Abstract
We report a case of subcutaneous phaeohyphomycosis due to Wallemia sebi in a 43-year-old-female, the first case reported since 1950. The lesion presented as a nonhealing ulcer on the dorsum of the left foot. Diagnosis was based on histological demonstration of the fungus and its recovery in culture.
CASE REPORT
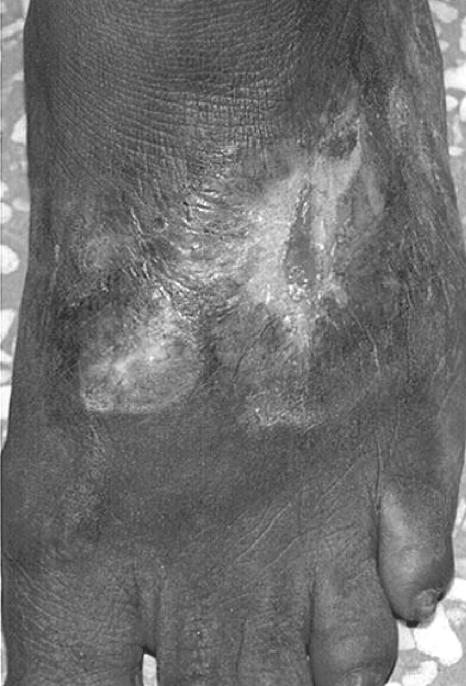
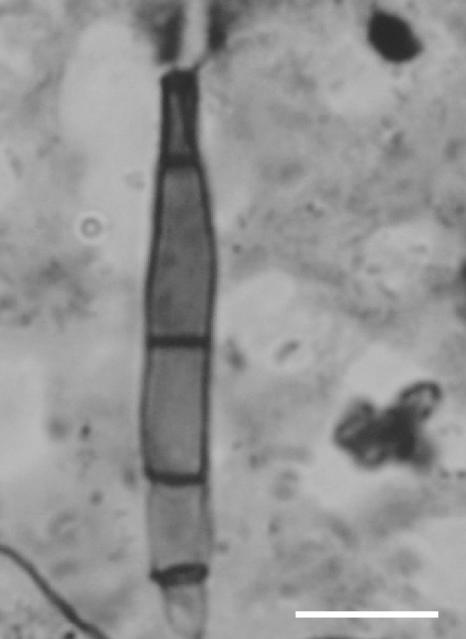
A 43-year-old housewife, resident of Varanasi in the State of Uttar Pradesh in northern India, presented with a nonhealing ulcer, with a ragged margin and covered with slough, on the dorsum of left foot (Fig. 1). The lesion was erythematous at its base, mildly warm (to touch), minimally tender, and of 8 months duration; it had started as an itchy papule that gradually progressed to its present size (8 by 6 cm). She could not recall any injury to the foot prior to development of the lesion. Smears of scrapings from the lesion stained by Gomori methenamine silver and Gram stains were negative for fungal structures and bacteria; a few polymorphonuclear neutrophils were seen. Ziehl-Neelsen-stained smears did not show any acid-fast bacilli. The patient was nondiabetic and human immunodeficiency virus negative. The patient had no other systemic or underlying disease. Routine hematological examination and chest X-ray were normal; X-ray of the foot did not reveal any bone erosion. A provisional clinical diagnosis of cutaneous tuberculosis/deep mycosis was made. A biopsy sample was taken from the lesion. Histopathological examination showed hyperplastic epidermis with ulceration. Deep dermis showed dense acute or chronic inflammatory granulation tissue lining an abscess cavity filled with necrotic material. Grocott-stained tissue sections revealed septate hyphae (Fig. 2). No acid-fast bacilli were seen in tissue sections stained with Ziehl-Neelsen stain. A portion of the biopsy sample was minced into tiny pieces, which were cultured on multiple slopes of Sabouraud dextrose agar (SDA; Difco Laboratories, Detroit, MI) containing chloramphenicol (0.05 mg/ml) and slopes of SDA with chloramphenicol and cycloheximide (0.5 mg/ml).
FIG. 1.
Ulcer with ragged margin due to Wallemia sebi on the dorsum of the left foot; peripheral areas show partial healing.
FIG. 2.
Groccot stain showing a septate hypha. Bar = 10 μm.
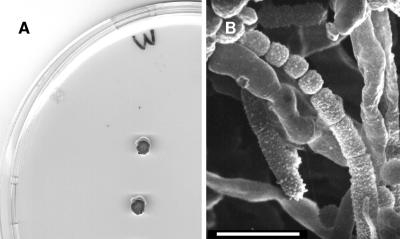
Cultures on SDA without cycloheximide yielded several colonies of a slow growing dematiaceous mold, which was identified as Wallemia sebi on the basis of a detailed study of its colonial and microscopic morphology (4). The colonies attained a diameter of 2 to 4 mm after 2 weeks at 25°C (Fig. 3A). They were elevated, irregular in shape, and orange-brown to blackish brown. Microscopic examination of fungus growth in lactophenol cotton blue mounts showed slender, cylindrical, and usually unbranched conidiophores. Conidiophores were smooth and subhyaline and possessed a swollen upper part from which a cylindrical, verrucose fertile hypha emerges and fragments to form conidia. Conidia were catenate, cubical, up to 2.5 μm in diameter, becoming spherical or subspherical, finely verruculose, subhyaline, and brown in mass (Fig. 3B). This isolate did not grow at 37°C, and growth at 35°C was extremely slow (up to 1.5-mm diameter after 2 weeks). A living culture of the isolate has been deposited in the Faculty of Medicine (Reus, Spain) as FMR 8645 and in the Centraalbureau voor Schimmelcultures (Utrecht, The Netherlands) as CBS 121954.
FIG. 3.
Wallemia sebi. (A) Colonies on SDA at 25°C after 2 weeks. (B) Conidia. Bar = 50 μm.
An in vitro antifungal susceptibility test of the isolate, performed according to the guidelines of CLSI (formerly NCCLS) (12) except for the incubation temperature (30°C in our case) and with Candida parapsilosis ATCC 23019 and Candida krusei ATCC 62258 as quality controls, showed the following MICs (in micrograms per milliliter) for the different antimycotics: itraconazole, 0.125; micafungin, 0.06; flucytosine, >64; voriconazole, 0.03; posaconazole, 0.25; terbinafine, <0.03; ketoconazole, <0.03; and amphotericin B, 2. Due to the extremely slow growth of the fungus, MICs could be read only after 7 days of incubation.
The patient was put on itraconazole 100 mg twice daily and asked to report after 3 weeks for evaluation. The patient did not return and was regrettably lost to follow-up.
Phaeohyphomycosis refers to infections of skin, subcutaneous tissues, and internal organs caused by dematiaceous (melanized) fungi that produce pigmented hyphae and/or yeast-like cells in culture and frequently in the infected tissue. Species of several genera of dematiaceous fungi, e.g., Alternaria, Bipolaris, Curvularia, Cladophialophora, Cladosporium, Exophiala, Exserohilum, Phaeoacremonium, or Phialophora, are commonly reported as agents of phaeohyphomycosis (4, 11). Wallemia sebi, another dematiaceous anamorphic fungus and a common causative agent of farmer's lung disease (10, 13-15, 18), is a rarely known agent of human infection reported in earlier literature between 1909 and 1950 (2, 3, 6, 9). Thus, it is considered of interest to report here a case of subcutaneous phaeohyphomycosis caused by W. sebi.
The class Wallemiomycetes and the order Wallemiales have recently been erected and considered as a sister group of the Basidiomycota to accommodate the single genus Wallemia (8, 19). This genus comprises three xerophilic species, which are phenotypically distinguished mainly by the size range of conidia and by the degree of their xerophily (19). Wallemia sebi is the only species capable of growth on media such as malt extract agar without additional solutes (NaCl, glucose) and shows the smallest conidia (1.5- to 2.5-μm diameter). It is the only species of Wallemia that has been involved in human infections.
Wallemia sebi is a mold with a world-wide distribution. It is common in indoor environments and has been isolated from jams, dates, bread, cakes, salted beans, maize flour, crystalline sugar, fish, bacon, fruits, soil, hay, and textiles. This fungus is also commonly found in agricultural environments in many parts of the world (1, 5, 7, 16, 17, 20). There are only a few known cases of cutaneous or subcutaneous infections in humans due to W. sebi reported in the earlier literature without specified clinical features (2, 3, 6, 9). The infections caused by this fungus were called “hemisporiosis” after the synonymous species Hemispora stellata. Subsequent to 1950, W. sebi has not been reported as an agent of human infection. The present case provides further evidence of a pathogenic role of this rarely known fungus.
One intriguing issue related to W. sebi infections is the fact that they have not been described since the 1950s. One explanation could be that these infections were previously underdiagnosed mainly due to the extremely slow growth of this fungus, which possibly led to discarding the isolates as laboratory contaminants. More difficult to explain is why this fungus has so far infected only immunocompetent patients. Other known pathogen molds, such as Fusarium, Acremonium, Scedosporium, etc., before the emergence of immunocompromised patients also caused localized infections in immunocompetent patients, but nowadays their spectra of action have changed substantially, affecting mainly the immunosuppressed hosts. Unfortunately, due to the rarity of W. sebi infections, there is hardly any data on their clinical features and treatment. In conclusion, W. sebi should be added to the relatively short list of basidiomycetous fungi that are known to cause infections in humans.
Footnotes
Published ahead of print on 3 January 2008.
REFERENCES
- 1.Allotey, J., M. F. Simpanya, and S. Mpuchane. 2001. Insect and mycoflora interactions in maize flour. Afr. J. Food Nutr. Sci. 13-8. [Google Scholar]
- 2.Auvrey, M. 1909. A propos d'une nouvelle mycose observée chez l'homme. Suppuration cervicale due à Hemispora stellata. Bull. Mem. Soc. Chir. Paris 20686. [Google Scholar]
- 3.Beurmann, L., M. de Clair, and H. Gourgerot. 1909. Une nouvelle mycose, l'hemisporose de la verge. Bull. Mem. Soc. Med. Hop. Paris 3911-917. [Google Scholar]
- 4.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 5.Eduard, W., J. Lacey, K. Karlsson, U. Palmgren, G. Strom, and G. Blomquist. 1990. Evaluation of methods for enumerating microorganisms in filter samples from highly contaminated occupational environments. Am. Ind. Hyg. Assoc. J. 51427-436. [DOI] [PubMed] [Google Scholar]
- 6.Gougerot, H., and M. Caraven. 1909. Mycose nouvelle: l'hemisporose, ostette hummaine primitive du tibia due a l'Hemispora stellata (non preliminaire). C. R. Soc. Biol. Paris 1174. [Google Scholar]
- 7.Hanhela, R., K. Louhelainen, and A.-L. Pasanen. 1995. Prevalence of microfungi in Finnish cow barns and some aspects of the occurrence of Wallemia sebi and Fusaria. Scand. J. Work Environ. Health 21223-228. [DOI] [PubMed] [Google Scholar]
- 8.Hibbett, D. S., M. Binder, J. F. Bischoff, M. Blackwell, P. F. Cannon, O. E. Eriksson, S. Huhndorf, T. James, P. M. Kirk, R. Lucking, H. T. Lumbsch, F. Lutzoni, P. B. Matheny, D. J. McLaughlin, M. J. Powell, S. Redhead, C. L. Schoch, J. W. Spatafora, J. A. Stalpers, R. Vilgalys, M. C. Aime, A. Aptroot, R. Bauer, D. Begerow, G. L. Benny, L. A. Castlebury, P. W. Crous, Y. C. Dai, W. Gams, D. M. Geiser, G. W. Griffith, C. Gueidan, D. L. Hawksworth, G. Hestmark, K. Hosaka, R. A. Humber, K. D. Hyde, J. E. Ironside, U. Koljalg, C. P. Kurtzman, K. H. Larsson, R. Lichtwardt, J. Longcore, J. Miadlikowska, A. Miller, J. M. Moncalvo, S. Mozley-Standridge, F. Oberwinkler, E. Parmasto, V. Reeb, J. D. Rogers, C. Roux, L. Ryvarden, J. P. Sampaio, A. Schussler, J. Sugiyama, R. G. Thorn, L. Tibell, W. A. Untereiner, C. Walker, Z. Wang, A. Weir, M. Weiss, M. M. White, K. Winka, Y. J. Yao, and N. Zhang. 2007. A higher-level phylogenetic classification of the fungi. Mycol. Res. 111509-547. [DOI] [PubMed] [Google Scholar]
- 9.Janke, D. 1950. Zur Kenntniss der Hemisporose. Arch. Dermatol. Syphil. 19095-113. [PubMed] [Google Scholar]
- 10.Lappalainen, S., A.-L. Pasanen, M. Reiman, and P. Kalliokoski. 1998. Serum IgG antibodies against Wallemia sebi and Fusarium species in Finnish farmers. Ann. Allergy Asthma Immunol. 81585-592. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto, T., and L. Ajello. 1998. Agents of phaeohyphomycosis, p. 503-524. In L. Ajello and R. J. Hay (ed.), Topley & Wilson's microbiology and microbial infections. Arnold, London, United Kingdom.
- 12.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 13.Reboux, G., R. Piarroux, F. Mauny, A. Madroszyk, L. Millon, K. Bardonnet, and J.-C. Dalphin. 2001. Role of molds in farmer's lung disease in Eastern France. Am. J. Respir. Crit. Care Med. 1631534-1539. [DOI] [PubMed] [Google Scholar]
- 14.Roussel, S., G. Reboux, J.-C. Dalphin, J.-J. Laplante, and R. Piarroux. 2005. Evaluation of salting as a hay preservative against farmer's lung disease agents. Ann. Agric. Environ. Med. 12217-221. [PubMed] [Google Scholar]
- 15.Sakamoto, T., S. Torii, M. Yamada, A. Urisu, H. Iguchi, M. Ueda, and Y. Matsuda. 1989. Allergenic and antigenic activities of the osmophilic fungus Wallemia sebi asthmatic patients. Arerugi 38352-359. [PubMed] [Google Scholar]
- 16.Udagawa, S.-I., and S. Uchiyama. 2004. Three new hyphomycetes isolated from soil and feather debris. Can. J. Bot. 761637-1646. [Google Scholar]
- 17.Vindelov, J., and N. Arneberg. 2001. Interactions between Zygosaccharomyces mellis and Wallemia sebi in diluted molasses. Int. J. Food Microbiol. 6373-79. [DOI] [PubMed] [Google Scholar]
- 18.Wood, G. M., P. J. Mann, D. F. Lewis, W. J. Reid, and M. O. Moss. 1990. Studies on a toxic metabolite from the mould Wallemia. Food Addit. Contam. 769-77. [DOI] [PubMed] [Google Scholar]
- 19.Zalar, P., G. S. de Hoog, H.-J. Schroers, J. M. Frank, and N. Gunde-Cimeman. 2005. Taxonomy and phylogeny of the osmophilic genus Wallemia (Wallemiomycetes and Wallemiales, cl. et ord. nov.). Antonie van Leeuwenhoek 87311-328. [DOI] [PubMed] [Google Scholar]
- 20.Zeng, Q.-Y., S.-O. Westermark, A. Rasmunson-Lestander, and X.-R. Wang. 2004. Detection and quantification of Wallemia sebi in aerosols by real-time PCR, conventional PCR, and cultivation. Appl. Environ. Microbiol. 707295-7302. [DOI] [PMC free article] [PubMed] [Google Scholar]