Abstract
Leukocyte interactions with vascular endothelium during inflammation occur through discrete steps involving selectin-mediated leukocyte rolling and subsequent firm adhesion mediated by members of the integrin and Ig families of adhesion molecules. To identify functional synergy between selectin and Ig family members, mice deficient in both L-selectin and intercellular adhesion molecule 1 (ICAM-1) were generated. Leukocyte rolling velocities in cremaster muscle venules were increased significantly in ICAM-1-deficient mice during both trauma- and tumor necrosis factor α-induced inflammation, but rolling leukocyte flux was not reduced. Elimination of ICAM-1 expression in L-selectin-deficient mice resulted in a sharp reduction in the flux of rolling leukocytes during tumor necrosis factor α-induced inflammation. The observed differences in leukocyte rolling behavior demonstrated that ICAM-1 expression was required for optimal P- and L-selectin-mediated rolling. Increased leukocyte rolling velocities presumably translated into decreased tissue emigration because circulating neutrophil, monocyte, and lymphocyte numbers were increased markedly in L-selectin/ICAM-1-deficient mice. Furthermore, neutrophil emigration during acute peritonitis was reduced by 80% in the double-deficient mice compared with either L-selectin or ICAM-1-deficient mice. Thus, members of the selectin and Ig families function synergistically to mediate optimal leukocyte rolling in vivo, which is essential for the generation of effective inflammatory responses.
The recruitment of leukocytes into sites of acute and chronic inflammation involves leukocyte interactions with vascular endothelium under conditions of shear flow. Leukocyte-endothelial cell interactions are envisioned as a series of discrete steps using distinct constitutive or inducible adhesion molecules. Specifically, it is thought that the selectins mediate leukocyte capture and rolling along the vessel wall whereas interactions between integrins and Ig superfamily members arrest rolling cells and mediate firm adhesion that leads to migration into sites of inflammation (1–3). L-selectin (CD62L) is expressed constitutively by most leukocytes whereas P-selectin (CD62P) and E-selectin (CD62E) are expressed by activated endothelial cells and primarily mediate neutrophil and monocyte rolling (4). In addition, leukocytes express the β2 integrins, including lymphocyte function-associated antigen 1 (CD11a/CD18), which interact with members of the Ig superfamily expressed by activated endothelial cells including intercellular adhesion molecule 1 (ICAM-1) (CD54). Although lymphocyte function-associated antigen 1/ICAM-1 interactions do not support leukocyte rolling, recent studies have shown that, under in vitro conditions of low shear flow, α4β1 (CD49d/CD29) and α4β7 integrins, as well as hyaluronan receptors (CD44) expressed by leukocytes, can mediate rolling (5–8). The in vivo relevance of these observations is unknown.
The generation of gene-targeted mice deficient in expression of L-, P-, or E-selectin has provided considerable insight into the molecular interactions that occur during inflammation in vivo (9–11). L-selectin-deficient (L-selectin−/−) mice have decreased trauma-induced rolling of leukocytes in the exteriorized mesentery and have decreased rolling in cremaster muscle venules after tumor necrosis factor (TNF)-α treatment (9, 12, 13). Leukocyte recruitment into sites of inflammation is decreased markedly in L-selectin−/− mice (9, 14, 15). P-selectin−/− mice display decreased trauma-induced leukocyte rolling in the exteriorized mesentery at early time points and decreased leukocyte entry early during inflammatory responses (11–13, 16). E-selectin−/− mice have increased leukocyte rolling flux fractions (number of rolling leukocytes expressed as a percent of all leukocytes traveling through each microvessel) and increased leukocyte rolling velocities in cremaster venules after TNF-α treatment (12). Although several inflammatory responses are normal in E-selectin−/− mice, treatment with a P-selectin function-blocking mAb dramatically reduces inflammation in these mice when compared with similarly treated wild-type mice (10). Mice deficient in both E- and P-selectin display a virtual absence of leukocyte rolling for at least 3 hr after TNF-α treatment (17) but have normal neutrophil emigration after 24 hr of peritonitis (17, 18). Taken together, these findings demonstrate that, although L-, P-, and E-selectin have distinct roles, the selectins support optimal leukocyte rolling during inflammation through synergistic and overlapping functions.
ICAM-1 is constitutively expressed by endothelial cells and is up-regulated rapidly during inflammation, resulting in increased leukocyte–endothelial cell adhesion (19). ICAM-1−/− mice have significantly reduced numbers of infiltrating neutrophils during some inflammatory responses (14, 20, 21). ICAM-1 interactions with β2 integrins do not support leukocyte rolling in vitro (22, 23), and both trauma- and TNF-α-induced leukocyte rolling flux is normal in exteriorized cremaster muscle venules of ICAM-1−/− mice (24). Although mean leukocyte rolling velocities in ICAM-1−/− mice are similar to those of wild-type mice over a 2-hr time period (24), leukocyte rolling velocities have not been examined in detail in these mice. Recent studies in P-selectin/ICAM-1−/− mice suggest a complex role for ICAM-1 in leukocyte rolling when P-selectin is absent (24, 25). P-selectin/ICAM-1−/− mice display a profound decrease in trauma-induced leukocyte rolling that persists much longer than in mice deficient in P-selectin alone (24). This results in an almost complete lack of neutrophil emigration into an inflamed peritoneum at early time points (25). The significant reduction of rolling in P-selectin/ICAM-1−/− mice therefore prompted us to determine the mechanism by which ICAM-1 absence affects leukocyte rolling and inflammation.
To further define the cascade of interactions among families of adhesion molecules during leukocyte–endothelial cell interactions in vivo, mice deficient in both L-selectin and ICAM-1 were generated. The phenotype of these mice demonstrates a physiologically significant role for ICAM-1 in regulating leukocyte rolling flux fractions and rolling velocities at sites of inflammation.
MATERIALS AND METHODS
Animals.
Mice deficient in L-selectin were produced as described (9). ICAM-1−/− mice (20) were obtained from The Jackson Laboratory. These ICAM-1−/− mice express residual amounts of ICAM-1 splice variants in the thymus and spleen but not in other organs (26). Mice lacking both L-selectin and ICAM-1 were generated by crossing F1 offspring from crosses of homozygous L-selectin−/− mice with homozygous ICAM-1−/− mice. Lack of L-selectin surface expression was confirmed by direct immunofluorescence staining of blood leukocytes with the LAM1-116 antibody (27). Presence of the mutated ICAM-1 gene was verified by PCR analysis of DNA from tail biopsies. The L-selectin/ICAM-1−/− mice were healthy and fertile and did not display any evidence of infection or disease. All mice were backcrossed between 5 and 10 generations onto the C57BL/6 background. Mice were 7–12 weeks old for all experiments, and age-matched wild-type littermates or C57BL/6 mice (The Jackson Laboratory) were used as controls. All mice were housed in a specific pathogen-free barrier facility and were screened regularly for pathogens. All studies and procedures were approved by the Animal Care and Use Committee of Duke University Medical Center and the Animal Research Committee of the University of Virginia.
Intravital Microscopy.
Mice were anesthetized with an 100-mg/kg i.p. injection of ketamine hydrochloride (Ketalar, Parke-Davis) after pretreatment with sodium pentobarbital (30 mg/kg i.p., Nembutal, Abbott) and atropine (0.1 mg/kg i.p., Elkins-Sinn, Cherry Hill, NJ). The mice were kept at 37°C and received ≈0.2 ml/hr diluted pentobarbital in saline i.v. to maintain anesthesia and a neutral fluid balance. For TNF-α studies, mice were pretreated with 0.5 μg of TNF-α (Genzyme) in 0.30 ml of isotonic saline injected intrascrotally 2.5 hr before surgery. The cremaster muscle was prepared for intravital microscopy as described (12, 24). In brief, the epididymis and testis were gently pinned to the side to expose the well-perfused cremaster microcirculation. The cremaster muscle was superfused with thermocontrolled (37°C) bicarbonate-buffered saline. Blood samples (10 μl each) were taken throughout the experiment from the carotid catheter at ≈45 min intervals to analyze systemic leukocyte concentrations. Differential leukocyte counts were obtained by Kimura stain of the blood samples.
Microscopic observations were made on an intravital microscope (Axioskop, Zeiss) with a saline immersion objective (SW 40/0.75 numerical aperture). Venules with diameters between 15 and 50 μm were observed, and video recordings were made through a charge-coupled device camera system (model VE-1000CD, Dage–MTI, Michigan City, IN) on a Panasonic S-VHS recorder (Panasonic, Secausus, NJ). Microvascular centerline red blood cell velocity was measured by using a dual photodiode and a digital on-line cross-correlation program (28). Centerline velocities were converted to mean blood flow velocities by multiplying with an empirical factor of 0.625 (29). Wall shear rates were estimated as 2.12 (8Vb/dvessel), where Vb is the mean blood flow velocity, dvessel is the in vivo diameter of the vessel, and 2.12 is a median empirical correction factor obtained from actual velocity profiles measured in microvessels in vivo (30). To reduce the influence of hemodynamic variables on leukocyte rolling velocities, only venules with calculated wall shear rates between 300 and 1000 s−1 were analyzed. Rolling velocity varies little over this range of shear rates (31).
Microvessel diameter and individual rolling leukocyte velocity were measured by using a digital image processing system. Freeze frame advancing was used to accurately monitor the movements of the individual rolling leukocytes. Each rolling leukocyte passing a line perpendicular to the vessel axis was followed for ≈150 μm downstream. The difference between the end points of the traveled distance was measured either on the monitor with a caliper or by using a custom digital image processing system (28). Rolling velocities for individual leukocytes were calculated by dividing this distance by the elapsed time period. Rolling leukocyte flux was determined by counting the number of leukocytes passing through each venule as described (9, 32, 33). Total leukocyte flux was estimated as the product of measured systemic leukocyte concentration and blood volume flow. Leukocyte rolling flux fraction is defined as the flux of rolling leukocytes as a percent of total leukocyte flux. By definition, leukocyte rolling flux fraction is independent of variations in systemic leukocyte counts.
Thioglycollate-Induced Peritonitis.
Thioglycollate solution (1 ml; 3% wt/vol; Sigma) was injected i.p. into mice and the leukocyte infiltrate was recovered by peritoneal lavage as described (15).
Statistical Analysis.
All data are shown as mean values ± SEM. ANOVA followed by Student’s t test was used to determine the level of significance of differences in population means. Distributions of leukocyte rolling velocities were compared by using a Kruskal–Wallis Multiple-Comparison Z-Value test with Bonferroni correction.
RESULTS
Phenotype of L-selectin/ICAM-1−/− Mice.
Mice deficient in L-selectin and ICAM-1 were bred to generate L-selectin/ICAM-1−/− mice homozygous at both loci. There were no obvious indications of pathology or disease susceptibility for any of the mice up to 1 year of age. A consistent finding was that both ICAM-1−/− and L-selectin/ICAM-1−/− mice were ≈20% larger and heavier than age-matched wild-type or L-selectin−/− mice (D.A.S. and T.F.T., unpublished observations).
The loss of both L-selectin and ICAM-1 led to elevated numbers of circulating neutrophils (580% of wild-type, P < 0.001), lymphocytes (200%, P < 0.001), and monocytes (640%, P < 0.001; Table 1). Mice deficient in L-selectin alone had significantly increased numbers of circulating monocytes (146% increase) whereas the loss of ICAM-1 significantly increased the numbers of circulating neutrophils by 190%, lymphocytes by 47%, monocytes by 223%, and eosinophils by 178% relative to wild-type littermates (Table 1) as published (20, 34). Eosinophil numbers were increased to a similar extent in both ICAM-1−/− and L-selectin/ICAM-1−/− mice.
Table 1.
Numbers of blood leukocytes in L-selectin/ICAM-1-deficient mice
| Cell lineage | No. of cells/ml (×10−5)
|
|||
|---|---|---|---|---|
| Wild type | L-selectin−/− | ICAM-1−/− | L-selectin/ICAM-1−/− | |
| Total | 41 ± 5 | 51 ± 8 | 75 ± 7* | 115 ± 8*†‡ |
| Neutrophil | 6.2 ± 1.1 | 8.6 ± 2.3 | 18 ± 3* | 36 ± 4*†‡ |
| Lymphocyte | 34 ± 4 | 39 ± 7 | 50 ± 6* | 68 ± 5*†‡ |
| Monocyte | 1.3 ± 0.3 | 3.2 ± 0.9* | 4.2 ± 0.7* | 8.3 ± 1.1*†‡ |
| Eosinophil | 0.9 ± 0.2 | 0.8 ± 0.5 | 2.5 ± 0.6* | 3.0 ± 0.4*† |
Differential leukocyte counts were performed on blood smear preparations following Wright–Giemsa staining. Values represent the mean ± SEM results from 13 wild-type, 9 L-selectin−/−, 23 ICAM-1−/−, and 37 L-selectin/ICAM-1−/− mice.
Differences between test and wild-type mice were significant, P < 0.05.
Differences between L-selectin−/− and L-selectin/ICAM-1−/− mice were significant, P < 0.01.
Differences between ICAM-1−/− and L-selectin/ICAM-1−/− mice were significant, P < 0.05.
Leukocyte Rolling.
The rolling behavior of leukocytes in L-selectin/ICAM-1−/− mice was assessed in venules of the cremaster muscle after surgical exteriorization. Surgical exteriorization initiates a mild inflammatory response resulting in leukocyte rolling within the venules but limited firm adhesion or transmigration of leukocytes. Estimated wall shear rates, microvessel diameters, and centerline blood flow velocities were similar among the four groups of experimental animals (Table 2 and data not shown). The properties of 65–365 rolling leukocytes were assessed in 13–73 venules of each mouse line at time points between 10 and 120 min after exteriorization of the cremaster muscle. The rolling flux fractions of leukocytes in cremaster venules of L-selectin/ICAM-1−/− mice were 35 ± 1% (mean ± SEM, 24 venules in seven mice) at times <30 min and 41 ± 1% at times >60 min (68 venules in seven mice). These values were within the normal range as also reported for ICAM-1−/− (t < 60 min, 50 ± 4%; t > 60 min, 47 ± 4%) and wild-type (≈45% for both time periods) mice (24). However, these results are surprising given the ≈50% decrease in frequency of rolling leukocytes previously observed in L-selectin−/− mice relative to wild-type mice at time points beyond 30 min (13). That the rolling flux fraction of leukocytes in L-selectin/ICAM-1−/− mice was similar to wild-type levels may result from the 4- to 6-fold increase in numbers of circulating neutrophils observed in L-selectin/ICAM-1−/− mice relative to wild-type or L-selectin−/− mice (Table 1). Circulating leukocyte counts are factored into the equation used for determining rolling flux fractions, thus controlling for higher or lower systemic counts. Neutrophils normally represent a minority of the circulating leukocytes, but most rolling leukocytes under these conditions are neutrophils (16). Therefore, a relative increase in neutrophil numbers in L-selectin/ICAM-1−/− mice may account for the unaltered flux fraction.
Table 2.
Leukocyte rolling velocities and wall shear rates in mouse cremaster venules
| Early (<30 min after exteriorization) | ||||
|---|---|---|---|---|
| Wild type | L-selectin−/− | ICAM-1−/− | L-selectin/ICAM-1−/− | |
| Rolling vel. (μm/s) | 36 ± 0.2 (65) | 19 ± 0.1 (105)* | 47 ± 0.3 (95) | 40 ± 0.2 (120) |
| Wall shear rate (s−1) | 608 ± 19 (13) | 575 ± 8 (19) | 551 ± 8 (21) | 654 ± 8 (24) |
| Late (>60 min after exteriorization) | ||||
| Rolling vel. (μm/s) | 38 ± 0.1 (140) | 24 ± 0.1 (365)* | 58 ± 0.4 (100)‡ | 64 ± 0.1 (340)†§ |
| Wall shear rate (s−1) | 560 ± 2 (73) | 580 ± 8 (28) | 564 ± 11 (20) | 559 ± 3 (68) |
| TNF-α (3 hr) | ||||
| Rolling vel. (μm/s) | 4.7 ± 0.6 (247) | 6.4 ± 0.5 (164) | 8.8 ± 0.8 (105)‡¶ | 8.2 ± 0.6 (150)‡ |
| Wall shear rate (s−1) | 526 ± 4 (56) | 529 ± 65 (33) | 461 ± 42 (14) | 491 ± 36 (28) |
Mean ± SEM of (n) vessels or cells, respectively. vel. = velocity. The TNF-α data from wild-type and L-selectin−/− mice were published previously (12).
Significantly different from all other genotypes at the same time pont (P < 0.01).
Significantly different from the same genotype at the <30 min time pont (P < 0.01).
Significantly different from wild-type at the same time point (P < 0.01),
(P < 0.05).
Significantly different from L-selectin−/− mice (P < 0.05).
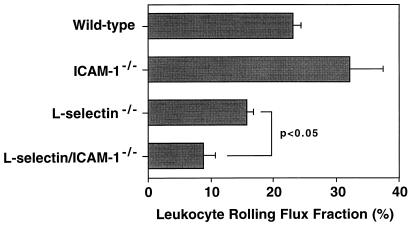
To determine the effects of L-selectin and ICAM-1 loss on leukocyte rolling during a more vigorous inflammatory response, adhesion molecule-deficient mice were pretreated with an intrascrotal injection of the pro-inflammatory cytokine TNF-α before the initiation of surgery. Hemodynamic parameters and estimated wall shear rates were similar among the four groups of experimental animals after TNF-α treatment (Table 2, and data not shown). There was a significant, 50% (P < 0.05) decrease in leukocyte rolling flux observed in L-selectin/ICAM-1−/− mice below the level seen in L-selectin−/− mice (Fig. 1). Consistent with published results (12, 24), the leukocyte rolling flux fraction was decreased by 34% in TNF-α-treated L-selectin−/− mice (P < 0.05) and was increased slightly in ICAM-1−/− mice relative to wild-type values (Fig. 1). Therefore, the combined loss of L-selectin and ICAM-1 significantly reduced the fraction of leukocytes rolling in response to TNF-induced inflammation.
Figure 1.
Leukocyte rolling flux fractions (percent of all leukocytes rolling) in cremaster venules of adhesion molecule-deficient mice after treatment with TNF-α. Mice were pretreated with an intrascrotal injection of TNF-α 2.5 hr before the initiation of surgery. Values represent mean rolling flux fractions ± SEM from 14–47 venules. The data from wild-type and L-selectin−/− mice are published (12). Differences between L-selectin−/− and L-selectin/ICAM-1−/− mice were significant (P < 0.05).
Leukocyte Rolling Velocities.
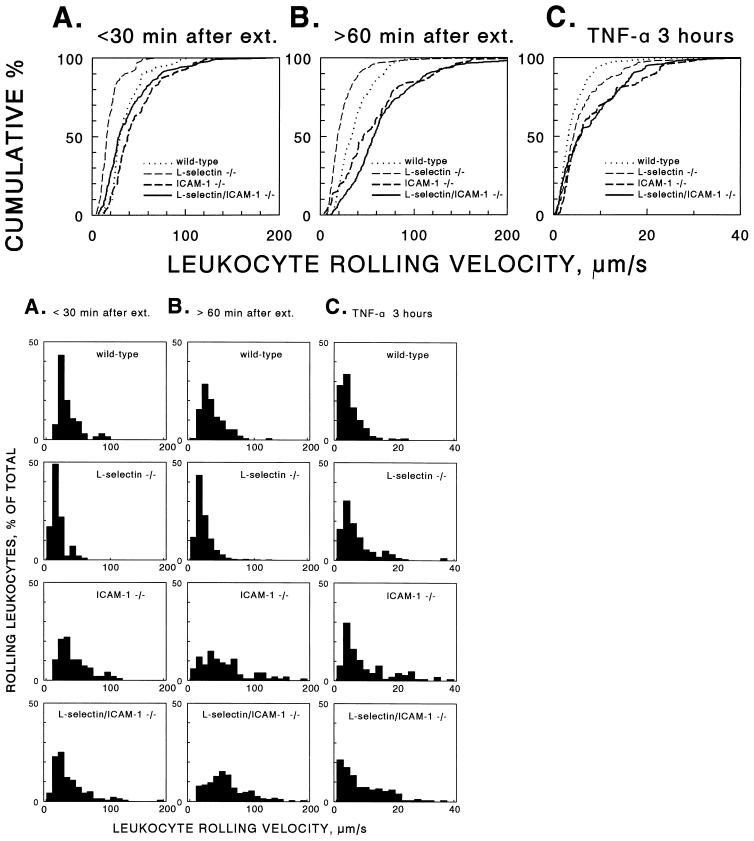
Leukocyte rolling velocities were measured to further investigate rolling behavior in ICAM-1−/− and L-selectin/ICAM-1−/− mice. During trauma-induced inflammation, P-selectin predominantly mediates rolling at early time points whereas L-selectin predominantly mediates rolling at later time points, as demonstrated by using L- and P-selectin-deficient mice (9, 16). Therefore, we grouped rolling velocity distributions of leukocytes into two time periods to reveal these differences: time points <30 min and time points >60 min. At early time points, leukocytes from ICAM-1−/− mice displayed slightly faster rolling velocities than wild-type mice, as indicated by a shift to the right of the cumulative velocity distribution (Fig. 2A, Table 2). Rolling velocities were significantly lower in L-selectin−/− mice at times <30 min (Fig. 2A). The molecular basis for this decrease in rolling velocity is that leukocyte rolling in L-selectin−/− mice completely depends on P-selectin under the conditions studied, as described (16). P-selectin mediates rolling at a slower characteristic velocity than L-selectin (16). The finding that rolling velocities in L-selectin/ICAM-1−/− mice were significantly higher than those of L-selectin−/− mice (Table 2) demonstrates that ICAM-1 is a necessary component for manifestation of the slower velocity of P-selectin-mediated rolling at early time points in vivo.
Figure 2.
Cumulative (Upper) and conventional histograms of the distribution of leukocyte rolling velocities in adhesion molecule-deficient mice. Rolling velocities were measured in hemodynamically similar cremaster venules after surgical exteriorization at times <30 min (A) and >60 min (B). (C) Mice also were pretreated with an intrascrotal injection of TNF-α 2.5 hr before the initiation of surgery. Cumulative frequency analysis (top row) ranks all individual cells analyzed in each mouse genotype from the cell with the highest rolling velocity (100%) to the lowest (<1%) on the y axis and plots these values as the percentage of cells that had a velocity lower than the value indicated on the x axis. Cumulative frequency analysis allows direct comparison between data sets and makes the distribution independent of bin size (Lower; 10-μm/s bins in A and B, 2-μm/s bins in C) used in conventional histograms. For numbers of venules and cells examined and statistical analysis of results see Table 2. The data from wild-type and L-selectin−/− mice treated with TNF-α are published (12).
Leukocyte rolling velocities in ICAM-1−/− mice at later times (t > 60 min) after trauma-induced inflammation were significantly faster than in wild-type mice, as indicated by a shift to the right of the cumulative velocity distribution (P < 0.05; Fig. 2B, Table 2). Because rolling at these time points is predominantly mediated by L-selectin with a contribution by P-selectin (9, 33), these results suggest that ICAM-1 expression also is required for optimal L-selectin-mediated rolling. Again, rolling velocities in L-selectin−/− mice at later time points were slower than in wild-type mice (Fig. 2B), which is consistent with the observation that residual rolling in L-selectin−/− mice is mediated by P-selectin under these conditions (16). However, rolling velocities in L-selectin/ICAM-1−/− mice were significantly higher (P < 0.01) than in wild-type or L-selectin−/− mice and higher than in ICAM-1−/− mice (Fig. 2B, Table 2). This finding again indicates that ICAM-1 expression retards the velocity of cells that roll via P-selectin. Moreover, the finding that rolling at later time points was affected more significantly (P < 0.01) by the combined loss of L-selectin and ICAM-1 than rolling at early time points indicates that the contribution of ICAM-1 to rolling is revealed most readily when the relative contribution of P-selectin to rolling is decreased. These differences in leukocyte rolling velocities verify that ICAM-1 expression is required for optimal P- and L-selectin-mediated rolling.
After TNF-α treatment, leukocyte rolling velocities were reduced 3- to 8-fold compared with untreated mice (Fig. 2C) as reported (12, 24). Nonetheless, differences in rolling velocities between groups of mice after TNF-α activation were similar to those observed for trauma-induced rolling. Among treated mice, rolling velocities were significantly higher in ICAM-1−/− mice compared with wild-type or L-selectin−/− mice (Table 2). As in all other groups, the combined loss of L-selectin and ICAM-1 did not alter the rolling velocity of leukocytes beyond that observed for ICAM-1 deficiency alone (Fig. 2C). These results directly demonstrate a role for ICAM-1 in the stabilization of leukocyte/endothelial cell interactions, which support leukocyte rolling at sites of inflammation.
Acute Neutrophil Emigration During Peritonitis.
The extent that increased velocity and/or decreased rolling flux fractions in L-selectin/ICAM-1−/− mice affected acute neutrophil entry into sites of inflammation was assessed in an experimental model of peritonitis. Small numbers of neutrophils were present in the peritoneum of all groups of mice before thioglycollate injection (Table 3). At 2 hr after thioglycollate injection, the vast majority of neutrophil entry into the inflamed peritoneum of L-selectin/ICAM-1−/− mice was inhibited compared with wild-type (by 92%, P < 0.007), ICAM-1−/− (by 80%, P < 0.001), and L-selectin−/− (by 83%, P < 0.05) mice (Table 3). L-selectin−/− and ICAM-1−/− mice each had significant reductions (55% and 61%, respectively) in the number of infiltrating neutrophils compared with wild-type mice (P < 0.05), as reported (9, 20). Thus, a concurrent L-selectin and ICAM-1 loss resulted in a far greater reduction of acute neutrophil transmigration than would have been predicted by an additive effect from loss of each individual adhesion molecule.
Table 3.
Emigration of neutrophils during thioglycollate-induced peritonitis
| Genotype | No. of neutrophils, ×10−4
|
|
|---|---|---|
| 0 hr | 2 hr | |
| Wild type | 0.9 ± 0.7 | 99.4 ± 23.5 |
| L-selectin−/− | 0.9 ± 0.9 | 44.8 ± 15.0* |
| ICAM-1−/− | 0.4 ± 0.4 | 38.3 ± 5.6* |
| L-selectin/ICAM-1−/− | <0.1 ± 0.1 | 7.8 ± 3.3*†‡ |
Values represent the mean ± SEM of results from 4–9 mice of each genotype at each time.
Differences between test and wild-type mice at 2 hr were significant, P < 0.05.
Differences between L-selectin−/− and L-selectin/ICAM-1−/− mice at 2 hr were significant, P < 0.05.
Differences between ICAM-1−/− and L-selectin/ICAM-1−/− mice at 2 hr were significant, P < 0.05.
DISCUSSION
The generation of mice deficient in both ICAM-1 and L-selectin has provided a powerful tool to further unravel the complex interactions that occur between the selectins and members of the Ig superfamily during leukocyte interactions with endothelial cells at sites of inflammation. Of primary importance are two findings. First, the frequency of rolling leukocytes in L-selectin−/− mice treated with TNF-α was decreased significantly by the additional loss of ICAM-1 expression (Fig. 1). This demonstrates a direct role for ICAM-1 in leukocyte rolling in inflamed vessels. Second, leukocyte rolling velocities were significantly greater in ICAM-1−/− mice than in wild-type mice (Fig. 2, Table 2). Greater leukocyte rolling velocities for ICAM-1−/− mice were notable at early time points (t < 30 min) and were quite significant at later time points (t > 60 min) after trauma-induced inflammation (Fig. 2 A and B, Table 2). Because P-selectin predominantly mediates trauma-induced rolling at early time points and both L-selectin and P-selectin mediate rolling at later time points in vivo (9, 16), the current studies indicate a role for ICAM-1 in regulating leukocyte rolling mediated by both P- and L-selectin. These results demonstrate that ICAM-1 expression influences selectin-mediated rolling of leukocytes in vivo, in addition to its well established roles in firm adhesion and transmigration of leukocytes at sites of inflammation (35).
The specific role of ICAM-1 during selectin-mediated rolling is clarified by comparing the phenotypes of ICAM-1−/− mice that also lack expression of either L- or P-selectin. Given that P-selectin primarily mediates rolling in untreated L-selectin−/− mice (13), the current findings in L-selectin/ICAM-1−/− mice indicate that leukocyte rolling through P-selectin does not absolutely require ICAM-1 expression. However, ICAM-1 expression was required for P-selectin to mediate rolling at characteristic velocities (Fig. 2). In P-selectin/ICAM-1−/− mice, rolling is absent for at least 2 hr after exteriorization of the cremaster muscle (24). Because L-selectin primarily mediates rolling in P-selectin−/− mice (13), the absence of rolling in P-selectin/ICAM-1−/− mice indicates that ICAM-1 expression is essential for leukocyte rolling through L-selectin. These conclusions are reinforced further by findings that P-selectin function-blocking antibodies inhibit leukocyte rolling flux by 88% in ICAM-1−/− mice after surgical trauma (24) but have little or no effect at time points >60 min in wild-type mice, a time period in which L-selectin predominantly mediates rolling (13). Conversely, blocking L-selectin function by a mAb significantly reduces the rolling flux fraction in wild-type mice (13) but has modest effects in ICAM-1−/− mice (24). Furthermore, L-selectin in combination with E-selectin supports rolling in P-selectin/ICAM-1−/− mice treated with TNF-α, but blocking E-selectin function in P-selectin/ICAM-1−/− or P-selectin−/− mice eliminates rolling (17, 24). Thus, L-selectin can only mediate leukocyte rolling in vivo when ICAM-1, P-selectin, E-selectin, or appropriate combinations of these receptors are expressed. By contrast, P-selectin can mediate leukocyte rolling in the absence of L-selectin and ICAM-1 expression, albeit at significantly faster velocities.
Studies (22) under in vitro conditions of shear flow have suggested that L-selectin ligands and ICAM-1 are engaged in series by leukocytes, rather than in parallel. However, the current in vivo results (Fig. 1, Table 2) clearly reveal that L-selectin and ICAM-1 function synergistically to mediate leukocyte endothelial interactions, including rolling. The requirement for ICAM-1 expression and function for optimal selectin-mediated rolling in vivo may relate to the relative densities of adhesion molecules and their ligands. Under physiologic conditions in vivo, relative receptor and/or ligand densities appropriately displayed on leukocytes or endothelial cells may be limiting for individual receptor/ligand pairs, thereby requiring cooperative interactions between groups of adhesion molecules. Although adhesion molecule requirements for optimal rolling may vary significantly between different vascular beds, the consequences from loss of both L-selectin and ICAM-1 were more than additive in the accumulation of neutrophils during acute peritonitis (Table 3). Thus, L-selectin interactions with a vascular ligand(s) may be insufficient to mediate stable leukocyte rolling under shear flow unless leukocyte interactions with vascular endothelial cells are supported by other adhesion molecules, such as ICAM-1 or P-selectin.
The generally accepted model of leukocyte-endothelial interactions suggests a multistep process, with each step mediated via different families of adhesion molecules. The current study demonstrates cooperation between the adhesion molecules that mediate leukocyte rolling and those that previously were presumed to mediate firm adhesion. Although the selectins are functionally dominant during rolling, ICAM-1/β2 integrin interactions stabilize selectin-mediated interactions between leukocytes and endothelial cells in vivo, allowing effective rolling and leukocyte entry into sites of inflammation. A requirement for ICAM-1 in stabilizing rolling may explain why anti-CD18 antibodies reduce leukocyte rolling in vivo at low shear rates (36). ICAM-1-mediated decreases in leukocyte rolling velocities are likely to increase the frequency of firm adhesions between leukocytes and endothelial cells in vivo (37, 38). Because the α4β7 and α4β1 integrins and CD44 can each support rolling in vitro under low shear flow conditions (6–8, 39), they also may contribute to leukocyte rolling in vivo and may regulate rolling velocities. Although integrin function predominates during firm adhesive interactions between cells, the selectins also may contribute to this process (40). Therefore, instead of rolling and firm adhesion representing separate processes mediated by different molecular mechanisms, rolling and firm adhesion are interrelated events mediated by interactions between numerous families of adhesion molecules. The present studies with L-selectin/ICAM-1−/− mice, along with previous studies with P-selectin/ICAM-1−/− mice (24, 25), support a paradigm in which selectins and ICAM-1 act through interdependent pathways to regulate leukocyte rolling, firm adhesion, and emigration.
Acknowledgments
We thank Dr. M. Tang, N. Green, and X-Q. Zhang for assistance with these experiments and the manuscript. This work was supported by National Institutes of Health Grants AI-26872, CA-54464, and HL-50985 (to T.F.T.) and HL-54136 (to K.L.). M.A.C. was supported by a stipend through a Special Opportunity Award of the Whitaker Foundation (to K.L.). D.A.S. was supported by National Institute of Allergy and Infectious Diseases–National Institutes of Health Training Program Grant AI-07217.
ABBREVIATIONS
- ICAM-1
intercellular adhesion molecule 1
- TNF-α
tumor necrosis factor α
References
- 1.Ley K, Tedder T F. J Immunol. 1995;155:525–528. [PubMed] [Google Scholar]
- 2.Butcher E C. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 3.Springer T A. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 4.Tedder T F, Steeber D A, Chen A, Engel P. FASEB J. 1995;9:866–873. [PubMed] [Google Scholar]
- 5.Alon R, Kassner P D, Carr M C, Finger E B, Hemler M E, Springer T A. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berlin C, Bargatze R F, Campbell J J, von Andrian U H, Szabo M C, Hasslen S R, Nelson R D, Berg E L, Erlandsen S L, Butcher E C. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 7.Clark R A, Alon R, Springer T A. J Cell Biol. 1996;134:1075–1087. doi: 10.1083/jcb.134.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeGrendele H C, Estess P, Picker L J, Siegelman M H. J Exp Med. 1996;183:1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbones M L, Ord D C, Ley K, Radich H, Maynard-Curry C, Capon D J, Tedder T F. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 10.Labow M A, Norton C R, Rumberger J M, Lombard-Gillooly K M, Shuster D J, Hubbard J, Bertko R, Knaack P A, Terry R W, Harbison M L, et al. Immunity. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 11.Mayadas T N, Johnson R C, Rayburn H, Hynes R O, Wagner D D. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 12.Kunkel E J, Ley K. Circ Res. 1996;79:1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- 13.Ley K E, Bullard D, Arbones M L, Bosse R, Vestweber D, Tedder T F, Beaudet A L. J Exp Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang M L K, Hale L P, Steeber D A, Tedder T F. J Immunol. 1997;158:5191–5199. [PubMed] [Google Scholar]
- 15.Tedder T F, Steeber D A, Pizcueta P. J Exp Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung U, Bullard D C, Tedder T F, Ley K. Am J Physiol. 1996;271:H2740–H2747. doi: 10.1152/ajpheart.1996.271.6.H2740. [DOI] [PubMed] [Google Scholar]
- 17.Bullard D C, Kunkel E J, Kubo H, Hicks M J, Lorenzo I, Doyle N A, Koerschuk C M, Ley K, Beaudet A L. J Exp Med. 1996;183:2329–2336. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frenette P S, Mayadas T N, Rayburn H, Hynes R O, Wagner D D. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 19.Dustin M L, Rothlein R, Bhan A K, Dinarello C A, Springer T A. J Immunol. 1986;137:245–253. [PubMed] [Google Scholar]
- 20.Sligh J E, Jr, Ballantyne C M, Rich S S, Hawkins H K, Smith C W, Bradley A, Beaudet A L. Proc Natl Acad Sci USA. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Gonzalo J A, St. Pierre Y, Williams I R, Kupper T S, Cotran R S, Springer T A, Guiterrez-Ramos J-C. J Exp Med. 1994;180:95–109. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence M B, Berg E L, Butcher E C, Springer T A. Eur J Immunol. 1995;25:1025–1031. doi: 10.1002/eji.1830250425. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence M B, Smith C W, Eskin S G, McIntire L V. Blood. 1990;75:227–237. [PubMed] [Google Scholar]
- 24.Kunkel E J, Jung U, Bullard D C, Norman K E, Wolitzky B A, Vestweber D, Beaudet A L, Ley K. J Exp Med. 1996;183:57–65. doi: 10.1084/jem.183.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bullard D C, Qin L, Lorenzo I, Quinlin W M, Doyle N A, Bosse R, Vestweber D, Doerschuk C M, Beaudet A L. J Clin Invest. 1995;95:1782–1788. doi: 10.1172/JCI117856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King P D, Sandberg E T, Selvakumar A, Fang P, Beaudet A L, Dupont B. J Immunol. 1996;154:6080–6093. [PubMed] [Google Scholar]
- 27.Steeber D A, Engel P, Miller A S, Sheetz M P, Tedder T F. J Immunol. 1997;159:952–963. [PubMed] [Google Scholar]
- 28.Pries A R. Int J Microcirc Clin Exp. 1988;7:327–345. [PubMed] [Google Scholar]
- 29.Lipowsky H H, Zweifach B W. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 30.Reneman R S, Woldhuis B, oude Egbrink M G A, Slaaf D W, Tangelder G J. In: Concentration and Velocity Profiles of Blood Cells in the Microcirculation. Hwang N H C, Turitto V T, Yen M R T, editors. New York: Plenum; 1992. p. 25. [Google Scholar]
- 31.Damiano E R, Westheider J, Tozeren A, Ley K. Circ Res. 1996;79:1122–1130. doi: 10.1161/01.res.79.6.1122. [DOI] [PubMed] [Google Scholar]
- 32.Ley K, Gaehtgens P. Circ Res. 1991;69:1034–1041. doi: 10.1161/01.res.69.4.1034. [DOI] [PubMed] [Google Scholar]
- 33.Ley K. Am J Physiol. 1994;267:H1017–H1023. doi: 10.1152/ajpheart.1994.267.3.H1017. [DOI] [PubMed] [Google Scholar]
- 34.Steeber D A, Green N E, Sato S, Tedder T F. J Immunol. 1996;157:1096–1106. [PubMed] [Google Scholar]
- 35.Argenbright L W, Letts L G, Rothlein R. J Leukocyte Biol. 1991;49:253–257. doi: 10.1002/jlb.49.3.253. [DOI] [PubMed] [Google Scholar]
- 36.Gaboury J P, Kubes P. Blood. 1994;83:345–350. [PubMed] [Google Scholar]
- 37.Gaboury J P, Anderson D C, Kubes P. Am J Physiol. 1994;266:H637–H642. doi: 10.1152/ajpheart.1994.266.2.H637. [DOI] [PubMed] [Google Scholar]
- 38.Kanwar S, Johnston B, Kubes P. Circ Res. 1995;77:879–887. doi: 10.1161/01.res.77.5.879. [DOI] [PubMed] [Google Scholar]
- 39.Alon R, Hammer D A, Springer T A. Nature (London) 1995;374:539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 40.Ley, K., Allietta, M., Bullard, D. C. & Morgan, S. J. (1998) Circ. Res., in press. [DOI] [PubMed]