Abstract
The serotypes and susceptibilities to penicillin, macrolides, and clindamycin of 1,655 invasive isolates of Streptococcus pneumoniae recovered between 1979 and 2004 were determined. A precipitous decrease of 61% in the number of isolates was found following 2000, the year of 7-valent protein-conjugated pneumococcal vaccine (PCV7) introduction (139 versus 55 per 2-year period prior to versus after 2000; P < 0.001). This decrease was 84% in children <5 years old (80 versus 13 per 2-year period; P < 0.001) and 18 to 23% in other age groups (P, not significant). PCV7 serotypes decreased by 76% overall (103 versus 25 per 2-year period; P < 0.001) and by 92% in children <5 years old (65 versus 5 per 2-year period; P < 0.001), with significant decreases in six of the seven PCV serotypes. Other serotypes, except for type 19A, decreased from 32 to 22 per 2-year period, while type 19A increased from 4 to 8 per 2-year period, although none of these changes reached significance. Drug resistance emerged slowly, with the first penicillin-intermediate strain isolated in 1980 and the first macrolide/lincosamide-resistant strain isolated in 1984. The first penicillin-resistant strain was isolated in 1993. Resistance increased steadily thereafter until 2003-2004, when 51.1% of isolates were penicillin nonsusceptible and 53.3% were macrolide resistant. Clindamycin resistance remained low until 2003-2004, when 26.7% of strains were resistant; this was associated with the emergence of multidrug-resistant type 19A strains. This study documents the emergence of resistance over a quarter century among invasive pneumococci in the Cleveland area, as well as the reduction in disease caused by PCV7 serotypes following the introduction of PCV7 in 2000.
Streptococcus pneumoniae is the most-common cause of bacterial respiratory infections, and infections by this pathogen are among those that are least likely to resolve without treatment. The pneumococcus is also a significant cause of bacteremia and meningitis, responsible prior to 2000 for approximately 3,000 cases of meningitis and 50,000 cases of bacteremia every year in the United States alone (1, 6, 13, 37).
In 2000, the 7-valent pneumococcal polysaccharide-diphtheria CRM197 protein conjugate vaccine (PCV7) (Prevnar; Wyeth Pharmaceuticals Inc., Philadelphia, PA) was introduced into wide pediatric use. The vaccine combines the seven most commonly invasive and often drug-resistant serotypes found in children: 19F, 14, 6B, 23F, 9V, 18C, and 4. Studies done prior to and immediately following the introduction of the vaccine showed protective efficacy against invasive disease caused by the seven vaccine (V) serotypes of 97.4% (93.9% in the intent-to-treat analysis) and 89.1% against all serotypes (4). A serotype-specific reduction in otitis media of 57 to 66.7% was also found, although there was an increase in non-vaccine-related (NV) serotypes (4, 9). A CDC study reported in 2003 showed statistically significant reductions in invasive disease caused by V serotypes and vaccine-related (VR) serotypes, from within the vaccine serogroups, of 78 and 50 percent, respectively (43). Reductions were seen in all age groups, but were most prominent in children under 2 years of age. A more-recently published CDC study showed a significant reduction in invasive disease associated with penicillin-nonsusceptible strains of all V serotypes in both children and older adults, but a large increase (>200%) in the incidence of infections caused by serotype 19A (15). Further studies have shown a “herd effect,” demonstrating that the vaccination of children less than 2 years of age reduces disease in older children and adults (10, 16). The carriage of V serotypes has been shown to fall following conjugate vaccine use, leading to a decrease in both disease caused by and transmission of V serotypes (8, 11, 22, 29).
This study examined the serotypes and antimicrobial susceptibilities of invasive S. pneumoniae isolates collected at the University Hospitals Case Medical Center (UHCMC) from 1979 to 2004.
MATERIALS AND METHODS
The isolates tested represented sequentially collected S. pneumoniae strains isolated from blood and cerebrospinal fluid (CSF) in the clinical microbiology laboratory of the UHCMC, a 900-bed tertiary care institution, from January 1979 through December 2004. Only one isolate per patient was included in the study. The identification of each isolate was confirmed by following standard procedures (24). Isolates were submitted to the Centers for Disease Control and Prevention (CDC) as part of the work of the Pneumococcal Surveillance Working Group from 1979 to 2000 (39). Surveillance was continued at UHCMC from 2001 to 2004.
Susceptibility testing was conducted by MIC determination by broth microdilution according to NCCLS (now CLSI)-recommended procedures (26), using in-house or commercial (Trek, Westlake, OH) frozen microdilution trays. All isolates were tested against penicillin and a macrolide (erythromycin through 1996 and azithromycin from 1997). Clindamycin susceptibility was determined against all macrolide-resistant isolates through 1996 and against all isolates from 1997; macrolide-susceptible isolates were regarded as clindamycin-susceptible through 1996. The testing ranges (in μg/ml) were as follows: penicillin, 0.015 to 8; erythromycin, 0.008 to 8; azithromycin, 0.015 to 32; and clindamycin, 0.015 to 2. Testing was done in cation-adjusted Mueller-Hinton broth supplemented with 5% lysed horse blood; inocula prepared in saline suspension equivalent to a 0.5 McFarland standard with aliquots were added to produce inoculum sizes of 3 × 105 to 7 × 105 CFU/ml. Periodic inoculum counts were determined during testing to ensure that the inocula remained within the acceptable range. The trays were incubated under ambient conditions at 35°C for 20 to 24 h, and the lowest concentration showing no growth read as the MIC. Macrolide-resistant, clindamycin-susceptible isolates were also tested for inducible clindamycin resistance by the erythromycin disk induction method, using erythromycin (15 μg) and clindamycin (2 μg) disks placed 10 mm apart (edge to edge) on plates (25, 41). Inducible clindamycin resistance is indicated by blunting of the clindamycin zone on the side where the erythromycin disk was placed, and inducibly clindamycin-resistant isolates are interpreted as clindamycin resistant. Quality control organisms specified by CLSI, including S. pneumoniae ATCC 49619, were used (27). The proportions of isolates that were susceptible, intermediate, and resistant to each agent were determined for the agents tested based on CLSI interpretive criteria (7, 28). Intermediate and resistant strains were grouped as nonsusceptible for some analyses. Multidrug-resistant (MDR) strains were defined as being nonsusceptible to all three drug classes tested. Selected macrolide-resistant strains were tested for the presence of erm(B) and mef(E) genes by PCR with primers and conditions previously described, with expected PCR products of 639 and 345 bp, respectively (25, 40-42).
Serotyping was performed by capsular swelling reaction analysis using group- and type-specific antisera (CDC produced or obtained from Statenserum Institute, Copenhagen, Denmark) (18).
Data analysis.
Data were primarily analyzed by 2-year periods in relation to the 2000 introduction of PCV7. Data on the five-county greater Cleveland area served by our hospital showed that the population was stable over the study period at approximately 2.15 million people based on the 1980, 1990, and 2000 censuses and estimates for 2001 to 2004 (30). The numbers of adult and pediatric beds were also relatively constant during the study period, so the proportions of patients in the greater Cleveland area admitted to our hospital were assumed to be constant for purposes of comparisons of incidence over time. Differences in incidence were analyzed in relation to the introduction of PCV7 in 2000, using the Poisson distribution to compare differences between observation periods; 95% confidence intervals (CI) were calculated for the Poisson estimates by the SMR exact procedure and were regarded as significant if the upper and lower bounds did not include 0 (17). Statistical analyses of significance were determined using the Fisher exact test for small sample sizes and the Chi-square test for larger sample sizes. P values of <0.05 were regarded as significant for single comparisons and were adjusted for multiple comparisons using the Bonferroni correction by dividing 0.05 by the number of comparisons made.
Data were analyzed for the periods before and after the introduction of PCV7 to examine changes in serotype distribution overall and stratified by age. Since the vaccine was introduced in 2000, some analyses were performed without including that year. Patient ages were grouped by age as <5 years, 5 to 18 years, 19 to 65 years, and >65 years of age. Comparisons were done by age group for the years 1979 to 1999 versus 2001 to 2004. The serotypes were analyzed individually and by their relationship to PCV7, with analysis of the serotypes included in PCV7 (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F), PCV7-related serotypes (other serotypes of serogroups 6, 9, 18, 19, and 23), and NV serotypes.
RESULTS
There were 1,655 S. pneumoniae strains collected from blood or CSF by the Clinical Microbiology Laboratory of UHCMC between January 1979 and December 2004. Seventy-two strains were isolated from CSF only, 43 from blood and CSF, 17 from blood and another site, and the remaining 1,523 from blood only. Serotypes were determined for all isolates; antimicrobial susceptibilities were determined for 1,591 isolates.
Prevaccine period.
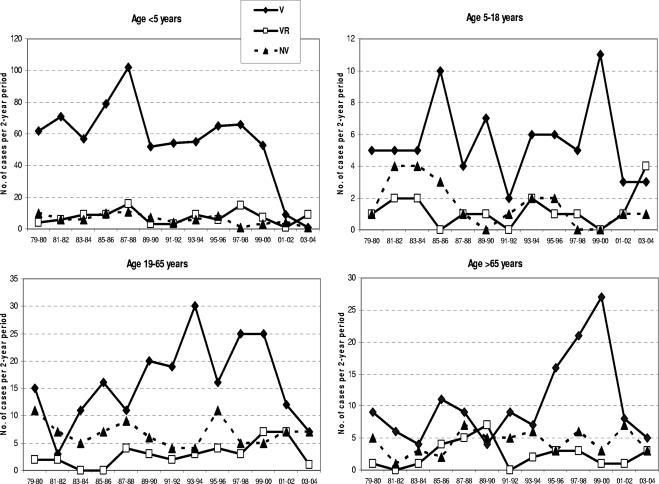
Between 1979 and 2000, there were 104 to 186 cases per 2-year period, with a mean of 139 cases per 2-year period (Table 1). During this pre-PCV7 period, 74.7% of isolates were V, 10.7% VR, and 14.6% NV serotypes. Analysis by age in the pre-PCV7 period showed that 62.7% of isolates were from children (56.6% <5 years and 6.2% 5 to 18 years) and 31.8% from adults (19.1% 19 to 65 years and 12.7% >65 years). V serotypes predominated in children, with 80.6% of isolates being V, 10.1% VR, and 9.3% NV serotypes. The incidence in children under 5 years of age was constant throughout the pre-PCV7 period, except for a peak in 1987-1988 (Fig. 1). Other than peaks in 1993-1994 and 1997 to 2000 in the 19- to 65- and >65-year age groups, respectively, the incidence in adults was also relatively constant throughout the pre-PCV7 period, with 65.0% of isolates being V, 11.3% VR, and 23.7% NV serotypes. The distribution of V, VR, and NV groups was constant in children but varied somewhat in adults, with V serotypes not always predominating (Fig. 1). The predominant serotypes pre-2000 were all V serotypes, with serotype 14 accounting for 22.3%, serotype 6B for 11.6%, and serotype 19F for 10.5% of isolates. The remaining V serotypes (4, 9V, 18C, and 23F) each accounted for 5 to 10% of all isolates, while two VR serotypes (6A and 19A) accounted for 1.7 and 4.8% of all isolates, respectively.
TABLE 1.
Incidence of invasive pneumococcal disease prior to 2000, year of vaccine introduction, and from 2001 to 2004, with percent change pre-2000 versus post-2000a
| Age (yrs) or group | Avg incidence/2-yr period
|
% Change pre-2000 vs post-2000 (95% CI) | P value | |
|---|---|---|---|---|
| Pre-2000 | Post-2000 | |||
| <5 | 80 | 13 | −84 (−72, −91) | <0.001 |
| 5-18 | 9 | 7 | −23 (−69, 60) | 0.12 |
| All children | 88 | 19 | −79 (−66, −87) | <0.001 |
| 19-65 | 26 | 21 | −20 (−51, 20) | 0.052 |
| >65 | 17 | 14 | −18 (−55, 38) | 0.08 |
| All adults | 42 | 34 | −20 (−44, 13) | 0.03 |
| All ages | 139 | 55 | −61 (−49, −70) | <0.001 |
Significant P value with Bonferroni correction for this data set is <0.007.
FIG. 1.
Distribution of isolates by PCV7 serotype relatedness and by age group, in 2-year increments during the surveillance period.
Postvaccine period.
After 2000, there was a precipitous decrease in cases of invasive pneumococcal disease overall, from 139 cases per 2-year period to 55 cases per 2-year period, representing a 61% decrease in incidence (95% CI, −49% to −70%; P < 0.001) (Table 1). There was also a shift in the predominant serotypes, with serotypes 14, 19A, and 19F now the most common individual serotypes and with NV serotypes much more prevalent (Table 2). Notably, serotype 19A increased in both incidence and proportion and serotype 3 in proportion. Serotype 19A strains increased in incidence from 4.4 isolates per 2-year period pre-2000 to 8 isolates per 2-year period post-2000 and in proportion from 3.1% pre-2000 to 14.7% post-2000. Serotype 3 strains increased in proportion from 2.2% pre-2000 to 5.5% post-2000, but its incidence was unchanged (2.9 versus 3.0 isolates per 2-year period for pre-2000 versus post-2000).
TABLE 2.
Serotype incidence pre- and post-2000 and percentage change between the two time periods for all cases and for children <5 years olda
| Serotype(s) (n = 1,655) | Incidence per 2-yr period
|
|||||||
|---|---|---|---|---|---|---|---|---|
| All cases
|
<5-yr age group
|
|||||||
| Pre-2000 | Post-2000 | % Change pre-2000 vs post-2000 (95% CI) | P value | Pre-2000 | Post-2000 | % Change pre-2000 vs post-2000 (95% CI) | P value | |
| All V | 103 | 25 | −76 (−64, −84) | <0.001 | 65 | 5 | −92 (−82, −97) | <0.001 |
| 19F | 15 | 6 | −62 (−13, −85) | 0.005 | 11 | 1 | −96 (−58, −100) | <0.001 |
| 14 | 31 | 7 | −77 (−53, −91) | <0.001 | 22 | 3 | −86 (−59, −97) | <0.001 |
| 6B | 16 | 2 | −88 (−54, −98) | <0.001 | 11 | 0 | −100 (−67, −100) | <0.001 |
| 23F | 11 | 3 | −73 (−20, −94) | 0.005 | 5 | 1 | −90 (−8, −100) | 0.01 |
| 9V | 11 | 5 | −59 (−85, 6) | 0.023 | 5 | 1 | −81 (−100, 5) | 0.027 |
| 18C | 9 | 1 | −95 (−38, −99) | 0.005 | 7 | 0 | −100 (−44, −100) | 0.005 |
| 4 | 10 | 2 | −80 (−28, −98) | 0.005 | 4 | 0 | −100 (−10, −100) | 0.017 |
| All VR | 15 | 14 | −5 (−48, 59) | 0.11 | 8 | 5 | −34 (−79, 50) | 0.099 |
| 6A | 5 | 5 | −6 (−69, 120) | 0.18 | 4 | 1 | −72 (−99, 55) | 0.099 |
| 19A | 4 | 8 | +87 (−20, 267) | 0.04 | 2 | 4 | +91 (−48, 388) | 0.099 |
| Other | 5 | 1 | −81 (−100, 9) | 0.03 | 2 | 0 | −100 (−100, 76) | 0.123 |
| All NV | 21 | 16 | −24 (−56, 24) | 0.052 | 7 | 3 | −64 (−94, 19) | 0.028 |
| All serotypes | 131 | 53 | −61 (−48, −70) | <0.001 | 80 | 13 | −84 (−72, −91) | <0.001 |
Significant P value with Bonferroni correction for this data set is <0.0036.
The decrease in incidence was highest in children and was 79% overall (P < 0.001) and 84% in children <5 years old (P < 0.001); there was also a decrease of 23% in children 5 to 18 years old, but this was not statistically significant (P = 0.12) (Table 1). In adults, the incidence decreased by 20% overall (P = 0.03, 95% CI −44 to 13); when stratified by age, the decrease was 20% for adults 19 to 65 years old (P = 0.052) and 19% for adults >65 years old (P = 0.08). These decreases in incidence in adults did not reach statistical significance but suggested that they might become significant if the trend continued.
When analyzed according to PCV7 V serotypes, the decrease in the incidence of V serotypes was statistically significant at 76% overall and 92% in children <5 years (Table 2). The incidence for individual V serotypes decreased 59 to 95% overall and 81 to 100% in children <5 years old, with the decreases for six of the seven V serotypes being statistically significant based on 95% CI ranges (serotypes 4, 6B, 14, 18C, 19F, and 23F) (Table 2). Although the incidence of VR serotypes decreased 5% overall and 34% in children <5 years old and that of NV serotypes by 24% overall and 64% in children <5 years old, the number of cases was small and the changes were not statistically significant. The incidence of individual VR serotypes decreased 72% in children <5 years old for serotype 6A and 100% for serotypes of serogroups 9, 18, and 23. The only serotype to increase in incidence was the VR serotype 19A, which increased 87% overall and 91% in children <5 years old. However, none of these differences among VR serotypes were statistically significant. No trends in individual serotypes were noted among NV serotypes.
The proportional distribution of V, VR, and NV groups showed little overall variation prior to 2000, with overall proportions of 74%, 11%, and 15%, respectively, although some variation in proportions and in peaks and valleys between individual 2-year periods was present when data were stratified by age (Fig. 1). After 2000, the V-serotype prevalence fell to 45% overall (P < 0.0001 versus pre-2000), 42.1% in adults (P < 0.0001) and 47.1% in children (P < 0.03). When the age groups were further stratified to young children (age, <5 years), children and adolescents (5 to 18 years), adults (19 to 65 years), and older adults (>65), the largest difference in the prevalence of V serotypes before and after 2000 was seen in children under age 5 (P < 0.0001) (Table 1). The differences between groups in the other age strata did not reach statistical significance.
Antimicrobial resistance.
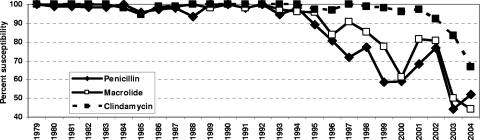
The antimicrobial susceptibilities for the three agents tested over the study period are summarized in Table 3 and Fig. 2. All isolates were susceptible to the three agents tested in 1979. Occasional penicillin-intermediate strains were found from 1980 through 1983, but all isolates were macrolide and clindamycin susceptible. Small numbers of penicillin-intermediate strains were seen from 1984 through 1992, with the first penicillin-resistant strain seen in 1993, when one of the 74 strains isolated that year was penicillin resistant (4.1% had intermediate susceptibility that year). Penicillin-intermediate and -resistant strains were prominent from 1995 onwards, with penicillin-resistant strains peaking in 2000 (36.3%) and 2004 (40.7%). After 2000, the number of penicillin-resistant isolates decreased, although the proportion of isolates that were resistant did not.
TABLE 3.
Penicillin, macrolide, and clindamycin susceptibility of isolates by year
| Year | No. of isolates | % of isolates with susceptibility toa:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Penicillin
|
Macrolideb
|
Clindamycin
|
||||||
| S | I | R | S | R | S | R | ||
| 1979 | 46 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 |
| 1980 | 95 | 98.9 | 1.1 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 |
| 1981 | 81 | 98.8 | 1.2 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 |
| 1982 | 62 | 98.4 | 1.6 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 |
| 1983 | 64 | 98.4 | 1.6 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 |
| 1984 | 55 | 100.0 | 0.0 | 0.0 | 98.2 | 1.8 | 98.2 | 1.8 |
| 1985 | 74 | 95.9 | 4.1 | 0.0 | 94.6 | 5.4 | 94.6 | 5.4 |
| 1986 | 77 | 97.4 | 2.6 | 0.0 | 98.7 | 1.3 | 98.7 | 1.3 |
| 1987 | 96 | 97.9 | 2.1 | 0.0 | 99.0 | 1.0 | 99.0 | 1.0 |
| 1988 | 77 | 93.5 | 6.5 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 |
| 1989 | 49 | 100.0 | 0.0 | 0.0 | 98.0 | 2.0 | 100.0 | 0.0 |
| 1990 | 59 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 |
| 1991 | 49 | 98.0 | 2.0 | 0.0 | 98.0 | 2.0 | 100.0 | 0.0 |
| 1992 | 50 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 |
| 1993 | 74 | 94.6 | 4.1 | 1.4 | 97.3 | 2.7 | 100.0 | 0.0 |
| 1994 | 54 | 98.1 | 1.9 | 0.0 | 96.3 | 3.7 | 100.0 | 0.0 |
| 1995 | 73 | 89.0 | 4.1 | 6.8 | 95.9 | 4.1 | 97.3 | 2.7 |
| 1996 | 62 | 80.6 | 11.3 | 8.1 | 83.9 | 16.1 | 96.8 | 3.2 |
| 1997 | 64 | 71.9 | 7.8 | 20.3 | 90.6 | 9.4 | 100.0 | 0.0 |
| 1998 | 88 | 77.3 | 9.1 | 13.6 | 85.2 | 14.8 | 98.9 | 1.1 |
| 1999 | 53 | 58.5 | 18.9 | 22.6 | 77.4 | 22.6 | 98.1 | 1.9 |
| 2000 | 80 | 58.8 | 5.0 | 36.3 | 61.3 | 38.8 | 96.3 | 3.8 |
| 2001 | 38 | 68.4 | 5.3 | 26.3 | 81.6 | 18.4 | 97.4 | 2.6 |
| 2002 | 26 | 76.9 | 7.7 | 15.4 | 80.8 | 19.2 | 92.3 | 7.7 |
| 2003 | 18 | 44.4 | 22.2 | 33.3 | 50.0 | 50.0 | 83.3 | 16.7 |
| 2004 | 27 | 51.9 | 7.4 | 40.7 | 44.4 | 55.6 | 66.7 | 33.3 |
| Overall | 1,591 | 88.9 | 4.3 | 6.8 | 92.2 | 7.8 | 98.1 | 1.9 |
S, susceptible; I, intermediate; R, resistant.
Erythromycin 1979 through 1996; azithromycin 1997 through 2004.
FIG. 2.
Penicillin, macrolide, and clindamycin susceptibility of isolates by year from 1979 to 2004.
The first macrolide-resistant strains were seen in 1984, with these isolates being cross-resistant to clindamycin, and occasional isolates resistant to both macrolides and clindamycin were seen from 1985 through 1987. The first macrolide-resistant, clindamycin-susceptible strain was seen in 1989, and all macrolide-resistant isolates were clindamycin susceptible through 1994. Thereafter, macrolide-resistant strains had variable clindamycin resistance, with macrolide-resistant, clindamycin-susceptible strains predominating. Macrolide-resistant strains were prominent from 1996 onwards, peaking in 2000 (38.8%) and 2004 (55.6%), while clindamycin-resistant strains only became prominent in 2002, peaking in 2004 (33.3%). All macrolide-resistant, clindamycin-susceptible strains were tested for inducible clindamycin resistance by the erythromycin-clindamycin double-disk method, and no inducibly clindamycin strains were detected. Additionally, six macrolide-resistant, clindamycin-susceptible strains isolated from when first detected in 1989 through 1996 were tested for the presence of erm(B) and mef(E) genes, and were all positive for mef(E) and negative for erm(B), documenting the presence of mef(E) from 1989. Overall, 93 macrolide-resistant, clindamycin-susceptible strains were found; these strains belonged to nine serotypes (4, 6A, 6B, 9V, 11, 14, 19A, 19F, and 23F), with serotype 14 the most frequent (44 isolates), peaking in 1999-2000 (25 isolates).
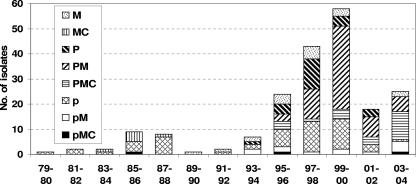
The results of analysis of the antimicrobial resistance patterns of the three agents tested are shown in Fig. 3 and Table 4. The most-frequent resistance pattern seen was penicillin and macrolide resistant and clindamycin susceptible, with this pattern peaking in 1999-2000; most of these isolates were serotypes 14 and 19F (Table 4). The second-most-frequent pattern seen was penicillin intermediate and macrolide/clindamycin susceptible, with this pattern being prominent in 1987-1988 and 1995 to 2000; the associated serotypes were 14, 6B, 23F, 9V, and 19A. Penicillin-resistant, macrolide/clindamycin-susceptible isolates were prominent in 1997-1998, associated with serotypes 6B, 23F, and 9V. Twenty-five MDR isolates, intermediate or resistant to all three agents, were detected during the study period. MDR strains were first detected in 1986, associated with serotype 9V, followed by serotypes 6B, 14, 19A, and 23F. This changed after 2000, with MDR serotype 19A becoming prominent in 2004. All MDR isolates were resistant to all three agents except for three that were penicillin intermediate (serotypes 6B, 9V, and 15A).
FIG. 3.
Incidence of invasive S. pneumoniae strains with nonsusceptibility to penicillin, macrolide, or clindamycin isolated during the study period, in 2-year increments (n = 200). p, penicillin intermediate; P, penicillin resistant; M, macrolide resistant; C, clindamycin resistant.
TABLE 4.
Susceptibility of isolates to penicillin, macrolide, and clindamycin by serotype
| Serotype(s) | No. of isolates with susceptibility(ies)a
|
Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Susc. | p | M | MC | pM | P | pMC | PM | PMC | ||
| All V | 995 | 32 | 15 | 6 | 3 | 23 | 2 | 59 | 10 | 1,145 |
| 19F | 140 | 3 | — | 2 | 2 | — | — | 13 | 3 | 163 |
| 14 | 291 | 7 | 8 | 1 | 1 | 2 | 35 | 5 | 350 | |
| 6B | 140 | 6 | 6 | 1 | — | 4 | 1 | 3 | 1 | 162 |
| 23F | 98 | 8 | — | — | — | 10 | — | 3 | 1 | 120 |
| 9V | 116 | 6 | — | — | — | 7 | 1 | 4 | — | 134 |
| 18C | 98 | — | — | 2 | — | — | — | — | — | 100 |
| 4 | 112 | 2 | 1 | — | — | — | — | 1 | — | 116 |
| All VR | 145 | 16 | 2 | 0 | 10 | 1 | 0 | 3 | 12 | 189 |
| 6A | 56 | 3 | 1 | — | 5 | 1 | — | 3 | — | 69 |
| 19A | 32 | 13 | 1 | — | 5 | — | — | — | 12 | 63 |
| 23 not F | 3 | — | — | — | — | — | — | — | — | 3 |
| 9 not V | 36 | — | — | — | — | — | — | — | — | 36 |
| 18 not C | 18 | — | — | — | — | — | — | — | — | 18 |
| All NV | 251 | 4 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 257 |
| 1 | 10 | — | — | — | — | — | — | — | — | 10 |
| 3 | 38 | — | — | — | — | — | — | — | — | 38 |
| 7 | 24 | 1 | — | — | — | — | — | — | — | 25 |
| Nontypeable | 6 | 1 | — | — | — | — | — | — | — | 7 |
| Other | 173 | 2 | 1 | — | — | — | 1 | — | — | 177 |
| Total | 1,391 | 52 | 18 | 6 | 13 | 24 | 3 | 62 | 22 | 1,591 |
—, no isolates; Susc., susceptible to all three drug classes; p, penicillin intermediate; P, penicillin resistant; M, macrolide resistant; C, clindamycin resistant.
DISCUSSION
Overall, during the course of over a quarter century of surveillance, the burden of invasive pneumococcal disease in patients at UHCMC was significant and relatively constant prior to the introduction of PCV7 in 2000, with a mean of 139 cases per 2-year period. During this period, 62.7% of cases occurred in children, 90.2% of whom were under 5 years of age and 73.2% under 2 years of age. Of the cases occurring in adults, 39.9% of patients were >65 years old. The predominant serotypes during this period were the seven serotypes included in PCV7, followed by serotypes 6A and 19A.
The incidence of invasive pneumococcal disease decreased by 61% overall after the introduction of PCV7 in 2000, with the mean number of cases decreasing from 139 to 55 per 2-year period (P < 0.001). This decrease was statistically significant and was driven by the 84% decrease in children under 5 years old. A decrease of 20% was seen in adults, but this did not reach statistical significance. When changes in individual serotypes were examined, the incidence of six of the seven serotypes included in the PCV7 vaccine decreased significantly, by 86 to 100%, again driven by decreases in children <5 years old. The incidences of VR serotypes other than serotype 19A and of NV serotypes decreased somewhat as well, but these changes did not reach statistical significance. The only serotype to increase in incidence was serotype 19A, but this change also did not reach statistical significance. Nevertheless, this trend needs to be monitored, as many of the serotype 19A strains are MDR.
The association between the introduction of PCV7 and the changes in pneumococcal disease observed in the population served by our institution follows a pattern similar to that seen with the introduction of polysaccharide-conjugated Haemophilus influenzae type b (Hib) vaccines between 1985 and 1990, where the number of children with Hib bacteremia decreased from 46 in 1985 to 10 to 15 per year from 1987 through 1990, with only occasional cases after 1991, the year protein-conjugated Hib vaccines were recommended for universal use in children (38).
Another noticeable trend over the study period was that isolates became increasingly resistant to the agents tested. Little resistance was seen through 1994, with penicillin-intermediate strains of V and VR serotypes predominating. Resistant strains became common from 1995, peaking in 1999-2000, with penicillin-intermediate strains of several serotypes and penicillin and macrolide-resistant serotype 14 strains predominating. This changed dramatically following the introduction of PCV7, with the number of resistant isolates decreasing dramatically in 2001-2002. This changed, however, in 2003-2004, with MDR isolates accounting for 24.4% of all isolates and 44% of resistant isolates; these isolates were mainly serotype 19A. It is interesting to note that the first MDR strains of S. pneumoniae were reported from South Africa and were also serotype 19A (12). The clonality of these original MDR strains, South Africa19A-13, sequence type (ST) 41 (21, 36), however, appears to be unrelated to that of recent U.S. MDR serotype 19A strains, with only occasional penicillin-susceptible ST41 strains found in the United States (31). Recent U.S. MDR serotype 19A strains belong to clonal complexes of ST 81, ST 156, ST 271, and ST 1296, which are highly related to international MDR clones Spain23F-1, Spain9V-3, Taiwan19F-14, and North Carolina6A-23, respectively (31). Another noteworthy international MDR clone is Spain23F-1, which is resistant to penicillin, chloramphenicol, and tetracycline. This clone was isolated at our institution in 1989 from the middle-ear fluid of a child with otitis media attending a day care center in the Cleveland area, and 52 carriers among 250 children attending this day care center were detected (35). The outbreak strain was shown to be the MDR Spain23F-1 clone (21), and this was the first recognition of the international spread of this clone to the United States. (23). This clone has since disseminated widely, both internationally (20, 32, 44) and within the United States (19, 44). Eight invasive isolates of 23F strains of this clone were found in our study, detected between 1996 and 1999 (data not shown).
Population-based data from the Active Bacterial Core surveillance of the Centers for Disease Control and Prevention, using data from seven U.S. geographic areas, showed that the rate of invasive pneumococcal disease dropped 29% overall, from an average of 24.3 cases per 100,000 persons in 1998 and 1999 to 17.3 per 100,000 in 2001 (43). The largest decline was in children under 2 years of age, with a 69% decrease in 2001; the rate of disease caused by V serotypes declined by 78%, and the rate of disease caused by VR serotypes by 50%. Disease rates also fell for adults; the rate of disease in 2001 was 32% lower for adults 20 to 39 years, 8% lower for those 40 to 64 years of age, and 18% lower for those 65 years or more. The rates of invasive pneumococcal disease continued to fall, with the rate being 42% lower in 2002 and 2003 and 48% lower in 2004 (15). A study of invasive pneumococcal disease in eight pediatric hospitals demonstrated that the annual number of invasive pneumococcal infections for children up to 24 months of age declined 58% in 2001 and 66% in 2002, with the greatest decrease observed for serotype 14 (14). Another study showed that as of April 2002 through March 2003, no cases of invasive V-serotype disease were seen in children <1 year of age, compared to 16 to 34 cases per year in the years before vaccine introduction, and similar reductions were seen in children <5 years of age (3). In a study of hospitalizations and ambulatory visits of families of 40 large United States employers due to all-cause and pneumococcal pneumonia from 1997 through 2004, Zhou and coworkers found that hospitalizations and ambulatory visits due to all-cause and pneumococcal pneumonia among children younger than 2 years declined significantly, with decreases of 41.1% to 57.6% (45). Projecting these data to the United States population, the total estimated direct medical expenditures for all-cause pneumonia-related hospitalizations and ambulatory visits of young children were reduced 45.3% (from an annual average of $688.2 million in 1997 to 1999 to $376.7 million in 2004). Our data are in agreement with changes reported in these studies of invasive pneumococcal disease related to the introduction of PCV7, although the decreases in adults did not reach statistical significance in our study.
Data from the CDC Active Bacterial Core surveillance program also measured disease caused by antibiotic-nonsusceptible pneumococci from 1996 through 2004 in eight surveillance areas (15). The rates of invasive disease caused by penicillin-nonsusceptible strains and strains not susceptible to multiple antibiotics peaked in 1999 and decreased by 57 to 59% by 2004. Among children under 2 years of age, disease caused by penicillin-nonsusceptible strains decreased by 81%, but a 313% increase was seen in disease caused by penicillin-nonsusceptible serotype 19A (from 2.0 to 8.3 per 100,000). Similar findings were made in our study, with resistance peaking in 1999-2000 and decreasing by 69% in 2001-2002 and 57% in 2003-2004; strains not susceptible to multiple antibiotics also peaked in 1999-2000 and decreased by 69% in 2001-2002 and 44% in 2003-2004. Additionally, our data showed that MDR serotype 19A strains also increased from two in 1999-2000 and one in 2001-2002 to nine in 2003-2004 (seven of these nine were isolated from children <5 years old). This is particularly disturbing as these serotype 19A strains are resistant to all oral agents administered to children, including amoxicillin, oral cephalosporins, macrolides, clindamycin, and trimethoprim-sulfamethoxazole (2, 15, 31, 33, 34).
The strengths of our study include its long period of observation in a single population, which may be unique among reports of pneumococcal disease in the United States, and the thorough examination of both resistance and serotype trends over that time period. The limitations of our study are that it was not population based and that data were analyzed assuming that the population base served by our institution was constant. As the patient population served by our hospital (that of the greater Cleveland area), the numbers of adult and pediatric beds, and the incidence of invasive pneumococcal disease in the pre-PCV7 era were relatively constant during 20 years, however, the effect of this limitation is not likely to be great. Additional limitations that may apply include variations in demographic data, including the racial and age makeup of the population, and the influence of the human immunodeficiency virus epidemic. Additionally, our study did not capture PCV7 vaccine use or antimicrobial treatment of patients, so direct correlations of these factors with the effects found cannot be made, and differentiation between primary invasive disease and treatment failure could not be assessed. However, CDC vaccination estimates for children 19 to 35 months of age show coverages for PCV7 (three doses) in Cuyahoga County, which includes about two-thirds of the population of the greater Cleveland area, of 54.0% in 2002, 67.3% in 2003, and 79.7% in 2004 (the national coverages were 40.8%, 68.1%, and 73.2% for these years) (5). Another limitation is that our institution serves proportionally more pediatric than adult patients in our area, so that children may be overrepresented in the overall effects compared to the national population-based data. Nevertheless, the trends found in our study reflect national trends, with, in fact, better vaccine efficacy than shown in population-based studies.
In conclusion, after the introduction of PCV7, the incidence of invasive pneumococcal disease precipitously decreased, associated with a decrease in the prevalence of V serotypes. This was most notable in children under 5 years old but was also apparent in all other age groups, providing possible evidence for a herd effect accompanying pediatric usage of the vaccine. However, antimicrobial resistance of S. pneumoniae emerged and, although initially decreasing after the introduction of PCV7, rose quickly thereafter, with serotype 19A strains resistant to penicillin, macrolides, and clindamycin now predominating. As these strains are resistant to all oral agents currently recommended for pediatric respiratory infections, the addition of serotype 19A to future vaccine formulations should be considered.
Acknowledgments
The assistance of Timothy Bailiff, Saundra Mathis, and Shantia Warren of the Streptococcus Laboratory, Respiratory Diseases Branch, Centers for Disease Control and Prevention, is gratefully acknowledged.
Financial support was provided in part by the Centers for Disease Control and Prevention. Michael R. Jacobs has served as consultant to or has received research grants from GlaxoSmithKline Pharmaceuticals and Wyeth Ayerst/Lederle Pharmaceuticals. Bernard Beall, Cynthia G. Whitney, Caryn E. Good, Saralee Bajaksouzian, and Anne R. Windau have no financial disclosures related to potential conflicts of interest.
Footnotes
Published ahead of print on 30 January 2008.
REFERENCES
- 1.Atkinson. W., J. Hamborsky, and S. Wolfe (ed.). 2006. Epidemiology and Prevention of Vaccine-Preventable Diseases, 9th Ed. Centers for Disease Control and Prevention, Atlanta, GA.
- 2.Beall, B., M. C. McEllistrem, R. E. Gertz, Jr., S. Wedel, D. J. Boxrud, A. L. Gonzalez, M. J. Medina, R. Pai, T. A. Thompson, L. H. Harrison, L. McGee, and C. G. Whitney. 2006. Pre- and postvaccination clonal compositions of invasive pneumococcal serotypes for isolates collected in the United States in 1999, 2001, and 2002. J. Clin. Microbiol. 44999-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, S., H. Shinefield, R. Baxter, R. Austrian, L. Bracken, J. Hansen, E. Lewis, and B. Fireman. 2004. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in northern California Kaiser Permanente. Pediatr. Infect. Dis. J. 23485-489. [DOI] [PubMed] [Google Scholar]
- 4.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19187-195. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2007. Immunization Coverage in the U.S. Centers for Disease Control and Prevention, Atlanta, GA. http://cdc.gov/vaccines/stats-surv/imz-coverage.htm#nis. Accessed 21 August 2007.
- 6.Centers for Disease Control and Prevention. 1996. Defining the public health impact of drug-resistant Streptococcus pneumoniae: report of a working group. MMWR Recommend. Rep. 451-20. [PubMed] [Google Scholar]
- 7.CLSI. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. CLSI M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Dagan, R., R. Melamed, M. Muallem, L. Piglansky, D. Greenberg, O. Abramson, P. M. Mendelman, N. Bohidar, and P. Yagupsky. 1996. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J. Infect. Dis. 1741271-1278. [DOI] [PubMed] [Google Scholar]
- 9.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, and P. H. Makela. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344403-409. [DOI] [PubMed] [Google Scholar]
- 10.Givon-Lavi, N., D. Fraser, and R. Dagan. 2003. Vaccination of day-care center attendees reduces carriage of Streptococcus pneumoniae among their younger siblings. Pediatr. Infect. Dis. J. 22524-532. [DOI] [PubMed] [Google Scholar]
- 11.Hammitt, L. L., D. L. Bruden, J. C. Butler, H. C. Baggett, D. A. Hurlburt, A. Reasonover, and T. W. Hennessy. 2006. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J. Infect. Dis. 1931487-1494. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs, M. R., H. J. Koornhof, R. M. Robins-Browne, C. M. Stevenson, Z. A. Vermaak, I. Freiman, G. B. Miller, M. A. Witcomb, M. Isaacson, J. I. Ward, and R. Austrian. 1978. Emergence of multiply resistant pneumococci. N. Engl. J. Med. 299735-740. [DOI] [PubMed] [Google Scholar]
- 13.Jones, M. E., D. C. Draghi, J. A. Karlowsky, D. F. Sahm, and J. S. Bradley. 2004. Prevalence of antimicrobial resistance in bacteria isolated from central nervous system specimens as reported by U.S. hospital laboratories from 2000 to 2002. Ann. Clin. Microbiol. Antimicrob. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan, S. L., E. O. Mason, Jr., E. R. Wald, G. E. Schutze, J. S. Bradley, T. Q. Tan, J. A. Hoffman, L. B. Givner, R. Yogev, and W. J. Barson. 2004. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 113443-449. [DOI] [PubMed] [Google Scholar]
- 15.Kyaw, M. H., R. Lynfield, W. Schaffner, A. S. Craig, J. Hadler, A. Reingold, A. R. Thomas, L. H. Harrison, N. M. Bennett, M. M. Farley, R. R. Facklam, J. H. Jorgensen, J. Besser, E. R. Zell, A. Schuchat, and C. G. Whitney. 2006. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 3541455-1463. [DOI] [PubMed] [Google Scholar]
- 16.Lexau, C. A., R. Lynfield, R. Danila, T. Pilishvili, R. Facklam, M. M. Farley, L. H. Harrison, W. Schaffner, A. Reingold, N. M. Bennett, J. Hadler, P. R. Cieslak, and C. G. Whitney. 2005. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 2942043-2051. [DOI] [PubMed] [Google Scholar]
- 17.Liddell, F. D. 1984. Simple exact analysis of the standardised mortality ratio. J. Epidemiol. Community Health 3885-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lund, E., and J. Henrichsen. 1978. Laboratory diagnosis, serology and epidemiology of Streptococcus pneumoniae, pp. 244-255. In T. Bergan and J. R. Norris (ed.), Methods in Microbiology, vol. 12. Academic Press, London, United Kingdom. [Google Scholar]
- 19.McDougal, L. K., R. Facklam, M. Reeves, S. Hunter, J. M. Swenson, B. C. Hill, and F. C. Tenover. 1992. Analysis of multiply antimicrobial-resistant isolates of Streptococcus pneumoniae from the United States. Antimicrob. Agents Chemother. 362176-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGee, L., K. P. Klugman, D. Friedland, and H. J. Lee. 1997. Spread of the Spanish multi-resistant serotype 23F clone of Streptococcus pneumoniae to Seoul, Korea. Microb. Drug Resist. 3253-257. [DOI] [PubMed] [Google Scholar]
- 21.McGee, L., L. McDougal, J. Zhou, B. G. Spratt, F. C. Tenover, R. George, R. Hakenbeck, W. Hryniewicz, J. C. Lefevre, A. Tomasz, and K. P. Klugman. 2001. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J. Clin. Microbiol. 392565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore, M. R., T. B. Hyde, T. W. Hennessy, D. J. Parks, A. L. Reasonover, M. Harker-Jones, J. Gove, D. L. Bruden, K. Rudolph, A. Parkinson, J. C. Butler, and A. Schuchat. 2004. Impact of a conjugate vaccine on community-wide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. J. Infect. Dis. 1902031-2038. [DOI] [PubMed] [Google Scholar]
- 23.Munoz, R., T. J. Coffey, M. Daniels, C. G. Dowson, G. Laible, J. Casal, R. Hakenbeck, M. Jacobs, J. M. Musser, B. G. Spratt, et al. 1991. Intercontinental spread of a multiresistant clone of serotype 23F Streptococcus pneumoniae. J. Infect. Dis. 164302-306. [DOI] [PubMed] [Google Scholar]
- 24.Murray, P. R., E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken. 2003. Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 25.Nagai, K., P. C. Appelbaum, T. A. Davies, L. M. Kelly, D. B. Hoellman, A. T. Andrasevic, L. Drukalska, W. Hryniewicz, M. R. Jacobs, J. Kolman, J. Miciuleviciene, M. Pana, L. Setchanova, M. K. Thege, H. Hupkova, J. Trupl, and P. Urbaskova. 2002. Susceptibilities to telithromycin and six other agents and prevalence of macrolide resistance due to L4 ribosomal protein mutation among 992 pneumococci from 10 central and Eastern European countries. Antimicrob. Agents Chemother. 46371-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCCLS. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 27.NCCLS. 2004. Performance standards for antimicrobial susceptibility testing; 14th informational supplement. NCCLS document M100-S14. NCCLS, Wayne, PA.
- 28.NCCLS/CLSI. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. CLSI document M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 29.Obaro, S. K., and S. A. Madhi. 2006. Bacterial pneumonia vaccines and childhood pneumonia: are we winning, refining, or redefining? Lancet Infect. Dis. 6150-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohio Department of Development. 2008. Ohio county profiles. Ohio Department of Development, Office of Policy Research and Strategic Planning, Columbus, OH. http://www.odod.state.oh.us/research/. Accessed 10 January 2008.
- 31.Pai, R., M. R. Moore, T. Pilishvili, R. E. Gertz, C. G. Whitney, and B. Beall. 2005. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J. Infect. Dis. 1921988-1995. [DOI] [PubMed] [Google Scholar]
- 32.Parry, C. M., N. M. Duong, J. Zhou, N. T. Mai, T. S. Diep, L. Q. Thinh, J. Wain, N. Van Vinh Chau, D. Griffiths, N. P. Day, N. J. White, T. T. Hien, B. G. Spratt, and J. J. Farrar. 2002. Emergence in Vietnam of Streptococcus pneumoniae resistant to multiple antimicrobial agents as a result of dissemination of the multiresistant Spain(23F)-1 clone. Antimicrob. Agents Chemother. 463512-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelton, S. I., H. Huot, J. A. Finkelstein, C. J. Bishop, K. K. Hsu, J. Kellenberg, S. S. Huang, R. Goldstein, and W. P. Hanage. 2007. Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 26468-472. [DOI] [PubMed] [Google Scholar]
- 34.Pichichero, M. E., and J. R. Casey. 2007. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA 2981772-1778. [DOI] [PubMed] [Google Scholar]
- 35.Reichler, M. R., A. A. Allphin, R. F. Breiman, J. R. Schreiber, J. E. Arnold, L. K. McDougal, R. R. Facklam, B. Boxerbaum, D. May, R. O. Walton, et al. 1992. The spread of multiply resistant Streptococcus pneumoniae at a day care center in Ohio. J. Infect. Dis. 1661346-1353. [DOI] [PubMed] [Google Scholar]
- 36.Reinert, R. R., M. R. Jacobs, P. C. Appelbaum, S. Bajaksouzian, S. Cordeiro, M. van der Linden, and A. Al-Lahham. 2005. Relationship between the original multiply resistant South African isolates of Streptococcus pneumoniae from 1977 to 1978 and contemporary international resistant clones. J. Clin. Microbiol. 436035-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson, K. A., W. Baughman, G. Rothrock, N. L. Barrett, M. Pass, C. Lexau, B. Damaske, K. Stefonek, B. Barnes, J. Patterson, E. R. Zell, A. Schuchat, and C. G. Whitney. 2001. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995-1998: opportunities for prevention in the conjugate vaccine era. JAMA 2851729-1735. [DOI] [PubMed] [Google Scholar]
- 38.Schreiber, J. R., B. Boxerbaum, and A. B. Morrissey. 1992. Decline of Haemophilus disease in Cleveland. Pediatr. Infect. Dis. J. 11779-780. [PubMed] [Google Scholar]
- 39.Spika, J. S., R. R. Facklam, B. D. Plikaytis, M. J. Oxtoby, et al. 1991. Antimicrobial resistance of Streptococcus pneumoniae in the United States, 1979-1987. J. Infect. Dis. 1631273-1278. [DOI] [PubMed] [Google Scholar]
- 40.Sutcliffe, J., T. Grebe, A. Tait-Kamradt, and L. Wondrack. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 402562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutcliffe, J., A. Tait-Kamradt, and L. Wondrack. 1996. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob. Agents Chemother. 401817-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tait-Kamradt, A., J. Clancy, M. Cronan, F. Dib-Hajj, L. Wondrack, W. Yuan, and J. Sutcliffe. 1997. mefE is necessary for the erythromycin-resistant M phenotype in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 412251-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 3481737-1746. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida, R., Y. Hirakata, M. Kaku, K. Tomono, S. Maesaki, Y. Yamada, S. Kamihira, M. R. Jacobs, P. C. Appelbaum, and S. Kohno. 1999. Genetic analysis of serotype 23F Streptococcus pneumoniae isolates from several countries by penicillin-binding protein gene fingerprinting and pulsed-field gel electrophoresis. Chemotherapy 45158-165. [DOI] [PubMed] [Google Scholar]
- 45.Zhou, F., M. H. Kyaw, A. Shefer, C. A. Winston, and J. P. Nuorti. 2007. Health care utilization for pneumonia in young children after routine pneumococcal conjugate vaccine use in the United States. Arch. Pediatr. Adolesc. Med. 1611162-1168. [DOI] [PubMed] [Google Scholar]