Abstract
An easy and reliable diagnostic method for malaria is highly desirable. We examined the recently introduced SD Bioline Malaria Antigen test, which detects Plasmodium lactate dehydrogenase, with the additional aid of the presence or absence of thrombocytopenia to diagnose vivax malaria. We enrolled 732 patients with clinically suspected malaria in an area where vivax malaria is endemic. We performed microscopic examination of thin film, applied the SD Bioline Malaria Antigen test, and checked platelet counts. One hundred ninety-five patients were smear positive for vivax malaria. The sensitivity of the SD Bioline Malaria Antigen test was 96.4%, and its specificity was 98.9%. We found that 95.4% of malaria patients had thrombocytopenia, and the proportion with malaria increased as platelet counts decreased. A positive SD Bioline Malaria Antigen test when thrombocytopenia was present showed a 100% positive predictive value for vivax malaria. In conclusion, the SD Bioline Malaria Antigen test is a rapid and accurate diagnostic method for vivax malaria, and a platelet count can facilitate a rapid diagnosis of malaria.
A prompt and accurate diagnosis is critical to the effective management and control of malaria. Microscopic examination of stained blood films has been the most widely used technique for detecting malarial parasites, but the correct interpretation of blood films requires considerable expertise, which may not be available at the periphery of the health care system (18). Recently, rapid antigen detection methods for diagnosing malaria based on the detection of Plasmodium lactate dehydrogenase (pLDH) have been developed. However, the accuracies of these tests in diagnosing vivax malaria in areas of endemicity have not been clearly established (8, 21).
A clinical diagnosis is widely used, in addition to microscopic examination, for diagnosing malaria. Although the symptoms of malaria are very nonspecific and overlap those of other febrile illness, thrombocytopenia has been reported to be associated with vivax malaria as well as with falciparum malaria (14, 20, 22) and is more common with malaria than with other febrile diseases (7, 15, 17). Recent studies have also demonstrated the high predictive value of thrombocytopenia in the diagnosis of malaria (1-3). However, as a substantial proportion of cases falciparum malaria was included in those studies, the predictive value of thrombocytopenia in the diagnosis of vivax malaria would have been limited.
In the Republic of Korea, Plasmodium vivax malaria has reemerged as an endemic disease following an extended period of effective control (6). Almost all of the reemerging malaria cases have been restricted to the northern part of Gyeonggi Province and the northwestern part of Gangwon Province near the demilitarized zone (6), where large numbers of soldiers are based. Because of this geographic distribution and the exposure of personnel during military service, most cases have been reported among military personnel since the beginning of the epidemic. Therefore, as part of the malaria control program of the Armed Forces Medical Command, Republic of Korea, chemoprophylaxis has been prescribed to military personnel assigned to areas where malaria is endemic (29). In addition to prevention, for the prompt diagnosis and treatment of malaria, a new rapid diagnostic test, the SD Bioline Malaria Antigen test, which is based on the detection of pLDH, has recently been introduced in military hospitals in which microscopic examinations are not necessarily available.
In this study, we evaluated results obtained with the SD Bioline Malaria Antigen test in a military hospital located in the northern part of Gyeonggi Province, which is in an area where malaria is endemic. Additionally, we assessed the predictive value of thrombocytopenia as an aid in the rapid initial diagnosis of vivax malaria.
MATERIALS AND METHODS
Patients and sample collection.
All consecutive patients who entered the Armed Forces Yangju Hospital with malaria-like symptoms, including fever with a duration of several days, were prospectively recruited from May 2005 to October 2006, with approval of the ethics committee of the Armed Forces Medical Command. The Armed Forces Yangju Hospital, as a referral military medical center, is responsible for the health of 75,000 soldiers in the northern part of the Gyeonggi Province, which is one of the most concentrated areas where malaria is endemic. All patients who had been treated for malaria in the previous month were excluded from the study. In total, 737 patients with clinical suspicion of malaria were eligible for this study. Of these patients, five (0.7%) were excluded because they either refused to enroll in the study or failed to complete the study protocol.
After informed consent was obtained, venous blood was drawn into EDTA-filled tubes to be used for routine examination for malaria, including automated complete blood counts. A drop of blood from the same tube as that used for microscopy was used for the SD Bioline Malaria Antigen test (Standard Diagnostics, Yongin, Republic of Korea). Different individuals were responsible for smear reading and performance of the SD Bioline Malaria Antigen test, and they were blinded to patients’ details and other test results.
Microscopic examination of blood smears.
Blood smears for malaria parasites were stained with Giemsa stain for 30 min at pH 7.2. The slides were reviewed by an experienced pathologist who had no information about the patient. The pathologist examined at least 300 high-power fields in thin smears before classifying the slides as being negative (12). Microscopic examination of blood films was considered to be the “gold standard” for diagnosing malaria in this study.
SD Bioline Malaria Antigen test.
All venous blood samples were tested with the SD Bioline Malaria Antigen test. This test strip can detect the presence of pLDH, an enzyme produced by both the sexual and asexual forms of the parasite using polyclonal antibodies directed against isoforms of the enzyme. Differentiation of malaria parasites is based on antigenic differences between the pLDH isoforms. The SD Bioline Malaria Antigen test was performed according to the manufacturer's instructions. Briefly, a drop of assay diluent (∼30 μl) and 4 drops (∼120 μl) of assay buffer were dispensed into the conjugate and washing wells, respectively. One drop of freshly collected blood (∼20 μl) was added to the conjugate well and mixed gently with the flat head of the dropper. After standing for 1 min, we placed the dipstick vertically into the conjugate wells, which contained the specimens, and left it in the well for 10 min. We then transferred the dipstick vertically from the conjugate wells into the washing wells and left it until it was cleared of blood and the procedure control band became clearly visible (within 10 to 15 min). We removed the dipstick from the washing well and read the results.
Blood cell counts.
Blood cell counts were performed automatically using the Cell-Dyn 1700 system (Abbott Laboratories, Abbott Park, IL). Thrombocytopenia was defined as a platelet count of <150,000 cells/mm3. Patients were divided into three groups based on platelet count: severe thrombocytopenia (<50,000 cells/mm3), moderate thrombocytopenia (50,000 to 100,000 cells/mm3), and mild thrombocytopenia (100,000 to 150,000 cells/mm3).
RESULTS
In total, 732 patients suspected of having malaria were included in the study. The patients ranged in age from 19 to 52 years, with a median age of 22 years, and all of the patients were male. The microscopic examination indicated that 194 (26.5%) patients were infected with malaria, and all cases were caused by Plasmodium vivax. The SD Bioline Malaria Antigen test detected malaria in 189 (25.8%) patients, and all of the results indicated the presence of species other than Plasmodium falciparum. The sensitivity of the SD Bioline Malaria Antigen test was 96.4% (95% confidence interval [CI], 92.4 to 98.4%), and its specificity was 99.6% (95% CI, 98.5 to 99.9%), with positive and negative predicted values of 98.9% (95% CI, 95.8 to 99.8%) and 98.7% (95% CI, 97.2 to 99.4%), respectively (Table 1). The accuracy of the test was 98.8%.
TABLE 1.
Comparison of the SD Bioline Malaria Antigen test and microscopic examination
| SD Bioline Malaria Antigen test result | No. of samples
|
||
|---|---|---|---|
| Microscopic examination
|
Total | ||
| Negative | Positive | ||
| Positive | 2 | 187 | 189 |
| Negative | 536 | 7 | 543 |
| Total | 538 | 194 | 732 |
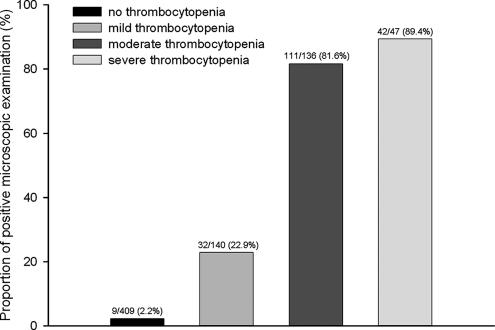
Thrombocytopenia was observed in 185 (95.4%) of 194 patients with malaria and 138 (26.7%) of 538 patients with febrile disease other than malaria. The odds ratio between thrombocytopenia and malaria infection was 59.6 (95% CI, 29.7 to 119.6). Malaria was diagnosed in 42 (89.4%) of 47 patients with severe thrombocytopenia, 111 (81.6%) of 136 patients with moderate thrombocytopenia, 32 (22.9%) of 140 patients with mild thrombocytopenia, and 9 (2.2%) of 409 patients with normal platelet counts. The proportion of malaria increased as platelet counts decreased (P < 0.001 by test for trends) (Fig. 1).
FIG. 1.
Results of microscopic examination according to severity of thrombocytopenia.
Seven patients with false-negative malaria antigen test results had thrombocytopenia, and two with false-positive results had normal platelet counts. On this basis, the positive predictive value of the SD Bioline Malaria Antigen test was 100% in patients with thrombocytopenia. Similarly, the negative predictive value was also 100% in those with normal platelet counts (Table 2).
TABLE 2.
Relationships among microscopic examination, the SD Bioline Malaria Antigen test, and thrombocytopenia
| SD Bioline Malaria Antigen test result | No. of samples
|
||
|---|---|---|---|
| Microscopic examination
|
Total | ||
| Negative | Positive | ||
| With thrombocytopenia | |||
| Negative | 138 | 7 | 145 |
| Positive | 0 | 178 | 178 |
| Total | 138 | 185 | 323 |
| Without thrombocytopenia | |||
| Negative | 398 | 0 | 398 |
| Positive | 2 | 9 | 11 |
| Total | 400 | 9 | 409 |
DISCUSSION
In the present study, we evaluated the accuracy of rapid antigen detection in diagnosing vivax malaria using the recently introduced SD Bioline Malaria Antigen test. This test showed high sensitivity (96.4%) and specificity (99.6%) in the diagnosis of vivax malaria, and when combined with an assessment of thrombocytopenia, it demonstrated a 100% positive predictive value.
Recent studies have verified the usefulness of rapid antigen diagnosis in falciparum malaria, while relatively inconsistent data have thrown doubt on its role in vivax malaria. The OptiMAL test, which detects pLDH, is the most widely evaluated rapid diagnostic test for vivax malaria. The test has shown a wide range of sensitivities for detecting clinically suspected cases of P. vivax in areas where vivax malaria is endemic (4, 9, 10, 23, 24). The ICT Malaria pf/pv test is another rapid diagnostic test that detects malarial histidine-rich protein 2. However, data on the ICT Malaria pf/pv test are scarce, and this test has shown unsatisfactory results for vivax malaria (10). The SD Bioline Malaria Antigen test showed a lower sensitivity for falciparum malaria than other rapid antigen tests (26), but the study could not give definite results for vivax malaria due to the small number of cases of vivax malaria. In this study, the SD Bioline Malaria Antigen test showed superior or at least comparable sensitivity and specificity to those of previously used methods in the rapid diagnosis of vivax malaria.
Thrombocytopenia is a well-recognized complication in falciparum malaria. In vivax malaria, however, thrombocytopenia is not well described, and some discrepancy regarding its incidence and severity exists. In a report on U.S. soldiers in 1969, 93% of patients had vivax malaria, but only 15% had thrombocytopenia (17), whereas 85.1% of Korean soldiers with vivax malaria had thrombocytopenia (22). Although profound thrombocytopenia is considered unusual in vivax malaria (7), severe thrombocytopenia has been reported in some cases (13, 16). In our patients, 185 (95.4%) malaria patients had thrombocytopenia, with severe cases (<50,000 platelets/μl) accounting for 21.6% of patients. The presence of thrombocytopenia is more common in malaria than in other febrile disease, but it has only a limited role in the prediction of malaria due to its low sensitivity and specificity (1). Our findings show that the presence of thrombocytopenia can have a role in the rapid diagnosis of vivax malaria in that all patients with a positive SD Bioline Malaria Antigen test when thrombocytopenia was present had a positive microscopic examination.
Two patients had false-positive results and seven patients had false-negative results for the SD Bioline Malaria Antigen test. In previous studies, recent malarial infection was shown to cause false-positive results (27, 28), and many patients with rheumatoid factor showed cross-reactivity with the ICT assay (11). However, the two patients with false-positive antigen test results in the present study did not have a history of previous malarial infection or rheumatoid arthritis. In addition, low parasitemias could also be a cause of false-positive antigen test results that were not detected by microscopy when parasitemias were below the threshold detection level (26). The chemoprophylaxis program in the Korean military, which prescribes hydroxychloroquine at 400 mg/week, may have influenced the accuracy of the test. If a patient takes medication before blood sampling, a false-negative result by microscopy can occur (9), which may cause false-negative results in antigen tests.
Several factors can explain the false-negative results found in the rapid antigen tests. Low parasite density is one of most widely explained factors which cause false-negative results (24). Although we had not counted the parasite density for all patients, we had checked it for 33 patients randomly by previously suggested methods (18). The median value was 2,700 parasites/μl (range, 35 to 29,100 parasites/μl). The SD Bioline Malaria Antigen test had a false-negative result for only one patient, and a sample from that patient had a parasite density of 35 parasites/μl, which was the lowest count of those tested. This suggests that parasite density can also affect the sensitivity of the SD Bioline Malaria Antigen test. In addition, phenotypic variations in parasites and host metabolic and immune factors have been reported to reduce target antigens or interfere with their binding in detecting antibodies (5, 19, 24). Chemoprophylaxis might also decrease pLDH (25).
Several limitations of this study must be considered. Only a thin smear was studied. To overcome this limitation, a pathologist observed more than 300 high-power fields on at least two slides before considering slides to be negative (12). In addition, all patients with negative smear results were followed up. All of them had improved febrile symptoms after treatment of other fever conditions, and none presented with malaria again within 1 month. However, this did not change the accuracy of the test. The study was carried out with soldiers, and almost all of them took chemoprophylaxis before presentation. Chemoprophylaxis can cause false-negative results by both microscopic examination and rapid antigen test. Finally, why all the patients with false-negative SD Bioline Malaria Antigen test results had thrombocytopenia and all patients with false-positive results had normal platelet counts is not clear. Further study is required to elucidate the relationship between rapid antigen tests and thrombocytopenia.
In conclusion, the SD Bioline Malaria Antigen test is a rapid and reliable method for diagnosing vivax malaria, and the predictive value of the test is increased by combination with the presence of thrombocytopenia. Therefore, the SD Bioline Malaria Antigen test with platelet counts can be used safely for the initial detection of vivax malaria infection, especially when microscopic examinations are not available, although confirmation of malaria diagnosis should always be made by examining stained blood smears.
Acknowledgments
We thank Gyu Bum Oh, who had served as a medical private at the Armed Forces Yangju Hospital, for data entry.
We have no relation with SD Incorporation, which manufactures the SD Bioline Malaria Antigen test dipstick used in this study, and we have not received any support from that incorporation.
The views, opinions, and/or findings contained in this article are those of the authors and do not reflect the official policy or position of the Ministry of National Defense or the Government of South Korea.
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Casalino, E., J. Le Bras, F. Chaussin, A. Fichelle, and E. Bouvet. 2002. Predictive factors of malaria in travelers to areas where malaria is endemic. Arch. Intern. Med. 1621625-1630. [DOI] [PubMed] [Google Scholar]
- 2.D'Acremont, V., P. Landry, I. Mueller, A. Pecoud, and B. Genton. 2002. Clinical and laboratory predictors of imported malaria in an outpatient setting: an aid to medical decision making in returning travelers with fever. Am. J. Trop. Med. Hyg. 66481-486. [DOI] [PubMed] [Google Scholar]
- 3.Erhart, L. M., K. Yingyuen, N. Chuanak, N. Buathong, A. Laoboonchai, R. S. Miller, S. R. Meshnick, R. A. Gasser, Jr., and C. Wongsrichanalai. 2004. Hematologic and clinical indices of malaria in a semi-immune population of western Thailand. Am. J. Trop. Med. Hyg. 708-14. [PubMed] [Google Scholar]
- 4.Ferro, B. E., I. J. Gonzalez, F. Carvajal, G. I. Palma, and N. G. Saravia. 2002. Performance of OptiMAL in the diagnosis of Plasmodium vivax and Plasmodium falciparum infections in a malaria referral center in Colombia. Mem. Inst. Oswaldo Cruz 97731-735. [DOI] [PubMed] [Google Scholar]
- 5.Fryauff, D. J., Purnomo, M. A. Sutamihardja, I. R. Elyazar, I. Susanti, Krisin, B. Subianto, and H. Marwoto. 2000. Performance of the OptiMAL assay for detection and identification of malaria infections in asymptomatic residents of Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 63139-145. [DOI] [PubMed] [Google Scholar]
- 6.Han, E. T., D. H. Lee, K. D. Park, W. S. Seok, Y. S. Kim, T. Tsuboi, E. H. Shin, and J. Y. Chai. 2006. Reemerging vivax malaria: changing patterns of annual incidence and control programs in the Republic of Korea. Kor. J. Parasitol. 44285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horstmann, R. D., M. Dietrich, U. Bienzle, and H. Rasche. 1981. Malaria-induced thrombocytopenia. Blut 42157-164. [DOI] [PubMed] [Google Scholar]
- 8.Iqbal, J., N. Khalid, and P. R. Hira. 2002. Comparison of two commercial assays with expert microscopy for confirmation of symptomatically diagnosed malaria. J. Clin. Microbiol. 404675-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal, J., A. Muneer, N. Khalid, and M. A. Ahmed. 2003. Performance of the OptiMAL test for malaria diagnosis among suspected malaria patients at the rural health centers. Am. J. Trop. Med. Hyg. 68624-628. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal, J., A. Sher, P. R. Hira, and R. Al-Owaish. 1999. Comparison of the OptiMAL test with PCR for diagnosis of malaria in immigrants. J. Clin. Microbiol. 373644-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iqbal, J., A. Sher, and A. Rab. 2000. Plasmodium falciparum histidine-rich protein 2-based immunocapture diagnostic assay for malaria: cross-reactivity with rheumatoid factors. J. Clin. Microbiol. 381184-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kain, K. C., M. A. Harrington, S. Tennyson, and J. S. Keystone. 1998. Imported malaria: prospective analysis of problems in diagnosis and management. Clin. Infect. Dis. 27142-149. [DOI] [PubMed] [Google Scholar]
- 13.Kakar, A., S. Bhoi, V. Prakash, and S. Kakar. 1999. Profound thrombocytopenia in Plasmodium vivax malaria. Diagn. Microbiol. Infect. Dis. 35243-244. [DOI] [PubMed] [Google Scholar]
- 14.Kotwal, R. S., R. B. Wenzel, R. A. Sterling, W. D. Porter, N. N. Jordan, and B. P. Petruccelli. 2005. An outbreak of malaria in US Army Rangers returning from Afghanistan. JAMA 293212-216. [DOI] [PubMed] [Google Scholar]
- 15.Kumar, A., and Shashirekha. 2006. Thrombocytopenia—an indicator of acute vivax malaria. Ind. J. Pathol. Microbiol. 49505-508. [PubMed] [Google Scholar]
- 16.Makkar, R. P., S. Mukhopadhyay, A. Monga, A. Monga, and A. K. Gupta. 2002. Plasmodium vivax malaria presenting with severe thrombocytopenia. Braz. J. Infect. Dis. 6263-265. [DOI] [PubMed] [Google Scholar]
- 17.Martelo, O. J., M. Smoller, and T. A. Saladin. 1969. Malaria in American soldiers. Arch. Intern. Med. 123383-387. [PubMed] [Google Scholar]
- 18.Moody, A. 2002. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 1566-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moody, A., A. Hunt-Cooke, E. Gabbett, and P. Chiodini. 2000. Performance of the OptiMAL malaria antigen capture dipstick for malaria diagnosis and treatment monitoring at the Hospital for Tropical Diseases, London. Br. J. Haematol. 109891-894. [DOI] [PubMed] [Google Scholar]
- 20.Newton, J. A., Jr., G. A. Schnepf, M. R. Wallace, H. O. Lobel, C. A. Kennedy, and E. C. Oldfield III. 1994. Malaria in US Marines returning from Somalia. JAMA 272397-399. [PubMed] [Google Scholar]
- 21.Ochola, L. B., P. Vounatsou, T. Smith, M. L. Mabaso, and C. R. Newton. 2006. The reliability of diagnostic techniques in the diagnosis and management of malaria in the absence of a gold standard. Lancet Infect. Dis. 6582-588. [DOI] [PubMed] [Google Scholar]
- 22.Oh, M. D., H. Shin, D. Shin, U. Kim, S. Lee, N. Kim, M. H. Choi, J. Y. Chai, and K. Choe. 2001. Clinical features of vivax malaria. Am. J. Trop. Med. Hyg. 65143-146. [DOI] [PubMed] [Google Scholar]
- 23.Palmer, C. J., J. A. Bonilla, D. A. Bruckner, E. D. Barnett, N. S. Miller, M. A. Haseeb, J. R. Masci, and W. M. Stauffer. 2003. Multicenter study to evaluate the OptiMAL test for rapid diagnosis of malaria in U.S. hospitals. J. Clin. Microbiol. 415178-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer, C. J., J. F. Lindo, W. I. Klaskala, J. A. Quesada, R. Kaminsky, M. K. Baum, and A. L. Ager. 1998. Evaluation of the OptiMAL test for rapid diagnosis of Plasmodium vivax and Plasmodium falciparum malaria. J. Clin. Microbiol. 36203-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piper, R., J. Lebras, L. Wentworth, A. Hunt-Cooke, S. Houze, P. Chiodini, and M. Makler. 1999. Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pLDH). Am. J. Trop. Med. Hyg. 60109-118. [DOI] [PubMed] [Google Scholar]
- 26.Ratsimbasoa, A., A. Randriamanantena, R. Raherinjafy, N. Rasoarilalao, and D. Menard. 2007. Which malaria rapid test for Madagascar? Field and laboratory evaluation of three tests and expert microscopy of samples from suspected malaria patients in Madagascar. Am. J. Trop. Med. Hyg. 76481-485. [PubMed] [Google Scholar]
- 27.Shiff, C. J., Z. Premji, and J. N. Minjas. 1993. The rapid manual ParaSight-F test. A new diagnostic tool for Plasmodium falciparum infection. Trans. R. Soc. Trop. Med. Hyg. 87646-648. [DOI] [PubMed] [Google Scholar]
- 28.Singh, N., N. Valecha, and V. P. Sharma. 1997. Malaria diagnosis by field workers using an immunochromatographic test. Trans. R. Soc. Trop. Med. Hyg. 91396-397. [DOI] [PubMed] [Google Scholar]
- 29.Yeom, J. S., S. H. Ryu, S. Oh, D. H. Choi, K. J. Song, Y. H. Oh, J. H. Lee, Y. A. Kim, S. Y. Ahn, H. Y. Yang, J. E. Cha, and J. W. Park. 2005. Evaluation of anti-malarial effects of mass chemoprophylaxis in the Republic of Korea army. J. Kor. Med. Sci. 20707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]