Abstract
We describe the development of nonsusceptibility to daptomycin and vancomycin during treatment for methicillin-resistant Staphylococcus aureus (MRSA) bacteremia associated with infective endocarditis and probable septic thrombophlebitis in a uremic patient. MRSA bacteremia persisted during glycopeptide and subsequent daptomycin treatment but cleared after 5 days' treatment with linezolid and fusidic acid.
CASE REPORT
We describe the first reported case in Taiwan of a patient with the loss of daptomycin susceptibility after a prolonged glycopeptide treatment. The primary infections in this patient were left-side infective endocarditis and probable septic thrombophlebitis.
A 66-year-old man was admitted due to fever and extending redness around the double-lumen catheter insertion site over the right inguinal area for 2 days. The patient had hepatitis C virus-related liver cirrhosis (Child-Plugh class C), which was complicated by a previous episode of spontaneous peritonitis, and end-stage renal disease caused by type 1 membranoproliferative glomerulonephritis. He received regular hemodialysis via a right-femur double-lumen catheter. An arteriovenous fistula with a vascular graft was constructed over the left forearm 10 days prior to his admission.
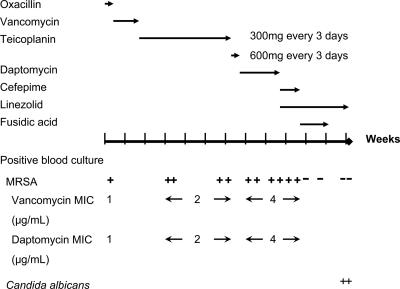
At admission, his body temperature was 38.2°C, his pulse rate was 117/min, and his blood pressure was 155/81 mm Hg. Redness and tenderness over the right inguinal area and swelling of the left arm were noted. Laboratory investigation showed a normal white blood cell count of 9,740 cells/mm3, with 90.8% segmentation. A vascular duplex ultrasound performed on admission revealed no fluid accumulation around the arteriovenous fistula graft. Two sets of blood culture performed on admission revealed methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin (1 g every 3 days, intravenously) was given starting on hospital day 3. Fever subsided thereafter. The trough level obtained after the patient was administered 25 mg of vancomycin/kg of body weight, followed by two other doses, was 13.34 μg/ml. Vancomycin was switched to teicoplanin (300 mg every 3 days, intravenously) on hospital day 12 due to a suspected drug rash. The vascular graft was removed on hospital day 26 due to suspicion of infection. Culture of blood obtained on hospital days 22, 24, 40, and 43 still revealed MRSA, although the patient remained afebrile throughout this period (Fig. 1). Transthoracic echocardiography was performed on hospital day 44 to search for the source of persistent bacteremia, and it revealed a 1.4-cm by 1.3-cm oscillating mass over the anterior mitral valve leaflet (Fig. 2).
FIG. 1.
Summary of the treatment course and MICs of all 11 MRSA bloodstream isolates. The blood culture results are indicated beneath the time line, with each positive result being indicated with a + and each negative result being indicated with a −. Numbers beside “Vancomycin MIC” and Daptomycin MIC” reflect the changing MICs of those drugs during the times indicated by arrows.
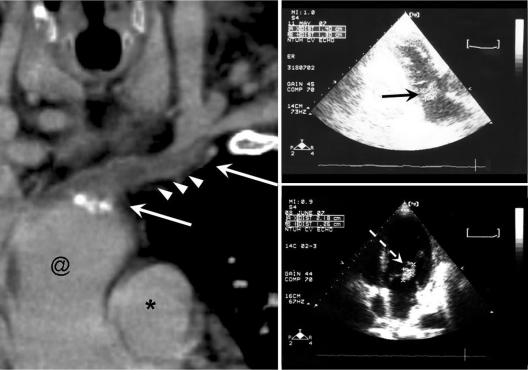
FIG. 2.
(Left) Computed tomographic image generated by curved multiplanar reconstruction showing thrombosis over the left brachiocephalic vein. Arrows, thrombus; arrowheads, peripheral edema of vascular wall; @, superior vena cava; *, aorta. (Right) Transthoracic echocardiographic images. The vegetation sizes were 1.40 cm by 1.30 cm on hospital day 44, when infective endocarditis was first diagnosed (upper right, black arrow), and 2.18 cm by 1.26 cm on hospital day 72, when the blood culture became negative (lower right, white arrow).
Due to poor wound healing after vascular graft removal and the development of left-extremity swelling, chest computed tomography was performed on hospital day 44, and it revealed thrombosis with mild perivascular edema over the left brachiocephalic vein (Fig. 2). Septic phlebitis was suspected. Daptomycin (6 mg/kg every other day) was given on hospital day 47 due to suspicion of glycopeptide treatment failure after the patient suffered a new spiking fever and persistent bacteremia. However, his fever continued to fluctuate, and a follow-up blood culture on hospital day 58, 14 days after daptomycin was started, remained positive for MRSA. The vancomycin MIC of the isolate from day 50 was 4 μg/ml, and the daptomycin MIC was 4 μg/ml as determined by the broth microdilution method, with the calcium concentration adjusted to 50 μg/ml according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (2). Linezolid (600 mg every 12 h, intravenously) was started on hospital day 61. Follow-up blood culture was sterile after 8 days of linezolid treatment. Fusidic acid (500 mg every 8 h, orally) was added on hospital day 69 and was discontinued on day 79 due to the deterioration of hepatic function. Follow-up blood cultures on hospital days 75, 83, and 85 revealed no vancomycin-intermediate Staphylococcus aureus (VISA). However, the patient died of an episode of septic shock with multiorgan failure caused by Candida albicans on hospital day 83.
MICs of all 11 isolates were determined simultaneously (i) by the broth microdilution method with Mueller-Hinton broth (BBL, Becton Dickinson, Sparks, MD) and an initial inoculum of 5 × 105 CFU/ml according to the CLSI guidelines and (ii) by the Etest (AB Biodisk, Solna, Sweden) according to the manufacturer's instructions (2). When daptomycin MICs were tested, the medium was Mueller-Hinton broth containing physiological levels of calcium (50 μg/ml), as recommended previously (6). S. aureus ATCC 29213 was used as a quality control strain in each run. S. aureus Mu3 and Mu50 strains were used in comparison with our VISA isolates. MICs were read after the incubation of microtiter plates and Mueller-Hinton agar plates in the Etest for 24 h at 37°C. The MICs for S. aureus ATCC 29213 were within CLSI control ranges (2).
All of the 11 MRSA isolates were susceptible to tigecycline (MIC, 0.25 μg/ml), fusidic acid (MIC, 0.12 μg/ml), and linezolid (MIC, 1 μg/ml) and resistant to rifampin (MIC, >32 μg/ml) by the broth microdilution method (Table 1). Ceftobiprole MICs for the 11 isolates were either 2 or 4 μg/ml. The Etest MICs of daptomycin (from 1 to 4 μg/ml) and tigecycline (from 0.094 to 0.25 μg/ml) for the 11 isolates were the same as those generated by the broth microdilution method except for those of vancomycin, which were twofold higher (from 1.5 to 8 μg/ml) (Table 1). Pulsotypes of the 11 isolates from the patient generated by pulsed-field gel electrophoresis analysis after the digestion of chromosomal DNA with XbaI were indistinguishable, suggesting that they belonged to a single clone (19). The means and standard deviations of cell wall thickness as determined by transmission electron microscopy and calculated from 30 cells for three isolates with MICs of both vancomycin and daptomycin of 1 μg/ml, 2 μg/ml, and 4 μg/ml were 22.85 ± 5.13, 25.50 ± 5.58, and 31.68 ± 10.71 nm, respectively (3). The cell wall thicknesses of the isolates were significantly increased with vancomycin and daptomycin MICs of 4 μg/ml as determined by Student's t test (P was 0.0001 in a comparison of results for isolates with MICs of 1 μg/ml and 4 μg/ml, respectively, and P was 0.0069 in a comparison of results for isolates with MICs of 2 μg/ml and 4 μg/ml, respectively) (3).
TABLE 1.
Antibiograms of the 11 isolates determined by the broth microdilution method and the Etest
| Isolate | Date of isolation (mo/day/yr) | MIC (μg/ml)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rifampin | Linezolid | Fusidic acid | Ceftobiprole | Vancomycin
|
Daptomycin
|
Tigecycline
|
|||||
| Broth microdilution method | Etest | Broth microdilution method | Etest | Broth microdilution method | Etest | ||||||
| 1 | 3/30/07 | >32 | 1 | 0.12 | 2 | 1 | 1.5 | 1 | 0.75 | 0.12 | 0.094 |
| 2 | 4/19/07 | >32 | 1 | 0.12 | 2 | 2 | 1.5 | 1 | 0.75 | 0.12 | 0.094 |
| 3 | 4/21/07 | >32 | 1 | 0.12 | 2 | 2 | 1.5 | 1 | 0.75 | 0.12 | 0.094 |
| 4 | 5/7/07 | >32 | 1 | 0.12 | 2 | 2 | 1.5 | 1 | 2 | 0.12 | 0.125 |
| 5 | 5/10/07 | >32 | 1 | 0.12 | 2 | 2 | 4 | 2 | 2 | 0.12 | 0.125 |
| 6 | 5/17/07 | >32 | 1 | 0.12 | 4 | 4 | 4 | 4 | 4 | 0.12 | 0.25 |
| 7 | 5/20/07 | >32 | 1 | 0.12 | 4 | 4 | 8 | 4 | 4 | 0.25 | 0.25 |
| 8 | 5/25/07 | >32 | 1 | 0.12 | 4 | 4 | 8 | 4 | 4 | 0.25 | 0.25 |
| 9 | 5/28/07 | >32 | 1 | 0.12 | 4 | 4 | 8 | 4 | 4 | 0.25 | 0.25 |
| 10 | 5/31/07 | >32 | 1 | 0.12 | 4 | 4 | 8 | 4 | 4 | 0.25 | 0.25 |
| 11 | 6/3/07 | >32 | 1 | 0.12 | 4 | 4 | 8 | 4 | 4 | 0.25 | 0.25 |
| Mu3 | 2 | 3 | |||||||||
| Mu50 | 4 | 10 | |||||||||
Isolates were tested with rifampin, linezolid, fusidic acid, and ceftobiprole by the broth microdilution method only.
Daptomycin is a cyclic lipopeptide antibiotic with rapid bactericidal activity against various gram-positive bacteria, including multidrug-resistant strains such as vancomycin-resistant enterococci, MRSA, VISA, and penicillin-resistant streptococci (7). Reduced susceptibility to daptomycin was considered rare due to its unique bactericidal activity, which occurs via calcium-dependent alternation of cytoplasmic membrane potential (7). In Taiwan, the first two clinically significant isolates of VISA were reported in 2004, although no obvious dissemination of these clones has been noted since (19).
The MRSA isolates from this patient showed a gradual elevation of vancomycin MICs during glycopeptide treatment, from 1 μg/ml, initially, to 2 μg/ml and finally 4 μg/ml soon after daptomycin treatment (Fig. 1; Table 1). We did not perform blood sampling before daptomycin use. Thus, it is not known whether the VISA isolates emerged after prolonged glycopeptide treatment or were selected soon after daptomycin exposure. The S. aureus isolates also showed increased MICs of ceftobiprole, another cell wall-inhibiting agent. From the data reported by Bogdanovich et al., two out of the five VISA isolates tested for ceftobiprole revealed higher ceftobiprole MICs; both VISA isolates showed a ceftobiprole MIC of 2 μg/ml (1). Although rarely reported, the ceftobiprole MIC might become elevated in some VISA strains. Further studies are needed to clarify this issue.
Reduced susceptibility to daptomycin by VISA has been correlated with vancomycin resistance and may be related to the increased thickness of the cell wall of S. aureus (4, 15). Electron microscopy results for our isolates also showed that increased cell wall thickness was correlated to decreased MICs of vancomycin and daptomycin, indicating susceptibility. Recently, a reduction in muramic acid O acetylation in the cell walls of non-daptomycin-susceptible VISA isolates has been demonstrated to be another possible mechanism of drug resistance (10).
Although rare, the emergence of a lack of daptomycin susceptibility during the treatment of high-grade MRSA bacteremia has been reported (8, 9, 11-13, 17) (Table 2). The prolonged use of daptomycin for deep-tissue infection or endovascular infection seems to be common in these cases (Table 1). It has been demonstrated that the accumulation of mutations over time is correlated with reduced drug susceptibility in laboratory-derived non-daptomycin-susceptible S. aureus cultured in sublethal concentrations of daptomycin (5). Daptomycin may not reach an adequate concentration in deep infected tissue or endovascular vegetations and may thus provide an environment which could promote the development of nonsusceptible S. aureus, especially when prolonged treatment is needed in these patients (18).
TABLE 2.
Summary of reported cases of MRSA bacteremia with failed daptomycin treatmenta
| Age of patient (yr)/gender | Underlying condition(s) | Source(s) of bacteremia | Daptomycin dosage | Time until development of nonsusceptibility after usage (days) | Vancomycin MIC (μg/ml) | Daptomycin MIC (μg/ml) | Salvage treatment | Outcome(s) | Molecular method for comparison | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 86/F | Knee prosthesis | Prosthetic joint infection, complication with epidural abscess and vertebral osteomyelitis | 6 mg/kg/day | 35 | NA | 4 | NA | NA | PFGE | 8 |
| 61/F | Cardiac surgery | Sternal osteomyelitis, vertebral osteomyelitis | 6 mg/kg/day | 42 | NA | 4 | NA | NA | PFGE | 9 |
| 91/M | Pacemaker implantation | Pacemaker wire infection | 7 mg/kg/day | 11 | 2 | 2 | NA | Died | PFGE | |
| NA | IVDU | Right-side IE | 1.5 mg/kg/q12h | 4 | NA | 5 | NA | NA | NA | 11 |
| 54/M | HCV-related liver cirrhosis | Portal vein septic thrombosis | 4 mg/kg/q12h | 27 | 1 | 2 | Linezolid, and then vancomycin plus gentamicin | Hospice care, microbiological success | NA | 12 |
| 61/M | AML post-HSCT with GVHD | Vertebral osteomyelitis and diskitis | 6 mg/kg/day | 20 | NA | 4 | Linezolid, and then vancomycin plus rifampin | Died of AML relapse; microbiological success | PFGE | 13 |
| 64/F | DM, breast cancer | Septic arthritis, leg abscess over surgical site | 6 mg/kg/day | —b | ≤2 | 4 | Linezolid | Survived, cured | NA | 17 |
| 66/M | HCV-related liver cirrhosis, ESRD | Left-side IE and probable septic phlebitis | 6 mg/kg/day | 0c | 4 | 4 | Linezolid | Died of candidemia; microbiological success | PFGE | Current study |
F, female; M, male; NA, not applicable; IVDU, intravenous-drug user; HCV, hepatitis C virus; AML, acute myeloblastic leukemia; HSCT, hemopoietic stem cell transplantation; GVHD, graft versus host disease; DM, diabetes mellitus; ESRD, end-stage renal disease; IE, infective endocarditis; q12h, every 12 h; PFGE, pulse-field gel electrophoresis.
—, for this case, the time period included 28 weeks over the course of 2 years.
Nonsusceptible at initiation.
It has been reported that heterogeneous daptomycin susceptibility in S. aureus may be induced after vancomycin usage even without daptomycin exposure (16). Sequential point mutations in S. aureus that occur during vancomycin treatment may lead to decreased susceptibility to daptomycin despite a lack of exposure to this agent (14). The increase of the daptomycin MICs in our case might be induced by both the prolonged usage of glycopeptides and the subsequent daptomycin exposure that selected the nonsusceptible isolates from the heterogeneous daptomycin-susceptible isolates.
In conclusion, daptomycin susceptibilities should be determined especially when this agent is being used as a salvage therapy for MRSA and/or VISA bacteremia with a difficult-to-eradicate focus.
Footnotes
Published ahead of print on 16 January 2008.
REFERENCES
- 1.Bogdanovich, T., L. M. Ednie, S. Shapiro, and P. C. Appelbaum. 2005. Antistaphylococcal activity of ceftobiprole, a new broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 494210-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7, 7th ed. CLSI, Wayne, PA.
- 3.Cui, L., H. Murakami, K. Kuwahara-Arai, H. Hanaki, and K. Hiramatsu. 2000. Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50. Antimicrob. Agents Chemother. 442276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui, L., E. Tominaga, H.-M. Neoh, and K. Hiramatsu. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 501079-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman, L., J. D. Alder, and J. A. Silverman. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 502137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2000. Daptomycin susceptibility tests: interpretive criteria, quality control, and effect of calcium on in vitro tests. Diagn. Microbiol. Infect. Dis. 3851-58. [DOI] [PubMed] [Google Scholar]
- 7.Hancock, R. E. 2005. Mechanisms of action of newer antibiotics for gram-positive pathogens. Lancet Infect. Dis. 5209-218. [DOI] [PubMed] [Google Scholar]
- 8.Hayden, M. K., K. Rezai, R. A. Hayes, K. Lolans, J. P. Quinn, and R. A. Weinstein. 2005. Development of daptomycin resistance in vivo in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 435285-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirschwerk, D., C. C. Ginocchio, M. Bythrow, and S. Condon. 2006. Diminished susceptibility to daptomycin accompanied by clinical failure in a patient with methicillin-resistant Staphylococcus aureus bacteremia. Infect. Control Hosp. Epidemiol. 27315-317. [DOI] [PubMed] [Google Scholar]
- 10.Julian, K., K. Kosowska-Shick, C. Whitener, M. Roos, H. Labischinski, A. Rubio, L. Parent, L. Ednie, L. Koeth, T. Bogdanovich, and P. C. Appelbaum. 2007. Characterization of daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a patient with endocarditis. Antimicrob. Agents Chemother. 513445-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaatz, G. W., T. S. Lundstrom, and S. M. Seo. 2006. Mechanisms of daptomycin resistance in Staphylococcus aureus. Int. J. Antimicrob. Agents 28280-287. [DOI] [PubMed] [Google Scholar]
- 12.Mangili, A., I. Bica, D. R. Snydman, and D. H. Hamer. 2005. Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 401058-1060. [DOI] [PubMed] [Google Scholar]
- 13.Marty, F. M., W. W. Yeh, C. B. Wennersten, L. Venkataraman, E. Albano, E. P. Alyea, H. S. Gold, L. R. Baden, and S. K. Pillai. 2006. Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. J. Clin. Microbiol. 44595-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mwangi, M. M., S. W. Wu, Y. Zhou, K. Sieradzki, H. de Lencastre, P. Richardson, D. Bruce, E. Rubin, E. Myers, E. D. Siggia, and A. Tomasz. 2007. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc. Natl. Acad. Sci. USA 1049451-9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel, J. B., L. A. Jevitt, J. Hageman, L. C. McDonald, and F. C. Tenover. 2006. An association between reduced susceptibility to daptomycin and reduced susceptibility to vancomycin in Staphylococcus aureus. Clin. Infect. Dis. 421652-1653. [DOI] [PubMed] [Google Scholar]
- 16.Sakoulas, G., J. Alder, C. Thauvin-Eliopoulos, R. C. Moellering, Jr., and G. M. Eliopoulos. 2006. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob. Agents Chemother. 501581-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skiest, D. J. 2006. Treatment failure resulting from resistance of Staphylococcus aureus to daptomycin. J. Clin. Microbiol. 44655-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steenbergen, J. N., J. Alder, G. M. Thorne, and F. P. Tally. 2005. Daptomycin: a lipopeptide antibiotic for the treatment of serious gram-positive infections. J. Antimicrob. Chemother. 55283-288. [DOI] [PubMed] [Google Scholar]
- 19.Wang, J. L., S. P. Tseng, P. R. Hsueh, and K. Hiramatsu. 2004. Vancomycin heteroresistance in methicillin-resistant Staphylococcus aureus, Taiwan. Emerg. Infect. Dis. 101702-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]