Abstract
Mycobacterium tuberculosis isolates from different regions of Bulgaria were studied by a variety of molecular typing tools. Based on spacer oligonucleotide typing (spoligotyping), the 113 strains were subdivided into 35 spoligotypes: 5 unique profiles and 15 profiles shared by two to 29 strains; the Hunter-Gaston diversity index (HGI) was 0.9. Comparison with the international database SITVIT2 at the Institut Pasteur de Guadeloupe showed the presence of two globally distributed shared types, ST53 (25.7%) and ST47 (6.2%). Nineteen (16.8%) and six (5.3%) strains belonged to the ST125 (LAM/S subfamily) and ST41 (LAM7_TUR subfamily) types described in SITVIT2 as ubiquitous/rare and ubiquitous/common types, respectively. Seven spoligoprofiles (12 strains) were not found in the database; two of them constituted new shared types. The Beijing genotype strains were not found in the studied collection in spite of close contacts with Russia in the recent and historical past. Additional subtyping by IS6110-restriction fragment length polymorphism (RFLP) and 12-locus mycobacterial interspersed repetitive unit (MIRU)-variable number of tandem repeat analyses were performed within selected spoligotypes. In particular, MIRU typing showed better discrimination within ST125 than IS6110-RFLP typing (HGI = 0.83 versus 0.39). A high gradient for ST125 in Bulgaria compared to its negligible presence in the global database and neighboring countries leads us to suggest a Bulgarian phylogeographic specificity of this spoligotype. To conclude, this first study of the Bulgarian M. tuberculosis population demonstrated its heterogeneity and predominance of several worldwide-distributed and Balkan-specific spoligotypes.
Tuberculosis (TB) remains an important public health issue for Bulgaria, although genotypic data on the circulating Mycobacterium tuberculosis strains have not yet been published from this Balkan country. Although the number of new cases is showing a steady decline since 2001 (48.6/100,000), the TB incidence rate in Bulgaria is still sufficiently high (42.7/100,000 in 2005) (5). Geographically, Bulgaria is located in the region with contrasting epidemiological situation for TB. The southern neighbor, Greece, reported TB rates to have been gradually decreased, while the incidence was only 6.9/100,000 in 2005 (5). The reported TB rates for Romania and Turkey are significantly higher and have been increasing and reached 135.2/100,000 in Romania and 28.1/100,000 in Turkey in 2005 (5).
Recent advances in molecular techniques have enabled development of a variety of genotyping methods for the differentiation of clinical isolates of M. tuberculosis (15, 21). In particular, repetitive and insertion sequences have proven useful for studying both the epidemiology and the phylogeography of M. tuberculosis (1, 2, 4, 11-19, 24), and regularly updated genetic diversity databases are available for this pathogen (3, 4, 7, 11-13, 23).
The chromosomal locus, containing a large number of direct repeats (DRs) interspersed with unique spacer sequences, is the target of spoligotyping (i.e., spacer oligonucleotide typing) technique (10). This method has been widely applied to study the molecular epidemiology and evolutionary genetics of TB. Since the technique is PCR-based, it requires less DNA than conventional molecular typing, e.g., IS6110 restriction fragment length polymorphism (RFLP) analysis, which is the most widely applied and standardized molecular typing method (20, 21). The more recently introduced mycobacterial interspersed repetitive units (MIRU) typing (18) is based on variation in copy number in the variable number of tandem repeat (VNTR) loci, and, in its simplest version, it requires only basic PCR and agarose electrophoresis equipment. This relatively new method was shown with different strain samples to possess a higher discriminatory power than that of spoligotyping and only slightly below that of IS6110-RFLP typing (19), although this may vary depending on the local population structure (14, 24). The apparent advantage of the MIRU approach (compared to IS6110 typing) is its portability due to easy digitalization of the generated profiles.
This study presents the first insight into the population structure of M. tuberculosis in Bulgaria, as a necessary step toward an implementation and better understanding of molecular epidemiology of TB here. We further looked at our data on a global scale through comparison with the international SITVIT2 database.
MATERIALS AND METHODS
Bacterial isolates.
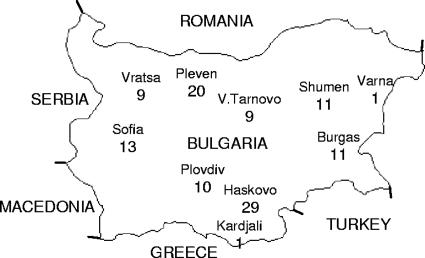
A total of 113 M. tuberculosis strains were recovered from 113 TB patients for whom no epidemiological links were detected by standard investigation. These patients were admitted to the TB hospitals in different regions of Bulgaria from 2004 to 2006 (Fig. 1). All patients were human immunodeficiency virus negative.
FIG. 1.
Map of Bulgaria showing the distribution of M. tuberculosis strains included in the present study (numbers indicate the absolute number per area of isolation).
DNA fingerprinting.
The DNA of the studied strains was extracted from 4- to 6-week-old Löwenstein-Jensen medium cultures according to the recommended method (20).
Spoligotyping was used to analyze a variation in the DR locus (i.e., the absence or presence of 43 different spacers) as described previously (10). The individual spoligotyping patterns were entered in an Excel spreadsheet and compared to those of the international database SITVIT2 (Institut Pasteur de Guadeloupe), which is an updated version of the published SpolDB 4.0 database (3). At the time of this comparison (4 September 2007), SITVIT2 contained a total of 2,880 shared types corresponding to 66,846 clinical isolates from 122 isolation countries and 166 countries of origin. Major phylogenetic clades were assigned according to signatures provided in SpolDB4 (3), which defines 62 genetic lineages and sublineages. These included specific signatures for various M. tuberculosis subspecies such as M. bovis, M. microti, M. caprae, M. pinipedii, and M. africanum, as well as rules for defining major lineages and sublineages for M. tuberculosis sensu stricto. The latter included the Central Asian (CAS) clade (2 sublineages), the East African Indian (EAI) clade (9 sublineages), the Haarlem (H) clade (34 sublineages), the Latin-American-Mediterranean (LAM) clade (12 sublineages), the “Manu” family (3 sublineages), the Beijing family, the S clade, the IS6110-low banding X clade (3 sublineages), and an ill-defined T clade (5 sublineages).
Diversity indices of the global distribution of spoligotypes were estimated by the formulas proposed by Filliol et al. (7). The two calculated qualifiers were spreading index (indicating type description as epidemic, common, recurrent, or rare) and matching code (indicating type description as endemic, localized, or ubiquitous).
IS6110-RFLP typing was performed mainly as previously described (20). Briefly, M. tuberculosis DNA was digested with PvuII, electrophoresed, Southern blotted, and hybridized with a digoxigenin-labeled 245-bp PCR-generated IS6110 probe. Each Southern blot included DNA of M. tuberculosis strain 14323 as an external molecular weight marker. The hybridization profiles were visualized as banding patterns on membrane by using an alkaline phosphatase (Roche Applied Science)-catalyzed colorimetric reaction.
MIRU-VNTR analysis was performed mainly as described by Supply et al. (19), using the Tth polymerase (Eurobio, France) for PCR. The amplicons were evaluated in the 1.5% standard (Quantum) agarose gels by using a 100-bp DNA ladder (Amersham Bioscience). The results from each of the 12 loci were combined into 12-digit allelic profiles. The H37Rv strain was run as an additional control for the performance of the method in our laboratory.
Statistical analysis.
The Hunter-Gaston index (HGI) was used to evaluate the discriminatory power of the typing method (9). The HGI is the probability that two strains consecutively taken from a given population will be placed into different types by a typing method; the lower the index value, the less discriminative the typing method. The HGI was calculated as described previously (9).
The most parsimonious network of the MIRU profiles was built by using the PARS routine of the PHYLIP 3.6 package (6). PARS is a general parsimony program that carries out the Wagner parsimony method with multiple states. It assumes that different characters and different lineages evolve independently, and changes to all other states are equally probable. This is applicable for describing the evolution of MIRU-VNTR loci treated as categorical variables.
RESULTS
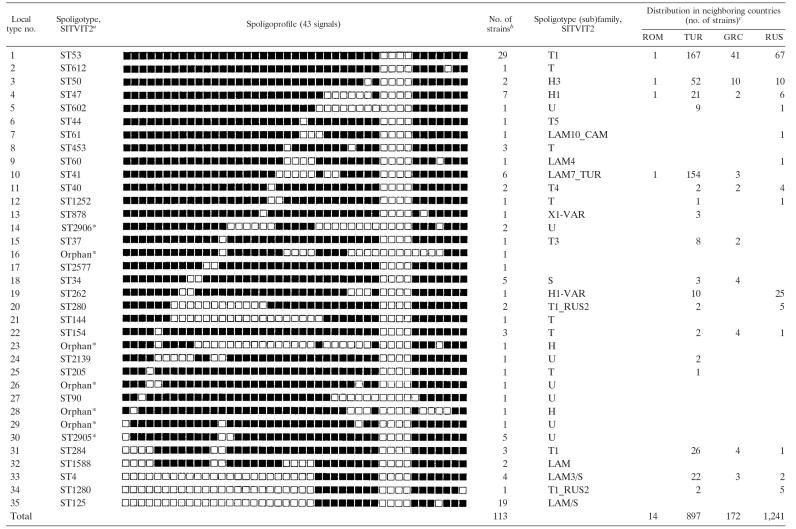
A total of 113 M. tuberculosis clinical isolates originating from different regions of Bulgaria (Fig. 1) were analyzed by spoligotyping. These isolates were subdivided into 35 spoligotypes: 5 unique profiles and 15 profiles shared by 2 to 29 strains (Table 1) . The HGI was sufficiently high (0.90), although the fact that the largest cluster comprised 25.7% of the studied strains indicates a somewhat limited utility of spoligotyping, at least, as a primary typing tool, in the Bulgarian setting.
TABLE 1.
Description of M. tuberculosis spoligotypes found in Bulgaria and compared to SITVIT2 database
*, A “new” profile not found in the SITVIT2 database.
This study.
Abbreviations: ROM, Romania; TUR, Turkey; GRC, Greece; RUS, Russia.
The spoligotype designation was attributed by online comparison of the obtained profiles presented in binary code to those included in the international SITVIT2 database (Institut Pasteur de Guadeloupe) (Table 1). This comparison showed a noticeable presence of two globally distributed ubiquitous/common shared types: ST53 (25.7%) and ST47 (6.2%). Nineteen (16.8%) and six (5.4%) strains belonged to ST125 (LAM/S subfamily) and ST41 (LAM7_TUR subfamily) that can be defined as ubiquitous/rare and ubiquitous/common types, respectively. Seven spoligoprofiles (12 strains) were not found in the SITVIT2 database and were designated as “new”; two of them constituted the new shared types ST2905 and ST2906, while the other five remained orphans (Table 1).
Additional secondary subtyping by IS6110-RFLP and MIRU-VNTR methods was done for two types: ST125, which was found in a high proportion in Bulgaria but is rare worldwide, and ST41, which is phylogeographically specific to and prevalent in neighboring Turkey (24).
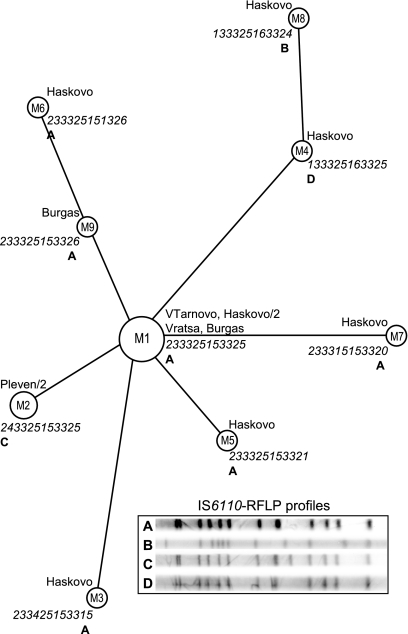
Most of the isolates of ST125 showed a similar 12-band IS6110 pattern, whereas three variants representing one or two strains differed mainly in one or two bands (Fig. 2). MIRU typing discriminated the same strains of spoligotype ST125 into nine profiles (Fig. 2). Two strains with unique IS6110 profiles D and B also had the unique MIRU profiles M4 and M8 (Fig. 2). The other 10 strains with identical IS6110-RFLP profile A were differentiated by MIRU typing into six types (a cluster of the five identical strains and five singletons [Fig. 2]). The MIRU40 locus was most polymorphic in these strains, showing five allelic variants. Thus, the HGIMIRU (0.83) was much higher than the HGIIS6110 (0.39) for the ST125 strains, demonstrating the utility of the MIRU typing even in its classical, 12-locus version.
FIG. 2.
MIRU-based network of the spoligotype ST125 strains. Each circle, node, or tip is described by MIRU type number (inside a circle), strain origin/number of strains (if more than one), MIRU 12-digit profile (in italics), and IS6110-RFLP one-letter profile designation (in boldface). MIRU type numbering within ST125 was done only for convenience of analysis and discussion. The circle size is roughly proportional to the number of strains sharing a respective MIRU profile.
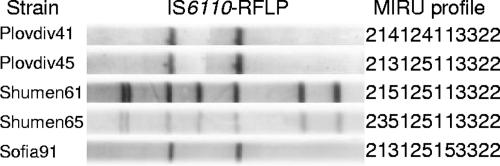
Five strains of ST41 available for secondary typing fall into two IS6110-RFLP groups, one having a two-band profile and the other with an additional five bands (Fig. 3). For these five strains, the classical 12-locus MIRU typing also provided greater discrimination by subdividing them into five variants (Fig. 3).
FIG. 3.
MIRU and IS6110-RFLP profiles of the spoligotype ST41 strains.
DISCUSSION
This study has been undertaken in order to gain a first insight into the population structure of M. tuberculosis in Bulgaria. Spoligotyping was used as a primary typing tool because of its easy use and straightforward coding and interpretation of results; furthermore, the availability of the international database permitted us to view our results in the context of the globally and locally circulating M. tuberculosis clones. We further attempted to differentiate within particular spoligotypes by the secondary subtyping methods IS6110-RFLP and MIRU-VNTR typing.
A large proportion of the studied strains belonged to spoligotype ST53 (Fig. 1 and Table 1). This worldwide-distributed spoligotype represents 6.0% of strains in SITVIT2. In our sample it constituted a much higher proportion (25.7%). Comparison to geographical and historical neighboring countries revealed that this spoligotype is present in almost as high a proportion in Turkey (18.6%) and Greece (23.8%) but not in Russia (5.4%) (Table 1). Consequently, Bulgarian ST53 strains were likely brought to Bulgaria as a result of the Balkan intraregional human movement. For example, a significant increase in human exchange between Bulgaria and Turkey has been noted since the latter 1980s (22). However, a similar and high proportion of these strains not only in Bulgaria and Turkey but also in Greece leads us to hypothesize a historically relatively more distant time for their importation here driven by the medieval expansion of the Ottoman Empire (8; history map of Europe, year 1600 [http://www.euratlas.com/big/big1600.htm]).
The other three frequently found spoligotypes in our collection were ST47, ST41, and ST125 (Table 1). In particular, ST41 belongs to the LAM7_TUR subfamily; it is phylogeographically specific to Turkey (24), and only rare ST41 isolates have been described in Greece and Romania. It may be possible that ST41 has reached its high rate in Turkey during the course of the 20th century and has not yet penetrated to the neighboring countries in significant proportions.
A comparison of the 12-locus MIRU and IS6110-RFLP profiles of the strains of spoligotype ST125 revealed a higher discriminatory power for the MIRU method (Fig. 2). The MIRU-based phylogenetic network suggested M1 type as a core type to which most other types could be directly linked. This analysis is limited by a small sample size; however, the fact that most of ST125 strains were found in the core type leads us to suggest the ancestral position of this MIRU profile. A star-like MIRU network of ST125, together with a predominating core type, suggest (i) a relatively distant presence of ST125 in Bulgaria (in particular, southern Bulgaria) and (ii) its recent dissemination. On the other hand, a comparison with the global database SITVIT2 revealed a high gradient for ST125 in Bulgaria and its negligible presence elsewhere (Table 1). A similarity of the IS6110-RFLP profiles confirmed a true relatedness of these strains, whereas the great diversity of the 12-MIRU loci suggested a long-term evolution of this spoligotype in Bulgaria. These findings lead us to suggest a Bulgarian phylogeographic specificity of the spoligotype ST125.
Although a detailed comparison with drug susceptibility data was beyond the scope of the present study, we noted a high rate of multidrug-resistant (MDR) strains in the studied Bulgarian sample (21.6%). For example, it has been suggested that current transmission of MDR-TB in Russia is greatly influenced by the ongoing dissemination of the Beijing family strains (16). In our study, several spoligotypes were shared by Bulgarian and Russian strains (Table 1), a finding that is readily explained by close links and extensive human movement between the two countries until the end of the 20th century. Nevertheless, the Beijing genotype was not identified in the studied strains from Bulgaria. Consequently, the current situation with MDR-TB in Bulgaria cannot be explained by global dissemination of the Beijing genotype, which apparently has not yet reached this country.
In conclusion, we present here the first molecular snapshot of the M. tuberculosis strains circulating in Bulgaria. We have demonstrated a heterogeneity of the Bulgarian M. tuberculosis population that appears to be dominated by several worldwide-distributed and Balkan-specific spoligotypes.
Acknowledgments
We are grateful to Thierry Zozio and Christophe Demay for help with the comparison of spoligotype patterns using the updated SITVIT2 database of the Pasteur Institute of Guadeloupe.
This study was supported by NATO's Public Diplomacy Division in the framework of Science for Peace program (grant SFP-982319).
Footnotes
Published ahead of print on 16 January 2008.
REFERENCES
- 1.Al-Hajoj, S. A., T. Zozio, F. Al-Rabiah, V. Mohammad, M. Al-Nasser, C. Sola, and N. Rastogi. 2007. First insight into the population structure of Mycobacterium tuberculosis in Saudi Arabia. J. Clin. Microbiol. 452467-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brudey, K., I. Filliol, S. Ferdinand, V. Guernier, P. Duval, B. Maubert, C. Sola, and N. Rastogi. 2006. Long-term population-based genotyping study of Mycobacterium tuberculosis complex isolates in the French departments of the Americas. J. Clin. Microbiol. 44183-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brudey, K., J. R. Driscoll, L. Rigouts, W. M. Prodinger, A. Gori, S. A. Al-Hajoj, C. Allix, L. Aristimuno, J. Arora, V. Baumanis, L. Binder, P. Cafrune, A. Cataldi, S. Cheong, R. Diel, C. Ellermeier, J. T. Evans, M. Fauville-Dufaux, S. Ferdinand, D. Garcia de Viedma, C. Garzelli, L. Gazzola, H. M. Gomes, M. C. Gutierrez, P. M. Hawkey, P. D. van Helden, G. V. Kadival, B. N. Kreiswirth, K. Kremer, M. Kubin, S. P. Kulkarni, B. Liens, T. Lillebaek, H. M. Ly, C. Martin, I. Mokrousov, O. Narvskaya, Y. F. Ngeow, L. Naumann, S. Niemann, I. Parwati, M. Z. Rahim, V. Rasolofo-Razanamparany, T. Rasolonavalona, M. L. Rossetti, S. Rusch-Gerdes, A. Sajduda, S. Samper, I. Shemyakin, U. B. Singh, A. Somoskovi, R. Skuce, D. van Soolingen, E. M. Streicher, P. N. Suffys, E. Tortoli, T. Tracevska, V. Vincent, T. C. Victor, R. Warren, S. F. Yap, K. Zaman, F. Portaels, N. Rastogi, and C. Sola. 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Sahly, H. M., J. A. Wright, H. Soini, T. T. Bui, N. Williams-Bouyer, P. Escalante, J. M. Musser, and E. A. Graviss. 2004. Recurrent tuberculosis in Houston, Texas: a population-based study. Int. J. Tuberc. Lung Dis. 8333-340. [PubMed] [Google Scholar]
- 5.EuroTB-Institut de Veille Sanitaire. 2007. Surveillance of Tuberculosis in Europe: Report on tuberculosis cases notified in 2005. EuroTB-Institut de Veille Sanitaire, Saint-Maurice, France.
- 6.Felsenstein, J. 2004. PHYLIP (phylogeny inference package), version 3.6b. Department of Genome Sciences, University of Washington, Seattle.
- 7.Filliol, I., J. R. Driscoll, D. van Soolingen, B. N. Kreiswirth, K. Kremer, G. Valetudie, D. A. Dang, R. Barlow, D. Banerjee, P. J. Bifani, K. Brudey, A. Cataldi, R. C. Cooksey, D. V. Cousins, J. W. Dale, O. A. Dellagostin, F. Drobniewski, G. Engelmann, S. Ferdinand, D. Gascoyne-Binzi, M. Gordon, M. C. Gutierrez, W. H. Haas, H. Heersma, E. Kassa-Kelembho, M. L. Ho, A. Makristathis, C. Mammina, G. Martin, P. Moström, I. Mokrousov, V. Narbonne, O. Narvskaya, A. Nastasi, S. N. Niobe-Eyangoh, J. W. Pape, V. Rasolofo-Razanamparany, M. Ridell, M. L. Rossetti, F. Stauffer, P. N. Suffys, H. Takiff, J. Texier-Maugein, V. Vincent, J. H. de Waard, C. Sola, and N. Rastogi. 2003. Snapshot of moving and expanding clones of Mycobacterium tuberculosis and their global distribution assessed by spoligotyping in an international study. J. Clin. Microbiol. 411963-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper, P. L. 2003. Forced population transfers in early Ottoman imperial strategy: a comparative approach. Ph.D. thesis. Princeton University, Princeton, NJ.
- 9.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson' s index of diversity. J. Clin. Microbiol. 262465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Rapid detection and simultaneous strain differentiation of Mycobacterium tuberculosis for diagnosis and tuberculosis control. J. Clin. Microbiol. 35907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kremer, K., J. R. Glynn, T. Lillebaek, S. Niemann, N. E. Kurepina, B. N. Kreiswirth, P. J. Bifani, and D. van Soolingen. 2004. Definition of the Beijing/W lineage of Mycobacterium tuberculosis on the basis of genetic markers. J. Clin. Microbiol. 424040-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mokrousov, I. 2007. Towards a quantitative perception of human-microbial co-evolution. Front. Biosci. 124818-4825. [DOI] [PubMed] [Google Scholar]
- 13.Mokrousov, I., H. M. Ly, T. Otten, N. N. Lan, B. Vyshnevskyi, S. Hoffner, and O. Narvskaya. 2005. Origin and primary dispersal of the Mycobacterium tuberculosis Beijing genotype: clues from human phylogeography. Genome Res. 151357-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mokrousov, I., O. Narvskaya, E. Limeschenko, A. Vyazovaya, T. Otten, and B. Vyshnevskiy. 2004. Analysis of the allelic diversity of the mycobacterial interspersed repetitive units in Mycobacterium tuberculosis strains of the Beijing family: practical implications and evolutionary considerations. J. Clin. Microbiol. 422438-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moström, P., M. Gordon, C. Sola, M. Ridell, and N. Rastogi. 2002. Methods used in the molecular epidemiology of tuberculosis. Clin. Microbiol. Infect. 8694-704. [DOI] [PubMed] [Google Scholar]
- 16.Narvskaya, O., I. Mokrousov, T. Otten, and B. Vishnevsky. 2005. Molecular markers: application for studies of the Mycobacterium tuberculosis population in Russia, p. 111-125. In M. M. Read (ed.), Trends in DNA fingerprinting research. Nova Science Publishers, New York, NY.
- 17.Sola, C., I. Filliol, E. Legrand, I. Mokrousov, and N. Rastogi. 2001. Mycobacterium tuberculosis phylogeny reconstruction based on combined numerical analysis with IS1081, IS6110, VNTR, and DR-based spoligotyping suggests the existence of two new phylogeographical clades. J. Mol. Evol. 53680-689. [DOI] [PubMed] [Google Scholar]
- 18.Supply, P., E. Mazars, S. Lesjean, V. Vincent, B. Gicquel, and C. Locht. 2000. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol. Microbiol. 36762-771. [DOI] [PubMed] [Google Scholar]
- 19.Supply, P., S. Lesjean, E. Savine, K. Kremer, D. van Soolingen, and C. Locht. 2001. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 393563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Embden, J., M. Cave, J. Crawford, J. Dale, K. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. Shinnick, and P. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendation for a standardized methodology. J. Clin. Microbiol. 31406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Soolingen, D. 2001. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J. Intern. Med. 2491-26. [DOI] [PubMed] [Google Scholar]
- 22.Vasileva, D. 1992. Bulgarian Turkish emigration and return. Int. Migr. Rev. 26342-352. [PubMed] [Google Scholar]
- 23.Weniger, T., D. Harmsen, P. Supply, and S. Niemann. 2007. Mycobacteria MIRU-VNTRplus: online database and analysis tool for MIRU, spoligo, and regions of difference data. Abstr. 107th Gen. Meet. Am. Soc. Microbiol., abstr. U-024, p. 688-689.
- 24.Zozio, T., C. Allix, S. Gunal, Z. Saribas, A. Alp, R. Durmaz, M. Fauville-Dufaux, N. Rastogi, and C. Sola. 2005. Genotyping of Mycobacterium tuberculosis clinical isolates in two cities of Turkey: description of a new family of genotypes that is phylogeographically specific for Asia Minor. BMC Microbiol. 544. [DOI] [PMC free article] [PubMed] [Google Scholar]