Abstract
A quantitative real-time PCR assay targeting the pcrA gene, encoding the catalytic subunit of perchlorate reductase, detected pcrA genes from perchlorate-reducing bacteria in three different genera and from soil microbial communities. Partial pcrA sequences indicated differences in the composition of perchlorate-reducing bacterial communities following exposure to different electron donors.
Perchlorate (ClO4−) is a widespread environmental contaminant that disrupts thyroid gland function (11, 21). According to a recent U.S. Environmental Protection Agency report, contamination of groundwater, surface water, and soil by perchlorate has been detected in 35 states, with California reporting the largest number of detections (22). Bacterial strains capable of respiratory perchlorate reduction, a process that produces innocuous chloride, have been isolated from a variety of sources (4-6, 9, 15, 17, 24, 25, 28). Although these perchlorate-reducing bacteria (PRB) appear to be ubiquitous (5), our knowledge of their population dynamics in the environment is very limited.
Estimating the abundance and growth of PRB can be helpful in assessing the potential for and optimization of biological treatment, a promising technology for perchlorate remediation. Quantitative information, however, is limited mostly to pure-culture studies (9, 15, 25), with few techniques available for enumerating PRB within larger microbial communities. Existing culture-dependent most-probable-number methods require a several-month incubation time to develop estimates of numbers of PRB in soil and water samples (5, 27).
More rapid detection could be achieved by targeting functional genes common to this bacterial group, such as genes encoding perchlorate reductase (pcrABCD) (3) and chlorite dismutase (cld) (1, 2). These two enzymes catalyze reactions of perchlorate to chlorite (ClO2−) (8) and chlorite to chloride (23), respectively. A nested PCR assay targeting the cld gene has been developed and applied to environmental samples (2). However, the cld gene is not specific to PRB, because non-PRB such as chlorate (ClO3−)-reducing bacteria also possess cld genes (18, 26). Targeting the perchlorate reductase is more appropriate for detecting PRB, because the pcr gene appears to be present exclusively in PRB, and the enzyme catalyzes the rate-limiting step in perchlorate reduction (15). A slot-blot hybridization probe has been designed for the pcrA gene, which encodes the catalytic subunit of perchlorate reductase (3), but the method has not been applied to environmental samples.
This study aimed to design a real-time quantitative PCR (qPCR) assay, based on the pcrA gene, for quantitatively detecting PRB in environmental samples. To our best knowledge, this is the first report of qPCR assay developed to detect PRB. Partial pcrA sequences for PRB isolates and enrichment cultures were determined to collect more information about pcrA sequences, as well as to examine the members of communities associated with reduction of perchlorate.
Primer design.
To identify conserved regions, deduced PcrA protein sequences from Dechloromonas agitata strain CKB (GenBank accession AAO49008) and Dechloromonas aromatica strain RCB (AAZ47315; http://genome.jgi-psf.org/finished_microbes/decar/decar.home.html) were aligned using Clustal W (19) (Fig. 1). Several other molybdoenzyme sequences from the dimethyl sulfoxide (DMSO) reductase family were included in order to identify unique PcrA sequence regions (Fig. 1). This enzyme group includes those with important roles in anaerobic respiration, specifically, respiratory reduction of oxyanions, such as nitrate, selenate, arsenate, and chlorate, in addition to perchlorate (10). Studies of the diversity and abundance of respiratory nitrate-reducing bacteria possessing narG (13, 14) suggest that specific molybdoenzyme sequences can be selectively detected despite being members of a larger, broader superfamily of sequences. Similar approaches could be applied to studying the diversity and abundance of PRB.
FIG. 1.
Multiple-sequence alignment for perchlorate reductase and related molybdoenzymes. The region encompassed by the primers used for the qPCR assay is shown, and the residues within the PcrA protein sequences corresponding to the primer DNA sequences are boxed. Positions with identical residues in all seven sequences are shaded black, and positions with identical residues in all sequences except NarG are shaded gray. The conserved aspartyl residue that provides a side chain ligand to Mo in the molybdenum cofactor corresponds to NarG residue Asp-223 (7). The NarG sequence contains a 34-residue region that is not present in the other sequences (indicated by three dots). PcrA, perchlorate reductase (sequence 1, D. agitata CKB [GenBank accession number AY180108]; sequence 2, D. aromatica RCB [AAZ47315]); ClrA, chlorate reductase (Ideonella dechloratans ATCC 51718; CAD97447); SerA, selenate reductase (Thauera selenatis AX; AJ007744); DdhA, dimethylsulfide dehydrogenase (Rhodovulum sulfidophilum SH1; AF453479); EbdA, ethylbenzene dehydrogenase (Azoarcus sp. strain EB1; AF337952); and NarG, nitrate reductase (E. coli K-12; NP 415742).
A primer pair, pcrA320F (5′-GCGCCCACCACTACATGTAYGGNCC-3′) and pcrA598R (5′-GGTGGTCGCCGTACCARTCRAA-3′), was selected using CODEHOP (20) along with inspection of the sequences and the degrees of genetic code degeneracy. The primer sequences correspond to nucleotide positions 320 to 344 and 577 to 598 of the D. agitata CKB pcrA gene.
Detection of pcrA genes in perchlorate-reducing cultures.
Detection of PRB by qPCR using the designed pcrA primers was confirmed with DNA from pure cultures of five PRB strains from four genera, Dechloromonas, Azospira, Azospirillum, and Dechlorospirillum, representing most of the previously identified PRB (4, 5). The assay was also tested with DNA extracted from Yolo silt loam soil enriched with 0.25 mM perchlorate and either acetate (YA) or hydrogen (YH), provided as electron donors (12). Two non-PRB were also tested, including the chlorate-reducing Pseudomonas sp. strain PK (presumably containing the clrA gene, encoding the molybdoenzyme chlorate reductase) (3, 5) and the nitrate-reducing Escherichia coli strain K-12 (which produces several molybdoenzymes).
Pure culture and soil enrichment DNA was extracted using an UltraClean microbial DNA kit (MoBio laboratories, Carlsbad, CA) and a FastDNA spin kit for soil (using 0.5 g soil) (MP Biomedicals, Solon, OH), respectively. Five nanograms of each DNA sample was added to a qPCR mixture (15 μl as a final reaction volume) containing 1× SYBR Ex Taq premix (TaKaRa Bio USA, Madison, WI) and primers (0.2 μM each). PCR was performed with a 7300 real-time PCR system (Applied Biosystems, Foster City, CA) with thermal cycling of 95°C for 1 min followed by 35 cycles of 95°C for 5 s and 60°C for 31 s. The absence of nonspecific PCR products was confirmed both by dissociation curve analysis and by 1.5% agarose gel electrophoresis.
Although the primers were designed using the only two available pcrA sequences, both from the genus Dechloromonas, substantial amplifications were also observed with three other PRB, including Azospirillum sp. strain TTI and Dechlorospirillum sp. strain WD (Table 1). The primer pair did not detect Azospira suillum PS (Table 1), which appears to possess the pcrA gene (3); therefore, the primer sequences will be improved when the pcrA sequence of this strain is available. No amplification was observed with the negative controls.
TABLE 1.
qPCR detection of pcrA genes in PRB strains, soil enriched with perchlorate, and non-PRB strains
| Source | Perchlorate reductionb | qPCR detectionc |
|---|---|---|
| Dechloromonas agitata strain CKB | + | + |
| Dechloromonas sp. strain MissR | + | + |
| Azospira suillum strain PS | + | − |
| Azospirillum sp. strain TTI | + | + |
| Dechlorospirillum sp. strain WD | + | + |
| Pseudomonas sp. strain PK | − | − |
| E. coli K-12 | − | − |
| YAa | + | + |
| YHa | + | + |
Yolo silt loam soil enriched in a mineral liquid medium containing 2.5 mM ClO4− with either 10 mM acetate under N2 headspace (YA) or 10 mM bicarbonate under H2 headspace (YH) for 4 to 5 months (12).
Ability to reduce perchlorate to chloride.
Detection above the detection limit (nine copies/reaction).
Amplification was also observed in DNA extracted from YA and YH soil enrichment cultures (Table 1), in which 16S rRNA genes identical to Dechlorospirillum sp. and Azospirillum sp. were previously detected (12).
Partial pcrA gene sequences.
pcrA amplicons from Dechloromonas sp. strains CKB and MissR, Dechlorospirillum sp. strain WD, Azospirillum sp. strain TTI, and the soil enrichment cultures, YA and YH, were cloned by using a TOPO TA cloning kit (Invitrogen, Carlsbad, CA). Positive clones were identified following screening with M13 universal primers. For the enrichment cultures that likely contained multiple strains of PRB, the M13 PCR products of 20 positive clones were subjected to restriction fragment length polymorphisms using the restriction endonuclease HhaI. The digestion patterns were examined by performing gel electrophoresis with 3% low-melting-point agarose gel (Fisher Scientific, Fair Lawn, NJ) in 1× Tris-borate-EDTA buffer at 6 V/cm and 4°C. Plasmids were extracted from the pcrA clones with distinct restriction fragment length polymorphisms and from those of PRB pure cultures by using a Plasmid Minikit (Qiagen, Valencia, CA). Inserts were sequenced at the University of California, Davis, DNA sequencing facility (Davis, CA).
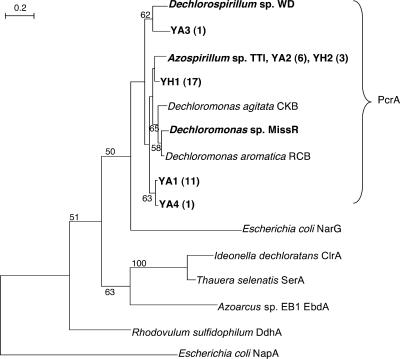
A phylogenetic tree of the deduced PcrA protein sequences (92 amino acids), and the corresponding sequences of the enzymes in the DMSO reductase family, was constructed by using the neighbor-joining method (16) (Fig. 2). The PcrA sequences of PRB isolates and soil enrichments were closely related to each other but distinct from other molybdoenzymes in the DMSO reductase family. Within the PcrA cluster, the three Dechloromonas sp. PcrA sequences formed a tight group, indicating that they are closely related.
FIG. 2.
Phylogenetic tree of deduced PcrA protein sequences and reference sequences. Bootstrap values above 50 obtained from 100 resamplings are shown at each node. The sequences obtained in this study are in bold type. The numbers of clones with identical sequences, in total 19 and 20 clones from the soil enrichments YA and YH, respectively, are in parentheses. The GenBank accession numbers for reference sequences are in the legend to Fig. 1, except for E. coli NapA (AAC75266). Bar, 0.2 changes per amino acid.
The enrichment culture PcrA sequences YA3 and YH2 were closely related to those from the Dechlorospirillum and Azospirillum pure cultures, respectively, as anticipated from the previous identification of the corresponding 16S rRNA gene sequences in these samples (12). In contrast, the YA2 PcrA sequence was identical to that from the Azospirillum sp. pure culture, although we did not detect Azospirillum sp. 16S rRNA gene sequences in the YA enrichment (12). More sequence data are needed to verify the similarity and disparity of sequence phylogenies between PcrA and16S rRNA genes.
A total of five and two different PcrA sequences were obtained from the YA and YH enrichments, respectively. Only one pcrA clone sequence was identical between the YA and YH enrichments, suggesting that different electron donors may enrich different PRB.
Plasmid standard curve for quantification.
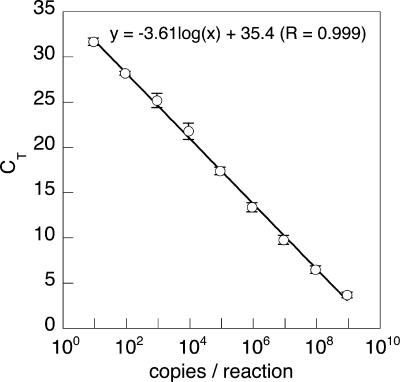
A standard curve was constructed relating gene copy numbers to qPCR threshold cycle using a plasmid with the cloned D. agitata CKB (ATCC 700666) pcrA gene. The copy number of the plasmid was calculated based on the DNA concentration determined by measuring absorbance at 260 nm. Five microliters of 10-fold serial dilutions of the plasmid solution was added to a qPCR mixture, and qPCR was performed as described above. The curve relating gene copy numbers and qPCR threshold cycles was strongly linear (R2 = 0.99) over 9 orders of magnitude (Fig. 3). The detection limit was approximately nine copies/reaction.
FIG. 3.
Standard curve relating pcrA gene copy numbers and qPCR threshold cycles (CT) using a plasmid containing the D. agitata pcrA gene (means ± standard deviations; n = 6).
Quantification of pcrA genes in soil samples.
The pcrA genes were amplified in samples of a previously unexposed Yolo silt loam soil and in an undescribed soil collected from a perchlorate-contaminated site in California (soil B). In addition, two sets of anaerobic unsaturated microcosms using these two soils were exposed to perchlorate and amended with either acetate or hydrogen, as a commonly used organic electron donor and an inorganic electron donor for PRB, respectively. Approximately 1.0 and 0.2 μmol/g dry soil−1 of perchlorate was reduced in the Yolo soil microcosms and the soil B microcosms, respectively, in the presence of either acetate and nitrogen gas (Yolo soil only) or bicarbonate and hydrogen gas (both Yolo soil and soil B) (12; M. Nozawa-Inoue, M. Jien, K. Yang, D. E. Rolston, K. R. Hristova, and K. M. Scow, unpublished data). Five microliters of 100×-diluted soil DNA, corresponding to 2 to 10 ng DNA (a semiquantitative estimate on agarose gel in comparison with serial dilutions of a known concentration of lambda DNA), was analyzed by qPCR. Prior to the analysis, several dilution rates were compared for each soil. The 100× dilution was the lowest dilution that exhibited the least inhibition while producing the most consistent results between triplicate reactions; this dilution was used for quantification. The pcrA copy numbers were calculated based on the standard curve described above, assuming 100% of DNA recovery from soil DNA extraction and no inhibition from the soil matrices. Although pcrA genes were not detected in any of the soils before treatment, 104 to 105 copies of pcrA genes per gram dry soil were successfully detected in samples after perchlorate reduction (Table 2), presumably due to the growth of PRB. Though a smaller amount of perchlorate was reduced, soil B had a larger copy number of pcrA genes than did Yolo loam soil. On possible explanation for this difference is that the PRB in Yolo soil had a higher level of perchlorate reduction activity on a per-cell basis.
TABLE 2.
pcrA copy numbers in soil microcosm samples
| Soil | Treatment | No. of pcrA copies/g dry soila |
|---|---|---|
| Yolo silt loam | None | <DL |
| ClO4−/acetate | (3.4 ± 2.1) × 104 | |
| ClO4−/H2 | (9.6 ± 6.0) × 104 | |
| Soil B | None | <DL |
| ClO4−/H2 | (4.5 ± 2.7) × 105 |
<DL, under the detection limit.
The developed qPCR assay targeting pcrA genes was able to quantify the abundance of pcrA genes, presumably reflecting the PRB population, in environmental samples. The sequence information collected using the pcrA gene primers and also different environmental conditions enriched for sequences associated with different types of perchlorate-reducing bacteria. The assay may help to estimate cell densities of naturally occurring organisms potentially involved in perchlorate reduction if the copy numbers of pcrA genes in PRB strains are known. Estimates of cell densities can be useful for optimizing biological treatment of perchlorate, including in bioreactors and in situ bioremediation. More information on the diversity and abundance of these microorganisms possessing or expressing pcr may also provide new insights into the PRB ecology of the environment, information not readily available from culture-based studies.
Nucleotide sequence accession numbers.
The sequences in this study have been deposited in the GenBank database under accession numbers EU273890 to EU273898.
Acknowledgments
We thank John D. Coates and J. Ian VanTrump for providing strains PS, PK, WD, and TTI.
This research was supported by UC Discovery Grant bio06-10600 from the University of California with corporate sponsorship from Microbial Insights, Inc., and by grant number 5 P42 ES04699 from the National Institute of Environmental Health Sciences (NIEHS), NIH.
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the aforementioned agencies.
Footnotes
Published ahead of print on 1 February 2008.
REFERENCES
- 1.Bender, K. S., S. A. O'Connor, R. Chakraborty, J. D. Coates, and L. A. Achenbach. 2002. Sequencing and transcriptional analysis of the chlorite dismutase gene of Dechloromonas agitata and its use as a metabolic probe. Appl. Environ. Microbiol. 68:4820-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender, K. S., M. R. Rice, W. H. Fugate, J. D. Coates, and L. A. Achenbach. 2004. Metabolic primers for detection of (per)chlorate-reducing bacteria in the environment and phylogenetic analysis of cld gene sequences. Appl. Environ. Microbiol. 70:5651-5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender, K. S., C. Shang, R. Chakraborty, S. M. Belchik, J. D. Coates, and L. A. Achenbach. 2005. Identification, characterization, and classification of genes encoding perchlorate reductase. J. Bacteriol. 187:5090-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruce, R. A., L. A. Achenbach, and J. D. Coates. 1999. Reduction of (per)chlorate by a novel organism isolated from paper mill waste. Environ. Microbiol. 1:319-329. [DOI] [PubMed] [Google Scholar]
- 5.Coates, J. D., U. Michaelidou, R. A. Bruce, S. M. O'Connor, J. N. Crespi, and L. A. Achenbach. 1999. Ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl. Environ. Microbiol. 65:5234-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman, D. C., and W. T. Frankenberger. 1999. Bacterial reduction of perchlorate and nitrate in water. J. Environ. Qual. 28:1018-1024. [Google Scholar]
- 7.Jormakka, M., D. Richardson, B. Byrne, and S. Iwata. 2004. Architecture of NarGH reveals a structural classification of Mo-bisMGD enzymes. Structure 12:95-104. [DOI] [PubMed] [Google Scholar]
- 8.Kengen, S. W. M., G. B. Rikken, W. R. Hagen, C. G. van Ginkel, and A. J. M. Stams. 1999. Purification and characterization of (per)chlorate reductase from the chlorate-respiring strain GR-1. J. Bacteriol. 181:6706-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logan, B. E., H. S. Zhang, P. Mulvaney, M. G. Milner, I. M. Head, and R. F. Unz. 2001. Kinetics of perchlorate- and chlorate-respiring bacteria. Appl. Environ. Microbiol. 67:2499-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEwan, A. G., J. P. Ridge, C. A. McDevitt, and P. Hugenholtz. 2002. The DMSO reductase family of microbial molybdenum enzymes; molecular properties and role in the dissimilatory reduction of toxic elements. Geomicrobiol. J. 19:3-21. [Google Scholar]
- 11.Motzer, W. E. 2001. Perchlorate: problems, detection, and solutions. Environ. Forensics 2:301-311. [Google Scholar]
- 12.Nozawa-Inoue, M., K. M. Scow, and D. E. Rolston. 2005. Reduction of perchlorate and nitrate by microbial communities in vadose soil. Appl. Environ. Microbiol. 71:3928-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philippot, L. 2005. Tracking nitrate reducers and denitrifiers in the environment. Biochem. Soc. Trans. 33:200-204. [DOI] [PubMed] [Google Scholar]
- 14.Philippot, L. 2006. Use of functional genes to quantify denitrifiers in the environment. Biochem. Soc. Trans. 34:101-103. [DOI] [PubMed] [Google Scholar]
- 15.Rikken, G. B., A. G. M. Kroon, and C. G. Van Ginkel. 1996. Transformation of (per)chlorate into chloride by a newly isolated bacterium: reduction and dismutation. Appl. Microbiol. Biotechnol. 45:420-426. [Google Scholar]
- 16.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 17.Shrout, J. D., T. E. Scheetz, T. L. Casavant, and G. F. Parkin. 2005. Isolation and characterization of autotrophic, hydrogen-utilizing, perchlorate-reducing bacteria. Appl. Microbiol. Biotechnol. 67:261-268. [DOI] [PubMed] [Google Scholar]
- 18.Stenklo, K., H. D. Thorell, H. Bergius, R. Aasa, and T. Nilsson. 2001. Chlorite dismutase from Ideonella dechloratans. J. Biol. Inorg. Chem. 6:601-607. [DOI] [PubMed] [Google Scholar]
- 19.Thompson, J., D. Higgins, and T. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorell, H. D., T. K. Stenklo, J. Karlsson, and T. Nilsson. 2003. A gene cluster for chlorate metabolism in Ideonella dechloratans. Appl. Environ. Microbiol. 69:5585-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbansky, E. T. 2002. Perchlorate as an environmental contaminant. Environ. Sci. Pollut. Res. 9:187-192. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Environmental Protection Agency. 2005. Known perchlorate releases in the U.S.—March 25, 2005. www.epa.gov/fedfac/pdf/detect0305.pdf.
- 23.van Ginkel, C. G., G. B. Rikken, A. G. M. Kroon, and S. W. M. Kengen. 1996. Purification and characterization of chlorite dismutase: a novel oxygen-generating enzyme. Arch. Microbiol. 166:321-326. [DOI] [PubMed] [Google Scholar]
- 24.Wallace, W., T. Ward, A. Breen, and H. Attaway. 1996. Identification of an anaerobic bacterium which reduces perchlorate and chlorate as Wolinella succinogenes. J. Ind. Microbiol. 16:68-72. [Google Scholar]
- 25.Waller, A. S., E. E. Cox, and E. A. Edwards. 2004. Perchlorate-reducing microorganisms isolated from contaminated sites. Environ. Microbiol. 6:517-527. [DOI] [PubMed] [Google Scholar]
- 26.Wolterink, A., A. B. Jonker, S. W. M. Kengen, and A. J. M. Stams. 2002. Pseudomonas chloritidismutans sp. nov., a nondenitrifying, chlorate-reducing bacterium. Int. J. Syst. Evol. Microbiol. 52:2183-2190. [DOI] [PubMed] [Google Scholar]
- 27.Wu, J., R. F. Unz, H. Zhang, and B. E. Logan. 2001. Persistence of perchlorate and the relative numbers of perchlorate- and chlorate-respiring microorganisms in natural waters, soils, and wastewater. Bioremediat. J. 5:119-130. [Google Scholar]
- 28.Zhang, H. S., M. A. Bruns, and B. E. Logan. 2002. Perchlorate reduction by a novel chemolithoautotrophic, hydrogen-oxidizing bacterium. Environ. Microbiol. 4:570-576. [DOI] [PubMed] [Google Scholar]