Abstract
In 2005, Candida nivariensis, a yeast species genetically related to Candida glabrata, was described following its isolation from three patients in a single Spanish hospital. Between 2005 and 2006, 16 fungal isolates with phenotypic similarities to C. nivariensis were submitted to the United Kingdom Mycology Reference Laboratory for identification. The strains originated from various clinical specimens, including deep, usually sterile sites, from patients at 12 different hospitals in the United Kingdom. PCR amplification and sequencing of the D1D2 and internal transcribed spacer 1 (ITS1) regions of the nuclear ribosomal gene cassette confirmed that these isolates from the United Kingdom are genetically identical to C. nivariensis. Biochemically, C. glabrata and C. nivariensis are distinguished by their differential abilities to assimilate trehalose. However, in contrast to the original published findings, we found that C. glabrata isolates, but not C. nivariensis isolates, are capable of assimilating this substrate. Antifungal susceptibility tests revealed that C. nivariensis isolates are less susceptible than C. glabrata isolates to itraconazole, fluconazole, and voriconazole and to have significantly higher flucytosine MICs than C. glabrata strains. Finally, C. nivariensis could be rapidly distinguished from the other common pathogenic fungus species by pyrosequencing of the ITS2 region. In the light of these data, we believe that C. nivariensis should be regarded as a clinically important emerging pathogenic fungus.
Invasive fungal infections caused by Candida spp. remain major causes of morbidity and mortality in the immunocompromised host (18, 20, 26), and more than 150 species of yeast have now been associated with human pathologies (4, 9). Although Candida albicans remains the predominant agent of nosocomial infections, an increasing number of infections have been attributed to non-Candida albicans species, with C. glabrata, C. parapsilosis, C. tropicalis, C. lusitaniae, and C. krusei emerging over recent years as significant opportunistic pathogens (13, 16, 21, 25). Given the inherently variable antifungal susceptibility profiles of different Candida spp., correct identification to the species level is often critical for informed therapeutic decisions.
The principal Candida spp. associated with human disease are readily identified by conventional mycological methods, which rely upon a combination of morphological features coupled with the abilities of the organisms to ferment selected sugars or assimilate a variety of carbon and nitrogen sources (12). However, an increasing number of less common yeast species that are difficult to identify by phenotypic methods have been reported from human infections (12), and certain Candida spp. (e.g., C. orthopsilosis and C. metapsilosis [22]) can be unambiguously identified only by molecular techniques. In addition, several new potentially pathogenic Candida spp. have recently been described on the basis of atypical fermentation and assimilation profiles coupled with unique DNA sequences. One such example, C. nivariensis, was described in 2005 following its isolation from three patients in a single Spanish hospital over a 3-year period. C. nivariensis was shown to be a close genetic relative of C. glabrata and was suggested to be a possible new opportunistic fungus (1). On the basis of the sequence similarities of the C. nivariensis strain with the sequence of a strain isolated from flowers in Canada (11), it was suggested that the C. nivariensis infection or colonization of the three patients might have been acquired from the hospital garden or potted plants.
In 2005 and 2006, 16 fungal isolates with phenotypic similarities to C. nivariensis were submitted to the United Kingdom Mycology Reference Laboratory (MRL) for routine identification. In the study described here we confirmed that these isolates, many of which were cultured from deep, usually sterile body sites, are identical to C. nivariensis and show that C. nivariensis can be rapidly distinguished from the other common pathogenic fungal species by pyrosequencing of the internal transcribed spacer 2 (ITS2) region. Our results also indicate that C. nivariensis isolates often exhibit multidrug resistance to azole antifungal agents and have MICs similar to or even more elevated than those for C. glabrata. Thus, we propose that C. nivariensis be regarded as a clinically important emerging pathogenic yeast.
MATERIALS AND METHODS
Antifungal agents.
Antifungal drugs were obtained from their respective manufacturers as standard powders. Amphotericin B (Sigma Chemical Co., St Louis, MO) and voriconazole (Pfizer Central Research, Sandwich, United Kingdom) were dissolved in dimethyl sulfoxide. Itraconazole (Janssen Research Foundation, Beerse, Belgium) and posaconazole (Schering Corporation) were prepared in polyethylene glycol 400 with heating to 70°C. Caspofungin (Merck Sharp & Dohme, Hoddlesdon, United Kingdom), flucytosine (Sigma Chemical Co.), and fluconazole (Pfizer Central Research) were resuspended in sterile water. Serial twofold dilutions of the various drugs were prepared in RPMI 1640 medium (with l-glutamine, without bicarbonate; Sigma Chemical Co) and buffered to pH 7.0 by using a 0.165 M solution of morpholinepropanesulfonic acid (Sigma Chemical Co).
Fungal isolates.
Clinical isolates with phenotypic similarities to C. nivariensis and recent clinical isolates of C. glabrata that were included for comparison had been submitted to the MRL for routine identification and were stored in sterile water at room temperature. Reference isolates of various Candida species were from the National Collection of Pathogenic Fungi (NCPF) and had been preserved in liquid nitrogen. All isolates were subcultured twice on plates of Oxoid Sabouraud dextrose agar containing 0.5% (wt/vol) chloramphenicol (Unipath Limited, Basingstoke, England). The cultures were incubated for 24 h at 35°C before they were tested.
Conventional yeast identification methods.
The clinical isolates included in this study were all subjected to the conventional identification methods employed by the MRL. These methods were as follows: after initial germ-tube testing, the isolates were subjected to testing with the Auxacolor2 identification kit (Sanofi Diagnostics Pasteur, Paris, France) exactly as described previously (6). The isolates were also cultured on Dalmau plates (Oxoid cornmeal agar supplemented with 1% Tween 80, with a sterile coverslip placed over a single streak of the organism) to establish the additional morphological characteristics required to obtain complete Auxacolor2 profiles. All C. nivariensis isolates were then also tested in the API 20C system (bioMerieux, Marcy l'Etoile, France), again, exactly as described previously (7).
Molecular methods.
Genomic DNA was prepared from the yeast cultures after 2 days of incubation on Sabouraud agar by use of the Whatman FTA filter paper technology exactly as described previously (5, 12). Amplification of a region of the large-subunit gene (LSU) and the ITS1 region was performed with the primers described in references 10 and 24, respectively. Amplification of a fragment of the ITS2 region prior to pyrosequencing was performed with the primers supplied with the PyroMark fungi ASR kit (Biotage, Sweden). In all cases, PCR amplification (100-μl reaction volumes) was performed in the presence of 200 μM of each deoxynucleoside triphosphate, 250 nM of the appropriate primers, 2 U of HotStar Taq polymerase (Qiagen, Valencia, CA), and a single FTA filter punch sample. Following enzyme activation at 94°C for 15 min, the reaction mixtures were subjected to 40 thermal cycles of 94°C (15 s), 55°C (15 s), and 72°C (90 s) on a GeneAmp PCR system 9700 thermocycler (Applied Biosystems, Foster City, CA). The success of the amplification was evaluated by electrophoresis of a fraction of the total amplification products in 1.2% (wt/vol) agarose gels run for 45 min at 120 V in Tris-borate buffer.
For conventional sequencing of the LSU and ITS1 PCR products, the contents of the PCR mixtures were adjusted so that the final concentration of polyethylene glycol 8000 was 10% (wt/vol) and that of MgCl2 was 10 mM and were centrifuged at 12,000 rpm for 10 min in a benchtop centrifuge. The resulting DNA pellets were washed in 75% ethanol, air dried, resuspended in sterile water, and subjected to automatic sequencing by use of the commercial service available at the Advanced Biotechnology Centre (Imperial College, London). The ITS2 amplification products were subjected to pyrosequencing with the reagents supplied with the Pyrogold SQA kit by using a PyroMark ID pyrosequencer (Biotage, Sweden).
The organisms were identified by using BLAST searches of their sequences against the fungal sequences in existing DNA databases (3) and multiple-sequence alignments (Clustal W, version 1.82) (23) by using a database of formally identified organisms compiled by the MRL. For phylogenetic analyses, sequence alignments were performed with the Clustal W program (version 1.82), and the final alignments were edited by hand. Phylogenetic inferences were made from distance trees constructed by using the Kimura two-parameter measure and neighbor joining obtained by use of the Phylip package (version 3.5) (8). The trees were unrooted and were not scaled. The final consensus trees generated from each data set were drawn by using the TreeView program (17), and bootstrap values (1,000 repetitions) greater than 50% are indicated.
Broth microdilution determination of yeast MICs.
MICs were determined according to CLSI (formerly NCCLS) methodologies (15) in round-bottomed 96-well plates with fungus blastospore suspensions prepared in saline and then diluted into RPMI 1640 and adjusted to final concentrations of 2.5 × 103 CFU/ml. The inoculated plates were incubated for 48 h at 35°C. The MICs were read at 48 h as the concentration of drug that elicited 100% inhibition of growth (for amphotericin B) or significant (approximately 80%) inhibition of growth compared with the growth of a drug-free control (for itraconazole, fluconazole, flucytosine, voriconazole, posaconazole, and caspofungin). It should be noted that use of 50% inhibition as an endpoint for the reading of the MICs of itraconazole, fluconazole, flucytosine, voriconazole, posaconazole, and caspofungin, as suggested in CLSI document M27-A2, did not significantly affect either the final recorded MICs or the interpretation of the resistance or susceptibility of the various test organisms.
Determination of MFCs.
Minimum fungicidal concentrations (MFCs) were determined after 48 h of incubation by removing 10 μl of the contents from wells showing no visible growth and spreading them onto Sabouraud dextrose agar plates. The plates were then incubated for 48 h, and the MFCs were determined as the lowest drug concentrations which killed 95% of the inoculum. MIC and MFC ranges and the drug concentrations required to inhibit or kill 50% of the isolates (MIC50s and MFC50s, respectively) and 90% of the isolates (MIC90s and MFC90s, respectively) were determined.
RESULTS
Isolation and identification of C. nivariensis strains from clinical samples in the United Kingdom.
Between 2005 and 2006, 16 primary isolates of yeast (including three sequential isolates from a single patient) were submitted to the MRL for identification (Table 1). While these yeast strains had been isolated from a variety of samples and specimen types, more than 50% of the isolates (9/16) had originated from deep, usually sterile body sites, suggesting that they were responsible for invasive infections. In agreement with the published description of C. nivariensis (1), the isolates from the United Kingdom were unable to produce germ tubes, pseudohyphae, chlamydospores, or ascospores in culture and yielded white colonies, in contrast to the purple colonies usually exhibited by C. glabrata strains, on CHROMagar (data not shown). All 16 isolates of C. nivariensis shared identical carbohydrate assimilation profiles by both the Auxacolor2 and API 20C system tests, which demonstrated that they had the ability to assimilate only glucose. This is in marked contrast to the published description of C. nivariensis, which stated that while both C. nivariensis and C. glabrata could assimilate trehalose, C. nivariensis strains but not C. glabrata strains were able to ferment trehalose (1). It seems likely that this discrepancy reflects an error in the published description, since all available published data, including those in the databases supplied with commercial yeast identification kits, agree that C. glabrata isolates are capable of assimilating and fermenting trehalose (see, for example, reference 4).
TABLE 1.
Fungal strains employed in this studya
| Organism | NCPF strain no. | Other collection no. | Yr of isolation | Isolation site | Clinical details | Hospital | EMBL accession nos. |
|---|---|---|---|---|---|---|---|
| C. nivariensis | 8842 | 2006 | Mouth | Oral candidosis | Oxford | AM745268, AM745269 | |
| C. nivariensis | 8843 | 2006 | Pelvic collection | Not stated | Oxford | AM745270, AM745271 | |
| C. nivariensis | 8844 | 2005 | Blood culture | Neutropenia, AML | UCL | AM745272, AM745273 | |
| C. nivariensis | 8845 | 2005 | Mouth | Oral candidosis | Plymouth | AM745274, AM745275 | |
| C. nivariensis | C. nivariensis 5 | 2005 | Not stated | Pneumonia | Leicester | NA | |
| C. nivariensis | 8846b | AM745276, AM745277 | |||||
| C. nivariensis | C. nivariensis 7 | 2005 | Pelvic abscess | Not stated | Weston-s-Mare | NA | |
| C. nivariensis | 8847 | 2005 | Ascitic fluid | Malignancy | Cambridge | AM745278, AM745279 | |
| C. nivariensis | C. nivariensis 9b | AM745280, AM745281 | |||||
| C. nivariensis | 8848 | 2006 | Mouth | Oral candidosis, neutropenia | Leeds | AM745282, AM745283 | |
| C. nivariensis | 8849 | 2005 | Exit site swab | CAPD | Sheffield | AM745284, AM745285 | |
| C. nivariensis | 8850 | 2006 | Peritoneal fluid | Peritonitis | Exeter | AM745286, AM745287 | |
| C. nivariensis | 8851 | 2006 | Lung biopsy | Not stated | Sheffield | AM745288, AM745289 | |
| C. nivariensis | 8852 | 2006 | Blood culture | Not stated | Newcastle | AM745290, AM745291 | |
| C. nivariensis | 8853 | 2006 | Not stated | Pneumonia | Salisbury | AM745292, AM745293 | |
| C. nivariensis | C. nivariensis 16 | 2006 | Blood culture | Not stated | Barnet | NA | |
| C. glabrataT | 3309 | CBS13, ATCC 2001 | AM745294, AM745295 | ||||
| C. glabrata | C. glabrata 1 | 2006 | Not stated | Pneumonia | Salisbury | AM745312, AM745313 | |
| C. glabrata | C. glabrata 2 | 2006 | Blood culture | Not stated | Dublin | AM745314, AM745315 | |
| C. glabrata | C. glabrata 3 | 2006 | BAL | Not stated | Cumbria | AM745316, AM745317 | |
| C. glabrata | C. glabrata 4 | 2006 | Blood culture | Febrile | Monmouth | AM745318, AM745319 | |
| C. glabrata | C. glabrata 5 | 2006 | Not stated | Febrile | Newport | AM745320, AM745321 | |
| C. glabrata | C. glabrata 6 | 2006 | Blood culture | Not stated | Bristol | AM745322, AM745323 | |
| C. glabrata | C. glabrata 7 | 2006 | Blood culture | Not stated | Cambridge | AM745324, AM745325 | |
| C. glabrata | C. glabrata 8 | 2006 | Drain fluid | Not stated | Sheffield | AM745326, AM745327 | |
| C. glabrata | C. glabrata 9 | 2006 | Blood culture | Pneumonia, breast cancer | Bath | AM745328, AM745329 | |
| C. glabrata | C. glabrata 10 | 2006 | Blood culture | Not stated | Hull | AM745330, AM745331 | |
| C. glabrata | C. glabrata 11 | 2006 | Blood culture | Renal dialysis | Cumbria | AM745332, AM745333 | |
| C. glabrata | C. glabrata 12 | 2006 | Sputum | Not stated | Gloucester | AM745334, AM745335 | |
| C. glabrata | C. glabrata 13 | 2006 | Urine | Sepsis | Salisbury | AM745336, AM745337 | |
| C. albicans | 3281 | 1981 | Not stated | Not stated | Bristol | AM745296, AM745297 | |
| C. parapsilosis | 8334 | CBS604, ATCC 22019 | AM745298, AM745299 | ||||
| C. norvegensis T | 3861 | CBS1922, ATCC 22977 | AM745300, AM745301 | ||||
| C. inconspicua T | 3859 | CBS180, ATCC 16783 | AM745302, AM745303 | ||||
| C. krusei | 3953 | CBS573, ATCC 6258 | AM745304, AM745305 | ||||
| C. lipolytica | 8630c | AM745306, AM745307 | |||||
| C. kefyr | 8678c | AM745308, AM745309 | |||||
| C. zeylanoides | 8426c | AM745310, AM745311 |
Abbreviations: NA, not analyzed; AML, acute myeloid leukemia; CAPD, continuous ambulatory peritoneal dialysis; UCL, University College Hospital, London, United Kingdom.
The isolate was from the same patient from whom isolate C. nivariensis 5 was recovered.
New York State Department of Health Proficiency testing program isolate.
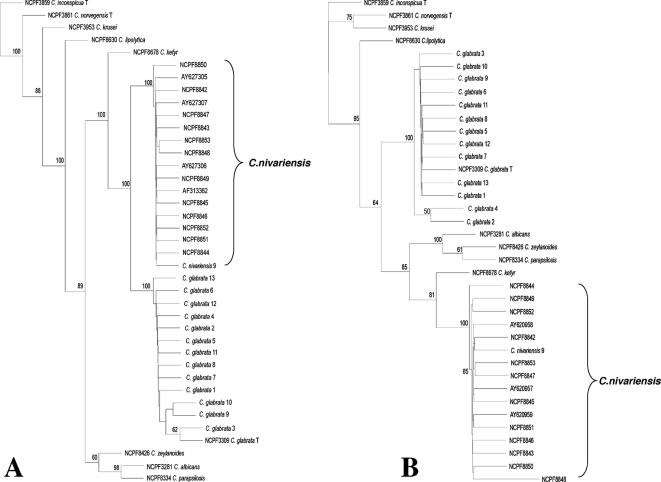
Phylogenetic analyses of sequences corresponding to the D1D2 and the ITS1 portions of the nuclear ribosomal repeat region confirmed that these isolates from the United Kingdom are genetically indistinguishable from previously described C. nivariensis strains (Fig. 1). All of the C. nivariensis strains from the United Kingdom clustered with good bootstrap support with the previously described isolates as a single monospecific clade that was separate from C. glabrata and from the other Candida species tested.
FIG. 1.
Phylogenetic analysis of the LSU (A) and ITS1 (B) data sets. Unrooted neighbor-joining consensus trees are drawn. Bootstrap values above 50% are indicated. The EMBL accession and NCPF numbers are listed in Table 1. The sequences with EMBL accession numbers AY627305, AY927306, and AY627307 correspond to the D1D2 sequences of the three original C. nivariensis isolates described previously (1), and EMBL accession numbers AY620957, AY620958, and AY620959 correspond to the ITS1 sequences of the three original C. nivariensis isolates described previously (1). EMBL accession number AF313362 corresponds to the D1D2 sequence of a potential C. nivariensis isolate from flowers in Canada (11).
Rapid identification of C. nivariensis by pyrosequencing.
Although the absolute numbers of isolates for a given species received at the United Kingdom MRL are likely to be biased due to the reference nature of its activities (isolates submitted to the MRL for identification are often those that have failed to be identified by the referring laboratories), over the period of 2005 and 2006, C. nivariensis was the 17th most common yeast species identified at the MRL (12) (data not shown). In the light of the relative prevalence of this organism, especially from clinical material suggestive of invasive candidosis (Table 1), we sought to establish a rapid molecular identification system that would distinguish C. nivariensis from other Candida spp. that are largely unreactive in carbohydrate assimilation-based identification strategies.
To this end, various Candida spp., including the C. nivariensis strains from the United Kingdom, were submitted to pyrosequencing by use of the PyroMark fungi ASR kit (Biotage), which analyzes a short region of ITS2. All C. nivariensis isolates shared an identical 20-bp signature sequence in this highly discriminatory region that was distinct from the sequences of all other Candida spp. tested and that is unique among the fungal species represented in the currently available ITS2 databases (Table 2; data not shown).
TABLE 2.
Species-specific ITS2 signature sequences obtained by pyrosequencing
| Organism | Sequencea |
|---|---|
| C. nivariensis | GTCAAACTTA AAGGTTCCTG |
| C. glabrata | GTCAAACTTA AAGacgtCTG |
| C. kefyr | GTCAAACTTT gAGagTttTG |
| C. lipolytica | GTCAAACTTA AAaGaaCaac |
| C. albicans | GTCAAAgTTt gAaGaTatac |
| C. parapsilosis | GTCgAAtTTg gAaGaagtTt |
| C. krusei | GTCgAgCTTt ttGttgtCTc |
| C. inconspicua | GTCgAgCTTg AttaaagtTc |
| Consensus sequence | GTC.A..TT............ |
Conserved bases between a given Candida sp. and C. nivariensis are given in uppercase and boldface.
Candida nivariensis exhibits significant resistance to azole antifungal agents.
To further evaluate the clinical significance of C. nivariensis as a potential human pathogen, the MICs of a range of antifungal agents currently employed for the treatment of invasive fungal infections were determined for C. nivariensis and C. glabrata (Table 3) by using methodologies accepted by the CLSI (15). In addition, in the light of the contradictory nature of the data concerning the relevance of MIC data versus that of MFC data in predicting clinical outcome (19), endpoint plating of MIC assays was also performed to allow the establishment of MFC ranges (see Materials and Methods). The 13 randomly selected recent clinical isolates of C. glabrata were used for comparison (Table 1, C. glabrata 1 to 13). However, recent antifungal data indicate that between 5 and 10% of C. glabrata isolates exhibit very high MICs with respect to the some of the azole antifungals, at least in vitro (www.hpa.org.uk/publications/PublicationDisplay.asp?PublicationID=65; MRL, unpublished data; see Discussion). Thus, MIC data were also compiled for nine such highly resistant C. glabrata isolates that had previously undergone antifungal susceptibility testing as part of the routine activities of the MRL (C. glabrata azole-resistant strains; Table 3). It should be noted that these azole-resistant strains had previously been confirmed to be true C. glabrata isolates by a combination of Auxacolor2 and API 20C system testing, conventional sequencing of the D1D2 region, and pyrosequencing of ITS2 (data not shown).
TABLE 3.
MIC and MFC ranges for C. nivariensis and C. glabrata
| Organism | Amphotericin B
|
Itraconazole
|
Voriconazole
|
Fluconazole
|
Posaconazole
|
Flucytosine
|
Caspofungin
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (μg/ml) | MIC90 (μg/ml) | MFC50 (μg/ml) | MFC90 (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | MFC50 (μg/ml) | MFC90 (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | MFC50 (μg/ml) | MFC90 (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | MFC50 (μg/ml) | MFC90 (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | MFC50 (μg/ml) | MFC90 (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | MFC50 (μg/ml) | MFC90 (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | MFC50 (μg/ml) | MFC90 (μg/ml) | |
| C. nivariensis (n = 13) | 0.5 | 0.5 | 0.5 | 2 | 8a | >16 | >16 | >16 | 0.5 | 4 | >16 | >16 | 64 | >64 | >64 | >64 | 1 | 2 | >16 | >16 | 0.25 | 0.5 | 2 | 32 | 0.5 | 1 | 2 | 2 |
| C. glabrata (n = 13) | 0.5 | 1 | 0.5 | 2 | 1 | 1 | >16 | >16 | 0.5 | 0.5 | >16 | >16 | 8 | 32 | >64 | >64 | 0.5 | 4 | >16 | >16 | <0.125 | <0.125 | <0.125 | <0.125 | 1 | 2 | 2 | >64 |
| C. glabrata (azole- resistant strainsb) | 0.5 | 1 | NAc | NA | >16 | >16 | NA | NA | 2 | 8 | NA | NA | 64 | >64 | NA | NA | 4 | >16 | NA | NA | <0.125 | <0.125 | NA | NA | 1 | 1 | NA | NA |
Values in boldface indicate significant differences in MIC or MFC values between C. nivariensis and C. glabrata.
Data were compiled from unpublished results of routine MIC testing by the MRL of nine clinical isolates of C. glabrata that exhibited the most significant resistance to azole antifungal agents.
NA, MFC data are not available for these isolates.
C. nivariensis exhibited significant in vitro resistance to itraconazole, voriconazole, and fluconazole (as assessed by both the MIC and the MFC ranges; Table 3). This resistance was slightly (for fluconazole and voriconazole) or significantly (for itraconazole) greater with C. nivariensis than with representative isolates of C. glabrata. Indeed, the C. nivariensis MIC/MFC ranges with fluconazole, itraconazole, and voriconazole were very similar to those observed with the highly resistant subset of C. glabrata strains that were specifically selected for comparison (Table 3). Interestingly, azole cross-resistance (as judged by the MICs) extended to include posaconazole with the subset of azole-resistant C. glabrata isolates, but the same was not found for the C. nivariensis strains. Other notable differences in the respective antifungal susceptibility profiles were observed with flucytosine, for which C. nivariensis but not C. glabrata exhibited significantly elevated MIC and MFC values, and caspofungin, for which a minority of C. glabrata strains demonstrated very high MFC values (see the MFC90 of caspofungin in Table 3).
DISCUSSION
We have presented here a detailed characterization of 16 isolates of C. nivariensis received at the United Kingdom MRL over a 12-month period. These strains from the United Kingdom had been isolated from a variety of clinical specimens (including deep, usually sterile body sites) at 12 different hospitals in the United Kingdom. These data indicate that C. nivariensis is potentially clinically widespread and is probably significantly more relevant in terms of human disease than was suggested when the species was originally described (1). Our own epidemiological data indicate that it was the 17th most common yeast species referred to the MRL between 2005 and 2006 (12) (data not shown).
The potential clinical significance of C. nivariensis was further underscored by antifungal susceptibility testing, which revealed the C. nivariensis isolates from the United Kingdom to have elevated itraconazole, voriconazole, and fluconazole MICs (Table 3). Indeed, the MICs of the azole antifungal agents for C. nivariensis were significantly higher than those for standard C. glabrata isolates tested with the same antifungal agents and were equivalent to the MICs observed for a subset of the most azole-resistant C. glabrata strains (Table 3). Furthermore, when the MICs observed here are correlated with the established breakpoints for resistance for the various antifungal agents, C. nivariensis isolates are at least as resistant as C. glabrata isolates to itraconazole and are more resistant than C. glabrata to fluconazole and voriconazole (Table 4) (www.hpa.org.uk/publications/PublicationDisplay.asp?PublicationID=65).
TABLE 4.
Percentage of Candida species intermediate or resistant to amphotericin B, flucytosine, fluconazole, itraconazole, voriconazole, and caspofungin tested at the MRL in 2005 (or 2005 to 2006 for C. nivariensis)a
| Organism | Amphotericin B
|
Flucytosine
|
Fluconazole
|
Itraconazole
|
Voriconazole
|
Caspofungin
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % R | n | % I | % R | n | % I | % R | n | % I | % R | n | % I | % R | n | % I | % R | |
| C. albicans | 1,425 | 0 | 127 | 0 | 2 | 1,816 | 1 | 1 | 1,727 | 1 | 1 | 1,375 | 0 | 0 | 908 | 0 | 0 |
| C. krusei | 74 | 0 | 15 | 47 | 0 | 82 | 77 | 23 | 74 | 82 | 10 | 74 | 3 | 3 | 52 | 0 | 0 |
| C. glabrata | 726 | 0 | 116 | 0 | 2 | 883 | 32 | 12 | 751 | 41 | 56 | 751 | 5 | 7 | 500 | 0 | 0 |
| C. nivariensis | 13 | 0 | 13 | 0 | 0 | 13 | 8 | 54 | 13 | 31 | 62 | 12 | 50 | 0 | 13 | 0 | 0 |
Data are adapted from MRL unpublished records and reference 4. Values in boldface indicate organism-antifungal agent combinations in which significant resistance is apparent. Abbreviations: n, number of isolates tested; R, resistant; I, intermediate.
Our own records indicate that C. nivariensis was not identified in clinical specimens prior to 2005, lending support for the proposal that it might be considered an emerging pathogen. Certainly, from the elevated MICs observed here for C. nivariensis with the azoles (see above), it is plausible that prophylactic azole antifungal use might have contributed to the proliferation of this species, as has been suggested for other azole-resistant Candida species, including C. glabrata (13, 14, 16, 21).
However, we do not believe that C. nivariensis has emerged as a pathogen only over the last 3 years. Effectively, all C. nivariensis isolates studied to date demonstrate the ability to assimilate only glucose among the carbohydrate sources present in most commercially available yeast identification kits. Several other pathogenic yeast species, including C. norvegensis, C. lipolytica, C. krusei, C. inconspicua, and C. zeylanoides, share identical assimilation profiles in Auxacolor2 tests. C. norvegensis, C. lipolytica, C. krusei, and C. zeylanoides can potentially be distinguished from C. nivariensis by their abilities to produce pseudohyphae under the appropriate growth conditions, which form a part of the full Auxacolor2 profile. C. inconspicua, however, like C. nivariensis, is unable to form pseudohyphae or true hyphae and can be distinguished from the latter only with further specialized tests. Thus, we believe that C. nivariensis is likely to have been confused with some of these other largely unreactive fungi (or, indeed, C. glabrata), explaining in part why the emergence of this organism has closely followed the recent introduction of molecular methods for yeast identification. Nevertheless, it is still likely that the azole cross-resistance of C. nivariensis will ensure that the relative prevalence of this species in clinical specimens will continue to increase.
Future studies will involve the retrospective molecular analysis of unreactive yeast isolates that have been submitted to the MRL in order to establish when C. nivariensis isolates first became associated in significant numbers with clinical specimens and in an attempt to assess how rapidly this organism may be emerging as a leading pathogenic yeast. Toward this aim, we have demonstrated that C. nivariensis can be rapidly identified by pyrosequencing of a short region of ITS2. We believe that pyrosequencing has distinct advantages over the recently described C. nivariensis-specific PCR (2) for the rapid identification and epidemiological surveillance of this organism. Since all Candida species tested to date yielded unique species-specific pyrosequencing sequence signatures over the 20 bp of the ITS2 region analyzed (this study; MRL, unpublished data), this method will potentially identify any of the unreactive yeast species with biochemical profiles similar to the profile of C. nivariensis rather than specifically detect only this organism.
In conclusion, in the light of the potential clinical significance of C. nivariensis coupled with the patterns of azole cross-resistance observed here, we believe that C. nivariensis should be added to the increasing number of pathogenic yeast species that require molecular identification.
Acknowledgments
We thank all our clinical colleagues from around the United Kingdom for continuing to submit their interesting isolates to us for identification. We are grateful to Biotage for providing pyrosequencing reagents, Whatman International for supplying the FTA cards and wash reagents used for this study, and the other members of the MRL for their assistance and interest.
Footnotes
Published ahead of print on 16 January 2008.
REFERENCES
- 1.Alcoba-Florez, J., S. Mendez-Alvarez, J. Cano, J. Guarro, E. Perez-Roth, and M. del Pilar Arevalo. 2005. Phenotypic and molecular characterisation of Candida nivariensis sp. nov., a possible new opportunistic fungus. J. Clin. Microbiol. 434107-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcoba-Florez, J., M. del Pilar Arevalo, F. J. Gonzalez-Paredes, J. Cano, J. Gurro, E. Perez-Roth, and S. Mendez-Alvarez. 2005. PCR protocol for specific identification of Candida nivariensis, a recently described pathogenic yeast. J. Clin. Microbiol. 436194-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altshul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programmes. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett, J. A., R. W. Payne, and D. Yarrow (ed.). 2000. Yeasts: characteristics and identification, 3rd ed. Cambridge University Press, Cambridge, United Kingdom.
- 5.Borman, A. M., C. J. Linton, S.-J. Miles, C. K. Campbell, and E. M. Johnson. 2006. Ultra-rapid preparation of total genomic DNA from isolates of yeast and mould using Whatman FTA filter paper technology-a re-usable DNA archiving system. Med. Mycol. 44389-398. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, C. K., K. G. Davey, A. D. Holmes, A. Szekely, and D. W. Warnock. 1999. Comparison of the API Candida system with the AUXACOLOR2 system for identification of common yeast pathogens. J. Clin. Microbiol. 37821-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davey, K. G., P. M. Chant, C. S. Downer, C. K. Campbell, and D. W. Warnock. 1995. Evaluation of the AUXACOLOR2 system, a new method of clinical yeast identification. J. Clin. Pathol. 48807-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felsenstein, J. 1989. PHYLIP—phylogeny interface package (version 3.2). Cladistics 5154-166. [Google Scholar]
- 9.Fromtling, R. A. 1995. Mycology, p. 697-877. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, DC.
- 10.Issakainen, J., J. Jalava, J. Saari, and C. K. Campbell. 1997. Relationship of Scedosporium prolificans with Petriella confirmed by partial LSU rDNA sequences Mycol. Res. 1031179-1184. [Google Scholar]
- 11.Lachance, M. A., W. T. Starmer, C. A. Rosa, J. M. Bowles, J. S. F. Barker, and D. H. Janzen. 2001. Biogeography of yeasts of ephemeral flowers and their insects. FEMS Yeast Res. 11-8. [DOI] [PubMed] [Google Scholar]
- 12.Linton, C. J., A. M. Borman, G. Cheung, A. D. Holmes, A. Szekely, M. D. Palmer, P. D. Bridge, C. K. Campbell, and E. M. Johnson. 2007. Molecular identification of unusual pathogenic yeast isolates by large ribosomal subunit gene sequencing: 2 years experience at the United Kingdom Mycology Reference Laboratory. J. Clin. Microbiol. 451152-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marr, K. A. 2004. Invasive Candida infections: the changing epidemiology. Oncology 149-14. [PubMed] [Google Scholar]
- 14.Marr, K. A., K. Seidel, T. C. White, and R. A. Bowden. 2000. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J. Infect. Dis. 181309-316. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution susceptibility testing of yeasts: approved standard, 2nd ed. Document M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 16.Nucci, M., and K. A. Marr. 2005. Emerging fungal diseases. Clin. Infect. Dis. 41521-526. [DOI] [PubMed] [Google Scholar]
- 17.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12357-358. [DOI] [PubMed] [Google Scholar]
- 18.Pfaller, M. A., R. N. Jones, S. A. Messer, M. B. Edmond, and R. P. Wenzel. 1998. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn. Microbiol. Infect. Dis. 31327-332. [DOI] [PubMed] [Google Scholar]
- 19.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruhnke, M. 2006. Epidemiology of Candida albicans infections and role of non-Candida albicans yeasts. Curr. Drug Targets 7495-504. [DOI] [PubMed] [Google Scholar]
- 21.Snydman, D. R. 2003. Shifting patterns in the epidemiology of nosocomial Candida infections. Chest 123500S-503S. [DOI] [PubMed] [Google Scholar]
- 22.Tavanti, A., A. D. Davidson, N. A. Gow, M. C. Maiden, and F. C. Odds. 2005. Candida orthopsilosis and Candida metapsilosis sp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 224673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White, T. J., T. Burns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., San Diego, CA.
- 25.Wingard, J. R. 1994. Infections due to resistant Candida species in patients with cancer who are receiving chemotherapy. Clin. Infect. Dis. 19(Suppl. 1)49-53. [DOI] [PubMed] [Google Scholar]
- 26.Wright, W. L., and R. P. Wenzel. 1997. Nosocomial Candida. Epidemiology, transmission and prevention. Infect. Dis. Clin. N. Am. 11411-425. [DOI] [PubMed] [Google Scholar]