Abstract
Skatole (3-methylindole) is a malodorous chemical in stored swine manure and is implicated as a component of foul-tasting pork. Definitive evidence for the skatole pathway is lacking. Deuterium-labeled substrates were employed to resolve this pathway in the acetogenic bacterium Clostridium drakei and Clostridium scatologenes and to determine if a similar pathway is used by microorganisms present in stored swine manure. Indoleacetic acid (IAA) was synthesized from tryptophan by both bacteria, and skatole was synthesized from both IAA and tryptophan. Microorganisms in swine manure produced skatole and other oxidation products from tryptophan, but IAA yielded only skatole. A catabolic mechanism for the synthesis of skatole is proposed.
Storage of swine manure is associated with the generation of a number of malodorous compounds (18, 22, 30), and the production of odor associated with concentrated livestock facilities creates a nuisance and has resulted in considerable conflicts between producers and rural neighbors. These odors are produced as a result of anaerobic degradation of materials present in manure and include sulfides, organic acids, ammonia, phenols, amines, and other volatile compounds (30). One of the more malodorous compounds identified in swine manure odor is skatole (3-methylindole). Skatole has also been implicated as an off-flavor component of pig meat (referred to as “boar taint”) (4, 6, 17, 19) and as a contributing factor in acute bovine pulmonary edema and emphysema (15, 23, 27).
Although the production of skatole has been attributed to the bacterial degradation of the amino acid tryptophan, the pathway by which skatole is produced from tryptophan has not been elucidated. The primary metabolite of tryptophan fermentation is indole (26, 27), and skatole is produced from tryptophan and indoleacetic acid (IAA) by pig fecal slurries (13). However, very few bacterial isolates have been shown to produce skatole. A Lactobacillus species from the rumen has been reported to produce skatole from IAA but not directly from tryptophan (28, 29). Clostridium scatologenes and the acetogen Clostridium drakei (originally isolated as C. scatologenes SL1) (14, 16) have also been reported to produce skatole (13, 14) and were selected for resolving the skatole catabolic pathway by using deuterium-labeled substrates and a combination of gas chromatography-mass spectrometry (GC-MS) and matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS).
Bacterial strains, cultivation conditions, and swine manure.
C. drakei SL1 DSM 12750 and C. scatologenes ATCC 25775 were utilized in this study. Preparation of media and inoculations were performed under anaerobic conditions using the method of Hungate as modified by Bryant (2). The basic medium for culturing C. drakei and C. scatologenes contained macrominerals, microminerals, buffers, reducing agents, and other components in routine growth medium (RGM) (9) and was supplemented with 1% (wt/vol) tryptone and either 1% (wt/vol) tryptophan or 1% (wt/vol) IAA. Glucose was added to a 0.2% (wt/vol) final concentration when indicated. The bacterial strains were also cultivated on anoxic brain heart infusion (BHI) medium (25) and MRS medium (Difco, Detroit, MI). RGM-tryptone medium was supplemented with l-[2′,3′,5′,6′,7′-2H5]tryptophan or [2,2-2H2]indole-3-acetic acid (Sigma-Aldrich, St. Louis, MO) for studies with deuterated substrates. All incubations were at 37°C. Aliquots of culture media were removed at various times of incubation for measurement of skatole; cells were separated by centrifugation (14,500 × g, 2 min), and the supernatant fluid was used for skatole assays. Results are representative of at least duplicate experiments.
Swine manure samples were obtained from a manure storage pit at a swine facility near Peoria, IL, where the feeder pigs were fed a corn-soybean-based diet. Samples from manure storage pits were collected using a NASCO (Fort Atkinson, WI) tank sampler and transported back to the laboratory. Slurry was transferred to tubes under anaerobic conditions, and deuterated substrates were added to the cultures and allowed to incubate at 37°C. Aliquots were removed at 24 h for analyses. Results are representative of at least duplicate experiments.
GC-MS.
Aliquots (0.5 ml) of cultures were removed and centrifuged (14,000 × g, 25°C) for 1 min. The supernatant fluid was recovered and then extracted with 0.2 ml ethyl acetate and centrifuged again. The ethyl acetate layer was recovered and evaporated to dryness on an N2 line. Samples were then dissolved in dry methanol (1 ml) and twice evaporated to dryness on an N2 line. The residues were dissolved in methanolic hydrochloric acid (0.1 M, 1 ml) and heated in sealed tubes for 10 min at 50°C on a reaction block (8). After cooling, the solvent was removed by evaporation and the residue was dissolved in acetonitrile (typically 200 μl). GC-MS analysis was performed on an Agilent (Santa Clara, CA) 8890N gas chromatograph interfaced with an Agilent 5973N mass-selective detector configured in electron impact (EI) mode and a Hewlett Packard (Santa Clara, CA) 7683 series autoinjector. Chromatography was on a Hewlett Packard DB-5ms column (30 m by 0.2 mm) using helium as the carrier. The oven temperature was ramped over a linear gradient from 150 to 300°C at 10°C per min. Mass spectra were recorded in positive-ion mode over the range m/z 60 to 550. Injector and detector/interface temperatures were 275 and 300°C, respectively. Data analysis was done off-line using HP Chemstation. The compounds listed in Table 1 were identified by reference to two structural databases (Wiley 7.0 and NIST).
TABLE 1.
Degradation products of deuterium-labeled tryptophan or IAA in swine manure slurrya
| Metabolite | Retention time (min) | Isotopic label
|
|
|---|---|---|---|
| D5-Trp | D2-IAA | ||
| Skatole | 3.65 | + | + |
| 3-Methylene-indole-2-one | 3.75 | + | − |
| 2,3-Dioxo-skatole | 3.80 | + | − |
| 1,3-Dihydroindole-2-one (oxindole) | 4.40 | + | − |
| 3-Methylindole-2-one | 4.55 | + | − |
| 2,3-Dihydroindole-1-carboxaldehyde | 5.40 | + | − |
The slurry incubated at 37°C for 24 h.
MALDI-TOF MS.
Samples were methylated with acidic methanol as described above and mixed with a saturated solution of matrix (α-cyano-4-hydroxycinnamic acid) in methanol. Samples were then transferred to a standard 7-by-7 MALDI-TOF MS stainless steel target and allowed to evaporate to dryness under a lamp at room temperature. Spectra were recorded on a Bruker-Daltonic (Billerica, MA) Omniflex instrument in reflectron mode. Ion sources 1 and 2 were set to 19.0 and 14.0 kV, respectively, with lens and reflector voltages of 9.20 and 20.00 kV, respectively. A 200-ns pulsed-ion extraction was used with matrix suppression up to 100 Da. Laser excitation was at 337.1 nm, typically at 60% of 150 μJ maximum output, and 80 shots were accumulated.
Production of skatole and IAA.
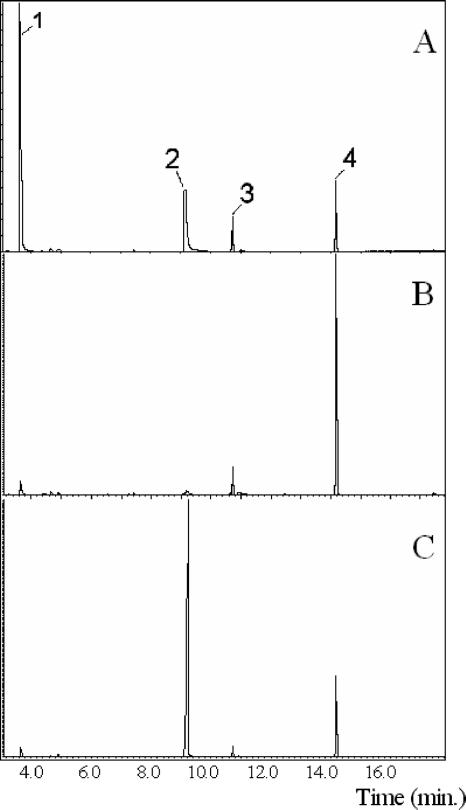
Both skatole and IAA were produced by C. drakei when cultivated in RGM supplemented with tryptophan (Fig. 1A and B). Neither IAA nor skatole was detected in uninoculated controls. Growth was routinely completed by 24 h, and skatole production was observed by 48 h, with concentrations of 0.1 to 0.5 mM skatole produced. Skatole production decreased and IAA production increased when cells were cultured in RGM that was supplemented with both tryptophan and glucose (Fig. 1C), suggesting that skatole production might be regulated. This inhibition was also noted during growth in BHI and MRS media (data not shown). C. scatologenes yielded results similar to those obtained with C. drakei (data not shown). These initial results were consistent with previous findings that suggested the productions of skatole and IAA are metabolically linked (3, 10, 13).
FIG. 1.
GC analysis of ethyl acetate-extracted metabolites from C. drakei. RGM was supplemented with the following substrates (times indicate when samples were removed for analysis): tryptophan, 72 h (A); tryptophan, 24 h (B); or tryptophan plus glucose, 72 h (C). Peaks: 1, skatole; 2, IAA; 3, palmitic acid; and 4, stearic acid. (These fatty acids were present in the culture medium.)
Fate of deuterated substrates.
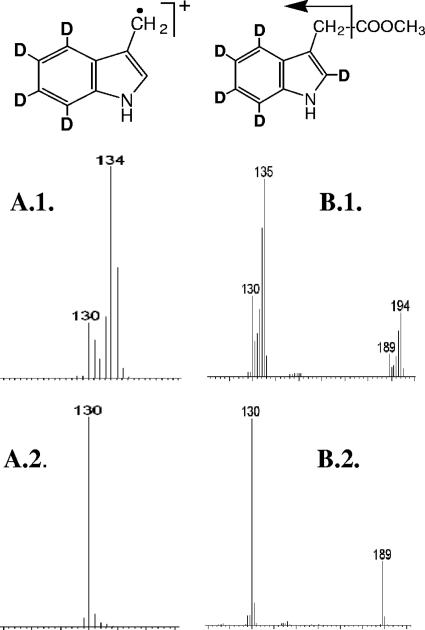
Four of the five deuterium ions of [2′,3′,5′,6′,7′-2H5]tryptophan were recovered in the skatole radical, as evidenced by a shift in the spectrum of the radical ion from m/z 130 to m/z 134 (Fig. 2A. 1). Furthermore, all five deuterium ions of [2′,3′,5′,6′,7′-2H5]tryptophan were recovered in IAA (m/z 189 to m/z 194 [Fig. 2B.1]), indicating that IAA was formed via the deamination and decarboxylation of the tryptophan side chain. All five deuterium atoms of [2′,3′,5′,6′,7′-2H5]tryptophan were also recovered in the skatole molecule (as evidenced by the shift of m/z 129.6 to m/z 134.6 [data not shown]).
FIG. 2.
Skatole and IAA produced from [2′,4′,5′,6′,7′-2H5]tryptophan by C. drakei. Ethyl acetate extracts from cultures grown on [2′,4′,5′,6′,7′-2H5]]tryptophan (A.1 and B.1) or unlabeled tryptophan (A.2 and B.2) were analyzed by GC-MS. The EI-MS spectra are from the skatole peaks (retention time [RT] = 3.6 min) (A.1 and A.2) and the IAA peaks (RT = 10.9 min) (B.1 and B.2). Unlabeled IAA is characterized by a molecular ion at m/z 189, and unlabeled skatole is characterized by an m/z 130 radical ion. Four deuterium incorporations are evident for the labeled skatole radical ion (m/z 134) (A.1), and five deuterium incorporations are evident for the labeled IAA molecular ion (m/z 194) (B.1). The [M-59]+ ion at m/z 130 in spectrum B.2 arises from a decarboxylation fragmentation of m/z 189 and also shows the expected incorporation of five deuteriums (m/z 135) (B.1). Chemical structures for the deuterated skatole radical ion (m/z 134) and the IAA molecular ion (m/z 194) are shown at the top. Similar results were obtained with C. scatologenes (data not shown).
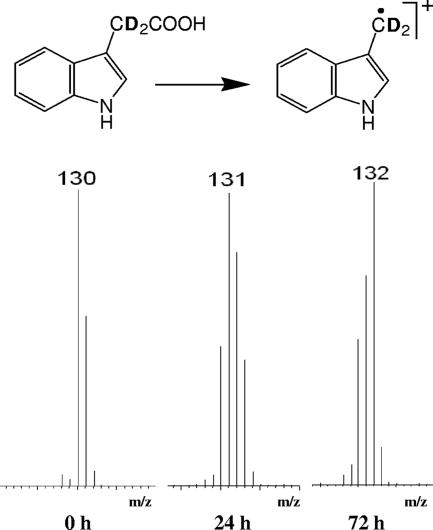
Deuterated skatole radical ion (m/z 132) accumulated with time when C. drakei was cultivated in the presence of [2,2′-2H2]indole-3-acetic acid (Fig. 3). Analogous results were obtained with C. scatologenes (data not shown).
FIG. 3.
Time-dependent conversion of [2,2′-2H2]IAA to skatole. Cultures were grown in the presence of [2,2′-2H2]IAA and harvested at the times indicated. Extracts were methylated and analyzed by GC-MS in EI mode. Mass spectra for the skatole peak (3.85 min) are shown. The unlabeled skatole radical ion, [M+H]·, is at m/z 130. The ions at m/z 131 and m/z 132 arise from a time-dependent metabolic incorporation of deuterium from [2,2′-2H2]IAA into [2,2′-2H2]skatole. The structures of the side-chain-labeled [2,2′-2H2]IAA and the resulting deuterium-labeled skatole radical are shown at the top.
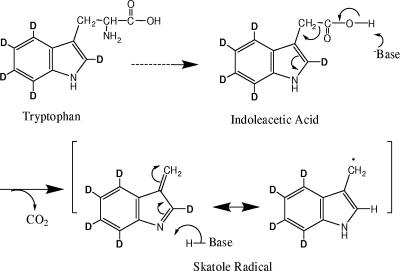
Proposed mechanism for the degradation of tryptophan to skatole by C. drakei and C. scatologenes.
The observations outlined above with deuterated substrates conclusively demonstrated that (i) skatole was produced from tryptophan and (ii) IAA was an intermediate in the formation of skatole. Based on the collective GC-MS and MALDI-TOF MS analyses, a mechanism is proposed by which IAA is formed from tryptophan and subsequently converted to skatole (Fig. 4 and data not shown). The tryptophan side chain is initially deaminated to indolepyruvate, a reaction that also occurs during the production of indole (20). Indolepyruvate is then converted to IAA. A subsequent electron shift and decarboxylation yield the skatole radical (Fig. 4). The formation of the skatole radical was confirmed by EI ion fragmentation data (data not shown).
FIG. 4.
Proposed mechanism for the formation of 2H5-IAA acid (D5-IAA) from 2H5-tryptophan and subsequent decarboxylation to the 2H4-skatole radical. The mechanism also elucidates the formation of 2H2-skatole from 2H2-IAA (Fig. 3).
The mechanism resolved in this study for C. drakei and C. scatologenes is consistent with hypothetical skatole pathways that have been previously suggested (10, 21, 26). The pathway for the catabolic conversion of tryptophan to skatole may be of importance for the clostridial species tested. For example, C. drakei is an acetogen and the CO2 produced during the decarboxylation of IAA may be used as a CO2 equivalent in the acetyl coenzyme A “Wood-Ljungdahl” pathway, as has been documented for the decarboxylation of various benzoates by the acetogen Moorella thermoacetica (7, 11, 12). Alternatively, IAA may be toxic to the clostridial species and conversion to skatole may detoxify IAA.
Catabolism of tryptophan and IAA by microorganisms in swine manure.
Deuterated tryptophan and IAA were transformed to skatole when incubated with swine manure (Table 1). Tryptophan was also oxidized to a variety of other end products, but IAA was converted only to skatole. This finding is of interest because indolic compounds, including IAA, are common metabolic end products from a variety of gastrointestinal anaerobic bacteria (1, 3) and may therefore be substrates for skatole production during storage of swine manure.
The chemical nature of swine manure odor is complex (18, 22, 30), and it is important to identify the biochemical pathways involved in biosynthesis of these compounds. Information on pathway intermediates might augment potential intervention strategies (e.g., via the development and employment of metabolic inhibitors) that seek to reduce the production of skatole in stored manure. To date, no bacterial species that produces skatole has been isolated from swine manure. Our current efforts seek to resolve the microbial ecology of swine manure (5, 24) and to isolate bacteria that produce skatole and other odorous compounds.
Acknowledgments
We acknowledge Rhonda Zeltwanger and Trina Hartman for excellent technical assistance and Kinchell Dorner (University of Western Kentucky) for the culture of C. scatologenes.
Product names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by USDA implies no approval of the product to the exclusion of others that may also be suitable.
Footnotes
Published ahead of print on 25 January 2008.
REFERENCES
- 1.Attwood, G., D. Li, D. Pacheco, and M. Tavendale. 2006. Production of indolic compounds by rumen bacteria isolated from grazing ruminants. J. Appl. Microbiol. 100:1261-1271. [DOI] [PubMed] [Google Scholar]
- 2.Bryant, M. P. 1972. Commentary on the Hungate technique for culture of anaerobic bacteria. Am. J. Clin. Nutr. 25:1324-1328. [DOI] [PubMed] [Google Scholar]
- 3.Chung, K. T., G. M. Anderson, and G. E. Fulk. 1975. Formation of indoleacetic acid by intestinal anaerobes. J. Bacteriol. 124:573-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claus, R., U. Weiler, and A. Herzog. 1994. Physiological aspects of androsterone and skatole formation in the boar—a review with experimental data. Meat Sci. 38:289-305. [DOI] [PubMed] [Google Scholar]
- 5.Cotta, M. A., T. R. Whitehead, and R. L. Zeltwanger. 2003. Isolation, characterization and comparison of bacteria from swine faeces and manure storage pits. Environ. Microbiol. 5:737-745. [DOI] [PubMed] [Google Scholar]
- 6.Deslandes, B., C. Garieyp, and A. Houde. 2001. Review of microbiological and biochemical effects of skatole on animal production. Livest. Prod. Sci. 71:193-200. [Google Scholar]
- 7.Drake, H. L., K. Küsel, and C. Matthies. 2006. Acetogenic prokaryotes, p. 354-420. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt. (ed.), The prokaryotes, 3rd ed., vol. 2. Springer-Verlag, New York, NY. [Google Scholar]
- 8.Grunwald C., M. Vendrell, and B. B. Stowe. 1967. Evaluation of gas and other chromatographic separations of indolic methyl esters. Anal. Biochem. 20:484-494. [DOI] [PubMed] [Google Scholar]
- 9.Hespell, R. B., R. Wolf, and R. J. Bothast. 1987. Fermentation of xylans by Butyrivibrio fibrisolvens and other ruminal bacteria. Appl. Environ. Microbiol. 53:2849-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honeyfield, D. C., and J. R. Carlson. 1990. Assay for the enzymatic conversion of indoleacetic acid to 3-methylindole in a ruminal Lactobacillus species. Appl. Environ. Microbiol. 56:724-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu, T., S. L. Daniel, M. F. Lux, and H. L. Drake. 1990. Biotransformations of carboxylated aromatic compounds by the acetogen Clostridium thermoaceticum: generation of growth-supportive CO2 equivalents under CO2-limited conditions. J. Bacteriol. 172:212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu, T., M. F. Lux, and H. L. Drake. 1990. Expression of an aromatic-dependent decarboxylase which provides growth-essential CO2 equivalents for the acetogenic (Wood) pathway of Clostridium thermoaceticum. J. Bacteriol. 172:5901-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen, M. T., R. P. Cox, and B. B. Jensen. 1995. 3-Methylindole (skatole) and indole production by mixed populations of pig fecal bacteria. Appl. Environ. Microbiol. 61:3180-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusel, K., T. Dorsch, G. Acker, E. Stackebrandt, and H. L. Drake. 2000. Clostridium scatologenes strain SL1 isolated as an acetogenic bacterium from acidic sediments. Int. J. Syst. Evol. Microbiol. 50:537-546. [DOI] [PubMed] [Google Scholar]
- 15.Linden, A., D. Desmatch, S. Vandeput, M. L. van de Weerdt, and P. Lekeux. 1996. Effect of serotonergic blockade on calf pulmonary function after the intravenous administration of 3-methylindole. J. Comp. Pathol. 114:361-371. [DOI] [PubMed] [Google Scholar]
- 16.Liou, J. S.-C., D. L. Balkwill, G. R. Drake, and R. S. Tanner. 2005. Clostridium carboxidivorans sp. nov., a solvent-producing clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov. Int. J. Syst. Evol. Microbiol. 55:2085-2091. [DOI] [PubMed] [Google Scholar]
- 17.Lundstrom, K., B. Malmfors, G. Malmfors, S. Stern, H. Peterson, A. B. Mortensen, and S. E. Sorensen. 1988. Skatole, androstenone and taint in boars fed two different diets. Livest. Prod. Sci. 18:55-67. [Google Scholar]
- 18.Mackie, R. I., P. G. Stroot, and V. H. Varel. 1998. Biochemical identification and biological origin of key odor components in livestock waste. J. Anim. Sci. 76:1331-1342. [DOI] [PubMed] [Google Scholar]
- 19.Peleran, J. C., and G. F. Bories. 1985. Gas chromatographic determination and mass spectrometric confirmation of traces of indole and 3-methylindole (skatole) in pig back fat. J. Chromatogr. 324:469-474. [DOI] [PubMed] [Google Scholar]
- 20.Powers, J. C. 1968. The mass spectrometry of simple indoles. J. Org. Chem. 33:2044-2050. [Google Scholar]
- 21.Reinecke, D. M., and R. S. Bandurski. 1985. Metabolic conversion of 14C-indole-3-acetic acid to 14C-oxindole-3-acetic acid. Biochem. Biophys. Res. Commun. 103:429-433. [DOI] [PubMed] [Google Scholar]
- 22.Spoelstra, S. F. 1980. Origin of objectionable odorous components in piggery wastes and the possibility of applying indicator components for studying odour development. Agric. Environ. 5:241-260. [Google Scholar]
- 23.Thorton-Manning, J. R., W. K. Nichols, B. W. Manning, G. L. Skiles, and G. S. Yost. 1993. Metabolism and bioactivation of 3-methylindole by Clara cells, alveolar macrophages, and subcellular fractions from rabbit lungs. Toxicol. Appl. Pharmacol. 122:182-190. [DOI] [PubMed] [Google Scholar]
- 24.Whitehead, T. R., and M. A. Cotta. 2001. Characterization and comparison of microbial populations in swine feces and manure storage pits by 16S DNA gene sequence analyses. Anaerobe 7:181-187. [Google Scholar]
- 25.Whitehead, T. R., and H. J. Flint. 1995. Heterologous expression of an endoglucanase gene (endA) from the ruminal anaerobe Ruminococcus flavefaciens 17 in Streptococcus bovis and Streptococcus sanguis. FEMS Microbiol. Lett. 126:165-170. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama, M. T., and J. R. Carlson. 1974. Dissimilation of tryptophan and related indolic compounds by ruminal microorganisms in vitro. Appl. Microbiol. 27:540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokoyama, M. T., and J. R. Carlson. 1979. Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole. Am. J. Clin. Nutr. 32:173-178. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama, M. T., and J. R. Carlson. 1981. Production of skatole and para-cresol by a rumen Lactobacillus sp. Appl. Environ. Microbiol. 41:71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokoyama, M. T., J. R. Carlson, and L. V. Holdeman. 1977. Isolation and characteristics of a skatole-producing Lactobacillus sp. from the bovine rumen. Appl. Environ. Microbiol. 34:837-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zahn, J. A., J. L. Hatfield, Y. S. Do, A. A. DiSpirito, D. A. Laird, and R. L. Pfeiffer. 1997. Characterization of volatile organic emissions and wastes from swine production facilities. J. Environ. Qual. 26:1687-1696. [Google Scholar]