Abstract
In recent years, clusters of Pneumocystis jirovecii (formerly Pneumocystis carinii) pneumonia (PCP) among immunocompromised individuals have been reported. Mostly, the source of infections was suspected to be within the clinical settings when transplant recipients and PCP patients shared hospital facilities. We report on a cluster of 16 renal transplant recipients positive for P. jirovecii. None of them received anti-Pneumocystis prophylaxis prior to P. jirovecii detection. Epidemiological studies revealed that 15 of them had received kidney transplants at a German university hospital and attended the same inpatient and outpatient clinic from January through September 2006. Multilocus sequence typing (MLST) was performed on the following genes: ITS1, β-tub, 26S, and mt26S. P. jirovecii DNA was available from 14 patients and showed identical MLST types among these renal transplant recipients. Surprisingly, one patient who was treated at a different nephrological center and reported no personal contact with patients from the renal transplantation cluster harbored an identical P. jirovecii MLST type. Three HIV-positive patients and one bone-marrow-transplanted hematologic malignancy patient—treated at different medical centers—were used as controls, and different MLST types were revealed. Interestingly, in three of the four previously described regions, new alleles were detected, and one new polymorphism was observed in the mt26S region. The epidemiological data and the genotyping results strongly suggest a nosocomial patient-to-patient transmission of P. jirovecii as the predominant transmission route. Therefore, strict segregation and isolation of P. jirovecii-positive/suspected patients in clinical settings seems warranted.
Pneumocystis jirovecii (formerly Pneumocystis carinii) is an opportunistic fungus causing serious and even life-threatening pneumonia (P. jirovecii pneumonia [PCP]) in immunocompromised individuals. PCP is still one of the most common initial AIDS manifestations but has also been described for malignancy patients, transplant recipients, and patients receiving immunosuppressive therapy.
The epidemiology and pathogenesis of P. jirovecii remain poorly understood. The source and reservoir of the infection in humans have not yet been established. Currently animal and human studies favor an airborne transmission route for PCP. Thereby, person-to-person spread is the most likely mode of acquisition of new infections (27). The incubation period in humans is supposed to range from 3 to 12 weeks (3, 22). Host specificity suggests that the reservoir of P. jirovecii is limited to humans. In newer studies, PCP is speculated to result from a de novo infection rather than from reactivation of a latent childhood infection (14, 13). Asymptomatic/unapparent carriage by immunocompromised or immunocompetent persons may serve as an infectious reservoir.
Since P. jirovecii cannot be grown in in vitro culture, microscopy of appropriate clinical samples has been the mainstay of laboratory diagnosis of pneumocystosis. More-sensitive methods, such as PCR, are increasingly used for routine diagnosis and are able to detect even low numbers of P. jirovecii organisms in asymptomatic but colonized persons (16, 18, 23, 24, 26). To address clinical and epidemiological issues of PCP and to assess the hypothesis of person-to-person transmission, several molecular typing methods are in use and a large number of gene loci have been examined. These methods are based on PCR amplification and sequencing of variable genome regions or the use of allele-specific PCRs and hybridizations. Therefore, molecular typing of single-strand-conformation polymorphisms (7, 11) and multiple-locus DNA sequence typing (MLST) (8) were used successfully. Epidemiologic studies showed a broad genetic diversity of Pneumocystis types in the population independent of season, geographical location, and human immunodeficiency virus (HIV) status (7). Furthermore, coinfections with two or more different molecular types were reported (15).
Here we report a cluster of 16 renal transplant recipients positive by microscopy for P. jirovecii in respiratory secretions from January through September 2006. Fifteen of them had received kidney transplants at the same hospital and attended the same inpatient and outpatient wards. One patient of this cluster was treated at a different hospital, and no personal contact with the other patients could be identified. In the previous 2 years (2004 and 2005), only two cases of PCP were observed in this population (kidney transplant recipients). In response to the sudden increase in PCP incidence, epidemiological data were evaluated and MLST sequencing of four regions (the internal transcribed spacer 1 of the nuclear rRNA gene operon [ITS1], the gene encoding β-tubulin [β-tub], the intron of the nuclear 26S rRNA gene [26S], and the variable region of the mitochondrial 26S rRNA gene [mt26S]) was performed using P. jirovecii DNA available from 14 patients. Three HIV patients and one bone-marrow-transplanted hematologic malignancy patient treated at three additional medical centers were used as control patients. Interestingly, in three of the four previously described regions, new alleles were detected by MLST typing, and one new polymorphism was observed in the mt26S region.
MATERIALS AND METHODS
Patients and hospital settings.
In total, 20 patients were studied (Table 1). Sixteen patients were renal transplant recipients (patients 1 to 16). Four unrelated PCP cases from three different hospitals were used as control patients (patients 17 to 20). Patient 17 was a bone-marrow-transplanted hematologic malignancy patient, and patients 18 to 20 were HIV infected. P. jirovecii DNA was recovered from bronchoalveolar lavage (BAL) fluid specimens obtained from January through September 2006. Patients 1 to 9 and patients 11 to 16 received kidney transplants at a German renal transplantation unit (termed clinic 1, ward A) and also attended the outpatient ward of the renal transplantation unit at clinic 1, ward A. BAL samples from patients 1, 3 to 8, and 11 to 16 were obtained during hospitalization, also at clinic 1, wards A to E, or at the Departments of Internal Medicine (clinic 2). Patients 2 and 9 were hospitalized at two different medical centers in two different towns (termed clinics 3 and 4, with distances from clinic 1 of about 120 km and 70 km, respectively) when PCP diagnosis was confirmed in BAL specimens. Patient 10 received a kidney transplant at clinic 5, and P. jirovecii was isolated from BAL samples obtained during hospitalization at clinic 6. In none of the four fatalities during the outbreak was the immediate cause of death attributable to PCP. Patients 18 and 20 attended clinic 6, patient 17 attended the pediatric unit of clinic 7, and patient 19 attended clinic 8.
TABLE 1.
Data for patients and controls
| Patient group and no. (gendera) | Age (yr) | Clinical statusb | All clinics attended since transplantationc | Place of hospitalization when infection detectedd | Date of first detectione |
|---|---|---|---|---|---|
| Transplantation cluster | |||||
| 1 (m) | 62 | Renal transplantation | Clinic 1, ward A; clinic 1, ward B | Clinic 1, ward B | 01/05/2006 |
| 2 (m) | 45 | Renal transplantation | Clinic 1, ward A; clinic 2, clinic 3 | Clinic 3 | 04/19/2006 |
| 3 (m) | 45 | Renal transplantation | Clinic 1, ward A; clinic 1, ward C | Clinic 1, ward A | 04/26/2006 |
| 4 (f) | 27 | Renal transplantation | Clinic 1, ward A; clinic 1, ward D | Clinic 1, ward D | 04/27/2006 |
| 5 (m) | 65 | Renal transplantation | Clinic 1, ward A; clinic 1, ward E | Clinic 1, ward E | 05/29/2006 |
| 6 (f) | 55 | Renal transplantation | Clinic 1, ward A; clinic 1, ward B | Clinic 1, ward B | 06/02/2006 |
| 7 (f) | 41 | Renal transplantation | Clinic 1, ward A; clinic 2 | Clinic 2 | 06/14/2006 |
| 8 (m) | 51 | Renal transplantation | Clinic 1, ward A; clinic 1, ward C | Clinic 1, ward A | 06/19/2006 |
| 9 (m) | 68 | Renal transplantation | Clinic 1, ward A; clinic 4 | Clinic 4 | 06/20/2006 |
| 10 (m) | 49 | Renal transplantation | Clinic 5, clinic 6 | Clinic 6 | 06/22/2006 |
| 11 (f) | 48 | Renal transplantation | Clinic 1, ward A | Clinic 1, ward A | 06/23/2006 |
| 12 (m) | 38 | Renal transplantation | Clinic 1, ward A; clinic 1, ward E; clinic 1, ward B | Clinic 1, ward A | 06/29/2006 |
| 13 (f) | 58 | Renal transplantation | Clinic 1, ward A | Clinic 1, ward A | 06/29/2006 |
| 14 (m) | 68 | Renal transplantation | Clinic 1, ward A | Clinic 1, ward A | 07/10/2006 |
| 15 (f) | 41 | Renal transplantation | Clinic 1, ward A | Clinic 1, ward A | 07/21/2006 |
| 16 (f) | 67 | Renal transplantation | Clinic 1, ward A; clinic 1, ward B | Clinic 1, ward B | 08/31/2006 |
| Control patients | |||||
| 17 (f) | 1 | BMT | Clinic 7 | Clinic 7 | 03/21/2006 |
| 18 (m) | 38 | HIV+ | Clinic 6 | Clinic 6 | 07/18/2006 |
| 19 (m) | 26 | HIV+ | Clinic 7 | Clinic 8 | 08/09/2006 |
| 20 (m) | 32 | HIV+ | Clinic 6 | Clinic 6 | 08/21/2006 |
m, male; f, female.
BMT, bone marrow transplantation; HIV+, HIV positive.
Clinical facilities visited by all patients from the transplantation cluster and by control patients in the time period from the date of transplantation for patient 1 until the detection of P. jirovecii in specimens from patient 16.
Clinic where patient was hospitalized when P. jirovecii infection was detected.
Month/day/year of first detection of P. jirovecii infection.
In clinic 1, ward A, the outpatient and inpatient wards were on the same floor of the building, and patients shared hospital facilities such as waiting and treatment rooms. The Departments of Internal Medicine building (clinic 2 and clinic 8) were localized about 5 km away from the transplantation unit building (clinic 1) in the same town. The pediatric unit building (clinic 7) was also situated in the same town, about 6 km away from the transplantation unit building (clinic 1). Clinics 5 and 6 were located in different parts of the town of clinic 1.
Patients 1, 3 to 8, and 10 to 16 were termed a transplantation cluster, and patients 17 to 20 were termed control patients. BAL specimens from patients 2 and 9 were not available for typing.
None of the renal transplant recipients received anti-Pneumocystis prophylaxis prior to P. jirovecii detection. Data about the patient's immunological status and further clinical data were not available. The PCP diagnosis of all 20 patients except for patients 2 and 9 was established by microscopy of both Giemsa- and Grocott-stained BAL samples and confirmed by PCR at the Max von Pettenkofer-Institute as published recently (24, 23). Thus, BAL samples from patients 2 and 9 were not available for MLST typing. After institution of prophylaxis with trimethoprim-sulfamethoxazole, only one further case of PCP in a patient refusing PCP prophylaxis occurred.
PCR and sequencing.
DNA was extracted as described elsewhere (24). MLST was performed at the Bavarian LGL. Fragments of four regions (ITS1, 26S, mt26S, and β-tub) of the P. jirovecii genome were amplified and subsequently sequenced (8). Sequencing was performed at least twice on all samples.
PCRs were performed in a 50-μl volume with the HotStartTaq master mix (Qiagen, Hilden, Germany) as described by the manufacturer using 5 μl template DNA. To enhance PCR performance, 2.5% dimethyl sulfoxide was added. The final concentration of each primer was 0.4 μM. Primer sequences, PCR programs with annealing temperatures, and magnesium concentrations of each of the four reactions were used as described by Hauser et al. (8).
The size of the ITS1 PCR product/amplicon was 204 bp, and that of the 26S rRNA gene fragment/amplicon was 426 bp. The mt26S rRNA PCR yielded a fragment of 340 bp and the β-tub PCR a fragment of 309 bp.
The amplicons were purified and sequenced using the ABI PRISM 310 genetic analyzer instrument and the Big Dye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Darmstadt, Germany), following the manufacturer's instructions. Sequence analysis was performed with the DNAStar Lasergene software package (DNASTAR, Inc., Madison, WI).
RESULTS
Epidemiological data.
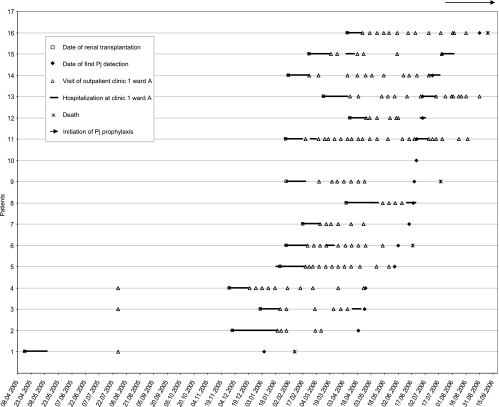
Patients' clinical data and hospitals visited by patients 1 to 16 during the outbreak are summarized in Table 1. All patients from the transplantation cluster except patient 10 visited the inpatient and outpatient department of the renal transplantation center (clinic 1, ward A), suggesting the source of infection to be within this ward. Calendar data of all visits at clinic 1, ward A, by patients 1 to 16 and dates of renal transplantation and first P. jirovecii detection are summarized in Fig. 1. Interestingly, patient 1 was not hospitalized at clinic 1, ward A, simultaneously with any of the following P. jirovecii cases but visited the outpatient ward on the same date that patients 3 and 4 visited it for the first time. Furthermore, patients 2 and 3 and patients 2 and 4 simultaneously visited clinic 1, ward A, when they received kidney transplants. Patient 10 received a kidney transplant at a different nephrological center (clinic 5) and was hospitalized at clinic 6 when P. jirovecii was detected. Both personal contact with patients from the transplantation cluster and any visits at clinic 1 were denied by patient 10. Epidemiological analysis of the outpatient clinic data from clinic 1, ward A, revealed no single medical assistant with contact with all patients of the transplantation cluster who might have transmitted P. jirovecii, as has been described previously for immunocompetent health care workers (28). One immunocompetent health care assistant working at clinic 1, ward A, showed signs of respiratory infection. BAL was collected in September 2006 and analyzed by microscopy and PCR but was found to be negative for P. jirovecii.
FIG. 1.
Transmission map of P. jirovecii among renal transplantation patients. x axis, date (day.month.year). Note on prophylaxis for P. jirovecii (Pj) infection: PCP prophylaxis using trimethoprim-sulfamethoxazole was initiated at clinic 1, ward A, for all kidney transplant recipients for at least 6 months after transplantation. Patients who were treated for rejection received PCP prophylaxis during immunosuppression. Patient no. 16 refused PCP prophylaxis.
As a result of the outbreak investigation presented here, PCP prophylaxis was initiated in July 2006 for all kidney transplant recipients using trimethoprim-sulfamethoxazole for at least 6 months after transplantation and during immunosuppressive therapy for patients who were treated for rejection. Thereafter, only one patient who refused PCP prophylaxis (patient 16) developed PCP.
MLST typing of variable regions of the P. jirovecii genome.
Known variable regions of the P. jirovecii genome from BAL specimens from the transplantation cluster and from the control patients were amplified and sequenced: ITS1, β-tub, 26S, and mt26S. Sequences were compared to published data (8) (Tables 2 and 3).
TABLE 2.
MLST polymorphisms in ITS1 and β-tub regions of DNA from P. jirovecii isolated from patients 1 to 20
| Patient no. | Nucleotide(s) in ITS1 region at position(s) (bp):
|
ITS1 allelea | Base in β-tub region at position (bp):
|
β-tub allelea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 8-10 | 11 | 17 | 22 | 46-47 | 54-62 | 71-72 | 111-113 | 24 | 282 | |||
| 1 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 2b | |||||||||||||
| 3 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 4 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 5 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 6 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 7 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 8 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 9b | |||||||||||||
| 10 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 11 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 12 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 13e | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | |||
| 14 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 15 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 16 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 17 | T | 2× T | A | T | T | TC | 10× T | GAGG | TTA | B | A | G | 1 |
| 18 | T | 2× T | A | T | T | TC | 9× T | GAGG | TTA | B1 | A | G | 1 |
| 19 | T | 3× T | A | T | C/Tc | TC | 10× T | GAGG | TTA | D1d | A | G | 1 |
| 20 | T | 3× T | A | T | T | TC | 10× T | GAGG | TTA | Dd | A | G | 1 |
Alleles were determined as described by Hauser et al. (8).
BAL samples from patients 2 and 9 were not available for MLST typing.
Sequence not clearly identifiable; possibly more than two types.
New allele, identified in this study.
There was no PCR product for β-tub DNA from P. jirovecii isolated from patient 13.
TABLE 3.
MLST polymorphisms in 26S and mt26S regions of DNA from P. jirovecii isolated from patients 1 to 20
| Patient no. | Nucleotide(s) in 26S at position(s) (bp):
|
26S allelea | Base(s) in mt26S at position(s) (bp):
|
mt26Sb allelea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 78 | 212 | 296 | 305 | 54-57 | 80e | 85 | 248 | 288 | |||
| 01 | A | A | A | 5× T | CT | 4d | 4× A | C | A | C | A | 7 |
| 02b | ||||||||||||
| 03 | A | A | A | 5× T | CT | 4d | 4× A | C | A | C | A | 7 |
| 04 | A | A | A | 5× T | CT | 4d | 4× A | C | A | C | A | 7 |
| 05 | A | A | A | 5× T | CT | 4d | 4× A | C | A | C | A | 7 |
| 06 | A | A | A | 5× T | CT | 4d | 4× A | C | A | C | A | 7 |
| 07 | A | A | A | 5× T | CT | 4d | 4× A | C | A | C | A | 7 |
| 08 | A | A | A | 5× T | CT | 4d | 4× A | C | A | C | A | 7 |
| 09b | ||||||||||||
| 10 | A | A | A | 5× T | CT | 4d | 4× A | C | A | C | A | 7 |
| 11 | A | A | A | 5× T | CT | 4d | 4× A | C | A | C | A | 7 |
| 12 | A | A | A | 5× T | CT | 4d | 4× A | C | A | C | A | 7 |
| 13 | A | A | A | 5× T | —f | 4d | 4× A | C | A | C | A | 7 |
| 14g | 4× A | C | A | C | A | 7 | ||||||
| 15 | A | A | A | 5× T | CT | 4d | 4× A | C | A | C | A | 7 |
| 16 | A | A | A | 5× T | CT | 4d | 4× A | C | A | C | A | 7 |
| 17 | A | A | A | 5× T | CT | 4d | 4× A | C | T | C | A | 8 |
| 18 | A | A | A | 6× T | C | 1 | 4× A | T | C | C | A | 15d |
| 19 | A | A | A | 6× T | C | 1 | 4× A | C | C | C | A | 2 |
| 20 | A | A | A | 5× T | CT | 4d | 4× A | C | C/Ac | C/Tc | A | >2 types (not identifiable) |
Alleles were determined as described at Hauser et al. (8).
BAL samples from patients 2 and 9 were not available for MLST typing.
Sequence not clearly identifiable; possibly more than two types.
New allele, identified in this study.
Polymorphism was described in this study for the first time at this position and was not taken into account for the alleles reported earlier by Hauser et al. (8).
—, sequence not clearly identifiable.
There was no PCR product for 26S DNA from P. jirovecii isolated from patient 14.
All observed polymorphism alleles in the ITS1 gene of P. jirovecii from the transplantation cluster were identical to the previously described allele B. For the control patients, two previously described alleles (B and B1) and two new alleles, termed allele D and allele D1, were observed. Sequencing of β-tub revealed allele 1 both for the transplantation cluster and for the control patients. With DNA from patient 13, no PCR product was detectable, probably because of the small amount of Pneumocystis-specific DNA in this sample. 26S region sequencing revealed one new allele for the transplantation cluster, termed allele 4. Using DNA from patient 14, no PCR product was detectable, probably also because of the small amount of DNA in this sample. For patients 17 and 20 from the control patient group, allele 4 also was observed, and for patients 18 and 19, sequencing revealed allele 1. In the mt26S region, one new polymorphism was observed at bp 80. Sequencing of the mt26S region from the transplantation cluster revealed allele 7. Sequencing of DNA from the control patients revealed allele 8, one new allele, termed allele 15, and allele 2 for patients 17, 18 and 19, respectively. In DNA from patient 20, two different bases were repeatedly observed at bp 248 and 288, indicating the presence of two alleles, most probably attributable to a coinfection with different types of P. jirovecii.
In conclusion, the MLST results for all studied patients from the transplantation cluster were identical, indicating an infection with the same P. jirovecii MLST type. Interestingly, patient 10, also treated at a different nephrological center (clinic 5) from that of the other 13 patients from this cluster, harbored the same P. jirovecii MLST type. In contrast, the four patients from the control patient group, coming from the same geographical region and analyzed during the same time period as the transplantation cluster, each harbored unique P. jirovecii MLST types.
DISCUSSION
In recent years, nosocomial clusters of PCP cases among immunocompromised individuals have been reported. Commonly, the source of infection was suspected to be within the clinical settings when transplant recipients and PCP patients shared hospital facilities (9). Person-to-person transmission was suggested as one possible mode of infection (9, 19, 28), even by immunocompetent contacts (e.g., hospital stuff members) transiently colonized by Pneumocystis organisms (2). Furthermore, the detection of Pneumocystis DNA in air samples from hospital rooms and private rooms of PCP patients indicates the possibility of aerosol spread (1, 21, 25), and an airborne mode of acquisition was also found with animal models (12). Interestingly, renal transplant recipients seem to be at especially high risk of getting both a symptomatic or an asymptomatic Pneumocystis infection. The risk of developing PCP for patients after renal transplantation who are not receiving prophylaxis is approximately 5%, and in recent years, six case reports of renal transplant recipient clusters have been published (3, 4, 6, 10, 11, 20, 22). In three of these studies, P. jirovecii DNA was analyzed by molecular typing and identical P. jirovecii genotypes were found among several patients, indicating an interhuman, nosocomial transmission of P. jirovecii.
In our study, P. jirovecii DNA obtained from respiratory secretions of 14 out of 16 renal transplantation recipients was MLST typed and compared to that of a control patient group of four immunocompromised patients. The different MLST types within the control patients and the unique MLST type of the transplantation cluster indicate that this is a suitable method of discriminating P. jirovecii clones. Moreover, sequencing of the mt26S region was the most discriminative due to its variability compared to sequencing of the other genes. In contrast, β-tub showed no variation between the different P. jirovecii sequences. This finding further demonstrates that each control patient was infected by an individual P. jirovecii clone and that the patients from the transplantation cluster were all infected by a single, undistinguishable clone. Whether patients 2 and 9 also belong to this cluster could not be determined by MLST because BAL specimens were not available, this but is likely because they were also visiting clinic 1, ward A, when PCP occurred. The fact that patients 1 to 7 and patients 9 to 16 were treated in the same inpatient and outpatient wards suggests close person-to-person contacts, which could support nosocomial transmission of P. jirovecii. Patients 2 to 7 and 9 to 16 were visiting clinic 1, ward A, during overlapping time periods, and thereby P. jirovecii might have been transmitted from person to person. Patient 1 was hospitalized at a different ward of clinic 1 when P. jirovecii was first detected but visited the outpatient ward A of clinic 1 once simultaneously with patients 3 and 4 before their renal transplantation and might have transmitted P. jirovecii to one of them, leading to an asymptomatic colonization. However, P. jirovecii also could have been transmitted from patient 1 to the following cases by contact persons, e.g., by transmission from an immunocompetent health care worker, as described previously (28), or by private contacts among renal transplantation patients in the hospital facilities or with immunocompetent relatives. Unfortunately, data on personal contacts were not available for this patient.
Interestingly, patient 10 showed the same MLST type of P. jirovecii, although no epidemiologically identifiable clinical contact with any of the transplantation cluster patients occurred. Considering that MLST was highly discriminative for the four control patients originally from the same geographical area and having been tested in the same time period by the same laboratories as the cluster patients, one might speculate that epidemiological contacts outside the clinical setting might account for this puzzling finding. Alternatively, although less likely, it is conceivable that the type or degree of therapeutic immunosuppression after renal transplantation might predispose for infection with a certain P. jirovecii MLST type during our study period.
None of the patients described in this study received PCP prophylaxis after kidney transplantation, possibly due to expected nephrological side effects of the treatment of choice, trimethoprim-sulfamethoxazole, e.g., a rise in serum creatinine or inhibition of renal potassium secretion, occasionally causing hyperkalemia. Nevertheless, the U.S. Public Health Service, the Infectious Diseases Society of America, and European guidelines suggest PCP prophylaxis for at least 4 months after kidney transplantation and for patients treated for graft rejection (5, 17). Interestingly, in the other large outbreak reported by de Boer et al. (4) as well, these guidelines were not observed by the respective nephrology department, supporting our conclusion to endorse the cited PCP prophylaxis recommendations. Moreover, initiation of PCP prophylaxis at clinic 1, ward A, stopped the PCP outbreak reported here; since then, only one further PCP case has occurred, in a patient refusing PCP prophylaxis.
Among the reported PCP outbreaks (Hocker et al. [n = 3] [11] and de Boer et al. [n = 22] [4]), the outbreak reported here is the second largest, comprising 16 patients. Moreover, it is the largest PCP outbreak reported so far which was caused by a single MLST type, since de Boer's outbreak was caused by at least two different MLST types (4). The two cluster studies by Olsson et al. (20) and Rabodonirina et al. (22) identified either no case of nosocomial person-to-person transmission or only a much smaller presumptive outbreak (n = 5), respectively.
The results of this study and previous studies of P. jirovecii outbreaks (4, 9, 11, 22) support the concept of nosocomial patient-to-patient transmission of P. jirovecii as one predominant transmission route. The transmission of PCP can be speculated to be highest before onset of clinical symptoms of PCP until the end of the first week of antipneumocystis therapy. Thus, a strict segregation of immunocompromised patients and P. jirovecii-positive patients or of PCP suspected-case patients in clinical settings seems warranted. Therefore, a strict isolation of P. jirovecii-positive patients and the use of face mask-type filtering facepiece 2 from early symptoms and PCP diagnosis to the middle of antipneumocystis therapy, when the risk of transmission is likely to be highest, should be recommended.
In conclusion, immunosuppressed renal transplant recipients need careful infection control measures, including trimethoprim-sulfamethoxazole PCP prophylaxis, according to national and international guidelines.
Acknowledgments
We thank Karin Tybus and Birgit Gross (Max von Pettenkofer-Institute) and Marion Lindermayer (Bavarian Health and Food Safety Authority) for excellent technical assistance.
Financial support: none.
Conflicts of interest: none.
Footnotes
Published ahead of print on 23 January 2008.
REFERENCES
- 1.Bartlett, M. S., S. H. Vermund, R. Jacobs, P. J. Durant, M. M. Shaw, J. W. Smith, X. Tang, J. J. Lu, B. Li, S. Jin, and C. H. Lee. 1997. Detection of Pneumocystis carinii DNA in air samples: likely environmental risk to susceptible persons. J. Clin. Microbiol. 352511-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chabe, M., E. Dei-Cas, C. Creusy, L. Fleurisse, N. Respaldiza, D. Camus, and I. Durand-Joly. 2004. Immunocompetent hosts as a reservoir of pneumocystis organisms: histological and RT-PCR data demonstrate active replication. Eur. J. Clin. Microbiol. Infect. Dis. 2389-97. [DOI] [PubMed] [Google Scholar]
- 3.Chave, J. P., S. David, J. P. Wauters, G. van Melle, and P. Francioli. 1991. Transmission of Pneumocystis carinii from AIDS patients to other immunosuppressed patients: a cluster of Pneumocystis carinii pneumonia in renal transplant recipients. AIDS 5927-932. [DOI] [PubMed] [Google Scholar]
- 4.de Boer, M. G., L. E. Bruijnesteijn van Coppenraet, A. Gaasbeek, S. P. Berger, L. B. Gelinck, H. C. van Houwelingen, P. van den Broek, E. J. Kuijper, F. P. Kroon, and J. P. Vandenbroucke. 2007. An outbreak of Pneumocystis jirovecii pneumonia with 1 predominant genotype among renal transplant recipients: interhuman transmission or a common environmental source? Clin. Infect. Dis. 441143-1149. [DOI] [PubMed] [Google Scholar]
- 5.EBPG Expert Group on Renal Transplantation. 2002. European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.7.1. Late infections. Pneumocystis carinii pneumonia. Nephrol. Dial. Transplant. 17(Suppl. 4)36-39. [PubMed] [Google Scholar]
- 6.Hardy, A. M., C. P. Wajszczuk, A. F. Suffredini, T. R. Hakala, and M. Ho. 1984. Pneumocystis carinii pneumonia in renal-transplant recipients treated with cyclosporine and steroids. J. Infect. Dis. 149143-147. [DOI] [PubMed] [Google Scholar]
- 7.Hauser, P. M., D. S. Blanc, P. Sudre, E. Senggen Manoloff, A. Nahimana, J. Bille, R. Weber, and P. Francioli. 2001. Genetic diversity of Pneumocystis carinii in HIV-positive and -negative patients as revealed by PCR-SSCP typing. AIDS 15461-466. [DOI] [PubMed] [Google Scholar]
- 8.Hauser, P. M., P. Francioli, J. Bille, A. Telenti, and D. S. Blanc. 1997. Typing of Pneumocystis carinii f. sp. hominis by single-strand conformation polymorphism of four genomic regions. J. Clin. Microbiol. 353086-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helweg-Larsen, J., A. G. Tsolaki, R. F. Miller, B. Lundgren, and A. E. Wakefield. 1998. Clusters of Pneumocystis carinii pneumonia: analysis of person-to-person transmission by genotyping. QJM 91813-820. [DOI] [PubMed] [Google Scholar]
- 10.Hennequin, C., B. Page, P. Roux, C. Legendre, and H. Kreis. 1995. Outbreak of Pneumocystis carinii pneumonia in a renal transplant unit. Eur. J. Clin. Microbiol. Infect. Dis. 14122-126. [DOI] [PubMed] [Google Scholar]
- 11.Hocker, B., C. Wendt, A. Nahimana, B. Tonshoff, and P. M. Hauser. 2005. Molecular evidence of Pneumocystis transmission in pediatric transplant unit. Emerg. Infect. Dis. 11330-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes, W. T. 1982. Natural mode of acquisition for de novo infection with Pneumocystis carinii. J. Infect. Dis. 145842-848. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, W. T. 1998. Current issues in the epidemiology, transmission, and reactivation of Pneumocystis carinii. Semin. Respir. Infect. 13283-288. [PubMed] [Google Scholar]
- 14.Kovacs, J. A., V. J. Gill, S. Meshnick, and H. Masur. 2001. New insights into transmission, diagnosis, and drug treatment of Pneumocystis carinii pneumonia. JAMA 2862450-2460. [DOI] [PubMed] [Google Scholar]
- 15.Manoloff, E. S., P. Francioli, P. Taffe, G. van Melle, J. Bille, and P. M. Hauser. 2003. Risk for Pneumocystis carinii transmission among patients with pneumonia: a molecular epidemiology study. Emerg. Infect. Dis. 9132-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maskell, N. A., D. J. Waine, A. Lindley, J. C. Pepperell, A. E. Wakefield, R. F. Miller, and R. J. Davies. 2003. Asymptomatic carriage of Pneumocystis jiroveci in subjects undergoing bronchoscopy: a prospective study. Thorax 58594-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masur, H., J. E. Kaplan, and K. K. Holmes. 2002. Guidelines for preventing opportunistic infections among HIV-infected persons—2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. Ann. Intern. Med. 137435-478. [DOI] [PubMed] [Google Scholar]
- 18.Medrano, F. J., M. Montes-Cano, M. Conde, C. de la Horra, N. Respaldiza, A. Gasch, M. J. Perez-Lozano, J. M. Varela, and E. J. Calderon. 2005. Pneumocystis jirovecii in general population. Emerg. Infect. Dis. 11245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, R. F., H. E. Ambrose, V. Novelli, and A. E. Wakefield. 2002. Probable mother-to-infant transmission of Pneumocystis carinii f. sp. hominis infection. J. Clin. Microbiol. 401555-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsson, M., B. M. Eriksson, K. Elvin, M. Strandberg, and M. Wahlgren. 2001. Genotypes of clustered cases of Pneumocystis carinii pneumonia. Scand. J. Infect. Dis. 33285-289. [DOI] [PubMed] [Google Scholar]
- 21.Olsson, M., C. Lidman, S. Latouche, A. Bjorkman, P. Roux, E. Linder, and M. Wahlgren. 1998. Identification of Pneumocystis carinii f. sp. hominis gene sequences in filtered air in hospital environments. J. Clin. Microbiol. 361737-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabodonirina, M., P. Vanhems, S. Couray-Targe, R.-P. Gillibert, C. Ganne, N. Nizard, C. Colin, J. Fabry, J. L. Touraine, G. van Melle, A. Nahimana, P. Francioli, and P. M. Hauser. 2004. Molecular evidence of interhuman transmission of Pneumocystis pneumonia among renal transplant recipients hospitalized with HIV-infected patients. Emerg. Infect. Dis. 101766-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sing, A., A. Roggenkamp, I. B. Autenrieth, and J. Heesemann. 1999. Pneumocystis carinii carriage in immunocompetent patients with primary pulmonary disorders as detected by single or nested PCR. J. Clin. Microbiol. 373409-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sing, A., K. Trebesius, A. Roggenkamp, H. Russmann, K. Tybus, F. Pfaff, J. R. Bogner, C. Emminger, and J. Heesemann. 2000. Evaluation of diagnostic value and epidemiological implications of PCR for Pneumocystis carinii in different immunosuppressed and immunocompetent patient groups. J. Clin. Microbiol. 381461-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sing, A., C. Wonhas, L. Bader, M. Luther, and J. Heesemann. 1999. Detection of Pneumocystis carinii DNA in the air filter of a ventilated patient with AIDS. Clin. Infect. Dis. 29952-953. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi, T., M. Goto, T. Endo, T. Nakamura, N. Yusa, N. Sato, and A. Iwamoto. 2002. Pneumocystis carinii carriage in immunocompromised patients with and without human immunodeficiency virus infection. J. Med. Microbiol. 51611-614. [DOI] [PubMed] [Google Scholar]
- 27.Thomas, C. F., Jr., and A. H. Limper. 2007. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat. Rev. Microbiol. 5298-308. [DOI] [PubMed] [Google Scholar]
- 28.Vargas, S. L., C. A. Ponce, F. Gigliotti, A. V. Ulloa, S. Prieto, M. P. Munoz, and W. T. Hughes. 2000. Transmission of Pneumocystis carinii DNA from a patient with P. carinii pneumonia to immunocompetent contact health care workers. J. Clin. Microbiol. 381536-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]