yellow-bellied sapsucker | 1/63 (1.59) | 0 | |||
| Least flycatcher | 1/422 (0.23) | 0 | |||
| Red-eyed vireo | 2/294 (0.76) | 0 | |||
| Blue jay | 9/1,575 (0.57) | 4 (0.25) | 3/10 | 2 (1) | |
| Bank swallow | 1/21 (4.76) | 0 | |||
| Black-capped chickadeea | 1/215 (0.46) | 1 (0.46) | 0/1 | 0 | |
| Brown creeper | 2/391 (0.51) | 2 (0.51) | 0/2 | 0 | |
| House wren | 10/266 (3.76) | 4 (1.50) | 1/13 | 0/1 | 1 |
| Winter wren | 4/155 (2.58) | 3 (1.93) | 3/7 | 1 (1) | |
| Golden-crowned kinglet | 1/513 (0.19) | 1 (0.19) | |||
| Ruby-crowned kinglet | 8/2,052 (0.39) | 1 (0.05) | 0/1 | 0 | |
| American robin | 11/416 (2.64) | 7 (1.68) | 6/16 | 4 (2) | |
| Gray-cheeked thrush | 10/177 (5.65) | 7 (3.95) | 1/10 | 0/1 | 1 |
| Hermit thrush | 45/898 (5.01) | 12 (1.34) | 0/7 | 0/7 | 0 |
| Swainson's thrush | 11/607 (1.81) | 7 (1.15) | 1/12 | 0/6 | 1 |
| Veery | 9/299 (3.01) | 2 (0.67) | 0/6 | 0/1 | 0 |
| Wood thrush | 3/229 (1.31) | 0 | |||
| Brown thrasher | 22/186 (11.83) | 7 (3.76) | 0/3 | 0/13 | 0 |
| Gray catbird | 9/1,092 (0.82) | 5 (0.46) | 0/7 | 0 | |
| European starling | 5/81 (6.17) | 2 (2.47) | 1/2 | 1 | |
| Cedar waxwing | 1/136 (0.73) | 1 (0.73) | 0/1 | 0 | |
| Blue-winged warbler | 1/45 (2.22) | 1 (2.22) | 0/1 | 0 | |
| Nashville warbler | 1/487 (0.21) | 0 | |||
| Yellow warbler | 2/1,477 (0.13) | 1 (0.06) | 1/1 | 1 | |
| Chestnut-sided warbler | 1/317 (0.31) | 1 (0.31) | 1/1 | 1 | |
| Magnolia warbler | 1/1,719 (0.06) | 0 | |||
| Black-throated blue warbler | 1/199 (0.05) | 1 (0.05) | 0/1 | 0 | |
| Yellow-rumped warbler | 1/1,642 (0.06) | 1 (0.06) | 0/1 | 0 | |
| Yellow palm warbler | 1/10 (10.00) | 1 (10.00) | 0/1 | 0 | |
| Bay-breasted warbler | 1/42 (2.38) | 0 | |||
| Ovenbird | 1/407 (0.24) | 0 | |||
| Northern waterthrush | 2/133 (1.50) | 2 (1.50) | 1/2 | 1 | |
| Mourning warbler | 2/125 (1.60) | 2 (1.60) | 0/1 | 0/1 | 0 |
| Common yellowthroat | 14/653 (2.14) | 11 (1.68) | 1/21 | 1 | |
| Canada warbler | 1/171 (0.58) | 1 (0.58) | 0/1 | 0 | |
| Hooded warbler | 1/24 (4.17) | 1 (4.17) | 0/1 | ||
| Northern cardinal | 1/119 (0.84) | 0 | |||
| Rose-breasted grosbeak | 1/534 (0.19) | 0 | |||
| Indigo bunting | 2/118 (1.69) | 2 (1.69) | 1/5 | 1 | |
| Eastern towhee | 4/78 (5.13) | 2 (2.56) | 0/2 | 0 | |
| Chipping sparrow | 2/1,733 (0.11) | 1 (0.05) | 0/2 | 0/1 | 0 |
| Nelson's sharp-tailed sparrow | 1/1 (100) | 0 | |||
| Field sparrow | 1/198 (0.50) | 1 (0.50) | 0/1 | 0 | |
| Fox sparrow | 4/82 (4.88) | 1 (1.22) | 0/6 | 0 | |
| White-throated sparrow | 92/4,674 (19.68) | 10 (0.21) | 1/13 | 0/5 | 1 |
| Eastern white-crowned sparrow | 9/1,331 (0.68) | 1 (0.07) | 0/1 | 0 | |
| Gambel's white-crowned sparrow | 2/33 (6.06) | 0 | |||
| Slate-colored junco | 17/1,357 (1.25) | 3 (0.22) | 1/1 | 0/3 | 1 |
| Lincoln's sparrow | 13/498 (2.61) | 2 (0.40) | 1/3 | 1 | |
| Song sparrow | 19/720 (2.64) | 5 (0.69) | 2/5 | 0/4 | 2 |
| Swamp sparrow | 18/482 (3.73) | 2 (0.41) | 2/4 | 1 (1) | |
| Brown-headed cowbird | 1/1,091 (0.09) | 0 | |||
| Red-winged blackbird | 19/1,747 (10.87) | 12 (0.69) | 3/28 | 3 | |
| Common grackle | 13/1,088 (0.92) | 8 (0.73) | 1/16 | 0/1 | 1 |
| Purple finch | 1/185 (0.54) | 0 | |||
| American goldfinch | 1/1,236 | 0 | |||
| House sparrow | 1/1,236 (0.08) | 0 | |||
| Total for all migratory species | 413/39,095 (1.06) | 138 (0.35) | 32/209 | 0/53 | 25 (5) |
This species was considered nonmigratory, and these data were not included in statistical analyses.
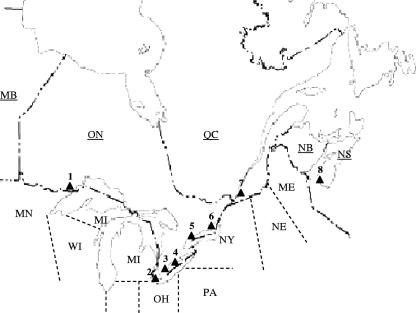
FIG. 1.
Locations of bird observatories that participated in the study. Abbreviations for the names of Canadian provinces and U.S. states, respectively, are as follows: MB, Manitoba; ON, Ontario; QC, Quebec; NB, New Brunswick; NS, Nova Scotia; and MN, Minnesota; WI, Wisconsin; MI, Michigan; OH, Ohio; PA, Pennsylvania; NY, New York; NE, New England states; and ME, Maine. Filled triangles indicate the locations of bird observatories participating in the study: 1, Thunder Cape; 2, Pelee Island; 3, Long Point (comprising the Old Cut, Breakwater, and Tip observatories); 4, Haldimand (comprising the Ruthven, Rock Point, and Selkirk observatories); 5, Tommy Thompson Park, Toronto; 6, Prince Edward Point; 7, McGill, Montreal; 8, Atlantic, Nova Scotia.
All bird observatories were provided with an instruction guide, including photographs on methods of searching for ticks. To assess the efficiency with which the bird observatory staff identified tick-carrying birds, five observers experienced in searching for and identifying ectoparasites carefully examined the heads of all birds captured at Prince Edward Point Bird Observatory during the period 2 to 6 May 2006 after they had been examined for ticks by bird observatory station staff. In all cases, the identification (ID) number of collected ticks and bird band numbers could be cross-referenced so that data on ticks and tick-borne pathogens could be related to data on the birds from which they were obtained.
Identifying potential risk factors for I. scapularis attachment to birds.
Bird species or genera may vary in their importance as hosts transporting I. scapularis because of variation in foraging or grooming behavior (11, 43, 46) and habitat associations (9, 17), as well as differences in innate or acquired immunity to ticks (5, 46). If so, their migration routes may identify risk areas for I. scapularis-borne zoonoses and I. scapularis establishment at the northern edge of the tick's range. Bird genus, as described by the American Ornithologists’ Union (http://www.aou.org/checklist/index.php3), was included as an explanatory variable in analyses. Each genus was included as a dummy variable and then collapsed stepwise into families, and then families were grouped according to statistical criteria described below.
We accounted for the date the bird was captured (coded as follows: 1 = earlier than 10 April; 2 = 11 to 30 April; 3 = 1 to 19 May; and 4 = later than 19 May), because the timing of migration relative to the normal seasonal activity of ticks in spring is likely to affect the frequency with which migrating birds encounter questing ticks (54).
We also accounted for potential confounding factors, including the following: (i) bird observatory ID number, because variations among bird observatories may occur due to different efforts by the staff or facilities to examine birds (i.e., sampling bias) or because some observatories occur on bird migration routes that are particularly important pathways for dissemination of ticks; and (ii) whether or not a known I. scapularis population occurs at the bird observatory where the bird was captured (as they do at Long Point and Prince Edward Point, Ontario). Birds at these observatories could have acquired an I. scapularis tick at the site of capture rather than prior to arrival.
Molecular analysis of ticks for B. burgdorferi infection.
All Ixodes ticks were tested for infection with B. burgdorferi by using extraction and real-time PCR methods as previously described (12, 39). DNA was extracted from ticks using Qiagen DNeasy 96 tissue kits (Qiagen, Inc., Mississauga, Ontario, Canada) according to the manufacturer's instructions, except that the ticks were incubated in lysis buffer containing proteinase K overnight at 56°C, which in our laboratory enhances the extraction of DNA from low numbers of pathogens in ticks. For each tick, successful extraction was confirmed using a PCR that uses primers (MM1 and MM2) specific for the tick 5.8S rRNA-28S rRNA intergenic spacer (IGS) as previously described (39). DNA extracts were screened for evidence of B. burgdorferi and A. phagocytophilum infection using a multiplex real-time PCR targeting their 23S rRNA and msp2 genes, respectively, as previously described (12).
Briefly, multiplex 25-μl reaction mixtures comprised TaqMan Universal Mastermix (Applied Biosystems, Foster City, CA) containing primers specific for B. burgdorferi 23S rRNA (Bb23Sf and Bb23Sr at 700 nM each), the TaqMan probe specific for the amplified segment (Bb23Sp-FAM at 175 nM), primers specific for the A. phagocytophilum msp2 gene (ApMSP2f and ApMSP2r at 900 nM each), the TaqMan probe specific for the amplified segment (ApMSP2p-HEX at 125 nM), and 2 μl of template DNA. Cycling conditions comprised incubation at 50°C for 2 min (to activate the enzyme AmpErase) and activation of AmpliTaq Gold polymerase (and denaturation of AmpErase) at 95°C for 10 min, followed by 40 cycles of a 15-s denaturation step at 95°C followed by a 1-min annealing-extension step at 60°C.
B. burgdorferi infection was then confirmed in positive samples using a real-time PCR targeting the OspA gene as previously described (39). Reaction mixtures comprised 2× TaqMan Universal Mastermix (Applied Biosystems) containing 900 nM primers (Osp A F and Osp A R) and the 250 nM OspA probe (the sequences are as detailed in reference 39). Cycling conditions comprised incubation at 50°C for 2 min (to activate AmpErase) and 10 min at 95°C (to denature AmpErase and activate AmpliTaq Gold polymerase), followed by 40 cycles of a 15-s denaturation step at 95°C followed by a 1-min annealing-extension step at 60°C. After amplification and real-time data acquisition, analysis was performed using the Sequence Detection System software (Applied Biosystems).
In-house evaluations of the sensitivity of the system, similar to those described in reference 12, identified the limit of detection of B. burgdorferi as 1 to 10 organisms per tick and thus comparable to traditional nested PCR assays (12).
Genotyping of B. burgdorferi.
We attempted to obtain ospC and rrs (16S)-rrl (23SA) IGS alleles for all samples that were positive for B. burgdorferi infection by real-time PCR.
A 522-bp region of the ospC gene of B. burgdorferi was amplified by a seminested PCR using inner primers OC6 (positive strand) and OC623 (negative strand) and internal primers OC6 (fluorescein conjugated) and OC602 (negative strand) that target conserved regions as previously described (6, 45). Amplicons were then probed with ospC-type specific probes by reverse line blotting (RLB) as previously described (6, 45). The amplicons were also sequenced in both directions using primers OC6 (positive strand) and OC602 (negative strand), and the complementary strands of each sequenced product were manually assembled to produce a single sequence. These sequences were then aligned against each other and with reference sequences downloaded from GenBank using the ClustalW algorithm implemented in MEGA, version 3.1 (26). The reference sequences were as follows: type A, AF029860; type B, AF029861; type C, AF029862; type D, AF029863; Type E, AF029864; type F, AF029865; type G, AF029867; type H, AF029868; type I, AF029869; type J, AF029870; type K, AF029871; type L, L42899; type M, U01892; type N, L42897; type O, X84778; type T, AF065143; type U, AF065144. The criterion for inclusion within an ospC type was ≥99% identity, and the criterion for exclusion from an ospC type was ≤90% identity (45).
The samples were also subjected to a nested PCR targeting a fragment of the rrs-rrl IGS of B. burgdorferi sensu lato as previously described (31). The products were sequenced in both directions using the inner primers, complementary strands were manually assembled, and then the sequences were aligned against each other and with reference sequences downloaded from GenBank. Phylogenetic analyses of the rrs-rrl IGS sequences were performed with the PAUP* 4.0b10 software program (55) using the neighbor-joining (NJ) and maximum-parsimony (MP) methods. The nucleotide substitution models for NJ analyses were determined using Modeltest (44). The best-fit evolutionary model was the transitional model with invariable sites (proportion of invariable sites, 0.8177) and equal base frequencies. For MP trees, we used parsimony-informative sites only and the heuristic search option with equal weighting, random sequence addition (20 replicates), and tree-bisection-reconnection branch swapping. Nonparametric bootstrapping with 1,000 replicates was carried out.
Statistical analyses.
The likelihood that a bird carried an I. scapularis tick was the outcome variable in logistic regression models in STATA, version 8.0 for Windows (STATACorp, College Station, TX). In these models, the explanatory variables were those described above: bird genus (as individual or grouped dummy variables as described above), capture date period, presence of I. scapularis populations, and bird observatory ID. The year of study was also considered as an explanatory variable. A process of backward and forward substitution and elimination obtained the most parsimonious multivariable model in which no variable could be removed without significantly affecting model deviance. Estimates for undercounting of ticks by the staff at Prince Edward Point Bird Observatory during the first week of May 2006 were calculated using McNemar's test statistic (34). We assumed that data on tick infestations of birds examined by station staff comprised “cases,” while data on tick infestations of birds after examination by station staff and experienced observers comprised “controls.”
The likelihood that a tick was PCR positive for a tick-borne pathogen was also investigated using logistic regression models (in STATA version 8.0) in which the explanatory variables were bird genus, capture period, bird observatory ID, level of engorgement of the tick (1 = no detectable blood meal; 2 = partially engorged; 3 = appearance of complete engorgement [39]) and tick instar. In these models, the bird ID was included as a random effect, because ticks from the same bird are not statistically independent (36). Separate models were used to further investigate factors associated with infection in ticks from birds captured at Long Point and Prince Edward Point in Ontario, where I. scapularis populations are established.
Nucleotide sequence accession numbers.
GenBank accession numbers of ospC sequences obtained in this study are EF634251 to EF634268, and those of IGS sequences obtained in this study are EF649781 to EF649791.
RESULTS
Birds captured and tick species collected.
A total of 39,629 birds were examined for ticks during the study, comprising species from 10 orders: Ciconiiformes (1 green heron), Anseriformes (1 Canada goose, 2 mallards, and 2 lesser scaup), Falconiformes (2 American kestrels, 1 Cooper's hawk, 1 merlin, and 20 sharp-shinned hawks), Charadriiformes (6 American woodcocks, 1 herring gull, 7 killdeers, and 8 ring-billed gulls), Columbiformes (155 mourning doves and 1 white-winged dove), Cuculiformes (16 black-billed cuckoos and 5 yellow-billed cuckoos), Strigiformes (3 northern saw-whet owls), Caprimulgiformes (1 whip-poor-will), Piciformes (46 downy woodpeckers, 4 hairy woodpeckers, 83 northern flickers, 1 pileated woodpecker, 25 red-bellied woodpeckers, and 11 red-headed woodpeckers), and Passeriformes (39,226 individuals of 136 species). Data from 39,095 birds were used in analysis after some species were excluded as winter residents at or close to the site of capture (e.g., some woodpeckers, gulls, and black-capped chickadees). Of these birds, 29,350 (75.1%) were captured at two Ontario bird observatories: Long Point and Prince Edward Point.
Of the 39,095 migratory birds examined by bird observatory staff, ticks were found on 413 individuals (1.06%; 95% confidence interval [CI] = 0.96 to 1.16), and a total of 897 ticks were submitted for identification (Table 1). Of these ticks, 305 were Ixodes dentatus (32 nymphs and 273 larvae), 263 were I. scapularis (208 nymphs and 55 larvae), 202 were Haemaphysalis leporispalustris (110 nymphs, 91 larvae, and 1 adult male), 44 were Ixodes muris (42 adult females and 2 nymphs), 30 were Ixodes brunneus (24 nymphs and 6 larvae), 17 were Amblyomma maculatum (8 nymphs and 9 larvae), 3 were Amblyomma inornatum (2 nymphs and 1 adult male), and the species of 33 ticks could not be determined because they were extensively damaged. The highest level of infestation was 45 I. dentatus larvae on a song sparrow, and the highest level of I. scapularis infestation was 9 ticks (5 larvae and 4 nymphs) on a Swainson's thrush captured at the Tommy Thompson (Toronto) bird observatory. Mixed infestations were found, the commonest being H. leporispalustris and I. dentatus, which were found together on 10 birds.
The I. scapularis ticks were collected from 138 of the 39,095 migratory birds examined (0.35%; 95% CI = 0.30 to 0.42), of which mean (median) infestations with nymphs and larvae and all I. scapularis ticks were 1.56 (1), 1.71 (1), and 1.66 (1), respectively (Table 1). I. scapularis ticks were found on birds captured at all bird observatories except McGill, Montreal. Of these ticks, 71 were nearly engorged (of which 14 [20%] were from birds captured at observatories with established I. scapularis populations); 129 were partially engorged (of which 22 [17%] were from birds captured at observatories with established I. scapularis populations); and 63 were slightly engorged (of which 52 [82%] were from birds captured at observatories with established I. scapularis populations). Of the 138 birds carrying an I. scapularis tick, 16 (11.8%) also carried a tick of another species (six H. leporispalustris ticks, four I. dentatus ticks, three I. brunneus ticks, two I. muris ticks, and one A. maculatum tick).
At Prince Edward Point Bird Observatory, 591 birds of 43 species (all members of Passeriformes except for two mourning doves, two downy woodpeckers, and one northern flicker) were examined by both bird observatory staff and observers experienced in identifying ticks during early May 2006. Station staff identified 22 (3.72%; exact binomial 95% CI = 2.35 to 5.58) of the birds as infested with ticks of any species, while the experienced observers found an additional 36 birds infested with ticks, i.e., a minimum of 58 (9.81%; 95% CI = 7.53 to 12.50) birds infested with ticks of any species, as well as additional ticks on 7 birds from which ticks were collected by station staff. Of those birds identified as tick infested by the station staff, 6 (1.01% of all birds examined; 95% CI = 0.37 to 2.20) carried an I. scapularis tick, while a further 7 I. scapularis-carrying birds were identified by the experienced observers; thus, a minimum 13 of 591 birds (2.20%; 95% CI = 1.18 to 3.73) carried an I. scapularis tick. Station staff undercounted ticks of any species by a factor of 0.33 (95% CI = 0.23 to 0.47; McNemar's χ2 = 38; P < 0.001) and undercounted I. scapularis by a factor of 0.46 (95% CI = 0.26 to 0.83; McNemar's χ2 = 7; P < 0.05). On four birds identified as carrying a total of six I. scapularis ticks by station staff, the experienced observers found an additional six I. scapularis ticks.
Risk factors for attachment of I. scapularis.
There was no difference among years in the proportion of birds carrying I. scapularis (χ2 = 0.01; df = 1; P > 0.1), and in the final most-parsimonious multivariable model, bird observatory ID, certain bird genera or families, and the date of capture remained significant determinants of the likelihood that a bird carried an I. scapularis tick (Table 2).
TABLE 2.
Variables that were significantly associated with parasitism of migratory birds by I. scapularis ticks in the most-parsimonious multivariable logistic regression modela
| Explanatory variable | Factorb | OR | 95% CI | Wald Z valued |
|---|---|---|---|---|
| Bird species/genus | Other bird genera combinedc | |||
| Wrens | 8.68 | 3.95-19.07 | 5.38*** | |
| Thrushes | 5.87 | 3.87-8.91 | 8.31*** | |
| Wood warblers | 4.66 | 2.48-8.73 | 4.81*** | |
| Mimids | 3.37 | 1.59-5.78 | 3.37** | |
| Capture date | Other capture datesc | |||
| Before 11 April | 2.33 | 1.12-4.86 | 2.25* | |
| After 19 May | 1.51 | 1.33-2.79 | 2.13* | |
| Bird observatory | Other bird observatoriesc | |||
| Atlantic, NS | 15.62 | 5.45-44.90 | 5.10*** | |
| Prince Edward Point, ON | 1.93 | 1.33-2.79 | 3.49*** |
OR, odds ratio.
NS, Nova Scotia; ON, Ontario.
Reference factor.
Significance is as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Accounting for station ID and date of capture, birds of four genera or families were more likely to carry an I. scapularis tick than birds of any other species or genus (Table 2). These were the mimids (family Mimidae, comprising northern mockingbirds, brown thrashers, and gray catbirds of the genera Mimus, Dumetella, and Toxostoma, respectively, although none of the seven northern mockingbirds captured carried I. scapularis), thrushes (Turdidae, of the genera Turdus, Hylocichla, and Catharus combined), wrens (the family Troglodytidae, although only members of the genus Troglodytes carried I. scapularis), and wood-warblers of the genera Wilsonia and Geothlypis combined (hooded, Wilson's, and Canada warblers and the common yellowthroat). These comprised 8.1%, 25.9%, 5.2%, and 9.6% of I. scapularis-infested birds, respectively (collectively, 48.8% of infested birds). A similar proportion of brown thrashers and gray catbirds carried I. scapularis (χ2 = 0.25; df = 1; P > 0.1), and a similar proportion of the three genera of thrushes carried an I. scapularis (χ2 = 2.61; df = 2; P > 0.1). There were no significant differences among other genera or families (χ2 = 36.2; df = 25; P > 0.05). Despite such interspecies differences, I. scapularis ticks were carried by a wide range of species (Table 1).
The prevalence of infested birds was significantly higher at Atlantic Bird Observatory and at Prince Edward Point bird observatory than at other bird observatories (Table 2), among which the prevalence of infested birds was not significantly different (χ2 = 7.2; df = 9; P > 0.1). Accounting for stations, occurrence of a tick population was not significantly associated with the prevalence of infested birds (χ2 = 1.4; df = 1; P > 0.1).
Infection in Ixodes ticks collected from birds.
A total of 262 I. scapularis ticks (209 nymphs and 53 larvae) were tested for B. burgdorferi and A. phagocytophilum infection (Table 3). One nymph came from a resident black-capped chickadee; otherwise, all were from migratory bird species. No larval I. scapularis ticks were found to be infected with either pathogen. Of the nymphal I. scapularis ticks collected from migratory birds, 32 (15.4%) were PCR positive for B. burgdorferi and 3 (1.4%) were PCR positive for A. phagocytophilum. The prevalence of B. burgdorferi-infected nymphs collected from birds at Long Point, Ontario (23/100; 23.0%) was significantly higher than the prevalence of nymphs collected at the other stations combined (9/109; 8.2%) (odds ratio [OR] = 2.79; 95% CI = 1.24 to 6.28; P < 0.05). There were no differences among years, bird genera, collection dates, and states of tick engorgement in the prevalence of B. burgdorferi infection (P > 0.1 for all data). However, when data from only Long Point and Prince Edward Point, Ontario (where I. scapularis populations are endemic), were analyzed, ticks that were partially or nearly completely engorged at Long Point were significantly less likely to be infected than those that were only slightly engorged (16/84 [19.0%] and 7/16 [43.7%], respectively; OR = 0.30; 95% CI = 0.10 to 0.93; P < 0.05) when accounting for the overall lower prevalence at Prince Edward Point (OR = 0.09; 95% CI = 0.02 to 0.54; P < 0.001) and the potential interaction among station and level of tick engorgement (OR = 4.79; 95% CI = 0.62 to 37.28; P > 0.1). At Prince Edward Point, partially or completely engorged ticks were no less likely to be infected than slightly engorged ticks (5/51 [9.8%] and 2/29 [6.9%], respectively; OR = 4.85; 95% CI = 0.63 to 37.53; P > 0.1).
TABLE 3.
Numbers of I. scapularis ticks and other tick species collected from birds that were identified as being infected with B. burgdorferi
| Bird observatory, province | Latitude | No. of birds examined | No. of infected ticks/no. of ticks collected (%)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
I. scapularis
|
I. muris
|
I. brunneus
|
I. dentatus
|
|||||||
| Nymphs | Larvae | Adult females | Nymphs | Nymphs | Larvae | Nymphs | Larvae | |||
| Thunder Cape, Ontario | 88°46′32″W | 2,644 | 0/4 (0) | 0/3 (0) | 0/2 (0) | 0/2 (0) | 0/2 (0) | 0/4 (0) | ||
| Pelee Island, Ontario | 82°39′28″W | 595 | 0/2 (0) | 0/1 (0) | 0/1 (0) | |||||
| Long Point, Ontarioa | 80°25′59″W to 80°34′36″W | 21,894 | 23/100 (23.00) | 0/33 (0) | 0/10 (0) | 0/1 (0) | 0/16 (0) | 0/5 (0) | 1/15 (6.67) | 0/128 (0) |
| Haldimand,a Ontario | 79°57′32″ to 79°33′15″W | 4,929 | 1/4 (25.00) | 0/8 (0) | 0/9 (0) | 0/2 (0) | 0/5 (0) | 0/24 (0) | ||
| Toronto, Ontario | 79°22′24″W | 724 | 0/15 (0) | 0/7 (0) | 0/2 (0) | 0/4 (0) | 0/14 (0) | |||
| Prince Edward Point, Ontario | 76°51′32″W | 7,566 | 7/80 (8.75) | 0/1 (0) | 0/17 (0) | 0/1 (0) | 0/5 (0) | 0/100 (0) | ||
| McGill, Quebec | 73°56′57″W | 659 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Atlantic, Nova Scotia | 65°44′57″W | 84 | 1/4 (25.00) | |||||||
| Total for all observatories | 39,095 | 32/209 (15.31) | 0/53 (0) | 0/38 (0) | 0/2 (0) | 0/23 (0) | 0/7 (0) | 1/31 (3.22) | 0/270 (0) | |
Long Point and Haldimand each comprise three bird observatories: Old Cut, Breakwater, and Tip; and Rockpoint, Selkirk, and Ruthven, respectively.
At Long Point bird observatory, migratory birds can spend 3 days at the “Tip” and “Breakwater” stations but 4 or more days at the inland “Old Cut” station (Bird Studies Canada, unpublished data), where food is more plentiful (16). Partially and fully engorged nymphs collected at the Tip and Breakwater stations are more likely, therefore, to have been acquired prior to arriving at Long Point than those collected from birds captured at Old Cut. There was no difference in the proportion of I. scapularis nymphs that were partially or almost fully engorged at these sites (47/55 [85.5%] at Tip and Breakwater and 31/39 [79.5%] at Old Cut; OR = 0.66; CI = 0.22 to 1.94; P > 0.4). The proportion of partially or almost fully engorged nymphs collected at Old Cut (8/31 [25.8%]) was higher than at Tip and Breakwater (7/48 [14.5%]), although this was not significant at the 5% level (OR = 2.55; CI = 0.96 to 6.78; P = 0.07).
A further 375 Ixodes ticks collected from the birds were tested, comprising 302 I. dentatus ticks (31 nymphs and 271 larvae), 43 I. muris ticks (2 nymphs and 41 adult females), and 30 I. brunneus ticks (24 nymphs and 6 larvae) (Table 3). Of these, only one I. dentatus larva, collected from a swamp sparrow, was PCR positive for B. burgdorferi. A further two I. dentatus nymphs, both collected from the same white-throated sparrow, were PCR positive for A. phagocytophilum. Of the 295 birds that carried an identified Ixodes tick, 28 (9.5%) carried B. burgdorferi-infected ticks. Of the 94 birds that carried more than one tick, 5 birds of four species simultaneously carried more than one B. burgdorferi-infected tick (Table 1).
B. burgdorferi genotypes in Ixodes ticks.
B. burgdorferi ospC PCR products were successfully amplified from 22 (21 I. scapularis nymphs and 1 I. dentatus nymph) out of 33 Borrelia-infected ticks. Of these, amplicons from 20 ticks annealed one or more probes in the RLB system; 16 B. burgdorferi-infected ticks carried only one ospC allele, and 8 carried two or more alleles (Table 4). Complete sequences were obtained from 18 of the ospC PCR products, including the two ticks that were not identified by the ospC probes in RLB. Each of the complete sequences obtained was 99.6% to 100% similar to one of the ospC sequences used for comparison. A wide range of ospC alleles was found. Twenty of the ospC alleles identified were of the types common in studies of questing nymphal I. scapularis and rodent reservoirs in the eastern United States (A, B, D, and K [3, 6, 45]), while 15 carried alleles that are less common in the northeastern United States (F, G, H, I, L, and O [3, 6, 55]) (Table 4). The ratio of common to rare ospC alleles in our study was 1.3 to 1, more similar to that seen in the study of Qiu et al. (45) using archived samples from ticks collected in studies throughout the eastern United States, in which this ratio was 0.7 to 1, than to that seen in the studies of Brisson and Dykuizen (2.5 to 1 [reference 6]) and Anderson and Norris (3.8 to 1 [reference 3]).
TABLE 4.
Alleles for ospC and IGS sequences of B. burgdorferi obtained from each tick (one row per tick) collected from birds that were PCR positive for B. burgdorferia
| Observatory | Presence of indicated allele of:
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ospC, determined by RLB and sequencingc
|
IGS, determined by sequencingd
|
||||||||||||||||
| A | B | D | F | G | H | I | K | L | O | 1 | 2 | 3 | 5 | 6 | 7 | NV | |
| Long Point, Ontariob | + | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − |
| − | − | − | − | + | − | − | + | − | + | − | − | − | − | + | − | − | |
| + | + | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | |
| − | + | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | |
| + | − | − | − | − | − | − | + | − | − | MX | MX | MX | MX | MX | MX | MX | |
| + | − | − | − | − | − | − | + | − | − | − | + | − | − | − | − | − | |
| + | + | − | − | − | − | − | + | + | − | MX | MX | MX | MX | MX | MX | MX | |
| + | − | − | − | + | − | − | − | − | + | − | − | − | − | + | − | − | |
| − | − | − | − | + | − | − | + | + | + | − | + | − | − | − | − | − | |
| + | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | |
| − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | + | − | |
| − | − | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | |
| − | − | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | |
| + | − | − | − | − | − | − | − | − | − | NP | NP | NP | NP | NP | NP | NP | |
| + | − | − | − | − | − | − | − | − | − | NP | NP | NP | NP | NP | NP | NP | |
| − | + | − | − | − | − | − | − | − | − | NP | NP | NP | NP | NP | NP | NP | |
| − | − | + | − | − | − | − | − | − | − | NP | NP | NP | NP | NP | NP | NP | |
| NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | − | − | + | − | − | − | − | |
| NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | − | + | − | − | − | − | − | |
| NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | + | − | − | − | − | − | − | |
| NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | − | − | − | + | − | − | − | |
| NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | MX | MX | MX | MX | MX | MX | MX | |
| Haldimand, Ontariob | NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | − | − | − | − | − | − | + |
| Prince Edward Point, | − | − | − | − | − | + | − | − | − | − | − | + | − | − | − | − | − |
| Ontario | − | − | − | − | − | − | − | + | − | − | − | + | − | − | − | − | − |
| − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | + | − | |
| − | − | + | + | + | − | − | + | − | − | MX | MX | MX | MX | MX | MX | MX | |
| NP | NP | NP | NP | NP | NP | NP | NP | NP | NP | + | − | − | − | − | − | − | |
| Atlantic, Nova Scotia | − | + | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − |
The IGS allele numbering scheme is that of Bunikis et al. (7). ospC alleles in bold are those that have been associated with disseminated infection in humans (51). NP, no PCR product was obtained; MX, polymorphic regions were seen in the sequences, suggesting mixed alleles; NV, a new IGS sequence variant that had almost equal identity to sequences of IGS alleles 2 and 4 (7).
Long Point and Haldimand each comprise three bird observatories: Old Cut, Breakwater, and Tip; and Rockpoint, Selkirk, and Ruthven, respectively.
For ospC, total numbers of alleles found were as follows: nine A alleles, five B alleles, two D alleles, one F allele, four G alleles, one H allele, two I alleles, seven K alleles, four L alleles, and three O alleles.
For the IGS, total numbers of alleles found were as follows: four occurrences of allele 1, seven of allele 2, four of allele 3, one of allele 5, two of allele 6, two of allele 7, and one of the new variant.
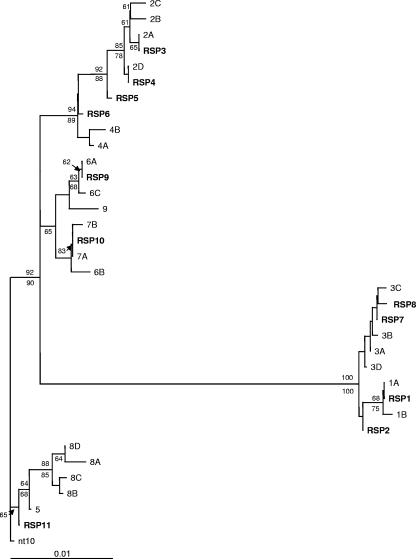
PCR products were obtained from 25 of 33 B. burgdorferi-infected ticks when the rrs-rrl IGS PCR was used. Eleven different sequences were obtained, which were identical to previously published sequences or were highly similar and clustered with them (Fig. 2). One exception was sequence RSP6 from a tick collected at Ruthven Park, Ontario, which was intermediate between sequences forming IGS allele groups 2 and 4 (Fig. 2) and thus could not be allocated to one of these groups (Table 4). The tree topology was the same using the NJ and MP methods. In all but one case, IGS and ospC alleles obtained from the same tick by either sequencing or RLB were consistent with the linkages between these sequences proposed by Bunikis et al. (7). The ratio of common IGS alleles (1, 2, 3, and 5, which are thought to be linked with ospC alleles A, K, B, and D, respectively [7]) to rare alleles in a study in Connecticut was 5.2 to 1 (20), while in our study that ratio was 3.2 to 1.
FIG. 2.
An unrooted neighbor-joining distance tree of rrs-rrl IGS of B. burgdorferi. Reference sequences were taken from the work of Bunikis et al. (7). Sequences obtained in this study are in bold with identifying numbers prefixed by “RSP.” Nonparametric bootstrap values for nodes with ≥65% support in both NJ and MP analyses are shown above and below the branches, respectively. Tree topologies involving nodes with ≥65% bootstrap support were identical in both NJ and MP analyses.
Two nymphs collected from the same American robin were both infected with B. burgdorferi carrying the same ospC allele (L) and the same IGS allele (2), and two nymphs from the same winter wren were both infected with B. burgdorferi carrying ospC allele B and IGS allele 3.
DISCUSSION
In this study we have estimated the proportion of northward-migrating birds in spring that carry I. scapularis ticks into and through Canada and, for the first time, made a detailed study of the prevalence of B. burgdorferi and A. phagocytophilum infection in these ticks and the genetic diversity of B. burgdorferi carried by them. A surprisingly high proportion of birds (up to 10%) carried ticks of at least one of seven species, and I. scapularis, the Lyme disease vector, was the second most common. Undercounting of ticks clearly took place. In the nested study at Prince Edward Point in 2006, experienced observers likely missed some ticks, yet their efforts more than doubled the prevalence obtained by bird observatory staff. Mean infestations of I. scapularis-carrying birds were also likely to be underestimates, because bird observatory staff did not collect all ticks from infested birds. Bird observatory staff were themselves likely sensitized to finding ticks during this study: they found I. scapularis on 1% of birds during the intensive study, but in the previous year, the same staff recorded finding only five I. scapularis ticks on the total 2,120 birds (0.2%) captured in May. Our estimate for the prevalence of infested birds is likely, therefore, to represent in the range of one-third to one-tenth of the true prevalence. If the true prevalence of I. scapularis-infested birds is between 0.9 and 3.5% and I. scapularis-infested birds carry a conservative mean 1.66 immature ticks of this species, then the 3 billion northward-migrating birds in eastern and central Canada in spring (excluding waterfowl [24]) could carry 50 to 175 million I. scapularis ticks into Canada per year. Therefore, it may not be unreasonable to suggest that migratory birds are responsible for the many adult I. scapularis ticks collected by passive surveillance from regions of Canada where reproducing populations of this tick are unknown (39). Precisely what this number means in terms of the density of ticks deposited in a particular location and the probability that these ticks can establish new populations remains to be determined. However, aggregation of migratory birds in time and space can occur for reasons such as inclement weather (reviewed in reference 33). Perhaps, therefore, the densities of migratory bird-borne I. scapularis could at times be high enough to establish new populations were such aggregations to occur in locations where temperature conditions, habitat, and host densities are suitable for the tick (29, 38).
From a public health perspective, it would be beneficial if we could predict the occurrence of adventitious I. scapularis and potential new I. scapularis populations from migratory bird pathways. However, our study suggests that this may not be possible at a resolution finer than that of the broad flyways for migratory birds in North America. First, I. scapularis ticks were collected from migratory birds from Nova Scotia to western Ontario. Second, while the prevalence of infestation with I. scapularis was particularly high for a limited number of species (thrushes, wrens, mimids, and some wood warblers), the geographic range of target breeding sites for all of these species is very wide in woodland and ecotonal habitats across Canada from the Rocky Mountains to the east coast (43). Third, greater numbers of ticks were carried by species other than thrushes, wrens, mimids, and wood warblers. Even though the prevalence of infestation was low for species such as red-winged blackbirds and white-throated sparrows, their sheer numbers compensated and may make these species particularly important in transporting I. scapularis: in the order of 110 million white-throated sparrows may nest in the boreal region of Canada alone (http://www.borealcanada.ca/research-research-data-e.php). Again, the geographic extent of the breeding range of these species is very wide in Canada (43). The wide geographic breeding range of I. scapularis-carrying migratory birds is consistent with the widespread geographical occurrence of I. scapularis in passive surveillance in Canada (39).
The wide range of species that carried I. scapularis ticks may in part be due to migratory birds relinquishing the associations with habitats they usually exhibit during the breeding season. Parasitism by I. scapularis very likely indicates that a bird has had a stopover in woodland or ecotonal habitats that are normally associated with I. scapularis (9). If so, on-migration individual red-winged blackbirds are using woodland and ecotonal habitats to an extent that does not occur during the breeding season (22, 52). Other risk factors identified in this study are mostly consistent with findings of previous studies: ground-feeding birds and birds migrating later than mid-May are more likely to acquire I. scapularis ticks (46, 54), although why birds in early April carried more nymphs is unclear. The high prevalence of infested birds at the Atlantic Bird Observatory in Nova Scotia may be consistent with most birds captured at this station having migrated through the regions of the northeastern United States where I. scapularis is particularly widespread (14), although many factors, such as different efforts and levels of competence in tick identification, could explain this finding.
The overall prevalence of B. burgdorferi infection in I. scapularis nymphs was similar to that seen in adventitious I. scapularis ticks collected across central and eastern Canada (12.5% (39). While many of the species we captured have been shown to be competent reservoirs in experimental studies or to amplify infection in feeding larvae during the breeding season (2, 46, 47), there was little evidence that birds migrating northward into Canada significantly amplified B. burgdorferi infection prevalence in I. scapularis ticks. Nymphal I. scapularis ticks are the instar and species most likely to have been infected prior to attachment to the birds. When data on nymphal I. scapularis ticks are excluded, only one tick of any Ixodid tick species and instar collected from 218 birds was PCR positive for B. burgdorferi.
There was evidence that the northward-migrating passerines as a whole may be zooprophylactic for B. burgdorferi. In spring, Long Point and bird observatories further east receive migratory passerines moving north through locations in the United States from Pennsylvania to the east coast (The Canadian Atlas of Bird-Banding [http://www.cws-scf.ec.gc.ca/publications/BBA-AOB/v1ed2/index_e.cfm]; Bird Studies Canada, unpublished data). The prevalence of B. burgdorferi infection in nymphs collected from birds at these observatories was 15.2% (7/46), and the prevalence was only 8.3% (2/24) in nymphs collected from birds at observatories east of Long Point. These prevalence values are much lower than those seen in most studies of questing nymphs from sites in the northeastern United States where the prevalence is generally greater than 30% (13, 20, 27, 45, 57) and rather more consistent with infection prevalence in questing nymphs in some areas of the Mississippi and Ohio valleys (8, 43, 58, 59). The significantly higher prevalence of infection in nymphs collected from birds at Long Point, where I. scapularis has been established for decades, could have been due to the high prevalence of infection (up to 40% depending on year and location [28]) in questing nymphs that could have attached to the birds as they landed at this site. Indeed, the B. burgdorferi infection prevalence in nymphs from birds at Long Point was higher in those recently attached (i.e., slightly engorged ticks) than in more fully engorged ticks, particularly those collected from birds at the Tip and Breakwater observatories, which were most likely to have attached to the birds in the United States en route to Canada. Most ticks that attached to birds at the Tip and Breakwater observatories at Long Point would likely be only slightly engorged, because the mean duration of residence at these stations is less than 4 days (Bird Studies Canada, unpublished data), whereas I. scapularis nymphs feed for approximately 4 days on their host (15).
Overall, therefore, the low B. burgdorferi infection prevalence in nymphs collected from the migratory birds is consistent with (i) ticks attaching to the birds at sites in the United States where infection prevalence is low, e.g., some areas of the Mississippi and Ohio valleys rather than in highly endemic areas of the northeastern United States, and/or (ii) infected ticks acquired in the northeastern United States being cleared of B. burgdorferi infections while feeding on the birds, as has been recorded when infected ticks feed on gray catbirds (46).
The latter hypothesis is more consistent with the following: (i) current knowledge on bird migration routes, (ii) geographic uniformity of B. burgdorferi infection prevalence in I. scapularis ticks submitted in passive surveillance from Ontario to the Atlantic Provinces in Canada, and (iii) an infection prevalence in these ticks that was at most one-half that commonly detected in questing nymphal I. scapularis in the northeastern United States (39).
Either way, how B. burgdorferi becomes established in newly established I. scapularis populations requires further study: any infective nymphs that survive to be adult females would be unlikely to feed on reservoir hosts, such as rodents, because their preferred hosts are reservoir-incompetent deer (56). It is most likely that larvae infected by the birds are the main route of introduction of infection, because these would become host-seeking nymphs infective for rodents and other competent reservoirs. However, there was little evidence that any of the birds in our sample were infective, and they carried few larvae, which are mostly active in late summer/early autumn when birds are migrating south. Northward-migrating birds in this case appear to be an inefficient means of introduction of infection, and consistent with this, field observations suggest that successful establishment of B. burgdorferi infection in a tick-host cycle may follow by years the establishment of the I. scapularis population itself at a new locality (e.g., Point Pelee, Ontario [L. R. Lindsay, unpublished data]).
Ticks from the migratory birds carried a wide range of alleles of the ospC and rrs-rrl IGS loci, including those associated with disseminated Lyme disease (51, 60, 62). The relative frequency of ospC and IGS alleles was somewhat different from that found in questing nymphal I. scapularis in recent studies in the northeastern United States (6, 20) and in infected rodents in Maryland (3). Alleles that were common in those studies were less dominant in the study of Qiu et al. (45) of archived ticks collected during the 1990s, suggesting that temporal shifts or geographic heterogeneity in the population structure of the bacterium may occur. In our study these common alleles were also less dominant, but alleles of ospC that are particularly rare in any study to date (L and O) were encountered in several ticks in our study. These differences in allele frequencies could be due to geographic or temporal variations in the B. burgdorferi population structure, but our findings could also support the hypothesis that some selection of B. burgdorferi genotypes may have occurred while the ticks fed on the birds. Further studies are needed to understand the role of birds in generating or maintaining genetic diversity in B. burgdorferi, the geographic and temporal variations in population structure, and any consequences for clinical presentation and diagnosis of Lyme borreliosis (60, 62).
The prevalence of infection by A. phagocytophilum in I. scapularis ticks carried on the birds was consistent with that found in questing nymphs in some studies in the northeastern United States (1, 27).
We have established estimates for the prevalence of northward-migrating land birds that carry I. scapularis ticks northward into Canada. Our study suggests that many bird species carry I. scapularis into Canada, and they are very likely to be important in expanding the northern geographic range of I. scapularis across a wide front in northeastern and north-central provinces. The ticks that these birds carry are infected with a wide range of genotypes of B. burgdorferi, including those that cause disseminated Lyme disease, but also with less common genotypes. The ticks are also infected with A. phagocytophilum. Clinicians should be aware that adventitious ticks, carried by the birds into a wide area of Canada, can be a source of human cases of Lyme disease and HGA, although the risk of infection is likely low for the following reasons: (i) their densities are likely to be low compared to that where reproducing populations of I. scapularis occur, and (ii) adult ticks are less likely to transmit Lyme disease than nymphs, because their larger size means they are more readily identified and removed when feeding on people, and thus, prevention of B. burgdorferi transmission is more likely (15, 39). The role of migratory birds in the introduction of B. burgdorferi into recently established I. scapularis populations is less certain, because (i) the birds carry few larvae, (ii) the birds do not seem to greatly amplify infection in the ticks they carry, and (iii) the birds may acquire ticks mostly from regions where the B. burgdorferi infection prevalence is low, or (iv) the birds are generally zooprophylactic, reducing infection prevalence in the ticks they introduce. Further studies are needed to understand the dynamic of introduction of B. burgdorferi into I. scapularis populations in Canada and the consequences of these processes for public health and control of Lyme disease.
Acknowledgments
This study was funded by the Climate Change Impacts and Adaptation Programme of Natural Resources Canada and by National Institutes of Health grant AR41511 to Ira Schwartz.
We sincerely thank the staff and volunteers at the Canadian Migration Monitoring Network stations who participated in the field work for this project (see Fig. 1).
Footnotes
Published ahead of print on 1 February 2008.
REFERENCES
- 1.Adelson, M. E., R. V. Rao, R. C. Tilton, K. Cabets, E. Eskow, L. Fein, J. L. Occi, and E. Mordechai. 2004. Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis ticks collected in Northern New Jersey. J. Clin. Microbiol. 42:2799-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. F., R. C. Johnson, L. A. Magnarelli, and F. W. Hyde. 1986. Involvement of birds in the epidemiology of the Lyme disease agent Borrelia burgdorferi. Infect. Immun. 51:394-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, J. M., and D. E. Norris. 2006. Genetic diversity of Borrelia burgdorferi sensu stricto in Peromyscus leucopus, the primary reservoir of Lyme disease in a region of endemicity in southern Maryland. Appl. Environ. Microbiol. 72:5331-5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker, I. K., G. A. Surgeoner, H. Artsob, S. A. McEwen, L. A. Elliott, G. D. Campbell, and J. T. Robinson. 1992. Distribution of the Lyme disease vector, Ixodes dammini (Acari: Ixodidae) and isolation of Borrelia burgdorferi in Ontario, Canada. J. Med. Entomol. 29:1011-1022. [DOI] [PubMed] [Google Scholar]
- 5.Battaly, G. R., and D. Fish. 1993. Relative importance of bird species as hosts for immature Ixodes dammini (Acari: Ixodidae) in a suburban residential landscape of southern New York State. J. Med. Entomol. 30:740-747. [DOI] [PubMed] [Google Scholar]
- 6.Brisson, D., and D. E. Dykhuizen. 2004. OspC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168:713-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunikis, J., U. Garpmo, J. Tsao, J. Berglund, D. Fish, and A. G. Barbour. 2004. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology 150:1741-1755. [DOI] [PubMed] [Google Scholar]
- 8.Caporale, D. A., C. M. Johnson, and B. J. Millard. 2005. Presence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) in southern Kettle Moraine State Forest, Wisconsin, and characterization of strain W97F51. J. Med. Entomol. 42:457-472. [DOI] [PubMed] [Google Scholar]
- 9.Carey, A. B., W. L. Krinsky, and A. J. Main. 1980. Ixodes dammini (Acari: Ixodidae) and associated ixodid ticks in South-central Connecticut, USA. J. Med. Entomol. 17:89-99. [DOI] [PubMed] [Google Scholar]
- 10.Clifford, C. M., G. Anastos, and A. Elbl. 1961. The larval ixodid ticks of the eastern United States. Misc. Publ. Entomol. Soc. Am. 2:215-244. [Google Scholar]
- 11.Comstedt, P., S. Bergstrom, B. Olsen, U. Garpmo, L. Marjavaara, H. Mejlon, A. G. Barbour, and J. Bunikis. 2006. Migratory passerine birds as reservoirs of Lyme borreliosis in Europe. Emerg. Infect. Dis. 12:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courtney, J. W., L. M. Kostelnik, N. S. Zeidner, and R. F. Massung. 2004. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J. Clin. Microbiol. 42:3164-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniels, T. J., T. M. Boccia, S. Varde, J. Marcus, J. Le, D. J. Bucher, R. C. Falco, and I. Schwartz. 1998. Geographic risk for Lyme disease and human granulocytic ehrlichiosis in southern New York State. Appl. Environ. Microbiol. 64:4663-4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennis, D. T., T. S. Nekomoto, J. C. Victor, W. S. Paul, and J. Piesman. 1998. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J. Med. Entomol. 35:629-638. [DOI] [PubMed] [Google Scholar]
- 15.des Vignes, F., J. Piesman, R. Heffernan, T. L. Schulze, K. C. Stafford III, and D. Fish. 2001. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J. Infect. Dis. 183:773-778. [DOI] [PubMed] [Google Scholar]
- 16.Dunn, E. H. 2002. A cross-Canada comparison of mass change in birds during migration stopover. Wilson Bull. 114:368-379. [Google Scholar]
- 17.Guerra, M., E. Walker, C. Jones, S. Paskewitz, M. R. Cortinas, A. Stancil, L. Beck, M. Bobo, and U. Kitron. 2002. Predicting the risk of Lyme disease: habitat suitability for Ixodes scapularis in the north central United States. Emerg. Infect. Dis. 8:289-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gylfe, A., S. Bergström, J. Lundström, and B. Olsen. 2000. Reactivation of Borrelia infection in birds. Nature 403:724-725. [DOI] [PubMed] [Google Scholar]
- 19.Hanincová, K., V. Taragelová, J. Koci, S. M. Schäfer, R. Hails, A. J. Ullmann, J. Piesman, M. Labuda, and K. Kurtenbach. 2003. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl. Environ. Microbiol. 69:2825-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanincová, K., K. Kurtenbach, M. Diuk-Wasser, B. Brei, and D. Fish. 2006. Epidemic spread of Lyme borreliosis, northeastern United States. Emerg. Infect. Dis. 12:604-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubálek, Z. 2004. An annotated checklist of pathogenic microorganisms associated with migratory birds. J. Wildl. Dis. 40:639-659. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman, K. 1996. Lives of North American birds. Peterson Natural History Companions, Houghton Mifflin Company, Boston, MA.
- 23.Keirans, J. E., H. J. Hutcheson, L. A. Durden, and J. S. H. Klompen. 1996. Ixodes (Ixodes) scapularis (Acari: Ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J. Med. Entomol. 33:297-318. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy, J. A., P. Dilworth-Christie, and A. J. Erskine. 1999. The Canadian breeding bird (mapping) census database. Technical report series no. 342. Canadian Wildlife Service, Environment Canada, Ottawa, Canada.
- 25.Klich, M., M. W. Lankester, and K. W. Wu. 1996. Spring migratory birds (Aves) extend the northern occurrence of blacklegged tick (Acari: Ixodidae). J. Med. Entomol. 33:581-585. [DOI] [PubMed] [Google Scholar]
- 26.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 27.Levin, M. L., F. des Vignes, and D. Fish. 1999. Disparity in the natural cycles of Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis. Emerg. Infect. Dis. 5:204-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindsay, L. R. 1995. Ph.D. thesis. University of Guelph, Guelph, Ontario, Canada.
- 29.Lindsay, L. R., S. W. Mathison, I. K. Barker, S. A. McEwen, and G. A. Surgeoner. 1999. Abundance of Ixodes scapularis (Acari: Ixodidae) larvae and nymphs in relation to host density and habitat on Long Point, Ontario. J. Med. Entomol. 36:243-254. [DOI] [PubMed] [Google Scholar]
- 30.Lindsay, R., H. Artsob, and I. Barker. 1998. Distribution of Ixodes pacificus and Ixodes scapularis re concurrent babesiosis and Lyme disease. Can. Commun. Dis. Rep. 24:121-122. [PubMed] [Google Scholar]
- 31.Liveris, D., S. Varde, R. Iyer, S. Koenig, S. Bittker, D. Cooper, D. McKenna, J. Nowakowski, R. B. Nadelman, G. P. Wormser, and I. Schwartz. 1999. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J. Clin. Microbiol. 37:565-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madhav, N. K., J. S. Brownstein, J. I. Tsao, and D. Fish. 2004. A dispersal model for the range expansion of blacklegged tick (Acari: Ixodidae). J. Med. Entomol. 41:842-852. [DOI] [PubMed] [Google Scholar]
- 33.Marra, P. P., C. M. Francis, R. S. Mulvihill, and F. R. Moore. 2005. The influence of climate on the timing and rate of spring bird migration. Oecologia 142:307-315. [DOI] [PubMed] [Google Scholar]
- 34.May, W. L., and W. D. Johnson. 1997. Confidence intervals for differences in correlated binary proportions. Stat. Med. 16:2127-2136. [DOI] [PubMed] [Google Scholar]
- 35.Morshed, M. G., J. D. Scott, K. Fernando, L. Beati, D. F. Mazerolle, G. Geddes, and L. A. Durden. 2005. Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J. Parasitol. 91:780-790. [DOI] [PubMed] [Google Scholar]
- 36.Neuhaus, J. M. 1992. Statistical methods for longitudinal and clustered designs with binary responses. Stat. Methods Med. Res. 1:249-273. [DOI] [PubMed] [Google Scholar]
- 37.Nicholls, T. H., and S. M. Callister. 1996. Lyme disease spirochaetes in ticks collected from birds in Midwestern United States. J. Med. Entomol. 33:379-384. [DOI] [PubMed] [Google Scholar]
- 38.Ogden, N. H., M. Bigras-Poulin, C. J. O'Callaghan, I. K. Barker, L. R. Lindsay, A. Maarouf, K. E. Smoyer-Tomic, D. Waltner-Toews, and D. Charron. 2005. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int. J. Parasitol. 35:375-389. [DOI] [PubMed] [Google Scholar]
- 39.Ogden, N. H., L. Trudel, H. Artsob, I. K. Barker, G. Beauchamp, D. Charron, M. A. Drebot, T. D. Galloway, R. O'Handley, R. A. Thompson, and L. R. Lindsay. 2006. Ixodes scapularis ticks collected by passive surveillance in Canada: analysis of geographic distribution and infection with the Lyme borreliosis agent Borrelia burgdorferi. J. Med. Entomol. 43:600-609. [DOI] [PubMed] [Google Scholar]
- 40.Ogden, N. H., A. Maarouf, I. K. Barker, M. Bigras-Poulin, L. R. Lindsay, M. G. Morshed, C. J. O'Callaghan, F. Ramay, D. Waltner-Toews, and D. F. Charron. 2006. Projections for range expansion of the Lyme disease vector Ixodes scapularis, in response to climate change. Int. J. Parasitol. 36:63-70. [DOI] [PubMed] [Google Scholar]
- 41.Parmesan, C., and G. Yohe. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37-42. [DOI] [PubMed] [Google Scholar]
- 42.Paskewitz, S. M., M. Vandermause, E. A. Belongia, and J. J. Kazmierczak. 2001. Ixodes scapularis (Acari: Ixodidae): abundance and rate of infection with Borrelia burgdorferi in four state parks in Wisconsin. J. Med. Entomol. 38:33-38. [DOI] [PubMed] [Google Scholar]
- 43.Poole, A. (ed.). 2005. The birds of North American online. Cornell Laboratory of Ornithology, Ithaca, NY. http://bna.birds.cornell.edu/BNA/.
- 44.Posada, D., and K. A. Crandall. 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14:817-818. [DOI] [PubMed] [Google Scholar]
- 45.Qiu, W. G., D. E. Dykhuizen, M. S. Acosta, and B. J. Luft. 2002. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the Northeastern United States. Genetics 160:833-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rand, P. W., E. H. Lacombe, R. P. Smith, Jr., and J. Ficker. 1998. Participation of birds (Aves) in the emergence of Lyme disease in Southern Maine. J. Med. Entomol. 35:270-276. [DOI] [PubMed] [Google Scholar]
- 47.Richter, D., A. Spielman, N. Komar, and F. R. Matuschka. 2000. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 6:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Root, T. L., J. T. Price, K. R. Hall, S. H. Schneider, C. Rosenzweig, and J. A. Pounds. 2003. Fingerprints of global warming on wild animals and plants. Nature 421:57-60. [DOI] [PubMed] [Google Scholar]
- 49.Scharf, W. 2004. Immature ticks on birds; temporal abundance and reinfestation. Northeast. Nat. 11:143-150. [Google Scholar]
- 50.Scott, J. D., K. Fernando, S. N. Banerjee, L. A. Durden, S. K. Byrne, M. Banerjee, R. B. Mann, and M. G. Morshed. 2001. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J. Med. Entomol. 38:493-500. [DOI] [PubMed] [Google Scholar]
- 51.Seinost, G., D. E. Dykhuizen, R. J. Dattwyler, W. T. Golde, J. J. Dunn, I. N. Wang, G. P. Wormser, M. E. Schriefer, and B. J. Luft. 1999. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect. Immun. 67:3518-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sibley, D. 2000. The North American bird guide. Pica Press, East Sussex, United Kingdom.
- 53.Smith, R. P., Jr., P. W. Rand, E. H. Lacombe, S. R. Morris, D. W. Holmes, and D. A. Caporale. 1996. Role of bird migration in the long-distance dispersal of Ixodes dammini, the vector of Lyme disease. J. Infect. Dis. 174:221-224. [DOI] [PubMed] [Google Scholar]
- 54.Stafford, K. C., III, V. C. Bladen, and L. A. Magnarelli. 1995. Ticks (Acari: Ixodidae) infesting wild birds (Aves) and white-footed mice in Lyme, CT. J. Med. Entomol. 32:453-466. [DOI] [PubMed] [Google Scholar]
- 55.Swofford, D. L. 2002. PAUP: phylogenetic analysis using parsimony (and other methods) 4.0 beta. Sinauer Associates, Sunderland, MA.
- 56.Thompson, C., A. Spielman, and P. J. Krause. 2001. Coinfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clin. Infect. Dis. 33:676-685. [DOI] [PubMed] [Google Scholar]
- 57.Tsao, J. I., J. T. Wootton, J. Bunikis, M. G. Luna, D. Fish, and A. G. Barbour. 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl. Acad. Sci. USA 101:18159-18164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker, E. D., T. W. Smith, J. DeWitt, D. C. Beaudo, and R. G. MLean. 1994. Prevalence of Borrelia burgdorferi in host-seeking ticks (Acari: Ixodidae) from a Lyme disease endemic area in northern Michigan. J. Med. Entomol. 31:524-528. [DOI] [PubMed] [Google Scholar]
- 59.Walker, E. D., M. G. Stobierski, M. L. Poplar, T. W. Smith, A. J. Murphy, P. C. Smith, S. M. Schmitt, T. M. Cooley, and C. M. Kramer. 1998. Geographic distribution of ticks (Acari: Ixodidae) in Michigan, with emphasis on Ixodes scapularis and Borrelia burgdorferi. J. Med. Entomol. 35:872-882. [DOI] [PubMed] [Google Scholar]
- 60.Wang, G., C. Ojaimi, H. Wu, V. Saksenberg, R. Lyer, D. Liveris, S. A. McClain, G. P. Wormser, and I. Schwartz. 2002. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J. Infect. Dis. 186:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weisbrod, A. R., and R. C. Johnson. 1989. Lyme disease and migrating birds in the Saint Croix River Valley. Appl. Environ. Microbiol. 55:1921-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wormser, G. P., D. Liveris, J. Nowakowski, R. B. Nadelman, L. F. Cavaliere, D. McKenna, D. Holmgren, and I. Schwartz. 1999. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J. Infect. Dis. 180:720-725. [DOI] [PubMed] [Google Scholar]