Abstract
Acinetobacter species other than Acinetobacter baumannii have rarely been reported to be associated with nosocomial outbreaks of bloodstream infections. Within a period of 1 week, seven Acinetobacter-like isolates were recovered from peripheral blood and catheter specimens of five patients at a neonatal intensive care unit (NICU) in a tertiary hospital in Turkey. All five patients had placement of central venous catheters and had received total parenteral nutrition before the onset of bacteremia. Two of the five patients died. Medical devices, tap water, aerators, water samples, various surfaces, intravenous fluids, and the hands of health care workers in the NICU were sampled and were culture negative for the bacterium. All seven of the isolates had identical biochemical reactions, antimicrobial susceptibility results, and pulsed-field gel electrophoresis patterns, indicating a clonal nosocomial outbreak. A panel of standard biochemical reaction profiles and three phenotypic commercial identification systems failed to identify these isolates. Phenotypically, the isolate differed from Acinetobacter ursingii by its hemolysis on sheep blood agar and its negative citrate utilization. Sequences of the full 16S rRNA gene, which contained at least three different gene copies with polymorphic sequences between nucleotide positions 70 and 206, were determined from the first recovered isolate. The complete 1,529- to 1,531-bp 16S rRNA gene sequences and partial 801-bp rpoB gene sequences had similarities of 99.5% and 97.2%, respectively, to an A. ursingii isolate. The DNA-DNA similarities of the strain against the type strain of A. ursingii were 64.7 and 68.7%, which were lower than the recommended threshold value of 70% for the definition of bacterial species. These data indicate that a novel Acinetobacter organism caused the nosocomial outbreak of bacteremia in the NICU unit. We propose the designation of Acinetobacter septicus sp. nov. for these isolates, with isolate AK001 as the type strain.
The genus Acinetobacter was proposed by Bouvet and Grimont in 1986 (3) and was recently expanded to 32 genomic species, including 17 species with a validated name (37). Acinetobacter species are nonmotile, nonfermentative, aerobic, gram-negative bacilli that are widely distributed in nature (2). Only 10 nomenspecies have been isolated in human specimens: A. baumannii, A. calcoaceticus, A. haemolyticus, A. johnsonii, A. junii, A. lwoffii, A. parvus, A. radioresistens, A. schindleri, and A. ursingii (12). The species most frequently isolated from humans is A. baumannii, which also appears to be the Acinetobacter species of greatest clinical importance.
Nosocomial infections are one of the most important causes of mortality and morbidity in hospitals, and numerous nosocomial outbreaks due to Acinetobacter species have been described. Risk factors associated with these infections include antibiotic exposure, length of stay in intensive care units, mechanical ventilations, and severity of underlying illness (1, 7, 9, 11, 21, 23, 39). Hospitalized patients can become “colonized” with Acinetobacter species, which may cause endemic problems in the hospital setting due to cross-transmission between patients (7, 11, 23). Acinetobacter species have been isolated from cases of pneumonia (6.9%), bloodstream infections (2.4%), surgical site infections (2.1%), and urinary tract infections (1.6%) according to U.S. National Nosocomial Infections Surveillance data from hospitals in 2003 (15). A. baumannii remains the main species associated with outbreaks of nosocomial infection. A genotypic analysis of Acinetobacter bloodstream infection isolates in a Turkish university hospital indicated that 80.5% were A. baumannii (1). Nosocomial infections caused by other Acinetobacter species have rarely been reported, although an outbreak of infections with A. calcoaceticus in burn patients has been reported (39). Acinetobacter genomic species 3 and 13TU have also been implicated in nosocomial infections (10), while A. johnsonii has been associated with catheter-related bacteremia (38). A sporadic case of bacteremia caused by A. ursingii has been reported (32). Most nosocomial Acinetobacter infections not caused by A. baumannii are seen in patients who are already suffering from severe underlying diseases, and their clinical significance remains defined (14, 22, 26, 42).
Here, we report a novel Acinetobacter species associated with a nosocomial outbreak of bacteremia in a neonatal intensive care unit (NICU). Within a period of 1 week, seven Acinetobacter-like isolates were recovered from peripheral blood and catheter specimens of five patients at a NICU in Gulhane Military Medical Academy Hospital in Turkey. All seven isolates had identical biochemical reactions, antimicrobial susceptibility results, and pulsed-field gel electrophoresis (PFGE) patterns, indicating a clonal nosocomial outbreak. Identification systems based on biochemical reactions failed to identify these isolates. Sequence analysis of full 16S rRNA and partial rpoB genes and DNA-DNA hybridization suggest that these isolates are new Acinetobacter species, and the designation of Acinetobacter septicus sp. nov. is proposed.
(This study was presented in part at the 107th General Meeting of the American Society for Microbiology, Toronto, Ontario, Canada, 21 to 25 May 2007.)
MATERIALS AND METHODS
Patients and participants.
Gulhane Military Medical Academy Hospital is a 1,500-bed teaching hospital in Ankara, Turkey. Three doctors, four nurses, and one nurse trainee work in the 15-bed NICU. Patients with low birth weight or respiratory dysfunction related to prematurity are generally admitted to this NICU. During 1 week in March 2006, a total of seven Acinetobacter isolates were recovered from five of eight newborn patients' blood specimens. Informed consent was obtained from the parents of all five patients. Clinical specimens were collected, and medical records were reviewed. Samples from medical devices, tap water, aerators, water samples, various surfaces, intravenous fluids, and the hands of health care workers were collected and cultured to evaluate potential risk factors and to find a source of the bacteremia.
Specimen processing and bacterial isolation.
Patients' blood samples were processed with the Bactec 9240 nonradiometric blood culture system (Becton Dickinson, Sparks, MD). When the positive blood cultures were confirmed to be gram-negative bacilli by Gram stain, they were subcultured onto MacConkey and 5% sheep blood agar for up to 3 days at 37°C. Four related and well-characterized Acinetobacter strains, including an Acinetobacter species (ATCC 410000; American Type Culture Collection [ATCC], Manassas, VA), A. lwoffii (ATCC 19002), A. ursingii (DSM 16037; Deutsche Sammlung von Mikroorganismen und Zellkulturen [DSMZ], Braunschweig, Germany), and one A. baumannii strain isolated at the Vanderbilt University Medical Center (7), were used as reference controls. Clinical isolates and reference strains were collected and saved in brain heart infusion broth containing 7.5% glycerol at −80°C for further study.
Phenotypic identification.
All isolates were presumptively identified by conventional methods including hemolysis on sheep blood agar; sugar fermentation; motility; catalase, oxidase, citrate, urease, indole, and H2S production; the API 20E system (bioMerieux Inc., Durham, NC); the Rapid NF Plus system (Remel Inc., Lenexa, KS); and the Biolog (Hayward, CA) GN2 system (24, 25, 41). The proposed type strain, AK001, was further identified based on a panel of standard biochemical methods at the DSMZ according to protocols described previously (3).
Antimicrobial susceptibility testing.
Susceptibilities of the isolates were determined by a disk diffusion method. The following antimicrobial agents (Oxoid, Basingstoke, Hampshire, United Kingdom) were tested: ciprofloxacin, trimethoprim-sulfamethoxazole, imipenem, meropenem, ceftazidime, ampicillin-sulbactam, amikacin, cefotaxime, gentamicin, piperacillin-tazobactam, and cefepime. Results were expressed as susceptible or resistant according to criteria recommended by the Clinical and Laboratory Standards Institute (5).
Genomic DNA analysis by PFGE.
PFGE typing of SmaI-digested DNA was performed according to a modification of a previously described method (7). Electrophoresis was performed with a run time of 18.5 h under a 1- to 17-s linear ramped pulse time by the contour-clamped homogeneous electric field method with a Bio-Rad CHEF DR II system (Bio-Rad, Hercules, CA). After PFGE, the gels were stained with ethidium bromide (0.5 μg/ml) and analyzed under UV transillumination using Quantity One software (Bio-Rad).
16S rRNA gene amplification, cloning, and sequencing.
A loopful of each purified bacterial isolate was put into 1 ml of distilled water. The suspension was vortexed, heated for 7 min at 95°C, and centrifuged at 8,000 × g for 15 s, and 1 μl of supernatant was used for PCR amplification. A highly conserved primer set (5′-TGG AGA GTT TGA TCC TGG CTC AG-3′ and 5′-AAG GAG GTG ATC CAR CCG CA-3′) spanning 5 to 1,553 nucleotides of the 16S rRNA gene was used to amplify the DNA fragment by PCR (41). The PCR products were directly used for sequence determination on an ABI Prism 3730 DNA sequencer (Applied Biosystems, Foster City, CA), as previously described (41). Furthermore, the PCR products were cloned into the pCR2.1 vector (TA cloning kits; Invitrogen Corp., Carlsbad, CA) according to the manufacturer's instructions. M13 universal forward and reverse primers and several additional 16S rRNA gene internal primers were used for sequencing of the cloned rRNA gene on the same DNA sequencer as previously described (41).
rpoB gene amplification and sequencing.
Amplification of the partial ribosomal polymerase B subunit (rpoB) gene (902 bp) was performed by using primers Ac696F and Ac1598R as previously described (30). In addition to the PCR primers, two internal primers, Ac1093R and Ac1055F, were used for sequencing (30). The sequencing products were purified using Centri-Sep spin columns (Princeton Separations, Adelphia, NJ) and were analyzed on the ABI Prism 3130 Avant genetic analyzer according to the manufacturer's instructions (Applied Biosystems).
Phylogenetic analysis.
Full sequences of the 16S rRNA gene were included for analysis. For the rpoB gene, the region from bp 2900 to 3700 (corresponding to A. baumannii rpoB gene positions of GenBank accession number DQ207471) of the rpoB gene was used. Both full 16S rRNA and partial rpoB gene sequences were analyzed using Ridom TraceEditPro software (version 1.1; Ridom GmbH, Würzburg, Germany). Multiple alignment, sequence similarities of the 16S rRNA and partial rpoB gene sequences, and neighbor-joining trees with bootstrap values were calculated using MEGA 3.1 software (27). Both full 16S rRNA and partial rpoB gene sequence trees were outgroup rooted with Pseudomonas aeruginosa strain PAO1 sequences.
DNA-DNA hybridization.
DNA-DNA hybridization of strain AK001 against A. ursingii (DSM 16037) was performed at the DSMZ. Bacterial DNA was isolated from logarithmic-phase cultures (Thermo Spectronic, Madison, WI) and was purified by chromatography on hydroxyapatite as described previously (4). DNA-DNA hybridization was carried out in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 67°C (8), according to modifications described previously (18), using a Cary 100 Bio UV/VIS spectrophotometer equipped with a Peltier-thermostatted 6-by-6 multicell changer and a temperature controller with an in situ temperature probe (Varian Inc., Palo Alto, CA). A threshold value of 70% DNA-DNA similarity was used for the definition of bacterial species according to ad hoc committee recommendations (44).
Nucleotide sequence accession numbers.
The sequences of the full 16S rRNA (three operons) and partial rpoB genes of A. septicus AK001 (DSM 19415) were deposited in the GenBank database under accession numbers EF611418 to EF611420 and EF611383.
RESULTS
There were eight patients in the NICU during the study period. A total of seven unusual Acinetobacter isolates, from five blood and two catheter tip samples, were recovered from a total of five newborns. All seven isolates had the same antibiotic susceptibility patterns and were susceptible to the antibiotics that were used in this study, except for ceftazidime and cefotaxime. Two of the five neonates died; the others recovered after a week's course of intravenous amikacin and meropenem (Table 1). The same Acinetobacter strain was not isolated from environmental and personnel samples, which were sampled at the same time period.
TABLE 1.
Demographic and clinical characteristics of five patients with bacteremia in the NICUa
| Case | Sex | Diagnosis on admission | Gestational age (wk) | Wt at birth (g) | Antibiotic treatment | Procedures | Method of Delivering | Prognosis | Dates (mo/day/yr) of blood culture (result) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | Wet lung, bacteremia | 38 | 3,290 | Amikacin, meropenem | TPN, PC | Cesarean section | Survived | 3/21/2006 (N), 3/22/2006 (P, AK001), 3/23/2006 (P, AK002), 3/24/2006 (P, AK003), 3/26/2006 (N) |
| 2 | Male | Premature, ARDS | 23 | 580 | Amikacin, meropenem | TPN, PC, intubation | Cesarean section | Died of ARDS | 3/20/2006 (N), 3/22/2006 (P, AK004), 3/25/2006 (N), 4/2/2006 (N) |
| 3 | Female | Hypoxic ischemic encephalopathy | 37 | 4,300 | Amikacin, meropenem | TPN, PC | Cesarean section | Survived | 3/23/2006 (N), 3/24/2006 (P, AK005), 3/26/2006 (N) |
| 4 | Female | Premature, ARDS, bacteremia | 32 | 1,720 | Amikacin, meropenem | TPN, PC | Cesarean section | Survived | 3/23/2006 (P, AK006), 3/26/2006 (N), 4/2/1006 (N) |
| 5 | Male | Premature, ARDS, intracranial hemorrhage | 25 | 700 | Amikacin, meropenem | TPN, PC | Cesarean section | Died of DIC | 3/16/2006 (N), 3/19/2006 (N), 3/24/2006 (P, AK007), 3/26/2007 (N) |
Abbreviations: TPN, total parenteral nutrition; PC, peripheral catheter; ARDS, acute respiratory distress syndrome; DIC, disseminated intravascular coagulation; N, negative; P, positive.
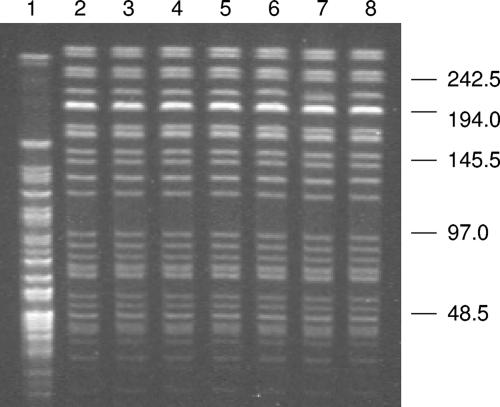
All seven strains had the same biochemical test results, including hemolysis on sheep blood agar, nonfermentative, nonmotile, catalase positive, oxidase negative, citrate-negative, urease negative, and indole and H2S production negative. A full panel of standard biochemical reactions were performed on the first isolate, AK001, which was a Gram stain-negative rod with a width and length of 0.7 to 0.9 and 1.0 to 2.5 μm, respectively. It produced hemolysis on sheep blood agar and was lysed by 3% KOH. It was positive for aminopeptidase and alcohol dehydrogenase but negative for conversion from NO3 to NO2. Other key biochemical reactions are listed in Table 2. This unusual Acinetobacter isolate was differentiated from A. ursingii by its hemolysis on sheep blood agar and negative citrate reaction (34, 36). Biochemical reactions were the same for all seven isolates by use of the bioMerieux API 20E, Remel Rapid NF Plus, and Biolog GN2 systems: all three systems failed to make an identification with acceptable similarity scores. The epidemiologic relatedness of the seven isolates was further analyzed by PFGE. For comparison, one A. baumannii strain, which was isolated from the burn unit at Vanderbilt University Hospital multiple times, was included (7). The PFGE patterns of the seven isolates were identical, suggesting that the isolates were epidemiologically related and clonal in origin (Fig. 1).
TABLE 2.
Phenotypic characteristics of unusual Acinetobacter isolate AK001 and A. ursingiia
| Phenotypic profile | Result for:
|
|
|---|---|---|
| AK001 | A. ursingiib | |
| Growth at: | ||
| 44°C | − | − |
| 41°C | − | − |
| 37°C | + | + |
| Acid from D-glucose | − | − |
| Gelatinase | − | − |
| Hemolysis of sheep blood | + | − |
| Utilization of: | ||
| dl-Lactate | + | + |
| dl-4-Aminobutyrate | − | − |
| trans-Aconitate | − | − |
| Citrate (Simmons) | − | + |
| Glutarate | + | 88 |
| l-Aspartate | + | 94 |
| Azelate | + | + |
| β-Alanine | ND | − |
| l-Histidine | − | − |
| d-Malate | + | + |
| Malonate | − | − |
| Histamine | − | − |
| l-Phenylalanine | − | − |
| Phenylacetate | − | − |
| l-Leucine | − | ND |
| l-Arginine | − | ND |
| l-Ornithine | − | ND |
FIG. 1.
PFGE patterns of SmaI-digested genomic DNA of Acinetobacter isolates. Lanes 1 to 8 are isolates of A. baumannii, AK001, AK002, AK003, AK004, AK005, AK006, and AK007, respectively. Molecular sizes are in kilobases.
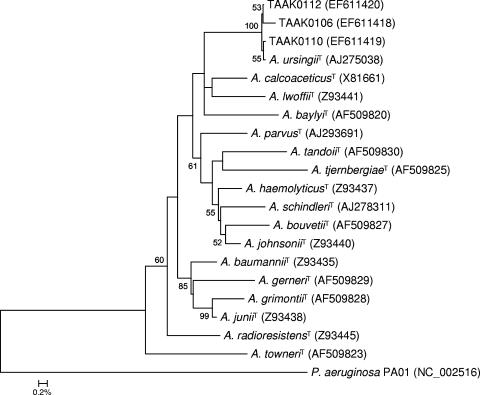
The 16S rRNA genes for all seven isolates were amplified by PCR, and the direct sequencing on amplicons was performed. Mixed sequences around nucleotide position 100 were repeatedly produced. Therefore, amplicons of strain AK001 were subcloned, and a total of 12 clones were selected and subjected to DNA sequencing. Sequencing resulted in three different operons of the 16S rRNA gene, named TAAK0106, TAAK0110, and TAAK0112, respectively, ranging from 1,529 to 1,531 nucleotides. The three full 16S rRNA gene sequences had 99.98% similarity among them and were closely related to an A. ursingii isolate (DSM 16037) (34, 36) at 99.5% similarity (Fig. 2). To further characterize the seven unknown Acinetobacter strains, partial 801-bp rpoB direct gene sequencing was applied, and the sequences were compared to the sequences of all 17 type strains. All seven unknown Acinetobacter strains showed identical rpoB sequences. Therefore, only AK001 was included in Fig. 3. The sequence of AK001 showed similarities of 97.2% to A. ursingii (DSM 16037) and 76.6 to 81.2% to other Acinetobacter species (Fig. 3).
FIG. 2.
Rooted neighbor-joining tree based on the almost-complete 16S rRNA gene sequence (1,346 bp) showing the phylogenetic relationship among all type strains of the genus Acinetobacter (n = 17) and three sequences from different clones of one representative strain (AK001) of the unusual Acinetobacter isolates. The scale bar indicates the evolutionary distance between sequences determined by measuring the lengths of the horizontal lines connecting two organisms. Numbers at nodes (shown if ≥50% within the consensus phylogenetic tree) indicate percentages of bootstrap support based on a neighbor-joining analysis of 1,000 resampled data sets. Pseudomonas aeruginosa strain PAO1 was used for outgroup rooting. GenBank accession numbers of downloaded sequences are in parentheses. Bar, 0.2% sequence divergence. T, type strain.
FIG. 3.
Rooted neighbor-joining tree based on partial rpoB gene sequences (801 bp) showing the phylogenetic relationship of strain AK001 among all type strains of the genus Acinetobacter (n = 17) and further Acinetobacter culture collection strains exhibiting unique 16S rRNA gene sequences. The scale bar indicates the evolutionary distance between sequences determined by measuring the lengths of the horizontal lines connecting two organisms. Numbers at nodes (shown if ≥50% within the consensus phylogenetic tree) indicate percentages of bootstrap support based on a neighbor-joining analysis of 1,000 resampled data sets. Pseudomonas aeruginosa strain PAO1 was used for outgroup rooting. GenBank accession numbers of submitted sequences are in parentheses. Bar, 5% sequence divergence. T, type strain. CIP, Collection de l'Institut Pasteur, Paris, France; LMG, Belgian Coordinated Collections of Microorganisms, Ghent, Belgium.
DNA-DNA hybridization of strain AK001 against A. ursingii (DSM 16037) was performed in duplicate, and the resulting DNA-DNA similarities were 64.7 and 68.7%, which were both lower than the recommended threshold value of 70% for the definition of bacterial species (44). Based on all physiological and chemotaxonomical data, we propose the designation of Acinetobacter septicus sp. nov. for the novel species in the Acinetobacter genus, with strain AK001 as the type strain.
DISCUSSION
In this report, we have described a cluster of five cases of bacteremia within a week in an NICU that were caused by a novel Acinetobacter species. Seven isolates were recovered from peripheral blood and catheter specimens of these five patients. Two of five patients died; the others recovered after receiving a week's course of intravenous amikacin and meropenem. All seven isolates presented identical biochemical reactions, antimicrobial susceptibility profiles, and PFGE patterns, indicating a clonal nosocomial outbreak.
Bacteremia among newborns in NICUs causes considerable mortality and morbidity and accounts for approximately 30% of hospital-acquired infections in this population (28). The incidence of bacterial infection in NICUs is estimated to be about 1 to 8 newborns per every 1,000 live births and 160 to 300 newborns per 1,000 live births in very-low-birth-weight newborns. Coagulase-negative staphylococci and Enterobacter species are the pathogens most commonly isolated from the NICU (31). Acinetobacter spp. are usually considered to be nonpathogenic to healthy individuals; however, especially in debilitated individuals and patients in intensive care units, they do cause nosocomial infections (13). Premature and low-birth-weight infants as well as the length of hospital stay are significant risks for developing infections (33). In this study, three patients were premature and had low birth weights.
Acinetobacter species are widely distributed in nature and in the hospital environment. It has been shown that the digestive tract of intensive care unit patients is an important epidemiologic reservoir in hospital outbreaks (6). Environmental contamination of various hospital items has often been identified, ranging from suctioning equipment to pillows and mattresses (13). Foreign bodies such as catheters play an important role in the pathogenic occurrence of A. lwoffi bacteremia (40, 43). Hand organism carriage by health care workers has been implicated in outbreaks of Acinetobacter infections (2). During an outbreak of A. baumannii bacteremia in an NICU in Taiwan, multiple A. baumannii isolates were recovered from hand washing samples, and some of them were epidemiologically related to those recovered from patients' blood, suggesting that the hospital environment was the potential reservoir and that transmission was possibly via the hands of health care workers (17). Higher device-associated infection rates and higher device utilization ratios in an ICU were reported in Turkey (20). In our event, all five patients had placement of central venous catheters and had received total parenteral nutrition before the onset of bacteremia. We suspected that cross-contamination of Acinetobacter via the hands of staff members was the likely source of this outbreak. Immediately after the cluster of bacteremia cases were observed, medical devices, tap water, aerators, water samples, various surfaces, intravenous fluids, and the hands of health care workers in the NICU were sampled and were culture negative for the bacterium. The route of transmission of this Acinetobacter bacteremia outbreak remains unknown.
Identification of Acinetobacter isolates to the species level has been problematic in clinical microbiology services. The majority of genospecies cannot be reliably separated by phenotypic tests (37). Some Acinetobacter species present inert biochemical reactions, which makes accurate identification based on phenotypic profiles difficult. In our study, standard biochemical reactions and three phenotypic identification systems, including the API 20E, RapID NF Plus, and Biolog GN2 systems (24, 25, 41), were unable to identify unusual Acinetobacter isolates to the species level. 16S rRNA gene sequencing determinations have been widely used to identify gram-negative bacilli, including Acinetobacter species (16, 41). However, this technique may fail to distinguish closely related genomic species of Acinetobacter (19, 29). Several studies have demonstrated the usefulness of rpoB gene sequences for the identification and taxonomic classification of various bacterial species including Acinetobacter (30, 35). Our data, based on both 16S rRNA and rpoB gene amplification and sequencing, indicated that the unusual Acinetobacter species was most closely related to A. ursingii, at similarities of 99.5% and 97.2%, respectively. Considering significant differences in several key biochemical reactions, a standard DNA-DNA hybridization method was used to further characterize and contrast the unusual Acinetobacter isolate from the A. ursingii type strain. The DNA-DNA hybridization of strain AK001 against the type strain of A. ursingii resulted in similarities that were below the recommended threshold value of 70% for the definition of bacterial species (44). It is worthwhile to point out that Acinetobacter species are a group of organisms which include naturally competent species and published genomes that reveal a large amount of mobile genes; DNA-DNA hybridization can be expected to vary significantly within bacterial species of this type.
Description of A. septicus sp. nov.
A. septicus was named to indicate its clinical relevance as an isolate causing sepsis in humans, especially newborns. The bacteria are gram-negative bacilli with inert biochemical activities. Two key phenotypic characteristics, hemolysis on sheep blood agar and negative citrate utilization, were the only differences between A. septicus and A. ursingii. Commercial biochemical identification systems were not useful to identify the bacterium. Genotypically, A. septicus is most closely related to A. ursingii based on nucleotide sequence analysis of both the 16S rRNA and rpoB genes. DNA-DNA hybridization against A. ursingii gave results below the recommended species delineation threshold. A. septicus has been found only in human blood and is considered to be a pathogen that caused a nosocomial sepsis outbreak in an NICU.
Description of the type strain.
The type strain of A. septicus is AK001 (DSM 19415). It was isolated from the blood of a newborn boy in Ankara, Turkey.
Acknowledgments
We thank Aylin Uskudar, Melinda McCormac, Shufang Meng, Susanne Verbarg, and Cathrin Sproer for their excellent technical assistance.
Footnotes
Published ahead of print on 26 December 2008.
REFERENCES
- 1.Alp, E., D. Esel, O. Yildiz, A. Voss, W. Melchers, and M. Doganay. 2006. Genotypic analysis of Acinetobacter bloodstream infection isolates in a Turkish university hospital. Scand. J. Infect. Dis. 38335-340. [DOI] [PubMed] [Google Scholar]
- 2.Bergogne-Berezin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouvet, P. J. M., and P. A. D. Grimont. 1986. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov. and emended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int. J. Syst. Bacteriol. 36228-240. [Google Scholar]
- 4.Cashion, P., M. A. Holder-Franklin, J. McCully, and M. Franklin. 1977. A rapid method for the base ratio determination of bacterial DNA. Anal. Biochem. 81461-466. [DOI] [PubMed] [Google Scholar]
- 5.CLSI. 2006. Performance standards for antimicrobial disk susceptibility tests. Approved standard, 9th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Corbella, X., M. Pujol, J. Ayats, M. Sendra, C. Ardanuy, M. A. Dominguez, J. Linares, J. Ariza, and F. Gudiol. 1996. Relevance of digestive tract colonization in the epidemiology of nosocomial infections due to multiresistant Acinetobacter baumannii. Clin. Infect. Dis. 23329-334. [DOI] [PubMed] [Google Scholar]
- 7.D'Agata, E. M., M. M. Gerrits, Y. W. Tang, M. Samore, and J. G. Kusters. 2001. Comparison of pulsed-field gel electrophoresis and amplified fragment-length polymorphism for epidemiological investigations of common nosocomial pathogens. Infect. Control Hosp. Epidemiol. 22550-554. [DOI] [PubMed] [Google Scholar]
- 8.De Ley, J., H. Cattoir, and A. Reynaerts. 1970. The quantitative measurement of DNA hybridization from renaturation rates. Eur. J. Biochem. 12133-142. [DOI] [PubMed] [Google Scholar]
- 9.De Vegas, E. Z., B. Nieves, M. Araque, E. Velasco, J. Ruiz, and J. Vila. 2006. Outbreak of infection with Acinetobacter strain RUH 1139 in an intensive care unit. Infect. Control Hosp. Epidemiol. 27397-403. [DOI] [PubMed] [Google Scholar]
- 10.Dijkshoorn, L., R. van Dalen, A. van Ooyen, D. Bijl, I. Tjernberg, M. F. Michel, and A. M. Horrevorts. 1993. Endemic Acinetobacter in intensive care units: epidemiology and clinical impact. J. Clin. Pathol. 46533-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dima, S., E. I. Kritsotakis, M. Roumbelaki, S. Metalidis, A. Karabinis, N. Maguina, F. Klouva, S. Levidiotou, E. Zakynthinos, J. Kioumis, and A. Gikas. 2007. Device-associated nosocomial infection rates in intensive care units in Greece. Infect. Control Hosp. Epidemiol. 28602-605. [DOI] [PubMed] [Google Scholar]
- 12.Dortet, L., P. Legrand, C. J. Soussy, and V. Cattoir. 2006. Bacterial identification, clinical significance, and antimicrobial susceptibilities of Acinetobacter ursingii and Acinetobacter schindleri, two frequently misidentified opportunistic pathogens. J. Clin. Microbiol. 444471-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fournier, P. E., and H. Richet. 2006. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 42692-699. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs, G. J., III, N. Jaffe, and L. K. Pickering. 1986. Acinetobacter calcoaceticus sepsis in children with malignancies. Pediatr. Infect. Dis. 5545-549. [DOI] [PubMed] [Google Scholar]
- 15.Gaynes, R., and J. R. Edwards. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41848-854. [DOI] [PubMed] [Google Scholar]
- 16.Harmsen, D., C. Singer, J. Rothgänger, T. Tønjum, G. S. de Hoog, H. Shah, J. Albert, and M. Frosch. 2001. Diagnostics of Neisseriaceae and Moraxellaceae by ribosomal DNA sequencing: ribosomal differentiation of medical microorganisms. J. Clin. Microbiol. 39936-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, Y. C., L. H. Su, T. L. Wu, H. S. Leu, W. S. Hsieh, T. M. Chang, and T. Y. Lin. 2002. Outbreak of Acinetobacter baumannii bacteremia in a neonatal intensive care unit: clinical implications and genotyping analysis. Pediatr. Infect. Dis. J. 211105-1109. [DOI] [PubMed] [Google Scholar]
- 18.Huss, V. A., H. Festl, and K. H. Schleifer. 1983. Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst. Appl. Microbiol. 4184-192. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim, A., P. Gerner-Smidt, and W. Liesack. 1997. Phylogenetic relationship of the twenty-one DNA groups of the genus Acinetobacter as revealed by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 47837-841. [DOI] [PubMed] [Google Scholar]
- 20.Inan, D., R. Saba, A. N. Yalcin, M. Yilmaz, G. Ongut, A. Ramazanoglu, and L. Mamikoglu. 2006. Device-associated nosocomial infection rates in Turkish medical-surgical intensive care units. Infect. Control Hosp. Epidemiol. 27343-348. [DOI] [PubMed] [Google Scholar]
- 21.Jeon, B. C., S. H. Jeong, I. K. Bae, S. B. Kwon, K. Lee, D. Young, J. H. Lee, J. S. Song, and S. H. Lee. 2005. Investigation of a nosocomial outbreak of imipenem-resistant Acinetobacter baumannii producing the OXA-23 β-lactamase in Korea. J. Clin. Microbiol. 432241-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joly-Guillou, M. L. 2005. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 11868-873. [DOI] [PubMed] [Google Scholar]
- 23.Kaul, R., J. A. Burt, L. Cork, H. Dedier, M. Garcia, C. Kennedy, J. Brunton, M. Krajden, and J. Conly. 1996. Investigation of a multiyear multiple critical care unit outbreak due to relatively drug-sensitive Acinetobacter baumannii: risk factors and attributable mortality. J. Infect. Dis. 1741279-1287. [DOI] [PubMed] [Google Scholar]
- 24.Kiska, D. L., A. Kerr, M. C. Jones, J. A. Caracciolo, B. Eskridge, M. Jordan, S. Miller, D. Hughes, N. King, and P. H. Gilligan. 1996. Accuracy of four commercial systems for identification of Burkholderia cepacia and other gram-negative nonfermenting bacilli recovered from patients with cystic fibrosis. J. Clin. Microbiol. 34886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitch, T. T., M. R. Jacobs, and P. C. Appelbaum. 1992. Evaluation of the 4-hour RapID NF Plus method for identification of 345 gram-negative nonfermentative rods. J. Clin. Microbiol. 301267-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ku, S. C., P. R. Hsueh, P. C. Yang, and K. T. Luh. 2000. Clinical and microbiological characteristics of bacteremia caused by Acinetobacter lwoffii. Eur. J. Clin. Microbiol. Infect. Dis. 19501-505. [DOI] [PubMed] [Google Scholar]
- 27.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5150-163. [DOI] [PubMed] [Google Scholar]
- 28.Larson, E. L., J. P. Cimiotti, J. Haas, M. Nesin, A. Allen, P. Della-Latta, and L. Saiman. 2005. Gram-negative bacilli associated with catheter-associated and non-catheter-associated bloodstream infections and hand carriage by healthcare workers in neonatal intensive care units. Pediatr. Crit. Care Med. 6457-461. [DOI] [PubMed] [Google Scholar]
- 29.La Scola, B., P. E. Fournier, P. Brouqui, and D. Raoult. 2001. Detection and culture of Bartonella quintana, Serratia marcescens, and Acinetobacter spp. from decontaminated human body lice. J. Clin. Microbiol. 391707-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.La Scola, B., V. A. Gundi, A. Khamis, and D. Raoult. 2006. Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J. Clin. Microbiol. 44827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lott, J. W. 2006. State of the science: neonatal bacterial infection in the early 21st century. J. Perinat. Neonat. Nurs. 2062-70. [DOI] [PubMed] [Google Scholar]
- 32.Loubinoux, J., L. Mihaila-Amrouche, A. Le Fleche, E. Pigne, G. Huchon, P. A. Grimont, and A. Bouvet. 2003. Bacteremia caused by Acinetobacter ursingii. J. Clin. Microbiol. 411337-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mireya, U. A., P. O. Marti, K. V. Xavier, L. O. Cristina, M. M. Miguel, and C. M. Magda. 2007. Nosocomial infections in paediatric and neonatal intensive care units. J. Infect. 54212-220. [DOI] [PubMed] [Google Scholar]
- 34.Nemec, A., T. De Baere, I. Tjernberg, M. Vaneechoutte, T. J. van der Reijden, and L. Dijkshoorn. 2001. Acinetobacter ursingii sp. nov. and Acinetobacter schindleri sp. nov., isolated from human clinical specimens. Int. J. Syst. Evol. Microbiol. 511891-1899. [DOI] [PubMed] [Google Scholar]
- 35.Nemec, A., L. Dijkshoorn, I. Cleenwerck, T. De Baere, D. Janssens, T. J. Van Der Reijden, P. Jezek, and M. Vaneechoutte. 2003. Acinetobacter parvus sp. nov., a small-colony-forming species isolated from human clinical specimens. Int. J. Syst. Evol. Microbiol. 531563-1567. [DOI] [PubMed] [Google Scholar]
- 36.Nemec, A., L. Dijkshoorn, and P. Jezek. 2000. Recognition of two novel phenons of the genus Acinetobacter among non-glucose-acidifying isolates from human specimens. J. Clin. Microbiol. 383937-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreckenberger, P. C., M. I. Daneshvar, and D. G. Hollis. 2007. Acinetobacter, Achromobacter, Chryseobacterium, Moraxella, and other nonfermentative gram-negative rods, p. 770-802. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed., vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 38.Seifert, H., A. Strate, A. Schulze, and G. Pulverer. 1993. Vascular catheter-related bloodstream infection due to Acinetobacter johnsonii (formerly Acinetobacter calcoaceticus var. lwoffi): report of 13 cases. Clin. Infect. Dis. 17632-636. [DOI] [PubMed] [Google Scholar]
- 39.Sherertz, R. J., and M. L. Sullivan. 1985. An outbreak of infections with Acinetobacter calcoaceticus in burn patients: contamination of patients' mattresses. J. Infect. Dis. 151252-258. [DOI] [PubMed] [Google Scholar]
- 40.Siau, H., K. Y. Yuen, P. L. Ho, S. S. Wong, and P. C. Woo. 1999. Acinetobacter bacteremia in Hong Kong: prospective study and review. Clin. Infect. Dis. 2826-30. [DOI] [PubMed] [Google Scholar]
- 41.Tang, Y. W., N. M. Ellis, M. K. Hopkins, D. H. Smith, D. E. Dodge, and D. H. Persing. 1998. Comparison of phenotypic and genotypic techniques for identification of unusual aerobic pathogenic gram-negative bacilli. J. Clin. Microbiol. 363674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tega, L., K. Raieta, D. Ottaviani, G. L. Russo, G. Blanco, and A. Carraturo. 2007. Catheter-related bacteremia and multidrug-resistant Acinetobacter lwoffii. Emerg. Infect. Dis. 13355-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valero, C., J. D. Garcia Palomo, P. Matorras, C. Fernandez-Mazarrasa, C. Gonzalez Fernandez, and M. C. Farinas. 2001. Acinetobacter bacteraemia in a teaching hospital, 1989-1998. Eur. J. Intern. Med. 12425-429. [DOI] [PubMed] [Google Scholar]
- 44.Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. Grimont, O. Kandler, M. I. Krichevsky, L. H. Moore, W. E. Moore, R. G. Murray, E. Stackebrandt, M. P. Starr, and H. G. Truper. 1987. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Microbiol. 37463-464. [Google Scholar]