Abstract
A tributyltin (TBT) luxAB transcriptional fusion in Escherichia coli revealed that a TBT-activated promoter is located upstream of two cotranscribed orphan genes, ygaV and ygaP. We demonstrate that transcription from the promoter upstream of ygaVP is constitutive in a ygaVP mutant, suggesting that YgaV is an autoregulated, TBT-inducible repressor.
Organotin compounds, such as tributyltin chloride (TBT), have been extensively used for over 50 years as preservation agents for wood and textiles and in antifouling paints for ship hulls. TBT was subsequently found to act as an endocrine disruptor, causing imposex in marine invertebrates (8, 15). However, though TBT use is now generally prohibited, the pollutant and its by-products still persist in many ecosystems.
Bacterial toxicity tests based on recombinant bioluminescent bacteria are widely used to screen for the presence of environmental pollutants (12). In order to obtain the appropriate indicator strains, one approach is to identify Escherichia coli genes that are transcriptionally regulated by cellular exposure to the potentially toxic agents (9). A luxAB gene transcription fusion library was constructed in E. coli and screened for bioluminescence in the absence and presence of exogenous TBT. One clone, called TBT3, whose luminescence was augmented in a dose-dependent manner upon exposure to TBT, was selected (3). As TBT has been reported to affect several processes in bacteria (17), we report here the E. coli chromosomal region and the regulator involved in the induction of luminescence in the TBT3 E. coli strain in an effort to better understand TBT effects on gene expression.
Localization of the transposon insertion in the chromosome of E. coli TBT3.
The modified Tn5 transposon used to create the luxAB chromosomal fusion of TBT3 harbors a single HindIII restriction site located after the tet gene (3). In order to study the sequences upstream of the insertion site of the transposon, chromosomal DNA of TBT3 was purified, digested with HindIII, and cloned into the plasmid pUC19. After transformation in E. coli TOP10 (Invitrogen), clones which grew on LB medium containing tetracycline were isolated and found to harbor the luxAB and tet genes along with the chromosomal sequences between the upstream HindIII restriction site and the beginning of the transposon (Fig. 1A).
FIG. 1.
Localization of the promoter activated after cellular TBT exposure in E. coli strain TBT3. (A) Map of the insertion region of the transposon in the chromosome of E. coli TBT3. H, HindIII site used for the cloning procedure. The double slash on the stpA arrow indicates the insertion site of the transposon in the stpA gene. The dotted line box delimits the region studied by deletion analysis, shown in panel B. (B) Localization of the promoter activated after cellular exposure to different TBT concentrations using deletion analysis of the upstream region of the luxAB insertion. The different regions of E. coli TBT3 tested for induction with TBT are represented by thick lines (numbers on each side indicate the base pair position from the transposon insertion). Increasing concentrations of TBT used (0, 0.1, 0.5, 1, 2, 5, and 10 μM) are indicated for each result by a rectangle with a gradient from white (0 μM) to black (10 μM). Experimental relative luminescence units/s (RLU/s) values represent the means of three independent experiments. Plasmid pBlux is a control plasmid devoid of an insert upstream of the luxCDABE reporter genes.
The HindIII fragment was sequenced, and the transposon insertion was located 72 bp before the stop codon of the stpA gene, with luxAB oriented in the opposite direction of stpA transcription and translation. Thus, the promoter activated by TBT is apparently located downstream of and in the opposite orientation from the stpA gene. Two genes, ygaV and ygaP, apparently comprising an operon, are located downstream of stpA and transcribed in the opposite direction of stpA. YgaV (P77295) is a hypothetical ArsR-like regulator of 99 amino acids, according to the UniProtKB/Swiss-Prot database (http://www.expasy.org/sprot/), whereas YgaP (P55734) is a membrane protein of 174 amino acids with rhodanese activity in vitro (1).
Localization of the promoter induced by TBT.
In order to localize the promoter activated by TBT in strain TBT3, we performed a deletion analysis of the region located upstream of the stpA insertion site of the transposon (and thus downstream of stpA). Eight plasmids, named pBTBT1 to -8, were constructed with different parts of the region upstream from the transposon insertion site in the E. coli chromosome. As a means of detecting transcriptional activity from these cloned chromosomal segments, we used the luciferase-encoding luxAB genes from Vibrio harveyi (luxABVh+) or the luxCDABE genes (luxCDABEVf+ [for possible subsequent use in TBT biosensors]) encoding the bacterial luciferase of Vibrio fischeri and genes for the provision of its aldehyde substrate, as transcriptional reporters. The plasmid pBTBT1 was generated by cloning a 3,520-bp fragment (reporter, luxABVh+), amplified with primer pair TBT1F-TBT1R and digested with HindIII (Table 1), into the HindIII site of plasmid pBΔptac. The plasmid pBTBT3 was constructed by cloning a 3,181-bp PCR fragment (primer pair TBT3F-TBT1R; reporter, luxABVh+) digested with NruI/HindIII and cloned into the NruI/HindIII sites of plasmid pBluxFi (5). The plasmids pBTBT2, pBTBT4, pBTBT5, pBTBT6, pBTBT7, and pBTBT8 were obtained by cloning the PCR fragments obtained with the primer pairs TBT2F-TBT2R, TBT4F-TBT2R, TBT5F-TBT2R, TBT6F-TBT2R, TBT7F-TBT2R, and TBT4F-TBT8R, respectively, in the correct orientation to create transcriptional fusions with luxCDABEVf in plasmid pBluxFi (a low-copy-number plasmid), previously linearized by NruI/EcoRI digestion. The plasmids were used to transform E. coli DH1 (CIP 104745; http://www.crbip.pasteur.fr). Induction of bioluminescence by TBT addition for each construct was tested as described by Durand et al. (7): a 16-h culture in minimum glucose medium (7) was grown with agitation (220 rpm) at 30°C for the plasmid containing the luxCDABEVf reporter operon and at 37°C for those with the luxABVh operon. All of the strains were subsequently diluted in fresh medium (1/10) and grown for a further 3 h. The A620 was then measured in a Hitachi model U-1800 spectrophotometer, and each strain was diluted to an A620 of 0.15. Then, 100 μl of each diluted culture was mixed in a 96-well microtiter plate with 50 μl of artificial seawater (control) or 50 μl artificial seawater (DSMZ medium 246; http://www.dsmz.de/media/media.htm) containing different concentrations of TBT (0.1 to 10 μM). After 1 h at 30°C, 25 μl of 210 μM decanal (mixed in deionized water with 1.6% isopropanol) was added to each well and bioluminescence was measured with a Microlumat L96V, EG G Berthold luminometer. A decrease of luminescence was generally observed at TBT concentrations of 5 μM and higher due to the toxicity of TBT. As depicted in Fig. 1B, the levels of TBT induction of bioluminescence increased in a dose-dependent manner for pBTBT1 and pBTBT2, while no induction was observed for pBTBT3, though the latter construct displayed residual bioluminescence possibly from plasmid read-through and/or a potential cryptic promoter (not shown) in the ygaP gene. These results show that the promoter activated by TBT is located upstream of the ygaVP genes and that these two genes are likely cotranscribed. Through an examination of bioluminescence levels produced from plasmids pBTBT4, pBTBT5, pBTBT6, pBTBT7, and pBTBT8, we were able to narrow down the sequences required for induction of bioluminescence by TBT. Except for pBTBT8, all constructs carried a promoter activated by TBT, even if TBT-inducible bioluminescence was lower in the case of pBTBT6 and pBTBT7. These results suggest that the deletions affect the overall activity of the TBT-inducible promoter, but not TBT-controlled regulation. The lack of bioluminescence observed with pBTBT8 implies that the transcriptional start site is located on the 109-bp fragment cloned in pBTBT7. Constructions using strain DH1 resulted in generally lower levels of bioluminescence than those obtained in strain TBT3. This observation can be explained by a strain difference in global gene expression and/or metabolism, as previously observed by Vijayendran et al. for two closely related strains of E. coli K-12: W3110 and MG1655 (16). In addition, bioluminescence differences between strain TBT3 and the plasmid constructions in strain DH1 can be explained by the fact that the Tn5::luxAB tet transposon insertion in strain TBT3 is located just inside the 3′ extremity of the stpA gene and thus possibly affected by stpA expression. Also, stpA is inactivated in TBT3, while in E. coli DH1 stpA is still functional, suggesting that StpA may play a role in ygaVP regulation, as it does for the bgl operon (19). However, as this regulation is independent of TBT concentration, and TBT inducibility of a ygaVPp::luxAB fusion was not found to be significantly different between an stpA+ strain and an stpA mutant strain (data not shown), it thus appears that StpA does not seem to be the major regulator involved in the induction of the ygaVP promoter by TBT.
TABLE 1.
Oligonucleotides used for PCR amplifications
| Primer name | Restriction site | Nucleotide sequencea |
|---|---|---|
| Deletion analysis | ||
| TBT1F | HindIII | 5′-aggtaaagctt-TGCACCCAGAACGCGTGAAT-3′ |
| TBT1R | HindIII | 5′-acagcaagctt-TTACGAGTGGTATTTGACGATGTTGGC-3′ |
| TBT2F | NruI | 5′-gagtatcgcga-TGCACCCAGAACGCGTGAAT-3′ |
| TBT2R | EcoRI | 5′-agttgaattcctcctcct-GCACTGGCCTGTAATTGCGTGA-3′ |
| TBT3F | NruI | 5′-tattatcgcga-TCACGCAATTACAGGCCAGTGC-3′ |
| TBT4F | NruI | 5′-ccaagtcgcga-TAATGAACGCCCAACCGAACC-3′ |
| TBT5F | NruI | 5′-actcttcgcga-TGATGACGATTCTCCAGAAACCCA-3′ |
| TBT6F | NruI | 5′-agttttcgcga-GCGTAGTAATTTTAGCGGAGGCTG-3′ |
| TBT7F | NruI | 5′-aatgttcgcga-TCATTTACGCTGCTGAGGCTGG-3′ |
| TBT8R | EcoRI | 5′-agttgaattcctcctcct-CCAGCCTCAGCAGCGTAAATGA-3′ |
| Complementation of ygaVP deletion | ||
| TBT9R | EcoRI | 5′-tattgaattcctcctcct-CATGCGGCGAAATGGTTGTC-3′ |
| TBT10R | EcoRI | 5′-aaaaaagaattccctcctta-GTCCGGCGTCGCTTCTCAA-3′ |
Lowercase letters indicate linker sequences. Underlined letters indicate the restriction sites. Boldface letters indicate a Shine-Dalgarno sequence added, when necessary, for luxC translation.
YgaV is a repressor regulated by TBT.
YgaV is a hypothetical ArsR-like regulator. The sequence alignment of YgaV, using ClustalW (6), with other small regulatory proteins also bearing an ArsR helix-turn-helix (HTH) motif (Fig. 2), shows that YgaV has greater identity (>30%) with HlyU (18) of Vibrio cholerae, NolR (13) of Rhizobium meliloti, PagR (10) of Bacillus anthracis, and SoxR (2, 14) of Pseudaminobacter salicylatoxidans than with the SmtB/ArsR regulatory proteins (4) (SmtB, ZiaR, ArsR1, ArsR2, ArsR, and CadC; <17% identities). Studies on induction of bioluminescence in E. coli TBT3 by various metals, metalloids, and organometal compounds (HgCl2, SnCl2, As2O3, and tributylgermanium) confirmed that YgaV is not a general metalloregulatory transcriptional protein, as no induction was observed (7). At present, the role of YgaV in the physiology of E. coli is unknown.
FIG. 2.
Multiple sequence alignments of YgaV with other small regulatory proteins bearing an ArsR DNA binding motif: SoxR_Rs, SoxR of Rhodovulum sulfidophilum (UniProtKB accession no. [UPacc] Q8GCH3); SoxR_Ps, SoxR of Pseudaminobacter salicylatoxidans (UPacc Q5ZQN5); SoxR_Pp, SoxR of Paracoccus pantotrophus (GenBank accession no. X79242); NolR_Rm, NolR of Rhizobium meliloti (UPacc P28267); NolR_Rl, NolR of Rhizobium leguminosarum (UPacc O54057); YgaV_Ec, YgaV of E. coli; HlyU_Vc, HlyU of Vibrio cholerae (UPacc P52695); SmtB_S7, SmtB of Synechococcus sp. strain PCC 7942 (UPacc P30340); ZiaR_S3, ZiaR of Synechococcus sp. strain PCC 6803 (UPacc Q55940); ArsR1_Ec, ArsR of E. coli encoded by plasmid R773 (UPacc P15905); ArsR2_Ec, ArsR of E. coli encoded by plasmid IncN R46 (UPacc P52144); ArsR_Ec, ArsR of E. coli encoded by chromosome (UPacc P37309); ArsR_Sa, ArsR of Staphylococcus aureus encoded by plasmid pI258 (UPacc P30338); PagR_Ba, PagR of Bacillus anthracis encoded by plasmid pXO1 (UPacc O31178); CadC_Sa, CadC of Staphylococcus aureus encoded by plasmid pI258 (UPacc P20047). Identical amino acids with YgaV are highlighted in black. Amino acids of the metal recognition site are shown in gray. The putative ArsR HTH is underlined.
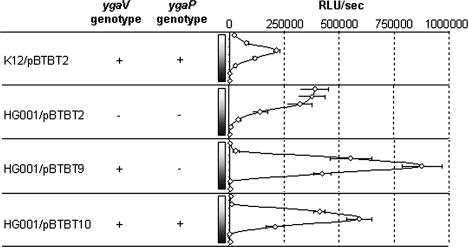
In order to determine whether YgaV is involved in the induction of bioluminescence by TBT in E. coli TBT3 and if it regulates its own expression, we replaced ygaVP in E. coli K-12 ATCC 23716 (http://www.lgcpromochem-atcc.com/) with a chloramphenicol resistance gene using the technique of allele replacement and the suicide plasmid pKNG101 (11). This strain was called HG001. Next, plasmid pBTBT2 was used to transform HG001 and the E. coli K-12 wild type to study induction of bioluminescence by TBT, as previously described. A fivefold TBT-inducible increase of bioluminescence in E. coli K-12/pBTBT2 was observed (Fig. 3), whereas bioluminescence was expressed constitutively in HG001/pBTBT2, even in the absence of TBT. These results suggest that ygaV likely represses its own expression in the absence of TBT. To confirm this hypothesis, we generated two additional constructs, pBTBT9 and pBTBT10, harboring ygaV and ygaV-ygaP, respectively, in order to study complementation of the mutant strain. The plasmids pBTBT9 and pBTBT10 were constructed in a similar manner to pBTBT2, except that the PCR product was amplified with primer pairs TBT1F-TBT9R and TBT1F-TBT10R, respectively.
FIG. 3.
Effect of the ygaVP deletion on the induction of bioluminescence by TBT. Increasing concentrations of TBT used (0, 0.1, 0.5, 1, 2, 5, and 10 μM) are indicated for each result by a rectangle with a gradient from white (0 μM) to black (10 μM). Values on the graph represent the means of three independent experiments for each concentration of TBT for each strain. RLU/s, relative luminescence units/s.
When luxCDABE was transcriptionally fused to ygaV alone (Fig. 3; HG001/pBTBT9), the bioluminescence level in strain HG001 without added TBT was as low as that observed for E. coli K-12/pBTBT2, while a dose-dependent TBT induction of bioluminescence was observed. These results strongly suggest that YgaV is a repressor which autoregulates its own expression at the transcriptional level. In the absence of TBT, YgaV acts as a repressor, while the presence of TBT abolishes YgaV-mediated repression. In the case of HG001/pBTBT10, the results were similar to what was observed with HG001/pBTBT9, except that the induction ratio (fold) was lower (about 60-fold) after addition of TBT. This difference could be explained by the bioluminescence level in the absence of TBT, which was higher when ygaVP is present than when ygaVP is absent.
The precise roles of YgaV and YgaP in bacterial physiology and TBT metabolism are unknown at this time, and their apparent conservation only among members of the Enterobacteriaceae (data not shown) remains an enigma. The growth of the mutant HG001 is not affected by the presence of 1 μM TBT compared to E. coli TBT3 and E. coli K-12 (see Fig. S1 in the supplemental material). TBT is likely not the natural inducer of ygaVP expression, as it is a manmade compound. However, TBT has been shown to react with sulfhydryl groups, and YgaP is an apparent membrane-associated protein that displays a sulfur transferase (rhodanese) activity (an activity often implicated in the detoxification of cyanides) in vitro, with a cysteine implicated in the catalytic site (1). It is possible that TBT reacts with this cysteine residue (position 64) of YgaP and negatively affects its structure and/or activity, ultimately leading to increased transcription of the ygaVP operon, possibly in addition to other rhodanese-encoding genes. Nonetheless, we demonstrate here that induction of bioluminescence in E. coli TBT3 by TBT is caused by relief of repression by YgaV of the transcription of luxABVh located downstream of the ygaVP operon.
Supplementary Material
Acknowledgments
We acknowledge Philippe Cornet (Solabia Company) for advice and the reviewers for excellent comments and suggestions.
This work was supported by grant CER 2000-2006, Action no. 15 (Section I), Research Program no. 18035 (Ville de La Roche sur Yon, Conseil Général de Vendée, Conseil Régional des Pays de la Loire, Ministère Français Chargé de la Recherche).
This publication is dedicated to Yves Thomas from the University of Nantes, on the occasion of his retirement.
Footnotes
Published ahead of print on 1 February 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Ahmed, F. 2003. Characterization of two novel proteins containing the rhodanese homology domain: YgaP and YbbB of Escherichia coli. Ph.D. thesis. Virginia Polytechnic Institute and State University, Blacksburg.
- 2.Bagchi, A., D. Roy, and P. Roy. 2005. Homology modeling of a transcriptional regulator SoxR of the lithotrophic sulfur oxidation (Sox) operon in alpha-proteobacteria. J. Biomol. Struct. Dyn. 22:571-577. [DOI] [PubMed] [Google Scholar]
- 3.Briscoe, S. F., C. Diorio, and M. S. DuBow. 1996. Luminescent biosensors for the detection of tributyltin and dimethyl sulfoxide and the elucidation of their mechanisms of toxicity, p. 645-655. In M. Moo-Young, W. A. Anderson, and A. M. Chakrabarty (ed.), Environmental biotechnology, principles and applications. Kluwer Academic Press, Dordrecht, The Netherlands.
- 4.Busenlehner, L. S., M. A. Pennella, and D. P. Giedroc. 2003. The SmtB/ArsR family of metalloregulatory transcriptional repressors: structural insights into prokaryotic metal resistance. FEMS Microbiol. Rev. 27:131-143. [DOI] [PubMed] [Google Scholar]
- 5.Charrier, T. 2006. Développement d'un biocapteur bactérien bioluminescent multicanal pour la détection de polluants dans l'environnement. Ph.D. thesis. University of Nantes, Nantes, France.
- 6.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31:3497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durand, M. J., G. Thouand, T. Dancheva-Ivanova, P. Vachon, and M. S. DuBow. 2003. Specific detection of organotin compounds with a recombinant luminescent bacteria. Chemosphere 52:103-111. [DOI] [PubMed] [Google Scholar]
- 8.Fent, K. 1996. Ecotoxicology of organotin compounds. Crit. Rev. Toxicol. 26:1-117. [PubMed] [Google Scholar]
- 9.Guzzo, A., and M. S. DuBow. 1991. Construction of stable, single-copy luciferase gene fusions in Escherichia coli. Arch. Microbiol. 156:444-448. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmaster, A. R., and T. M. Koehler. 1999. Autogenous regulation of the Bacillus anthracis pag operon. J. Bacteriol. 181:4485-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137-141. [DOI] [PubMed] [Google Scholar]
- 12.Köhler, S., S. Belkin, and R. D. Schmid. 2000. Reporter gene bioassays in environmental analysis. Fresenius J. Anal. Chem. 366:769-779. [DOI] [PubMed] [Google Scholar]
- 13.Kondorosi, E., M. Pierre, M. Cren, U. Haumann, M. Buire, B. Hoffmann, J. Schell, and A. J. Kondorosi. 1991. Identification of NolR, a negative transacting factor controlling the nod regulon in Rhizobium meliloti. J. Mol. Biol. 222:885-896. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyaya, P. N., C. Deb, C. Lahiri, and P. Roy. 2000. A soxA gene, encoding a diheme cytochrome c, and a sox locus, essential for sulfur oxidation in a new sulfur lithotrophic bacterium. J. Bacteriol. 182:4278-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith, B. S. 1981. Male characteristics on female mud snails caused by antifouling paints. J. Appl. Toxicol. 1:22-25. [DOI] [PubMed] [Google Scholar]
- 16.Vijayendran, C., T. Polen, V. F. Wendisch, K. Friehs, K. Niehaus, and E. Flaschel. 2007. The plasticity of global proteome and genome expression analyzed in closely related W3110 and MG1655 strains of a well-studied model organism, Escherichia coli-K12. J. Biotechnol. 128:747-761. [DOI] [PubMed] [Google Scholar]
- 17.White, J. S., J. M. Tobin, and J. J. Cooney. 1999. Organotin compounds and their interactions with microorganisms. Can. J. Microbiol. 45:541-554. [PubMed] [Google Scholar]
- 18.Williams, S. G., S. R. Attridge, and P. A. Manning. 1993. The transcriptional activator HlyU of Vibrio cholerae: nucleotide sequence and role in virulence gene expression. Mol. Microbiol. 9:751-760. [DOI] [PubMed] [Google Scholar]
- 19.Wolf, T., W. Janzen, C. Blum, and K. Schnetz. 2006. Differential dependence of StpA on H-NS in autoregulation of stpA and in regulation of bgl. J. Bacteriol. 188:6728-6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.