Abstract
The diversity of populations of yeast and lactic acid bacteria (LAB) in pig feeds fermented at 10, 15, or 20°C was characterized by rRNA gene sequencing of isolates. The feeds consisted of a cereal grain mix blended with wet wheat distillers' grains (WWDG feed), whey (W feed), or tap water (WAT feed). Fermentation proceeded for 5 days without disturbance, followed by 14 days of daily simulated feed outtakes, in which 80% of the contents were replaced with fresh feed mixtures. In WWDG feed, Pichia galeiformis became the dominant yeast species, independent of the fermentation temperature and feed change. The LAB population was dominated by Pediococcus pentosaceus at the start of the fermentation period. After 3 days, the Lactobacillus plantarum population started to increase in feeds at all temperatures. The diversity of LAB increased after the addition of fresh feed components. In W feed, Kluyveromyces marxianus dominated, but after the feed change, the population diversity increased. With increasing fermentation temperatures, there was a shift toward Pichia membranifaciens as the dominant species. L. plantarum was the most prevalent LAB in W feed. The WAT feed had a diverse microbial flora, and the yeast population changed throughout the whole fermentation period. Pichia anomala was the most prevalent yeast species, with increasing occurrence at higher fermentation temperatures. Pediococcus pentosaceus was the most prevalent LAB, but after the feed change, L. plantarum started to proliferate. The present study demonstrates that the species composition in fermented pig feed may vary considerably, even if viable cell counts indicate stable microbial populations.
The use of fermented wet feed for pigs has increased worldwide during the last decade, incorporating a variety of fermentation techniques and feedstuffs (6, 30). Fermented feed is produced by the incubation of solid material (e.g., cereal grains) together with a liquid phase (water or a coproduct from food or ethanol production, e.g., whey or wet wheat distillers' grains). During the incubation, fermentative microorganisms produce organic acids, which may reduce the pH to 3.5 to 4.5. The production of food and ethanol generates considerable amounts of coproducts. This effect has been exacerbated by the rapid increase in the production of biofuel ethanol from corn and other cereals. Using these by-products to produce liquid feed avoids wasteful disposal and can thus decrease costs and the environmental burden (5, 31). Whey and wet wheat distillers' grains may contain high numbers of microorganisms that can also positively stimulate the development of a beneficial microbial population in feed (20, 30).
The final hygienic and nutritional properties of the feed will depend on the substrates and fermentation process used (4, 20). Fermented wet feed reduces the pH and the number of coliform bacteria in the animal gastrointestinal tract (9, 24, 30). Daily weight gain, feed conversion ratios, and gastrointestinal health can also be improved (4, 12). Lactic acid bacteria (LAB) in fermented wet feed may have positive effects on the lower-gut microflora (37). The acidification of feed by the microbial metabolism may reduce the emptying rate of the stomach (22) and stimulate the secretion of proteolytic enzymes (11). The proliferation of spoilage organisms and food-borne pathogens can be prevented by low pH and high concentrations of lactic and acetic acids (2-4, 38). Lyberg et al. (20) found that the pH decreased to approximately 4.0 during the first 3 to 5 days of feed fermentation and that this reduction reduced the levels of enterobacteria in the fermented feed. The reduction of pH in liquid feeds by fermentation would therefore be a cost-effective method of reducing enteropathogens and spoilage organisms in the diet (9). Yeasts are sometimes considered undesirable in liquid diets, because they may confer off-flavors and taints that would affect the palatability of the feed (4). Yeasts may, on the other hand, inhibit mold growth (26) and may induce positive effects in the gastrointestinal tract (32). One concern is that yeast metabolism can convert starch into alcohol and carbon dioxide, which may result in high ethanol contents and losses of energy due to carbon dioxide production (5). However, in spite of high yeast CFU numbers during fermentation, we previously found only low levels of ethanol and small losses of weight in the feed (20).
With the exception of pathogens, the microbial community in fermented feed has been characterized only by the quantification of certain groups of fermenting microorganisms, e.g., LAB and yeasts (3, 6, 25). We recently performed an analysis of chemical parameters in different fermented feed mixtures, together with a quantitative analysis of the microbial populations (20). However, very little is known about the species-level identities of the organisms involved. Fluctuations in the composition of the microbial population that are not detected by quantification but that will still have an effect on the feed quality may thus occur. The aim of this study was to characterize and identify the LAB and yeasts involved in the fermentation processes for three different pig feeds that consisted of the same dry cereal base in combination with wet wheat distillers' grains, whey, or water as liquid components and that were fermented at different temperatures.
MATERIALS AND METHODS
Feed fermentations.
In a recent study (20), we quantified several groups of microorganisms in feed consisting of different materials and fermented at different temperatures. The cereal grain mix consisted of equal amounts of wheat, barley, and triticale. The dry cereal mix was stored at room temperature in paper bags. Wet wheat distillers' grains and whey were stored at 2°C but were transferred to room temperature 24 h before use to adapt the microbial flora to fermentation conditions. From these materials, liquid feeds were prepared. Briefly, cereal grains were mixed with wet wheat distillers' grains (spirits from Absolut, Åhus, Sweden) to obtain WWDG feed; whey (2 to 3 g of lactic acid liter−1, a maximum of 1 g of acetic acid liter−1, and butanediol or ethanol, pH 4.8; Milko, Bollnäs, Sweden), a coproduct from cheese production, to obtain W feed; or tap water to obtain WAT feed. Each kilogram of liquid feed contained approximately 25% dry matter. Mixtures were incubated at 10, 15, or 20°C. After the initial 5 days of fermentation, 80% of the contents were replaced with fresh liquids and cereal grains daily for the following 14 days, with 20% left each time as the inoculant for the fresh feed mix. The replacement was made to simulate feed outtakes. Sampling was done from the replaced material (20). We used quantification plates with de Man-Rogosa-Sharp (MRS) agar (Oxoid Ltd., Basingstoke, Hampshire, England) supplemented with 100 μg ml−1 Delvocid (active compound, natamycin; Gist-Brocades B.V., Ma Delft, The Netherlands) and quantification plates with malt extract agar (Oxoid Ltd., Basingstoke, Hampshire, England) supplemented with 100 μg ml−1 chloramphenicol (Sigma-Aldrich Inc., St. Louis, MO) to identify the LAB and yeasts, respectively, found in the fermented feed and in the raw materials during storage. Microbial population development was also characterized by analyses of the feed components (i.e., cereal grains, wet wheat distiller's grains, whey, and water) at the start of storage and after 20 days of storage. Cultivation-based quantification of microorganisms has been used previously as a standard method for the investigation of the quality of fermented feed (3, 6, 25). It is, at any rate, possible that the microbial diversity is underestimated by culture-dependent identification. In several ecosystems, additional species have been found by using culture-independent identification methods, although the results in general were in good agreement with those of culture-dependent methods (see, e.g., references 21 and 36).
Yeast and LAB sampling and strain conservation.
On each sampling occasion, 10 yeast colonies and 10 LAB colonies were selected randomly from the quantification plates (20) corresponding to the fermented feed and the feed component materials. Yeast colonies were inoculated into 4 ml of yeast extract-peptone-d-glucose (YPD) broth (yeast extract, 10 g liter−1 [Oxoid Ltd., Basingstoke, Hampshire, England]; bacteriological peptone, 20 g liter−1 [Oxoid Ltd., Basingstoke, Hampshire, England]; and d-glucose, 20 g liter−1 [VWR International Ltd., Poole, England]) and incubated on a rotary shaker at 25°C overnight. For strain conservation, 1 ml of the cell suspension was mixed with an equal volume of glycerol and the mixture was frozen at −70°C. LAB colonies were inoculated into 10 ml of MRS broth (Oxoid Ltd., Basingstoke, Hampshire, England) and incubated at 30°C for 24 h. Cells were harvested by centrifugation at 4,000 × g for 5 min, and the pellet was suspended in cryomedium (K2HPO4, 0.82 g liter−1; KH2PO4, 0.18 g liter−1; C6H5Na3O7·H2O, 0.67 g liter−1; MgSO4·7H2O, 0.25 g liter−1; and glycerol [Merck KGaA, Darmstadt, Germany] to 15% of the total volume) and frozen at −70°C.
DNA extraction, amplification, and identification.
Yeasts were harvested from 1 ml of the yeast extract-peptone-d-glucose overnight culture (see above) by centrifugation, and total DNA was extracted by shaking the pellet in a detergent buffer (7). LAB DNA was isolated by using the DNeasy tissue kit (Qiagen, Hilden, Germany). Genotypic differentiation was performed using repetitive DNA element PCR (rep-PCR) fingerprinting with the microsatellite primer (GTG)5 (MetaBion, Munich, Germany) (16). For yeasts, the PCR sample was mixed according to the recommendations of the Taq supplier (Takara Bio Inc., Shiga, Japan). LAB fingerprints were generated using PuReTaq ready-to-go PCR beads (Amersham Biosciences, Buckinghamshire, England) mixed with (GTG)5 primer and DNA according to the instructions in the supplier's manual. Amplification was performed in an MJ mini personal thermal cycler (Bio-Rad Laboratories Inc.). Reaction conditions for yeast fingerprints were initial denaturation at 94°C for 2 min followed by 29 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 2 min, with a final extension step at 72°C for 5 min; for LAB fingerprints, the reaction conditions were initial denaturation at 95°C for 7 min followed by 29 cycles of 90°C for 30 s, 95°C for 1 min, 40°C for 1 min, and 65°C for 4 min, with a final extension step at 65°C for 16 min. Amplification products were submitted to electrophoresis in 1% agarose gel in 0.5× Tris-borate-EDTA buffer, as follows: for yeasts, the settings were 5.1 V cm−1 and 80 mA for 120 min, and for LAB, they were 2.5 V cm−1 for 30 min followed by 1 V cm−1 for 16 h. PCR products were visualized by ethidium bromide staining, and images were recorded using a digital photo documentation system, Quantity One one-dimensional analysis software (Bio-Rad Laboratories Inc.). A standardized system for yeast identification, ID32C (bioMérieux, Marcy l'Etoile, France), was used for yeasts that did not give a fingerprint pattern with the microsatellite (GTG)5 primer. For sequence analyses of selected yeasts, the D1-D2 region (approximately 600 bp) in the 25S rRNA gene was amplified using primers NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) (35). For LAB sequence analysis, nearly the entire 16S rRNA gene was amplified using primers specific for the domain Bacteria: 16SS (5′-AGAGTTTGATCCTGGCTC-3′) and 16SR (5′-GGGAACGTATTCACCG-3′) (27). Reaction conditions for yeasts were as stated above, and those for LAB were as follows: initial denaturation at 94°C for 5 min, followed by 29 cycles of 94°C for 30 s, 49°C for 30 s, and 72°C for 2 min, with a final extension step at 72°C for 10 min. Electrophoresis settings were 5.6 V cm−1 and 80 mA for approximately 1 h for both yeasts and LAB. Resulting PCR products were purified using a PCR purification kit (Qiagen, Hilden, Germany). Purified fragments were sequenced by standard methods with primer NL4 for yeasts and with primer 16SS for LAB. Sequence comparison against the EMBL database (http://www.ebi.ac.uk) was performed using the NCBI BLAST2 program. The level of sequence similarity defining a positive match to a known species was set at 99% for yeast and 98% for LAB (1, 14).
Computer-assisted analysis of DNA patterns from the (GTG)5 primer.
Rep-PCR fingerprints for yeast and LAB were analyzed using GelCompar II version 4.5 software (Applied Maths, Kortrijk, Belgium). During gel image processing, band position tolerance and optimization were both set to 1%. A dendrogram was constructed using the Pearson correlation (0.0 to 100%) and the unweighted-pair group method with arithmetic means. From the dendrogram, yeasts and LAB at the Pearson correlation 80% homogeneity level were selected, and rRNA genes were sequenced to evaluate population diversity in the fermented feeds.
RESULTS
One isolate corresponding to each cluster of similar rep-PCR fingerprints (Fig. 1) was identified through sequence analysis and was thereafter considered to be representative of the whole cluster of isolates. In most cases, neighboring clusters in the dendrogram were also found to belong to the same species, indicating that the resolution of the clustering fingerprints was below the species level. However, in two cases, species were incorrectly incorporated into the Cryptococcus satoi or the Pichia membranifaciens group. These isolates were judged by rRNA gene sequencing to be unknown Cryptococcus spp. (see Tables 3 and 4). A group of yeast isolates did not yield fingerprints with the (GTG)5 primer (see, for instance, Fig. 1, lanes 11 and 12). These isolates were identified as Rhodotorula glutinis by using the ID32 kit. Also, the amplification of the D1-D2 region using the NL1 and NL4 primers did result in PCR products. The sequences of these products matched Rhodotorula glutinis sequences, in accordance with the results from the physiological identification.
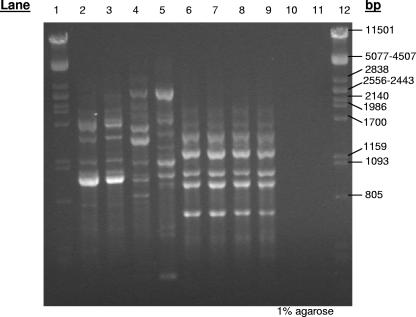
FIG. 1.
GTG fingerprints of yeast isolates (collected on days 0, 3, and 19) from the WAT feed fermented at 10°C. Isolates were grouped accordingly in a dendrogram constructed by GelCompar II version 4.5 software and further identified by rRNA gene sequencing using primers NL1 and NL4. Isolates corresponding to lanes 2 and 3 of this gel (identified as Cryptococcus strains) were grouped together, lane 4 (Kluyveromyces marxianus) and lane 5 (Torulaspora delbrueckii) correspond to individual isolates, and isolates corresponding to lanes 6 to 9 were grouped together and identified as Pichia anomala. For the isolates corresponding to lanes 10 and 11, no GTG fingerprints were obtained. Sequencing of the D1-D2 region identified these isolates as Rhodotorula glutinis. Lanes 1 and 12 represent molecular size markers from λ DNA digested with PstI.
TABLE 3.
Microbial counts and yeast and LAB species (identified by rRNA gene sequencing) in W feed mix during 19-day fermentations at 10, 15, and 20°Ca
| Species | No. of isolates on day 0 from sample fermented at:
|
No. of isolates on day 3 from sample fermented at:
|
No. of isolates on day 5 from sample fermented at:
|
No. of isolates on day 7 from sample fermented at:
|
No. of isolates on day 19 from sample fermented at:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10°C | 15°C | 20°C | 10°C | 15°C | 20°C | 10°C | 15°C | 20°C | 10°C | 15°C | 20°C | 10°C | 15°C | 20°C | |
| Yeast species | |||||||||||||||
| Cryptococcus sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1c | 0 | 0 | 0 | 0 | 0 |
| Kluyveromyces marxianus | 6 | 6 | 6 | 7 | 4 | 3 | 5 | 5 | 3 | 1 | 3 | 4 | 3 | 1 | 0 |
| Pichia galeiformis | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 4 | 0 | 0 |
| Pichia membranifaciens | 3 | 3 | 3 | 3 | 6 | 7 | 4 | 5 | 7 | 8 | 5 | 6 | 3 | 9 | 7 |
| Saccharomyces bayanus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Total no. of yeast CFU (log10 CFU g−1b) | 5.2 | 5.2 | 5.2 | 5.7 | 6.1 | 6.6 | 5.7 | 7.3 | 7.2 | 5.3 | 6.5 | 7.8 | 5.8 | 5.7 | 7.0 |
| LAB species | |||||||||||||||
| Lactobacillus plantarum | 10 | 10 | 10 | 8 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 9 | 9 | 9 | 8 |
| Lactobacillus sakei | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| Lactococcus lactis | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leuconostoc citreum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Total no. of LAB CFU (log10 CFU g−1b) | 7.0 | 7.0 | 7.0 | 9.5 | 8.9 | 8.8 | 10.1 | 10.5 | 10.7 | 10.5 | 11.5 | 11.4 | 11.8 | 12.4 | 12.7 |
Data are given as numbers of isolates of individual species among 10 isolates from each feed sample.
CFU values are from reference 20 and are mean values (n = 3).
Identity of <99% to the closest known species.
TABLE 4.
Microbial counts and yeast and LAB species (identified by rRNA gene sequencing) in WAT feed mix during 19-day fermentations at 10, 15, and 20°Ca
| Species | No. of isolates on day 0 from sample fermented at:
|
No. of isolates on day 3 from sample fermented at:
|
No. of isolates on day 5 from sample fermented at:
|
No. of isolates on day 7 from sample fermented at:
|
No. of isolates on day 19 from sample fermented at:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10°C | 15°C | 20°C | 10°C | 15°C | 20°C | 10°C | 15°C | 20°C | 10°C | 15°C | 20°C | 10°C | 15°C | 20°C | |
| Yeast species | |||||||||||||||
| Aureobasidium pullulans | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Candida allociferrii | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 0 |
| Cryptococcus adeliensis | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Cryptococcus festucosus | 1 | 1 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cryptococcus flavescens | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cryptococcus satoi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cryptococcus spp. | 0 | 0 | 0 | 1c | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1c | 0 | 0 |
| Issatchenkia orientalis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Kluyveromyces marxianus | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 1 | 0 | 0 |
| Pichia anomala | 2 | 6 | 6 | 3 | 6 | 4 | 2 | 7 | 7 | 3 | 3 | 4 | 5 | 8 | 7 |
| Pichia membranifaciens | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 |
| Rhodotorula glutinis | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Saccharomyces bayanus | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 5 | 0 | 0 | 0 |
| Saccharomyces cerevisiae | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Sporobolomyces ruberrimus | 3 | 3 | 3 | 3 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Torulaspora delbrueckii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Total no. of yeast CFU (log10 CFU g−1b) | 2.1 | 2.1 | 2.1 | 1.0 | 2.1 | 1.4 | 1.3 | 2.7 | 3.4 | 1.3 | 2.2 | 3.3 | 3.9 | 6.4 | 6.6 |
| LAB species | |||||||||||||||
| Lactobacillus curvatus | 0 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Lactobacillus plantarum | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 3 | 0 | 3 | 2 | 0 | 9 | 9 |
| Lactobacillus sakei | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Lactococcus garvieae | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lactococcus lactis | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leuconostoc citreum | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 |
| Pediococcus pentosaceus | 8 | 9 | 9 | 5 | 8 | 9 | 6 | 7 | 7 | 7 | 6 | 7 | 9 | 0 | 1 |
| Weissella cibaria | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total no. of LAB CFU (log10 CFU g−1b) | 3.3 | 3.3 | 3.3 | 6.6 | 8.9 | 8.8 | 7.6 | 4.7 | 9.3 | 9.3 | 4.6 | 11.2 | 9.7 | 12.7 | 11.8 |
Data are given as numbers of isolates of individual species among 10 isolates from each feed sample.
CFU values are from reference 20 and are mean values (n = 3).
Identity of <99% to the closest known species.
Microbial composition of feed components before and after storage.
Yeasts and LAB present in the different feed components before and after storage are shown in Table 1. No yeasts were found in the wet wheat distillers' grains prior to storage. However, after 20 days of storage at 2°C, Pichia galeiformis was isolated as the sole yeast. Both before and after storage, Pediococcus pentosaceus was the only LAB isolated from wet wheat distillers' grains. In whey, the yeast population consisted mostly of Pichia membranifaciens and Kluyveromyces marxianus. After 20 days of storage, the population was dominated by Kluyveromyces marxianus. The LAB population was dominated by Lactobacillus plantarum both before and after 20 days of storage. The cereal grain mix had a more diverse yeast flora, which consisted of Pichia anomala, Rhodotorula glutinis, Sporobolomyces ruberrimus, Aureobasidium pullulans, and Cryptococcus adeliensis. The LAB Pediococcus pentosaceus, Lactobacillus plantarum, Lactococcus lactis, and Lactococcus garvieae were identified in the cereal grain mix. The species composition of yeast and LAB populations did not change during grain mix storage (Table 1). No LAB or yeasts were found in the tap water.
TABLE 1.
Microbial counts and yeast and LAB species (identified by rRNA gene sequencing) in feed components before and after storagea
| Species | No. of isolates from wet wheat distillers' grain on day:
|
No. of isolates from whey on day:
|
No. of isolates from cereal grain mix on day:
|
|||
|---|---|---|---|---|---|---|
| 0 | 20 | 0 | 20 | 0 | 20 | |
| Yeast species | ||||||
| Aureobasidium pullulans | 0 | 0 | 0 | 0 | 1 | 1 |
| Cryptococcus adeliensis | 0 | 0 | 0 | 0 | 1 | 1 |
| Cryptococcus festucosus | 0 | 0 | 1 | 0 | 0 | 0 |
| Kluyveromyces marxianus | 0 | 0 | 4 | 7 | 0 | 1 |
| Pichia anomala | 0 | 0 | 0 | 0 | 4 | 3 |
| Pichia galeiformis | 0 | 10 | 0 | 0 | 0 | 0 |
| Pichia membranifaciens | 0 | 0 | 5 | 3 | 0 | 0 |
| Rhodotorula glutinis | 0 | 0 | 0 | 0 | 3 | 3 |
| Sporobolomyces ruberrimus | 0 | 0 | 0 | 0 | 1 | 1 |
| Total no. of yeast CFU (log10 CFU g−1b) | NDc | 3.4 | 5.2 | 5.1 | 2.5 | 2.8 |
| LAB species | ||||||
| Enterococcus faecium | 0 | 0 | 0 | 2 | 0 | 0 |
| Lactobacillus plantarum | 0 | 0 | 6 | 5 | 3 | 3 |
| Lactococcus garvieae | 0 | 0 | 0 | 0 | 1 | 1 |
| Lactococcus lactis | 0 | 0 | 0 | 1 | 2 | 1 |
| Pediococcus pentosaceus | 10 | 10 | 4 | 2 | 4 | 5 |
| Total no. of LAB CFU (log10 CFU g−1b) | 3.7 | 3.4 | 7.1 | 8.6 | 5.5 | 6.5 |
Data are given as numbers of isolates of individual species among 10 isolates from each feed sample.
CFU values are from reference 20 and are mean values (n = 3).
ND, not detected.
Microbial composition of WWDG feed mix.
During the first 7 days of fermentation of WWDG feed at 10°C, yeast counts were below the detection limit. At higher temperatures (15 and 20°C), yeasts were detected earlier during the fermentation (Table 2). Independent of the fermentation temperature, Pichia galeiformis was the most prevalent yeast in WWDG feed. This species was also the only yeast isolated from wet wheat distillers' grains after storage (Table 1). Apart from Pichia galeiformis, the growth of Pichia membranifaciens was detected in WWDG feed fermented at 10°C. Pichia anomala was isolated from samples incubated at 20°C for 19 days (Table 2). The LAB population in WWDG feed was dominated by Pediococcus pentosaceus during the initial 5 days of fermentation. After feed replacement, the Pediococcus pentosaceus population started to decrease, whereas that of Lactobacillus plantarum increased. At low temperatures (10 and 15°C), the LAB population at day 19 was still dominated by Pediococcus pentosaceus, whereas Lactobacillus plantarum was the dominating LAB at 20°C (Table 2).
TABLE 2.
Microbial counts and yeast and LAB species (identified by rRNA gene sequencing) in WWDG feed mix during 19-day fermentations at 10, 15, and 20°Ca
| Species | No. of isolates on day 0 from sample fermented at:
|
No. of isolates on day 3 from sample fermented at:
|
No. of isolates on day 5 from sample fermented at:
|
No. of isolates on day 7 from sample fermented at:
|
No. of isolates on day 19 from sample fermented at:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10°C | 15°C | 20°C | 10°C | 15°C | 20°C | 10°C | 15°C | 20°C | 10°C | 15°C | 20°C | 10°C | 15°C | 20°C | |
| Yeast species | |||||||||||||||
| Kluyveromyces marxianus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pichia anomala | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Pichia galeiformis | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 6 | 8 | 10 | 8 | 9 | 8 | 10 | 6 |
| Pichia membranifaciens | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 2 | 0 | 2 | 1 | 2 | 0 | 2 |
| Total no. of yeast CFU (log10 CFU g−1b) | NDc | ND | ND | ND | ND | 2.1 | ND | 2.1 | 3.1 | 2.0 | 2.1 | 2.1 | 3.3 | 3.1 | 5.2 |
| LAB species | |||||||||||||||
| Lactobacillus diolivorans | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Lactobacillus plantarum | 0 | 0 | 0 | 0 | 4 | 2 | 0 | 3 | 2 | 2 | 3 | 2 | 1 | 1 | 6 |
| Lactobacillus sakei | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Lactococcus lactis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| Leuconostoc citreum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 |
| Pediococcus pentosaceus | 10 | 10 | 10 | 10 | 6 | 8 | 9 | 7 | 8 | 8 | 4 | 8 | 4 | 6 | 3 |
| Total no. of LAB CFU (log10 CFU g−1b) | 5.5 | 5.5 | 5.5 | ND | 5.3 | 5.6 | 3.8 | 4.6 | 4.9 | 4.5 | 6.2 | 5.7 | 7.3 | 5.9 | 7.4 |
Data are given as numbers of isolates of individual species among 10 isolates from each sampling occasion.
CFU values are from reference 20 and are mean values (n = 3).
ND, not detected.
Microbial composition of W feed mix.
The yeast population in W feed mix consisted mainly of Kluyveromyces marxianus and Pichia membranifaciens (Table 3), which probably originated from the whey (Table 1). Kluyveromyces marxianus was the dominant species at the start of fermentation, regardless of the fermentation temperature. However, during fermentation at 15 and 20°C, the Pichia membranifaciens population increased to make this yeast the most prevalent species, and this prevalence persisted even after the feed replacement had started. In the W feed fermented at 10°C, Kluyveromyces marxianus was the dominant species prior to feed replacement. Upon the addition of new feed to the fermentation vessels, the population diversity changed, and no particular species was dominant on the last sampling occasion. The LAB population in W feed mix consisted mainly of Lactobacillus plantarum, which was dominant throughout the entire fermentation, independent of temperature and feed replacement (Table 3).
Microbial composition of WAT feed mix.
The diversity of yeast species in the WAT feed mix was greater than that in the WWDG and W feeds. Initially, Pichia anomala was the dominant species at both 15 and 20°C. After the first feed replacement (5 days), other species, e.g., Candida allociferrii and Kluyveromyces marxianus at 15°C and Saccharomyces bayanus at 20°C, were also detected. However, after a few days of fermentation with continuous feed replacement, Pichia anomala reassumed dominance. The yeast population observed throughout the whole period of fermentation at 10°C was more diverse than the yeast populations observed during fermentation at 15 and 20°C, but Pichia anomala was always present (Table 4). Among the LAB, Pediococcus pentosaceus was dominant in WAT feed, independent of temperature and feed replacement. The only exceptions were the feed samples from the 15 and 20°C fermentations on the last sampling occasion (day 19), in which Lactobacillus plantarum was dominant.
DISCUSSION
With the exception of pathogens, microbial populations in fermented pig feed have, until this study, been characterized only by the numbers of CFU of microorganisms of certain groups (LAB and yeasts, etc.) in the feed (see, e.g., references 3, 6, and 25). To the best of our knowledge, this is the first study that identifies the LAB and yeasts involved in cereal pig feed fermentation. Our study suggests that for each feed, characteristic associations of LAB and yeast species develop. The species compositions and numbers of CFU varied with substrates, temperatures, fermentation periods, and the addition of new feed components. The CFU values for yeasts in WAT and WWDG feeds were quite low at the start of fermentation but increased with increased fermentation temperatures and feed replacement. However, during the period of daily feed replacement, the CFU values were relatively stable (20). Interestingly, despite different feeds' yielding similar CFU values, the population diversity varied, particularly that in WAT feed. Generally, the populations of both yeast and LAB in feed fermented at 10°C were more diverse than those in feeds fermented at 15 and 20°C (Table 4).
In WWDG feed, the dominant population indicated that the use of wet wheat distillers' grains influenced the development of the microbial population in feed due to the microbial activity in the liquid. In W feed, the yeast CFU values were stable during the whole fermentation period, probably due to the high level of yeast in the whey substrate itself (20). The dominating yeast population in W feed completely shifted from Kluyveromyces marxianus to Pichia membranifaciens, with a faster change at higher fermentation temperatures. The presence of additional yeasts in feed may be beneficial. The growth of yeast may, for example, improve the protein composition of the feed, and Kluyveromyces marxianus has indeed been utilized as a single-cell protein source (33). Pichia membranifaciens and Pichia anomala, which were dominant in W and WAT feeds, respectively, have both been shown to inhibit molds during airtight storage of moist cereal grains (8). The LAB flora in WAT feed consisted mainly of Pediococcus pentosaceus but changed during fermentation and was more diverse in feed fermented at 10°C than in those fermented at 15 and 20°C. In W feed, the LAB population was completely dominated by Lactobacillus plantarum, indicating fast adaptation to or intrinsic preference for the nutritional and environmental conditions. In WWDG feed, the population diversity increased after feed replacement. Lactobacilli are widespread in nature, and many species have been found to have applications in the food and feed industries (17, 29). Strains of Lactobacillus plantarum and Pediococcus pentosaceus have been reported previously to have antifungal activity in the production of both grass silage (34) and sourdough bread (15), and it is possible that our isolates have similar characteristics. Lactobacillus spp. have been found to proliferate rapidly during the first days of the fermentation of pig feed, after which fairly constant numbers are maintained (10, 12, 20, 28). However, identifying yeasts and LAB to the species level demonstrated substantial changes within these apparently stable populations. Thus, CFU numbers alone cannot be regarded as a reliable indicator of population stability. It can be concluded that during the spontaneous fermentation of pig feed, each feed develops characteristic associations of LAB and yeast species. Some of the identified LAB and yeast species have been shown to have antimicrobial activity that may protect from colonization with potentially pathogenic microbes. However, due to considerable variation within the microbial populations of each feed, it may be difficult to assess the general impact of these populations on pig gut health. This finding suggests that better control of the fermentation conditions, in particular temperature, but also the use of starter cultures, is necessary in order to make use of the full potential of feed fermentation in the pig industry.
In WWDG feed, the LAB flora consisted mainly of Pediococcus pentosaceus during the whole fermentation period. This species was also the only LAB species isolated from wet wheat distillers' grains (Table 1). Absolut wet wheat distillers' grains were sterile at despatch (27), but spontaneous inoculation with microorganisms from the environment may occur during storage. Previously, with comparable isolation methods, Lactobacillus amylolyticus, Lactobacillus fermentum, Lactobacillus panis, and Lactobacillus pontis have been isolated from stored wet wheat distillers' grains (vodka from Absolut, Åhus, Sweden) (27), but in this study, the only LAB detected was Pediococcus pentosaceus. Due to different environmental conditions, various microorganisms may have been inoculated into the wet wheat distillers' grains after distillation. Fluctuations in environmental conditions during storage may select for different microflora in wet wheat distillers' grains, and this selection, in turn, is likely to yield feed with differences in microbial diversity, which may, thus, also have altered nutritional value. Variations in the microflora may affect the hygienic properties of feed, e.g., increasing pH may support increased levels of undesirable microbes, like various enterobacteria (28). We did not find any clostridia in our systems (20), but they may become a problem when raw materials that come in contact with soil are used (23).
During the fermentation of animal feed, the presence of yeasts is considered undesirable due to their metabolic activities. Yeast starch metabolism may result in energy losses in the feed due to alcohol and carbon dioxide production (5). However, we observed only low levels of ethanol and small weight losses during the fermentation of these feeds (20). The fermentation environment may be altered due to lactate assimilation by some of the dominant yeast species present in the different feeds (13). This effect may occur with Pichia anomala and Kluyveromyces marxianus, whereas Pichia galeiformis and Pichia membranifaciens have been described previously as having low and strain-variable levels of lactate assimilation, respectively (13). The reduction of pH occurs in W and WAT feeds during the first 3 to 7 days, with a faster reduction at higher fermentation temperatures (20). In W feed fermented at 10°C, the pH is not reduced to the same extent as that in other feeds, most probably due to reduced fermentation into organic acids by LAB at low temperatures. WWDG feed showed no further pH reduction during fermentation, probably because the pH of wheat distillers' grains was already low initially.
The lactic acid concentrations in W and WAT feeds were high, but the concentration in WWDG feed was significantly lower, independent of fermentation temperatures for each feed (20). Lactobacillus plantarum was the dominant LAB in W feed but was also present in WWDG and WAT feeds after 3 to 5 days of fermentation. Lactobacillus plantarum is a heterofermentative LAB that can produce ethanol, acetate, and carbon dioxide, in addition to lactic acid (29). This characteristic may explain the somewhat higher concentrations of acetic acid in W feed than in WAT feed (20). The acetic acid contents in both W and WAT feed were relatively low, but that in WWDG feed was significantly higher (20). The level of lactic acid and the proportion of lactic acid relative to acetic acid may influence the palatability of the feed, resulting in decreased feed intake by the pigs (5, 30). Pediococcus pentosaceus, the prevalent LAB in WAT and WWDG feeds, is homofermentative (29). This characteristic implies that this species cannot account for the high acetic acid content in WWDG feed. Thus, other acetate-producing microorganisms might have been present in the fermentations.
Feed fermentation is a spontaneous process, caused by the microbial population present in the feed components. Although the effects of fermented feed on the animals' health and nutrition are generally positive, they vary among different batches (4, 30). Our study provides a first insight into some of the factors that may influence this variability. Whereas these fermented feeds could be regarded as stable as assessed by traditional CFU methods for the determination of microbial dynamics, species identification demonstrated considerable variation within the microbial populations. Further studies are required to identify microorganisms that are appropriate to generate high-quality fermented feed and that can be used as starter cultures. This high-quality fermented feed should have a low pH (below 4.2) to minimize the pathogen load in the feed, a high content of lactic acid to reduce the number of pathogenic bacteria in the gut, a low content of acetic acid, and no biogenic amines, because high biogenic amine contents can reduce feed intake and may even be toxic. A high level of mineral availability, for instance, due to high phytase activity, is also desired (4, 12, 19).
Moreover, new diagnostic methods are required to monitor the quality of fermentation. Starter cultures may be established using dominant LAB identified in this study. It may also be interesting to determine whether it is possible to include appropriate yeasts in the starter culture. Although mainly undesired in fermented feed (5), yeasts can improve the protein content of the feed and prevent the growth of other undesired microorganisms (18, 26).
Acknowledgments
We are thankful to Inger Ohlsson, Carina Gossas, and Ulrika Edblad for their technical assistance.
The financial support from the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) and the thematic research program MicroDrive (http://microdrive.phosdev.se/) at the Faculty for Natural Resources and Agricultural Sciences, Swedish University of Agricultural Sciences, is gratefully acknowledged.
Footnotes
Published ahead of print on 25 January 2008.
REFERENCES
- 1.Amann, R. I., W. Ludwig, and K.-H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beal, J. D., C. A. Moran, A. Campbell, and P. H. Brooks. 2001. The survival of potentially pathogenic E coli in fermented liquid feed, p. 351-353. In J. E. Lindberg and B. Ogle (ed.), Digestive physiology in pigs. CABI Publishing, Wallingford, United Kingdom.
- 3.Beal, J. D., S. J. Niven, A. Campbell, and P. H. Brooks. 2002. The effect of temperature on the growth and persistence of Salmonella in fermented liquid pig feed. Int. J. Food Microbiol. 79:99-104. [DOI] [PubMed] [Google Scholar]
- 4.Brooks, P. H., J. D. Beal, and S. J. Niven. 2003. Liquid feeding of pigs. II. Potential for improving pig health and food safety. Anim. Sci. Pap. Rep. 21(Suppl. 1):23-29. [Google Scholar]
- 5.Brooks, P. H., J. D. Beal, and S. J. Niven. 2001. Liquid feeding of pigs: potential for reducing environmental impact and for improving productivity and food safety. Recent Adv. Anim. Nutr. Aust. 13:49-63. [Google Scholar]
- 6.Canibe, N., and B. B. Jensen. 2003. Fermented and nonfermented liquid feed to growing pigs: effect on aspects of gastrointestinal ecology and growth performance. J. Anim. Sci. 81:2019-2031. [DOI] [PubMed] [Google Scholar]
- 7.de Souza Liberal, A. T., E. A. da Silva Filho, J. O. F. de Morais, D. A. Simões, and M. A. de Morais, Jr. 2005. Contaminant yeast detection in industrial ethanol fermentation must by rDNA-PCR. Lett. Appl. Microbiol. 40:19-23. [DOI] [PubMed] [Google Scholar]
- 8.Druvefors, U. Ä., and J. Schnürer. 2005. Mold-inhibitory activity of different yeast species during airtight storage of wheat grain. FEMS Yeast Res. 5:373-378. [DOI] [PubMed] [Google Scholar]
- 9.Geary, T. M., P. H. Brooks, J. D. Beal, and A. Campbell. 1999. Effect on weaner pig performance and diet microbiology of feeding a liquid diet acidified to pH 4 with either lactic acid or through fermentation with Pediococcus acidilactici. J. Sci. Food Agric. 79:633-640. [Google Scholar]
- 10.Geary, T. M., P. H. Brooks, D. T. Morgan, A. Campbell, and P. J. Russell. 1996. Performance of weaner pigs fed ad libitum with liquid feed at different dry matter concentrations. J. Sci. Food Agric. 72:17-24. [Google Scholar]
- 11.Harada, E., M. Niiyama, and B. Syuto. 1986. Comparison of pancreatic exocrine secretion via endogenous secretin by intestinal infusion of hydrochloric acid and monocarboxylic acid in anesthetized piglets. Jpn. J. Physiol. 36:843-856. [DOI] [PubMed] [Google Scholar]
- 12.Jensen, B. B., and L. L. Mikkelsen. 1998. Feeding liquid diets to pigs, p. 107-126. In P. C. Garnsworthy and J. Wiseman (ed.), Recent advances in animal nutrition. Nottingham University Press, Nottingham, United Kingdom.
- 13.Kurtzman, C. P., and J. W. Fell. 1998. The yeast, a taxonomic study. Elsevier Science B.V., Amsterdam, The Netherlands.
- 14.Kurtzman, C. P., and C. J. Robnett. 1998. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek 73:331-371. [DOI] [PubMed] [Google Scholar]
- 15.Lavermicocca, P., F. Valerio, A. Evidente, S. Lazzaroni, A. Corsetti, and M. Gobbetti. 2000. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 66:4084-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieckfeldt, E., W. Meyer, and T. Börner. 1993. Rapid identification and differentiation of yeasts by DNA and PCR fingerprinting. J. Basic Microbiol. 33:413-426. [DOI] [PubMed] [Google Scholar]
- 17.Lindgren, S. E., and W. J. Dobrogosz. 1990. Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiol. Rev. 87:149-164. [DOI] [PubMed] [Google Scholar]
- 18.Litchfield, J. N. 1983. Single cell protein. Science 219:740-746. [DOI] [PubMed] [Google Scholar]
- 19.Lyberg, K., T. Lundh, C. Pedersen, and J. E. Lindberg. 2006. Influence of soaking, fermentation and phytase supplementation on nutrient digestibility in pigs fed a grower diet based on wheat and barley. Anim. Sci. 82:853-858. [Google Scholar]
- 20.Lyberg, K., M. Olstorpe, V. Passoth, J. Schnürer, and J. E. Lindberg. 5 November 2007, posting date. Biochemical and microbiological properties of a cereal mix fermented with whey, wet wheat distillers' grain or water at different temperatures. Anim. Feed Sci. Technol. doi: 10.1016/j.anifeedsci.2007.09.028. [DOI]
- 21.Masoud, W., L. B. Cesar, L. Jespersen, and M. Jakobsen. 2004. Yeast involved in fermentation of Coffea arabica in East Africa determined by genotyping and by direct denaturating gradient gel electrophoresis. Yeast 21:549-556. [DOI] [PubMed] [Google Scholar]
- 22.Mayer, E. A. 1994. The physiology of gastric storage and emptying, p. 929-976. In L. R. Johnson, D. H. Alpers, J. Christensen, E. D. Jacobson, and J. H. Walsh (ed.), Physiology of the gastrointestinal tract, vol. 1. Raven Press, New York, NY. [Google Scholar]
- 23.McDonald, P., A. R. Henderson, and S. J. E. Heron. 1991. The biochemistry of silage, 2nd ed. Chalcombe Publications, Marlow, United Kingdom.
- 24.Mikkelsen, L. L., and B. B. Jensen. 1998. Performance and microbial activity in the gastrointestinal tract of piglets fed fermented liquid feed at weaning. J. Anim. Feed Sci. 7:211-215. [Google Scholar]
- 25.Moran, C. A., R. H. J. Scholten, J. M. Tricarico, P. H. Brooks, and M. W. A. Verstegen. 2006. Fermentation of wheat: effects of backslopping different proportions of pre-fermented wheat on the microbial and chemical composition. Arch. Anim. Nutr. 60:158-169. [DOI] [PubMed] [Google Scholar]
- 26.Passoth, V., and J. Schnürer. 2003. Non-conventional yeast in antifungal application, p. 297-319. In J. H. de Winde (ed.), Functional genetics of industrial yeast. Springer-Verlag, Berlin, Germany.
- 27.Pedersen, C., H. Jonsson, J. E. Lindberg, and S. Roos. 2004. Microbiological characterization of wet wheat distillers' grain, with focus on isolation of lactobacilli with potential as probiotics. Appl. Environ. Microbiol. 70:1522-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell, P. J., T. M. Geary, P. H. Brooks, and A. Campbell. 1996. Performance, water use and effluent output of weaner pigs fed ad libitum with either dry pellets or liquid feed and the role of microbial activity in the liquid feed. J. Sci. Food Agric. 72:8-16. [Google Scholar]
- 29.Salminen, S., and A. von Wright. 1998. Lactic acid bacteria: microbiology and functional aspects. Marcel Dekker, Inc., New York, NY.
- 30.Scholten, R. H. J., C. M. C. van der Peet-Schweringa, M. W. A. Verstegen, L. A. den Hartoga, J. W. Schramab, and P. C. Vesseura. 1999. Fermented co-products and fermented compound diets for pigs: a review. Anim. Feed Sci. Technol. 82:1-19. [Google Scholar]
- 31.Scholten, R. H. J., and N. Verdoes. 1997. The Dutch benefit of a recycling rule. Pigs 13:16-17. [Google Scholar]
- 32.Schroeder, B., C. Winckler, K. Failing, and G. Breves. 2004. Studies on the time course of the effects of the probiotic yeast Saccharomyces boulardii on electrolyte transport in pig jejunum. Dig. Dis. Sci. 49:1311-1317. [DOI] [PubMed] [Google Scholar]
- 33.Schultz, N., L. Chang, A. Hauck, M. Reuss, and C. Syldatk. 2006. Microbial production of single-cell protein from deproteinized whey concentrates. Appl. Microbiol. Biotechnol. 69:515-520. [DOI] [PubMed] [Google Scholar]
- 34.Ström, K. 2005. Fungal inhibitory lactic acid bacteria characterization and application of Lactobacillus plantarum MiLAB 393. Doctoral thesis no. 2005:37. Swedish University of Agricultural Sciences, Uppsala, Sweden.
- 35.Valente, P., J. P. Ramos, and O. Leoncini. 1999. Sequencing as a tool in yeast molecular taxonomy. Can. J. Microbiol. 45:949-958. [DOI] [PubMed] [Google Scholar]
- 36.van Beek, S., and F. G. Priest. 2003. Bacterial diversity in scotch whisky fermentations as revealed by denaturing gradient gel electrophoresis. J. Am. Soc. Brew. Chem. 61:10-14. [Google Scholar]
- 37.Vanbelle, M., E. Teller, and M. Focant. 1990. Probiotics in animal nutrition: a review. Arch. Anim. Nutr. 40:543-567. [DOI] [PubMed] [Google Scholar]
- 38.van Winsen, R. L., L. J. A. Lipman, S. Biesterveld, B. A. P. Urlings, J. M. A. Snijders, and F. van Knapen. 2000. Mechanism of Salmonella reduction in fermented pig feed. J. Sci. Food Agric. 81:342-346. [Google Scholar]