Abstract
The presence of the genes engBF (endo-α-N-acetylgalactosaminidase) and afcA (1,2-α-l-fucosidase) was detected in several intestinal Bifidobacterium isolates. Two strains of Bifidobacterium bifidum contained both genes, and they were able to degrade high-molecular weight porcine mucin in vitro. The expression of both genes was highly induced in the presence of mucin.
The human intestine is covered with a protective mucus layer, which plays an important role in the mucosal barrier system and is crucial for preventing adhesion and binding by many pathogens, toxins, and other damaging agents present in the intestinal lumen (5, 13). The mucus mainly consists of water (ca. 95%) and glycoproteins (1 to 10%), as well as electrolytes, antibodies, and nucleic acids (13). Furthermore, this mucus has been reported to serve as a source of nutrients for bacterial growth (4). Thus, bacteria that are able to survive, multiply, and colonize within the mucus layer display an adaptative advantage to persist in the gastrointestinal tract. Nevertheless, the interactions of the gut microbiota, especially probiotic bacteria, with intestinal mucus are poorly understood.
Bifidobacterium species are common inhabitants of the gastrointestinal tract, and they have received special attention because of their health-promoting effects in humans. The long history of safe use of these bacteria in the functional food industry remains the best proof of their safety, as the risk of infection is assumed to be very low (18). Adherence to mucus is one of the main in vitro tests for the study of probiotic strains (2), and some bifidobacteria have been found to be highly adhesive (8); however, mucin degradation has been considered an undesirable characteristic of probiotics (21), since it is believed that it could favor alteration of the intestinal mucosal barrier. In this context, the aim of this work was to evaluate the presence of genes potentially involved in mucus degradation and the ability of several Bifidobacterium strains to grow in the presence of mucus as the only carbon source and to degrade it.
Two bifidobacterial exocellular glycosidases potentially acting on sugar chains of mucin glycoproteins have been described (9). They have been functionally characterized by using synthetic substrates, but their involvement in intestinal mucin degradation has never been studied before. The product of the engBF gene of Bifidobacterium longum JCM1217 is an endo-α-N-acetylgalactosaminidase (E.C. 3.2.1.97). This enzyme catalyzes the hydrolysis of the O-glycosidic α-linkages between galactosyl β-1-3 N-acetylgalactosamine and a serine or threonine residue in mucin-type glycoproteins (6, 9). On the other hand, 1,2-α-l-fucosidases (E.C. 3.2.1.63) release terminal α-linked l-fucose from oligosaccharides of glycoconjugates, including mucin glycoproteins. A member of this last family of enzymes is present in Bifidobacterium bifidum JCM1254 (10, 14) and is encoded by the gene afcA. Both proteins are about 1,960 amino acids in length and contain a signal peptide and a membrane anchor at the N and C termini, respectively. Thus, they are predicted to be displayed on the bacterial surface, thereby enabling the catalytic domains to gain access to extracellular substrates.
In the present work, the presence of engBF and afcA was tested in 22 strains of Bifidobacterium (Table 1) by using the primers Fuc-F (5′-TTCAACGAGGAGACGCTGTGGACCGG) and Fuc-R (5′-GCCAGTAGTTCATCTGGAGGTTCAC-3′) and the primers Nac-F (5′-CGTCAACTGGCAGGATGGCGCAATC-3′) and Nac-R (5′-CACCTTGAAGTGCTGGATGAACTTAG-3′), designed to amplify internal fragments of 1,072 and 943 bp from the sequences of afcA (AY303700) and engBF (AY836679), respectively. The annealing temperature for both amplifications was 45°C. The amplified DNA fragments are located in the conserved sequence coding for the catalytic domain of the enzymes. None of the genes were detected in the Bifidobacterium animalis, Bifidobacterium pseudocatenulatum, and Bifidobacterium breve strains tested. This could be due to the lack of similar genes in the genomes of these microorganisms or to a relatively low identity at the DNA level between these three species and the species B. longum and B. bifidum, from which the afcA and engBF genes were initially sequenced. The gene engBF was present in all of the strains of B. longum (14 isolates) and B. bifidum (2 isolates), but only the B. bifidum strains contained afcA and engBF (Table 1). All of the PCR products were sequenced, and the predicted protein sequences were subjected to BLASTp analysis (20) and compared with homologous sequences by using as a query the sequences of B. bifidum D119 and L22 (Fig. 1). Both AfcA and EngBF internal fragments displayed the highest homology scores with several proteins from the normal gut microbiota (Ruminococcus sp., Clostridium perfringens, Bacteroides sp., and Enterococcus faecalis; identities of 37 to 74% for EngBF and of 28 to 39% for AfcA), indicating the ubiquity of these mucin-degrading enzymes among the bacteria populating the intestine.
TABLE 1.
Microorganisms used in this study and amplification by PCR of the genes afcA and engBF
| Strain | Origin | PCR producta
|
|
|---|---|---|---|
| afcA | engBF | ||
| B. animalis IPLA4549b | Culture collection | − | − |
| B. bifidum D119b | Human feces | + | + |
| B. bifidum L22b | Human feces | + | + |
| B. longum C51 | Human feces | − | + |
| B. longum C61b | Human intestinal mucosa | − | + |
| B. longum C72 | Human feces | − | + |
| B. longum D12b | Human feces | − | + |
| B. longum H64 | Human feces | − | + |
| B. longum H92 | Human feces | − | + |
| B. longum L23 | Human feces | − | + |
| B. longum L43 | Human feces | − | + |
| B. longum L44 | Human feces | − | + |
| B. longum L45 | Human feces | − | + |
| B. longum M14 | Human intestinal mucosa | − | + |
| B. longum M25b | Human feces | − | + |
| B. longum M44 | Human feces | − | + |
| B. longum NCIMB8809b | Culture collectionc | − | + |
| B. pseudocatenulatum E114b | Human feces | − | − |
| B. pseudocatenulatum E514 | Human feces | − | − |
| B. pseudocatenulatum M115 | Human intestinal mucosa | − | − |
| B. pseudocatenulatum M63b | Human feces | − | − |
| B. breve NCIMB8807b | Culture collectionc | − | − |
Positive amplification (+) indicates that the sequence of the PCR product is a homolog of that held in the NCBI database (accession numbers AY303700 and AY836679).
Strain selected for mucin-degrading assays.
NCIMB, National Collection of Industrial, Marine, and Food Bacteria.
FIG. 1.
Phylogenetic relationship analysis of the internal amino acid sequences of AfcA (Y665 to V950) (a) and EngBF (A614 to I903) (b). Database accession numbers are in parentheses. Trees were constructed with the software MEGA4 (www.megasoftware.net), with the matrix of pair distances between sequences by using the neighbor-joining cluster algorithm. The numbers at the branches are bootstrap values (confidence limits). The bar scale refers to the number of amino acid substitutions per site.
For growth experiments, a selection of Bifidobacterium strains was used, taking into account the species and the presence or absence of glycosidase genes (Table 1). Cells were grown in a defined medium (BM [see the supplemental material]) to evaluate their ability to use mucin as a carbon source. Fresh stabilized fecal samples (1) obtained from a healthy adult donor who had not received antibiotics for the previous 6 months were used as the positive control. Bacterial cultures grown overnight in 10 ml MRSC medium under standard conditions (16), and stabilized fecal samples were washed with the same volume of sterile 50 mM pH 7.0 phosphate buffer and resuspended in 2 ml of sterile Ringer solution (0.25 strength; Oxoid). This suspension was used to inoculate at 2% (vol/vol) 10 ml of BM with or without mucin (3 g/liter mucin from porcine stomach type III [Sigma]), and samples were withdrawn after 24 and 48 h of incubation. Differences in growth were evident among the two strains of B. bifidum (D119 and L22), B. breve NCIMB8807, and B. longum NCIMB8809, all of them displaying the highest growth in the presence of mucin after 48 h (Fig. 2). None of the B. animalis and B. pseudocatenulatum strains lacking both glycosidase genes or the three B. longum human isolates harboring the engBF gene reached differences in optical density at 600 nm (OD600) of greater than 0.5. In relation to this, it has previously been shown that B. bifidum was the only species among 29 Bifidobacterium species tested that was able to ferment porcine gastric mucin (3). Also, an analysis of 18 different intestinal bacterial species showed that only Ruminococcus torques and B. bifidum were able to partially ferment mucin (19). It is noteworthy that in a recent study using human fecal samples as inocula to colonize porcine gastric mucin, the sequences most commonly recovered from mucin were from B. bifidum and Ruminococcus, suggesting the competence of these bacteria to colonize this specific substrate (12).
FIG. 2.
OD600s of cultures of Bifidobacterium strains reaching values higher than 0.5 and a fecal sample growing in BM in absence (a) or presence (b) of mucin. The difference in OD600 between the presence and absence of mucin for 10 Bifidobacterium strains is depicted for cultures at 48 h (c). Results are the average of three independent cultures, and the coefficients of variation ranged between 1 and 35%.
Mucin degradation was analyzed by gel permeation chromatography. Samples obtained after 48 h of incubation from BM containing mucin were centrifuged, and supernatants were collected to check polymer degradation. Samples were isocratically separated at 0.450 ml/min by using 0.1 M NaNO3 in two columns of TSK-Gel G3000PWXL and G5000PWXL (Supelco) placed in series as previously described (17). Several concentrations of dextran standards (Fluka) with different molecular masses (5 × 103, 5 × 104, 8 × 104, 2.7 × 105, 6.7 × 105, 1.4 × 106, and 4.9 × 106 Da) were run for molecular mass calibration and quantification. In BM containing mucin, a high-molecular-mass peak (∼5 × 105 Da) and several small peaks (<1 × 103 Da) were apparent in the chromatograms (Fig. 3a), whereas after 48 h of incubation with stabilized fecal samples, the high-molecular-mass peak completely disappeared and changes in the other peaks were also detected. Thus, we considered the decrease in the highest-molecular-mass peak to be an indicator of mucin degradation. Accordingly, most of the strains tested were unable to degrade mucin (Fig. 3b). However, the two B. bifidum strains were able to degrade more than 80% of the high-molecular-mass mucin glycoproteins after 48 h of incubation. Furthermore, B. longum NCIMB8809 and B. breve NCIMB8807 displayed an intermediate level of degradation compared with the other Bifidobacterium strains, in spite of the fact that none of the genes were present in the B. breve strain. Thus, probably other genes, not considered in the present work, or genes with a similar function but with low homology to those used in the present study could also be involved in the degradation of mucin by B. breve NCIMB8807.
FIG. 3.
Degradation of mucin by 10 Bifidobacterium strains after 48 h of growth in BM in the presence of mucin. (a) Chromatograms of BM containing 0.3% mucin, the supernatant of the positive mucin-degradation control (feces) after 48 h of incubation, and B. bifidum L22 culture supernatant after 48 h of incubation. (b) Amount of the highest (∼5 × 105 Da) peak. The dotted line indicates the maximum value of mucin initially present in the culture medium. For sample peak quantification (μg/ml), the linear regression equations (R2 = 0.994 ± 0.004) for each standard were calculated from at least three replicated measurements of each concentration. A linear regression equation (R2 = 0.995) was also calculated to determine the molecular masses (in daltons) of the sample peaks.
Considering the aforementioned results, the two B. bifidum strains analyzed in this study seem to possess a higher capacity to degrade intestinal mucin. To establish a correlation between this capacity and the likely involvement of afcA and engBF in mucin degradation, the influence of mucin and/or glucose on the expression levels of the afcA and engBF genes in strain B. bifidum L22 was assessed. The bacterium was grown in BM in the presence of 20 g/liter glucose and/or 3 g/liter mucin to an OD600 of 1 ± 0.2 as described above. Specific primers were designed by using the sequences obtained from the genes afcA (forward, 5′-ACACCGCCGTCAAGAAAGC-3′; reverse, 5′-CGATCTTCACGCGGTCGTA-3′) and engBF (forward, 5′-GCTCCCAGGCGCAGAAC-3′; reverse, 5′-TGTTGAGGGCGACCTTCTTG-3′) of B. bifidum L22. Lysis, total RNA extraction, and quantitative PCR analysis were carried out as previously described (7), with an ABI Prism 7500 machine (Applied Biosystems). Both genes were strongly induced in the presence of mucin and mucin plus glucose but not in the presence of glucose alone (Fig. 4). This suggests that these two mucin-degrading glycosidases are transcriptionally regulated in B. bifidum by the presence of mucin.
FIG. 4.
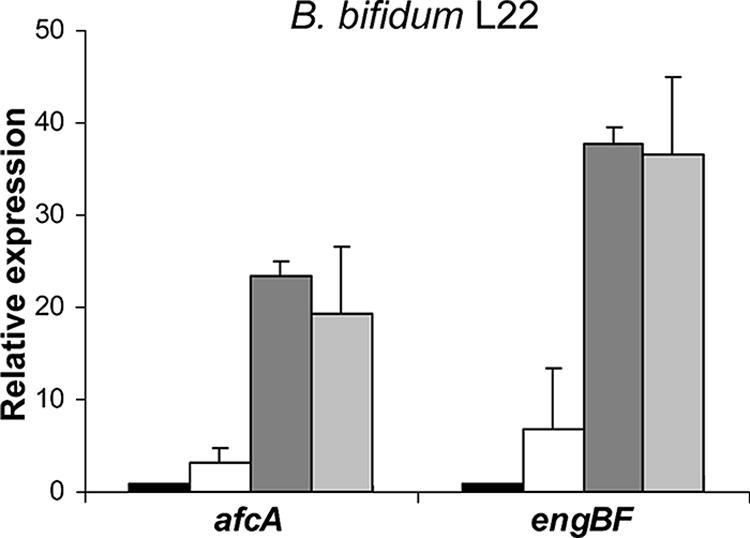
Levels of B. bifidum L22 afcA and engBF gene expression in four culture media, i.e., BM (black), BM plus 2% glucose (white), BM plus 0.3% mucin (dark gray), and BM plus 2% glucose plus 0.3% mucin (light gray). The expression levels shown are relative to those obtained in the control culture (BM). Experiments were carried out in duplicate and analyzed in duplicate in two independent PCR runs.
In summary, we have shown for the first time that several intestinal bifidobacteria, especially two B. bifidum isolates, are able to degrade intestinal mucin in vitro to different extents. This degradation capacity seems to have a correlation with the presence of two genes coding for extracellular glycosidases, afcA and engBF. Intraspecies differences were also detected in B. longum, since only strain NCIMB8809 was able to degrade mucin significantly; however, all B. longum strains have engBF, suggesting that the presence of this gene is not the only factor affecting the degradation capacity in question. In addition, B. breve NCIMB8807 is able to degrade mucin to some extent but it seems not to harbor these genes. The reactions catalyzed by the products of the afcA and engBF genes could represent the first step in the degradation of mucin sugars by B. longum and B. bifidum. The galactosyl β-1-3 N-acetylgalactosamine released extracellularly from mucin glycoconjugates by EngBF could be transported into the cell by a putative ABC transporter, entering the galacto-N-biose metabolic pathway recently described in B. longum (11, 15) and finally being metabolized through the glycolytic pathway or by amino sugar metabolism, thus serving as a carbon and energy source for bifidobacteria. Results shown here open an interesting debate on probiotic safety criteria regarding intestinal mucus degradation, which was until now considered a hazardous selection property.
Nucleotide sequence accession numbers.
The partial nucleotide sequences of afcA obtained in this study are available in the GenBank database under accession numbers EU260397 and EU260398 for positive strains appearing in the same order given in Table 1 from top to bottom. The partial nucleotide sequences of engBF obtained in this study are available in the GenBank database under accession numbers EU260399 to EU260414 for positive strains appearing in the same order given in Table 1 from top to bottom.
Supplementary Material
Acknowledgments
This work was financed by FEDER funds (European Union) and the Spanish Plan Nacional de I+D+i through projects AGL 2004-06088-CO2-01/ALI and AGL 2004-06727-CO2-01/ALI. M. Fernández-García was the recipient of a technician I3P contract from CSIC, and M. Gueimonde was funded by a Juan de la Cierva postdoctoral contract from the Spanish Ministry of Education and Science.
Baltasar Mayo is acknowledged for the kind supply of the strains used in this study.
Footnotes
Published ahead of print on 25 January 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Al-Tamimi, M. A. H. M., R. J. Palfram, J. M. Cooper, G. R. Gibson, and R. A. Rastall. 2006. In vitro fermentation of sugar beet arabinan and arabino-oligosaccharides by the human gut microflora. J. Appl. Microbiol. 100:407-414. [DOI] [PubMed] [Google Scholar]
- 2.Araya, M., L. Morelli, G. Reid, M. E. Sanders, and C. Stanton. 2006. Guidelines for the evaluation of probiotics in foods. Food and Agriculture Organization of the United Nations-World Health Organization report. ftp://ftp.fao.org/docrep/fao/009/a0512e/a0512e00.pdf.
- 3.Crociani, F., A. Alessandrini, M. M. Mucci, and B. Biavati. 1994. Degradation of complex carbohydrates by Bifidobacterium spp. Int. J. Food Microbiol. 24:199-210. [DOI] [PubMed] [Google Scholar]
- 4.Derrien, M., E. E. Vaughan, C. M. Plugge, and W. M. de Vos. 2004. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 54:1469-1476. [DOI] [PubMed] [Google Scholar]
- 5.Florey, H. 1955. Mucin and the protection of the body. Proc. R. Soc. Lond. B 143:144-158. [DOI] [PubMed] [Google Scholar]
- 6.Fujita, K., F. Oura, N. Nagamine, T. Katayama, J. Hiratake, K. Sakata, H. Kumagai, and K. Yamamoto. 2005. Identification and molecular cloning of a novel glycoside hydrolase family of core 1 type O-glycan-specific endo-α-N-acetylgalactosaminidase from Bifidobacterium longum. J. Biol. Chem. 280:37415-37422. [DOI] [PubMed] [Google Scholar]
- 7.Gueimonde, M., L. Noriega, A. Margolles, and C. G. de los Reyes-Gavilán. 2007. Induction of α-l-arabinofuranosidase activity by monomeric carbohydrates in Bifidobacterium longum and ubiquity of encoding genes. Arch. Microbiol. 187:145-153. [DOI] [PubMed] [Google Scholar]
- 8.Gueimonde, M., L. Noriega, A. Margolles, C. G. de los Reyes-Gavilán, and S. Salminen. 2005. Ability of Bifidobacterium strains with acquired resistance to bile to adhere to human intestinal mucus. Int. J. Food Microbiol. 101:341-346. [DOI] [PubMed] [Google Scholar]
- 9.Katayama, T., K. Fujita, and K. Yamamoto. 2005. Novel bifidobacterial glycosidases acting on sugar chains of mucin glycoproteins. J. Biosci. Bioeng. 99:457-465. [DOI] [PubMed] [Google Scholar]
- 10.Katayama, T., A. Sakuma, T. Kimura, Y. Makimura, J. Hiratake, K. Sakata, T. Yamanoi, H. Kumagai, and K. Yamamoto. 2004. Molecular cloning and characterization of Bifidobacterium bifidum 1,2-α-l-fucosidase (AfcA), a novel inverting glycosidase (glycosidase hydrolase family 95). J. Bacteriol. 186:4885-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitaoka, M., J. Tian, and M. Nishimoto. 2005. Novel putative galactose operon involving lacto-N-biose phosphorylase in Bifidobacterium longum. Appl. Environ. Microbiol. 71:3158-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitch, E. C., A. W. Walker, S. H. Duncan, G. Holtrop, and H. J. Flint. 2007. Selective colonization of insoluble substrates by human faecal bacteria. Environ. Microbiol. 9:667-679. [DOI] [PubMed] [Google Scholar]
- 13.Macfarlane, S., E. J. Woodmansey, and G. T. Macfarlane. 2005. Colonization of mucin by human intestinal bacteria and establishment of biofilm communities in a two-stage continuous culture system. Appl. Environ. Microbiol. 71:7483-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagae, M., A. Tsuchiya, T. Katayama, K. Yamamoto, S. Wakatsuki, and R. Kato. 2007. Structural basis of the catalytic reaction mechanism of novel 1,2-α-l-fucosidase from Bifidobacterium bifidum. J. Biol. Chem. 282:18497-18509. [DOI] [PubMed] [Google Scholar]
- 15.Nishimoto, M., and M. Kitaoka. 2007. Identification of N-acetylhexosamine 1-kinase in the complete lacto-N-biose I/galacto-N-biose metabolic pathway in Bifidobacterium longum. Appl. Environ. Microbiol. 73:6444-6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruas-Madiedo, P., A. Hernández-Barranco, A. Margolles, and C. G. de los Reyes-Gavilán. 2005. A bile salt-resistant derivative of Bifidobacterium animalis has an altered fermentation pattern when grown on glucose and maltose. Appl. Environ. Microbiol. 71:6564-6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruas-Madiedo, P., R. Tuinier, M. Kanning, and P. Zoon. 2002. Role of exopolysaccharides produced by Lactococcus lactis subsp. cremoris on the viscosity of fermented milks. Int. Dairy J. 12:689-695. [Google Scholar]
- 18.Salminen, S., A. von Wright, L. Morelli, P. Marteau, D. Brassart, W. M. de Vos, R. Fondén, M. Saxelin, K. Collins, G. Mogensen, S. E. Birkeland, and T. Mattila-Sandholm. 1998. Demonstration of safety of probiotics—a review. Int. J. Food Microbiol. 44:93-106. [DOI] [PubMed] [Google Scholar]
- 19.Salyers, A. A., S. E. West, J. R. Vercellotti, and T. D. Wilkins. 1977. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl. Environ. Microbiol. 34:529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye, J., S. McGinnis, and T. L. Madden. 2006. BLAST: improvements for better sequence analysis. Nucleic Acids Res. 34(Web Server Issue):W6-W9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou, J. S., P. K. Gopal, and H. S. Gill. 2001. Potential probiotic lactic acid bacteria Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019) do not degrade gastric mucin in vitro. Int. J. Food Microbiol. 63:81-90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





