Abstract
The diagnostic accuracy of an integron PCR method (Int-PCR) for detecting class 1 integrons (1,000, 1,200, and 1,600 bp) in the identification of antibiotic-resistant Salmonella strains was evaluated using 730 Salmonella isolates from pen floor samples collected from four swine production systems in Illinois. Three integron groupings were detected: 1,000 bp only, 1,600 bp only, and both 1,000 and 1,200 bp. The presence of any of the three class 1 integron groupings was associated with four-drug resistance (streptomycin, spectinomycin, sulfisoxazole, and tetracycline [St Spc Su Tet]). In addition, the presence of both the 1,000- and 1,200-bp integrons added resistance to ampicillin (Amp) and chloramphenicol (Cm), and the 1,600-bp integron added resistance to gentamicin (Gen) and kanamycin (Kan). DNA sequencing of integrons confirmed the presence of the aminoglycoside adenyl transferase (aadA) gene, conferring St Spc resistance in the 1,000-bp integron; the β-lactamase gene, conferring Amp resistance in the 1,200-bp integron; and the aadA and aadB genes, conferring St Spc Gen Kan resistance in the 1,600-bp integron. The 1,600-bp integron appears to have the 1,000-bp intergron as its core, with additional genetic material conferring additional antibiotic resistance. The diagnostic accuracy of Int-PCR in detecting resistance to individual antibiotics was limited by the presence of phenotypic resistance in isolates without integrons. However, Int-PCR had high diagnostic accuracy (sensitivity and specificity) in detecting multidrug resistance: 0.98 and 0.92, respectively, for St Spc Su Tet; 0.95 and 1.0 for Amp Cm St Spc Su Tet; and 1.0 and 0.99 for Gen Kan St Spc Su Tet. Thus, Int-PCR can be valuable in epidemiological surveys as a screening tool for the detection of multidrug-resistant Salmonella strains.
Epidemiological studies have implicated pork as being an important food-borne source of Salmonella enterica (2, 3). The emergence of antibiotic-resistant strains (22, 24), especially multidrug-resistant Salmonella enterica serovar Typhimurium DT104 (21, 27), has increased public health concerns. In pork production, antibiotic resistance may result from the routine addition of prophylactic antibiotics to feed (6, 12).
Mobile, horizontally transmissible genetic elements of bacteria such as plasmids and transposons may contain one or more integrons, gene cassettes inserted between two conserved flanking regions (11). Class 1 integrons can capture genes from the environment and incorporate them by site-specific recombination. When they are part of the genome of pathogenic organisms such as Salmonella (10, 8, 9, 17), Klebsiella (13, 26), Yersinia (23), and Escherichia coli (16), integrons may be responsible for the horizontal transfer of antibiotic resistance among other pathogenic bacteria. The presence of genes within integrons coding for antibiotic resistance and the association of antibiotic resistance phenotypes with the presence of integrons have been well documented (10, 16, 17, 25, 26).
Although the detection of class 1 integrons using PCR methods has become commonplace (5, 9, 10, 14, 19, 25), the diagnostic accuracy (e.g., sensitivity and specificity) of integron PCR (Int-PCR) methods in detecting antibiotic resistance has not been investigated. An accurate PCR-based diagnostic tool for the detection of antibiotic resistance could be useful in epidemiological investigations due to time and cost savings compared to commonly used antibiotic resistance panels. This study evaluated the diagnostic accuracy of a PCR technique for detecting class 1 integrons in identifying antibiotic resistance in Salmonella isolates obtained from swine production facilities.
MATERIALS AND METHODS
Four swine production units with a prior history of high Salmonella prevalence were studied. From May to September 2003, six to eight visits were made to each farm at approximately 2-week intervals. Samples of pen floor contents were collected from selected evenly spaced gestation pens and from all finishing pens.
Approximately 3 g of each pen floor sample was cultured for Salmonella by placing it into 30 ml of tetrathionate broth (Remel, Lenexa, KS) at the site of collection. Upon return to the laboratory on the same day, all samples were incubated at 37°C for 36 to 48 h. One hundred microliters of the culture from tetrathionate broth was then transferred from each tube into 9.9 ml of Rappaport's R-10 broth (Remel) and incubated at 37°C for 24 h. Each sample culture was then streaked onto a xylose lysine tergitol-4 agar plate (Remel) and incubated at 37°C for 24 h. A maximum of five red colonies with black centers were picked from each plate, streaked onto separate brilliant green agar (Remel), and incubated at 37°C for 24 h. Cultures with light pink colonies were streaked onto Trypticase soy agar (Difco, Detroit, MI) plates and incubated at 37°C for 24 h. A colony from each Trypticase soy agar plate was inoculated into 1 ml of Trypticase soy broth in freezer tubes that were incubated overnight at 37°C. After incubation, 50% glycerol (1:1 dilution of pure glycerol with water and autoclaved) was added to the Trypticase soy broth culture. The tubes were deep frozen at −80°C until they were retrieved for further laboratory procedures. After thawing, the identification of each isolate as Salmonella was confirmed by performing PCR for the invA gene (4), as described previously (20).
DNA was extracted from the Salmonella isolates using the QIAquick PCR purification kit (Qiagen Inc., Valencia, CA) and used as the template for the detection of integrons with intI primers in a PCR (Integrated DNA Technologies, Coralville, IA). The sense primer of intI was 5′-GGC ATC CAA GCA GCA AGC-3′, and the antisense primer was 5′-AAG CAG ACT TGA CCT GAT-3′ (15). Each 50-μl reaction mix contained 32 μl H2O, 5.0 μl template DNA, 2.5 U Taq polymerase (Takara Biol Inc., Japan), 5 μl 10× reaction buffer, 2 μl each 10 pmol primer, 2 μl 5 mM deoxynucleoside triphosphates, and 1.5 μl 50 mM MgCl2 deoxynucleoside triphosphates. Thermocycler run conditions included an initial denaturation step at 94°C for 3 min, 25 cycles each at 94°C for 30 s, primer annealing at 64°C for 30 s, and a extension step at 72°C for 1 min. The final extension time was 7 min at 72°C.
Antimicrobial susceptibility testing of Salmonella isolates was carried out using NARMS Sensititre panels according to the manufacturer's protocol (Trek Diagnostic Systems, Inc., OH). Antimicrobial breakpoints were selected according to Clinical Laboratory Standards Institute (formerly NCCLS) recommendations (18). The antibiotics selected and breakpoints for resistance were as follows: 32 μg/ml for ampicillin, 32 μg/ml-16 μg/ml for amoxicillin-clavulanic acid, 32 μg/ml for chloramphenicol, 32 μg/ml for cefoxitin, 16 μg/ml for gentamicin, 64 μg/ml for kanamycin, 512 μg/ml for sulfisoxazole, 64 μg/ml for streptomycin, and 16 μg/ml for tetracycline. The panel did not contain spectinomycin or sufficient dilutions of streptomycin. Thus, additional dilutions of spectinomycin (spectinomycin dihydrochloride; Sigma-Aldrich Co., MO) and streptomycin (streptomycin sulfate salt; Sigma-Aldrich Co., MO) were made in the laboratory, with breakpoints for resistance to streptomycin and spectinomycin at 64 μg/ml. The strains of organisms used for quality control were Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 29212, Enterococcus faecalis ATCC 51299, Staphylococcus aureus ATCC 29213, Pseudomonas aeruginosa ATCC 27853, and Streptococcus pneumoniae ATCC 49619. All plates were incubated for 18 h at 37°C and read using a Sensitouch reader (Trek Diagnostic Systems, Inc., OH).
A representative sample of Salmonella isolates was selected for nucleotide sequencing of integrons. The samples were chosen to represent all farms containing class 1 integrons in the study, various sampling periods and spatial locations within each farm, and different integron sizes. Every isolate with a single integron (1,000 or 1,600 bp) was purified using a QIAquick PCR purification kit (Qiagen Inc., Valencia, CA) following Int-PCR. For isolates with two integrons (1,000 and 1,200 bp), following Int-PCR and the separation of bands through electrophoresis, each integron was extracted using a QIAquick gel extraction kit (Qiagen Inc., Valencia, CA). Purified integron DNA samples were sequenced (Elim Biopharamaceuticals, CA) using intI primers. Forward and reverse sequences were formatted and aligned using DNASIS-MAX software, version 2.5 (Mirai-Bio, CA). The complete sequences were referenced in NCBI BLAST (http://www.ncbi.nih.gov/BLAST) for analogy with genes for antibiotic resistance in GenBank. Only sequences with similarities of ≥99% were considered to be identical.
The association of phenotypic resistance to each antibiotic with each detected integron group (no integrons, 1,000 bp, 1,000 bp plus 1,200 bp, and 1,600 bp) was identified using the Fisher exact test (α = 0.01, due to multiple tests) and quantified by calculating the phi coefficient (7). The sensitivities and specificities of Int-PCR for each integron group in detecting drug resistances were estimated (7); exact binomial 95% confidence intervals were calculated using EpiInfo, version 6.04d (Centers for Disease Control and Prevention, Atlanta, GA).
RESULTS
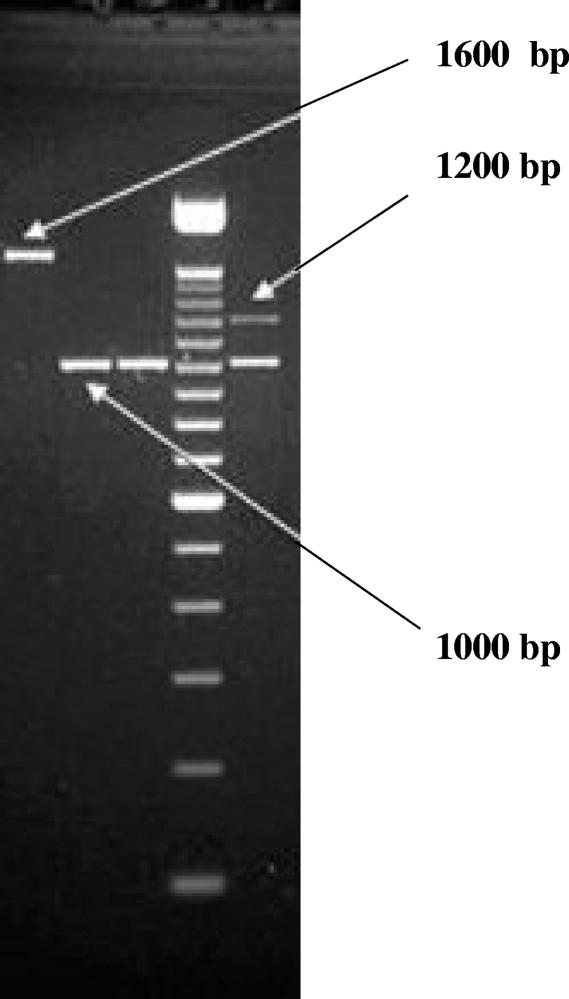
A total of 12,227 pen floor samples were collected. Class 1 integrons were detected in 338 of 730 Salmonella isolates (46%) on three of the four farms sampled. Isolates were classified into four groups based on the integrons present (Fig. 1): no integrons (54%), 1,000-bp integrons (39%), both 1,000- and 1,200-bp integrons (6%), and 1600-bp integrons (2%); 1,200-bp integrons did not occur independently of 1,000-bp integrons. Not all farms had all integron types.
FIG. 1.
Gel images showing the three integron size groups: 1,000 bp, 1,000 and 1,200 bp, and 1,600 bp.
The presence of any of the integron groups was highly associated with resistance to four drugs: streptomycin, sulfisoxazole, tetracycline, and spectinomycin (Table 1); 91% of isolates with any of the class 1 integrons had this multidrug resistance pattern. All isolates with 1,200-bp integrons were also resistant to both ampicillin and chloramphenicol. All isolates with 1,600-bp integrons were also resistant to gentamicin and kanamycin. However, some isolates without integrons were also resistant to the same antibiotics, most commonly tetracycline (31%), sulfisoxazole (20%), spectinomycin (16%), and chloramphenicol (15%). None of the isolates with no integrons were resistant to gentamicin; only 5% were resistant to kanamycin.
TABLE 1.
Distribution of integrons across farms and association with antibiotic resistance
| Integron group | Total no. of isolates | Farm(s) | No. (%) of isolates with resistance toa:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amox | Amp | Cef | Cm | Gen | Kan | St | Su | Tet | Spc | |||
| No Integrons | 392 | All | 2 (0.5) | 4 (1.0) | 3 (0.7) | 58 (14.8) | 0 | 21 (5.4) | 33 (8.4) | 78 (19.9) | 120 (30.6) | 64 (16.3) |
| 164 | D | 0 | 3 (1.8) | 0 | 4 (2.4) | 0 | 18 (11.0) | 28 (17.1) | 16 (9.8) | 52 (31.7) | 7 (4.3) | |
| 189 | K | 2 (1.1) | 1 (0.5) | 2 (1.1) | 54 (28.6) | 0 | 3 (1.6) | 5 (2.7) | 57 (30.2) | 66 (34.9) | 57 (30.2) | |
| 39 | R | 0 | 0 | 1 (2.6) | 0 | 0 | 0 | 0 | 5 (12.8) | 2 (5.1) | 0 | |
| 0 | S | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1,000 bp | 285 | All | 0 | 1 (0.4) | 0 | 2 (0.7) | 11 (3.9) | 0 | 268 (94.0) | 285 (100) | 270 (94.7) | 285 (100) |
| 142 | D | 0 | 1 (0.7) | 0 | 2 (1.4) | 11 (7.8) | 0 | 142 (100) | 142 (100) | 127 (89.4) | 142 (100) | |
| 25 | K | 0 | 0 | 0 | 0 | 0 | 0 | 20 (80) | 25 (100) | 25 (100) | 25 (100) | |
| 118 | S | 0 | 0 | 0 | 0 | 0 | 0 | 106 (90.0) | 118 (100) | 118 (100) | 118 (100) | |
| 1,000 + 1,200 bp | 41 | All | 0 | 41 (100) | 0 | 41 (100) | 0 | 0 | 41 (100) | 41 (100) | 41 (100) | 41 (100) |
| 40 | D | 0 | 40 (100) | 0 | 40 (100) | 0 | 0 | 40 (100) | 40 (100) | 40 (100) | 40 (100) | |
| 1 | K | 0 | 1 (100) | 0 | 1 (100) | 0 | 0 | 1 (100) | 1 (100) | 1 (100) | 1 (100) | |
| 1,600 bp | 12 | All | 0 | 0 | 0 | 0 | 11 (91.7) | 8 (66.7) | 12 (100) | 12 (100) | 12 (100) | 12 (100) |
| 12 | D | 0 | 0 | 0 | 0 | 11 (91.7) | 8 (66.7) | 12 (100) | 12 (100) | 12 (100) | 12 (100) | |
| Total | 730 | All | 2 (0.2) | 46 (6.3) | 3 (0.4) | 101 (13.8) | 22 (3.0) | 29 (4.0) | 354 (48.5) | 416 (57.0) | 443 (60.7) | 402 (55.1) |
For table rows with data for all farms, phi coefficients for resistances to amoxicillin, ampicillin, cefoxitin, chloramphenicol, gentamicin, kanamycin, streptomycin, sulfisoxazole, tetracycline, and spectinomycin were 0.05, −0.23, 0.06, 0.03, −0.19, 0.8, −0.86, −0.81, −0.66, and −0.84, respectively, for the no-integron group; −0.04, −0.20, −0.05, −0.30, −0.04, −0.16, 0.73, 0.70, 0.56, and 0.73 for the 1,000-bp group; −0.01, 0.94, −0.02, 0.61, −0.04, −0.05, 0.25, 0.21, 0.20, and 0.22 for the 1,000- and 1,200-bp group; and −0.01, −0.03, −0.01, −0.05, 0.67, 0.42, 0.13, 0.11, 0.10 (statistical significance at an α of 0.10), and 0.12 for the 1,600-bp group, with statistical significance at an a of 0.001 unless otherwise indicated. Amox, amoxicillin; Amp, ampicillin; Cef, cefoxitin; Cm, chloramphenicol; Gen, gentamicin; Kan, kanamycin; St, streptomycin; Su, sulfisoxazole; Tet, tetracycline; Spc, spectinomycin.
In detecting resistance to the four antibiotics with which all integron groups were associated, the sensitivity of Int-PCR test result of any integron present varied from 0.73 (tetracycline) to 0.91 (streptomycin); specificity varied from 0.95 to 1.0 (Table 2). For the antibiotic resistance patterns associated with the presence of both the 1,000- and 1,200-bp integrons, the sensitivities and specificities of Int-PCR were 0.91 and 0.57, respectively, for ampicillin and 0.43 and 0.53, respectively, for chloramphenicol; the specificity of Int-PCR increased to 1.0 for these antibiotics if the presence of both 1,000- and 1,200-bp integrons was used as the diagnostic criterion. For resistances added by the 1,600-bp integron, the sensitivity of Int-PCR in detecting gentamicin was 1.0 but was only 0.28 for kanamycin. The specificity of Int-PCR in detecting each of these resistances was 0.53 for the all-integron test criterion but increased to 0.99 if the presence of the 1,600-bp integron was the sole diagnostic criterion.
TABLE 2.
Sensitivities and specificities of Int-PCR diagnostics for detection of antibiotic resistancea
| Antibiotic | 1,000-bp group
|
1,000-bp + 1,200-bp group
|
1,600-bp group
|
Any integron
|
||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity (95% confidence interval) | Specificity (95% confidence interval) | Sensitivity (95% confidence interval) | Specificity (95% confidence interval) | Sensitivity (95% confidence interval) | Specificity (95% confidence interval) | Sensitivity (95% confidence interval) | Specificity (95% confidence interval) | |
| Amox | 0 (0.00-0.84) | 0.61 (0.57-0.64) | 0 (0.00-0.84) | 0.94 (0.92-0.96) | 0 (0.00-0.84) | 0.98 (0.97-0.99) | 0 (0.00-0.84) | 0.54 (0.53-0.61) |
| Amp | 0.02 (0.01-0.12) | 0.58 (0.55-0.62) | 0.89 (0.76-0.96) | 1 (0.99-1.00) | 0 (0.00-0.10) | 0.98 (0.97-0.99) | 0.91 (0.79-0.98) | 0.57 (0.53-0.61) |
| Cef | 0 (0.00-0.71) | 0.61 (0.57-0.64) | 0 (0.00-0.71) | 0.94 (0.92-0.96) | 0 (0.00-0.71) | 0.98 (0.97-0.99) | 0 (0.00-0.71) | 0.54 (0.50-0.57) |
| Cm | 0.02 (0.00-0.07) | 0.55 (0.51-0.59) | 0.41 (0.31-0.51) | 1 (0.99-1.00) | 0 (0.00-0.04) | 0.98 (0.97-0.99) | 0.43 (0.33-0.53) | 0.53 (0.49-0.57) |
| Gen | 0.5 (0.28-0.72) | 0.61 (0.58-0.65) | 0 (0.00-0.15) | 0.94 (0.92-0.96) | 0.5 (0.28-0.72) | 0.99 (0.99-1.00) | 1 (0.85-1.00) | 0.55 (0.51-0.59) |
| Kan | 0 (0.00-0.12) | 0.59 (0.56-0.63) | 0 (0.00-0.12) | 0.94 (0.92-0.96) | 0.27 (0.13-0.47) | 0.99 (0.99-1.00) | 0.28 (0.13-0.47) | 0.53 (0.49-0.57) |
| St | 0.76 (0.71-0.80) | 0.95 (0.93-0.97) | 0.12 (0.08-0.15) | 1 (0.99-1.00) | 0.03 (0.02-0.06) | 1 (0.99-1.00) | 0.91 (0.87-0.94) | 0.95 (0.93-0.97) |
| Su | 0.69 (0.64-0.73) | 1 (0.98-1.00) | 0.1 (0.07-0.13) | 1 (0.99-1.00) | 0.03 (0.01-0.05) | 1 (0.99-1.00) | 0.81 (0.77-0.83) | 1 (0.99-1.00) |
| Tet | 0.61 (0.56-0.66) | 0.95 (0.92-0.97) | 0.09 (0.07-0.12) | 1 (0.99-1.00) | 0.03 (0.01-0.05) | 1 (0.99-1.00) | 0.73 (0.69-0.77) | 0.95 (0.94-0.97) |
| Spc | 0.71 (0.66-0.75) | 1 (0.99-1.00) | 0.1 (0.07-0.14) | 1 (0.99-1.00) | 0.03 (0.02-0.05) | 1 (0.99-1.00) | 0.84 (0.80-0.88) | 1 (0.99-1.00) |
Amox, amoxicillin; Amp, ampicillin; Cef, cefoxitin; Cm, chloramphenicol; Gen, gentamicin; Kan, kanamycin; St, streptomycin; Su, sulfisoxazole; Tet, tetracycline; Spc, spectinomycin. Ninety-five-percent exact binomial confidence intervals are reported.
The sensitivities and specificities of the Int-PCR test to detect the four-drug (St Spc Su Tet) and six-drug (Amp Cm St Spc Su Tet and Gen Kan St Spc Su Tet) resistance patterns in the presence of any of the three integron groups were 0.98 and 0.92, 0.95 and 1.0, and 1.0 and 0.99, respectively.
There were 135 isolates with integrons selected for sequencing: 108 with the 1,000 bp integron only, 19 with both the 1,000- and 1200-bp integrons, and 8 with the 1,600-bp integron only (a total of 154 integrons sequenced). All sequences shared >99% homology with GenBank reference sequences. All 1,000-bp and 1,600-bp integrons contained the aminoglycoside adenyl transferase (aadA) gene, coding for streptomycin and spectinomycin resistance; 6 of these isolates had the aadA1 gene, and 129 had the aadA2 gene. The aadB gene, coding for gentamicin and kanamycin resistance, was identified in all 1,600-bp integrons. The β-lactamase (bla) gene, coding for ampicillin resistance, was identified in all 1,200-bp integrons.
DISCUSSION
In the present study, 46% of the isolates contained class 1 integrons (1,000, 1,200, and 1,600 bp in size), which is indicative of their high frequency of occurrence in Salmonella strains. As described previously (10, 16, 17, 26), a strong association of class 1 integrons (1,000, 1,000 and 1,200, and 1,600 bp) with identified resistance to specific antibiotics was demonstrated and attributed in part to the existence of resistance genes (aadA for streptomycin and spectinomycin, aadB for gentamicin and kanamycin, and β-lactamase for ampicillin) within these integrons. The gene sequencing and associated antibiotic resistance profiles of each integron group suggest that the 1,600-bp integron in part shares a common ancestor with the 1,000-bp integron plus additional genetic material containing the aadB gene. In contrast, the 1,200-bp integron containing the β-lactamase gene apparently has an independent evolutionary origin.
There were several resistance patterns identified that were not associated with genes present in integrons. All isolates with 1,000-bp and 1,600-bp integrons were resistant to sulfisoxazole and tetracycline, but genes for neither resistance were found on the integrons, almost one-third of isolates without class 1 integrons were resistant to tetracycline, and about 15% were resistant to sulfisoxazole, spectinomycin, and chloramphenicol. The genes carrying resistance to these antibiotics are not carried on class 1 integrons. Although kanamycin resistance was associated with the 1,600-bp integron and the gene coding for this resistance, aadB, has been identified on this integron, kanamycin resistance was also found in 5% of isolates not containing class 1 integrons. The tetB gene, conferring resistance to tetracycline, is carried on the Tn10 transposon (14). Sulfisoxazole resistance carried by the sulI gene is part of the 3′ conserved sequence (17), and chloramphenicol resistance, imparted by the S. enterica serovar Typhimurium flo gene, was not carried on integrons (1). The source of resistance to spectinomycin by isolates not carrying integrons is unknown.
Multiresistant S. enterica serovar Typhimurium DT104 strains are known to carry 1,000- and 1,200-bp integrons (9, 19). Although serotyping was not conducted here, the same six-drug resistance pattern, Amp Cm St Spc Su Tet, of the 1,000- and 1,200-bp isolates observed here has been identified previously for S. enterica serovar TyphimuriumDT104 strains containing 1,000- and 1,200-bp integrons (21).
The existence of antibiotic resistance genes outside of integrons in this study suggests that genes for resistance to some antibiotics existed and were spread by clonal proliferation in these populations for some time and that the incorporation of integrons and additional resistances into the genome in these populations occurred independently. The dual existence of antibiotic resistance genes within and outside integrons limits the sensitivity of the Int-PCR test.
The analysis of diagnostic accuracy indicates that Int-PCR with components for the detection of class 1 integrons of 1,000, 1,000 and 1,200, and 1,600 bp provides moderate to high sensitivity (0.73 to 0.91) in detecting individual resistances to streptomycin, sulfisoxazole, tetracycline, and spectinomycin, which are associated with the 1,000- and 1,600-bp integrons, and high sensitivity (0.91 to 1.0) in detecting resistance to ampicillin and gentamicin, which are associated with the 1,200- and 1,600-bp integrons, respectively. The low sensitivities of detection of resistance to kanamycin (0.28) and chloramphenicol (0.43) were due to the existence of resistance to these antibiotics in isolates lacking integrons. The specificity of Int-PCR was high (≥0.95) for the four antibiotic resistances associated with the 1,000- and 1,600-bp integrons (streptomycin, sulfisoxazole, tetracycline, and spectinomycin) but not for other antibiotic resistances, as would be expected.
Whereas Int-PCR had only moderate success as a diagnostic tool for detecting or ruling out some individual antibiotic resistances, its high sensitivity and specificity levels (>0.9) for detecting four-drug (St Spc Su Tet) and six-drug (Amp Cm St Spc Su Tet and Gen Kan St Spc Su Tet) resistance indicate that Int-PCR can be a valuable screening tool for identifying or ruling out multidrug resistance in Salmonella strains. Multiresistant S. enterica serovar Typhimurium DT104 strains are known to carry 1,000- and 1,200-bp integrons (9, 19). Although serotyping was not conducted here, the same six-drug resistance pattern, Amp Cm St Spc Su Tet, of the 1,000- and 1,200-bp isolates observed here was identified previously for S. enterica serovar Typhimurium DT104 strains containing 1,000- and 1200-bp integrons (21). Thus, in using Int-PCR, the detection of integrons can be followed by either Sensititre (phenotypic) testing or PCR techniques for the detection of specific genes encoding antibiotic resistances associated with class 1 integrons to verify resistance patterns.
One limitation of the present study is that Int-PCR was evaluated in only four swine production facilities over one summer season. The sensitivity of Int-PCR in detecting antibiotic resistance is dependent upon the incorporation of resistance genes within class 1 integrons. Thus, there could be between-population variability that has an impact on the sensitivity of Int-PCR. However, previously identified associations of class 1 integrons with multidrug-resistant phenotypes and genotypes across studies of Salmonella and other Enterobacteriaceae suggest that these results are not unique in the current setting. Thus, in epidemiological surveys, the use of Int-PCR can provide time and cost savings compared to running extensive antibiotic resistance panels on a large number of samples.
Acknowledgments
This work was supported by U.S. Department of Agriculture Project on Molecular Epidemiology of Salmonella Transmission in Different Swine Production Systems (1999) and U.S. Department of Agriculture Hatch funds for the Project on Using Integrons To Monitor Salmonella Transmission in Swine: Comparing Genotypes (2003).
Footnotes
Published ahead of print on 3 January 2008.
REFERENCES
- 1.Bolton, L. F., L. C. Kelley, M. D. Lee, P. J. Fedorka-Cray, and J. J. Maurer. 1999. Detection of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 based on a gene which confers cross-resistance to florfenicol and chloramphenicol. J. Clin. Microbiol. 371348-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryan, F. L. 1988. Risk of practices, procedures and processes that lead to the food borne disease. J. Food Prot. 51663-673. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz, U., B. Brodhun, S. O. Brockmann, C. M. Dreweck, R. Prager, H. Tschape, and A. Ammon. 2005. An outbreak of Salmonella Munchen in Germany associated with raw pork meat. J. Food Prot. 68273-276. [DOI] [PubMed] [Google Scholar]
- 4.Chiu, C. H., and J. T. Ou. 1996. Rapid identification of Salmonella serovars in feces by specific detection of virulence genes invA and spvC by an enrichment broth culture-multiplex PCR combination assay. J. Clin. Microbiol. 342619-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly, M., and S. Fanning. 2000. Characterization and chromosomal mapping of antimicrobial resistance genes in Salmonella enterica serotype Typhimurium. Appl. Environ. Microbiol. 664842-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewey, C. E., B. D. Cox, B. E. Straw, E. J. Bush, and S. Hurd. 1999. Swine Health Prod. 719-25. [Google Scholar]
- 7.Fleiss, J. L., B. Levin, and M. C. Paik. 2003. Statistical methods for rates and proportions, 3rd ed., p. 55-57. John Wiley & Sons, New York, NY.
- 8.Gebreyes, W. A., and C. Altier. 2002. Molecular characterization of multidrug-resistant Salmonella enterica subsp. enterica serovar Typhimurium isolates from swine. J. Clin. Microbiol. 402813-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebreyes, W. A., S. Thakur, P. R. Davies, J. A. Funk, and C. Altier. 2004. Trends in antimicrobial resistance, phage types and integrons among Salmonella serotypes from pigs, 1997-2000. J. Antimicrob. Chemother. 53997-1003. [DOI] [PubMed] [Google Scholar]
- 10.Guerra, B., S. Soto, S. Cal, and M. C. Mendoza. 2000. Antimicrobial resistance and spread of class 1 integrons among Salmonella serotypes. Antimicrob. Agents Chemother. 442166-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall, R. M., and C. M. Collins. 1998. Antibiotic resistance in gram-negative bacteria: the role of gene cassettes and integrons. Drug Resist. Updates 1109-119. [DOI] [PubMed] [Google Scholar]
- 12.Hayes, D. J., H. H. Jensen, and J. Fabiosa. 2002. Technology choice and the economic effects of a ban on the use of antimicrobial feed additives in swine rations. Food Control 1397-101. [Google Scholar]
- 13.Jones, L. A., A. J. McIver, M. J. Kim, W. D. Rawlinson, and P. A. White. 2005. The aadB gene cassette is associated with blaSHV genes in Klebsiella species producing extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 49794-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levesque, C., L. Piche, C. Larose, and P. H. Roy. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucey, B., D. Crowley, P. Moloney, B. Cryan, M. Daly, F. O'Halloran, E. J. Threlfall, and S. Fanning. 2000. Integron-like structures in Campylobacter spp. of humans and animal origin. Emerg. Infect. Dis. 650-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maguire, A. J., D. F. J. Brown, J. J. Gray, and U. Desselberger. 2001. Rapid screening technique for class 1 integrons in Enterobacteriaceae and nonfermenting gram-negative bacteria and its use in molecular epidemiology. Antimicrob. Agents Chemother. 451022-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miko, A., K. Pries, A. Schroeter, and R. Helmuth. 2005. Molecular mechanisms of resistance in multidrug-resistant serovars of Salmonella enterica isolated from foods in Germany. J. Antimicrob. Chemother. 561025-1033. [DOI] [PubMed] [Google Scholar]
- 18.NCCLS. 2001. Performance standards for antimicrobial susceptibility testing. Eleventh international supplement, 21(1). Veterinary antimicrobial susceptibility testing document M31-A2. NCCLS, Wayne, PA.
- 19.Nogrady, N., I. Gado, A. Toth, and J. Paszti. 2005. Antibiotic resistance and class 1 integron patterns of non-typhoidal human Salmonella serotypes isolated in Hungary in 2002 and 2003. Int. J. Antimicrob. Agents 26126-132. [DOI] [PubMed] [Google Scholar]
- 20.Nucera, D. M., C. W. Maddox, P. Hoien-Dalen, and R. M. Weigel. 2006. Comparison of API 20E and invA PCR for identification of Salmonella enterica isolates from swine production units. J. Clin. Microbiol. 443388-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandvang, D., F. M. Aarestrup, and L. B. Jensen. 1998. Characterisation of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica Typhimurium DT104. FEMS Microbiol. Lett. 16037-41. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan, J. J., and D. A. McDowell. 1998. Factors affecting the emergence of pathogens on foods. Meat Sci. 49S151-S167. [PubMed] [Google Scholar]
- 23.Soto, S. M., M. J. Lobato, and M. C. Mendoza. 2003. Class 1 integron-borne gene cassettes in multidrug-resistant Yersinia enterocolitica strains of different phenotypic and genetic types. Antimicrob. Agents Chemother. 47421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefani, S., and A. Agodi. 2000. Molecular epidemiology of antibiotic resistance. Int. J. Antimicrob. Agents 13143-153. [DOI] [PubMed] [Google Scholar]
- 25.Vo, A. T. T., E. van Duijkeren, A. C. Fluit, W. J. B. Wannet, A. J. Verbruggen, H. M. E. Maas, and W. Gaastra. 2006. Antibiotic resistance, integrons and Salmonella genomic island 1 among non-typhoidal Salmonella serovars in The Netherlands. Int. J. Antimicrob. Agents 28172-179. [DOI] [PubMed] [Google Scholar]
- 26.White, P. A., C. J. McIver, and W. D. Rawlinson. 2001. Integrons and gene cassettes in the Enterobacteriaceae. Antimicrob. Agents Chemother. 452658-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang, S. J., K. Y. Park, S. H. Kim, K. M. No, T. E. Besser, H. S. Yoo, S. H. Kim, B. K. Lee, and Y. H. Park. 2002. Antimicrobial resistance in Salmonella enterica serovars Enteritidis and Typhimurium isolated from animals in Korea: comparison of phenotypic and genotypic resistance characterization. Vet. Microbiol. 86295-301. [DOI] [PubMed] [Google Scholar]