Abstract
Telomere dysfunction has been proposed to contribute to the pathogenesis of Werner syndrome (WS), a premature-aging disorder. The WS protein WRN binds TRF2, a telomere-specific factor that protects chromosome ends. TRF2 possesses an amino-terminal domain that plays an essential role in preventing telomere shortening, as expression of TRF2ΔB, which lacks this domain, leads to the formation of telomeric circles, telomere shortening, and cell senescence. Our data show that the TRF2ΔB-induced telomeric-loop homologous-recombination pathway requires WRN helicase. In addition, we show that WRN represses the formation of spontaneous telomeric circles, as demonstrated by the increased levels of telomeric circles observed in telomerase-positive WS fibroblasts. The mechanism of circle formation in WS cells does not involve XRCC3 function. Circle formation in WS cells is reduced by reconstitution with wild-type WRN but not mutant forms lacking either exonuclease or helicase activity, demonstrating that both enzymatic activities of WRN are required to suppress telomeric-circle formation in normal cells expressing telomerase reverse transcriptase. Thus, WRN has a key protective function at telomeres which influences telomere topology and inhibits accelerated attrition of telomeres.
Werner syndrome (WS) is an autosomal recessive disease that is characterized by accelerated aging and the premature onset of several age-associated disorders such as cardiovascular disease and cancer (11). At the cellular level, WS cells show a significant level of genome instability and hypersensitivity to genotoxic agents such as camptothecin, 4-nitroquinoline-N-oxide, and mitomycin C (12, 24, 28-31). The disease is caused by loss-of-function mutations in the gene that encodes a member of the RecQ family of helicases (RecQ3 or WS protein WRN) (35). WRN, in contrast to other RecQ helicases, possesses a unique amino-terminal domain with exonuclease activity. The presence of both exonuclease and helicase activities indicates that WRN may be involved in the processing of double-stranded DNA molecules, and identification of proteins that interact with WRN has further suggested that this protein may have a function in DNA damage repair, replication, or recombination (27).
All of the physiological substrates for WRN have yet to be identified; however, one bona fide substrate for WRN is telomeric DNA (27). Human telomeres are composed of several kilobases of the repetitive hexamer TTAGGG and contain a 3′ single-stranded DNA extension that is thought to loop back and invade the proximal complementary strand, thereby leading to the establishment of a protective structure termed the telomeric loop (t-loop) (13, 23). The formation and maintenance of the t-loop are mediated by a multiprotein complex which includes telomere-specific proteins such as telomere repeat factor 1 (TRF1) and TRF2 that bind telomeres through a myb-like DNA binding domain located at the carboxy-terminal end (1, 3, 4). These proteins are believed to protect telomeres from end-to-end fusion and intra- or intertelomeric recombination events (33, 34). Disruption of this structure activates the DNA damage response pathway, which leads to cell cycle arrest and cell senescence or apoptosis (15, 16, 33). WRN has been shown to localize at telomeres, and recent studies have indicated that WRN may function in the resolution of secondary structures within telomeres during DNA replication, thus allowing the progression of the replication fork to chromosome ends (8, 25). The presence of WRN at chromosome ends is puzzling, as the exonuclease activity of WRN could potentially destroy the structural integrity of telomeres, thus affecting cell viability.
WRN has been shown to interact with TRF2 (22, 26), a protein that plays a critical role in telomere homeostasis. TRF2 is required to maintain the topology and length of telomeres. Expression of a dominant negative mutant form of TRF2 lacking the basic amino-terminal domain and the Myb DNA binding domain (TRF2ΔBΔM) in fibroblasts causes telomere fusion and leads to apoptosis (15, 33). In contrast, expression of a separation-of-function mutant form of TRF2 lacking the amino-terminal basic domain (TRF2ΔB) induces the increased formation of extrachromosomal telomeric circles and rapid telomere deletions, which elicit a DNA damage response and cell senescence (34). The process by which TRF2ΔB causes telomere circle formation and shortening has been proposed to involve t-loop homologous recombination (HR); however, the mechanism by which the amino terminus of TRF2 represses t-loop HR is unknown.
Here we demonstrate that, in contrast to normal fibroblasts, overexpression of TRF2ΔB in telomerase-positive WS fibroblasts fails to induce telomere shortening and cell senescence. Genetic complementation studies indicate that both the exonuclease and helicase activities of WRN are necessary to reconstitute TRF2ΔB-induced telomere shortening, telomere dysfunction-induced focus (TIF) formation, and cell senescence in WS fibroblasts. Interestingly, telomerase-positive WS cells display increased extrachromosomal telomeric circles in the absence of TRF2ΔB. Formation of telomeric circles sharply decrease in WS cells complemented with wild-type WRN but not the exonuclease or helicase mutant variant of WRN, while overexpression of TRF2ΔB results in a marked increase in telomeric-circle formation when WS cells are reconstituted with functional WRN. These data demonstrate that WRN plays a critical role in TRF2ΔB-mediated telomere shortening and senescence. Coimmunoprecipitation assays demonstrate that the amino terminus of TRF2 is required for WRN binding. Consistent with this observation, chromatin immunoprecipitation (ChIP) assays indicate that overexpression of TRF2ΔB may negatively influence WRN recruitment to telomeres. These results demonstrate that WRN is an important regulator of telomere topology in normal cells expressing telomerase reverse transcriptase (TERT) and is a required partner in TRF2ΔB-mediated telomere erosion and cell senescence.
MATERIALS AND METHODS
Cell lines.
Normal (AG06814, GM00319) and WS (AG00780 [arg369Stop] and AG03141 [gln748Stop]) fibroblasts were purchased from the Coriell Repository. ALT cells (CCL75.1) were purchased from ATCC. The colon carcinoma cell line HCT116 was obtained from K. Miyagawa and M. Katsura (University of Tokyo). Fibroblasts were immortalized by expression of hTERT (TERT catalytic subunit) by transduction with the recombinant lentivirus RRLsin.hCMV-hTERT. A lentivirus for the expression of hTERT was prepared as previously described (20). Cells were cultured in Dulbecco modified essential medium (DMEM) supplemented with 10% fetal calf serum and 1% penicillin-streptomycin and maintained at 37°C in a humidified incubator at 5% CO2 and atmospheric (21%) oxygen. Experiments on the activation of p53 were also performed with cells grown at 3% oxygen.
Expression vectors and lentiviral transduction of normal and WS fibroblasts.
cDNA coding for Flag-tagged wild-type TRF2, TRF2ΔB (which bears a deletion of amino acids 1 to 45), and TRF2ΔBΔM (which contains amino acids 45 to 454) were inserted into the pRRLsin.hCMV-Puro vector (20). Recombinant lentiviruses were produced as described in reference 20. For lentiviral infection, telomerase-positive normal and WS fibroblast cultures were trypsinized, seeded onto 10-cm culture dishes, and incubated for 24 h at 37°C. The viral supernatant was then added to cultures that were approximately 60 to 70% confluent and further incubated at 37°C. After 6 h, the viral supernatant was removed and the cells were washed twice and incubated in DMEM containing 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C. Transduced cells expressing Flag-TRF2, Flag-TRF2ΔB, and Flag-TRF2ΔBΔM were selected in medium supplemented with puromycin (5 μg/ml). The expression of the epitope-tagged proteins was analyzed by Western blot analysis with anti-Flag antibodies (Sigma). Construction of the lentiviruses for the expression of Flag-tagged wild-type WRN, the helicase mutant form (WRN-E84A), the exonuclease mutant form (WRN-K577M), and the helicase-exonuclease mutant form (WRN-E84A/K577M) was carried out by cloning the respective cDNAs into pRRLsin.hCMV-Puro vectors as described previously (20). For the reconstitution studies, to allow meaningful comparisons between the sets of lines being analyzed, the experiment was carried out as follows. First, WS fibroblasts were transduced with lentiviruses encoding wild-type and catalytically inactive forms of WRN and colonies were subjected to puromycin selection. Puromycin-resistant cell lines were analyzed by Western blotting to ensure that similar levels of expression of proteins would be compared and were then subjected to a second round of viral transduction with lentiviruses for the expression of TRF2, TRF2ΔB, or control lentivirus at a multiplicity of infection of 5. Under these experimental conditions, the efficiency of transduction of these cells is consistently higher than 95%, as reflected in the similar levels of TRF2 and TRF2ΔB expression. A second strategy with cloned cell lines was also employed to confirm the results of these experiments. Here, Flag-tagged wild-type and mutant WRN cDNAs were cloned into a lentivirus vector which does not carry a selectable marker (pRRLsin.hCMV). Colonies derived from six single clones were tested for expression, and matched (cloned) cell lines with similar levels of epitope-tagged protein expression were chosen for a second transduction with a control lentivirus or a lentivirus for the expression of TRF2 or TRF2ΔB (pRRLsin.hCMV-Puro-based vectors). Transduced cells were then placed under selection with puromycin and analyzed as described in Results. Both experimental approaches yielded identical results.
Cell growth curves and senescence-associated β-galactosidase (SA-βgal) assay.
Normal and WS fibroblasts were infected with a lentivirus for the expression of Flag-TRF2 or Flag-TRF2ΔB or a control lentivirus and cultured for 3 days in DMEM containing 5 μg/ml puromycin. Cells were plated in duplicate in 15-cm tissue culture dishes, and the DMEM was changed every 3 days. On the indicated days after transduction, cells were harvested and counted for the growth curve analyses. Transduced cells grown for 8 days were stained for SA-βgal activity as described by Dimri and colleagues (10). Student's t test was used to evaluate differences in means between two groups, and P < 0.05 was considered statistically significant.
Immunoprecipitation assay and antibodies.
Nuclear extracts were prepared as described in reference 20. Nuclear extracts (4 mg) from normal and WS fibroblasts expressing Flag-TRF2 or Flag-TRF2ΔB were incubated with Flag resin at 4°C for 90 min. After extensive washes, bound proteins were eluted with BCO buffer (17), resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and transferred to nitrocellulose. Western blot analyses were performed with antibodies against WRN (polyclonal BL1309; Bethyl Inc.), Mre11 (polyclonal SC-5859; Santa Cruz Biotechnology Inc.), Nbs1 (polyclonal SC-8580; Santa Cruz Biotechnology Inc.), Rad50 (polyclonal SC-20115; Santa Cruz Biotechnology Inc.), p53 (monoclonal SC-100; Santa Cruz Biotechnology Inc.), p53-phosphoserine15 (monoclonal 9284S; Cell Signaling), p21 (polyclonal SC-397; Santa Cruz Biotechnology Inc.), 53BP1 (sc-22760; Santa Cruz Biotechnology, Inc.), TRF1 (4E4, GTX 70304; GeneTex Inc.), and XRCC3 (NB100-180; Novus Biologicals). Anti-rabbit, anti-goat, and anti-mouse immunoglobulin G horseradish peroxidase-coupled antibodies were purchased from Promega and Santa Cruz Biotechnology Inc.
Neutral-neutral two-dimensional gel electrophoresis (2DGE).
Genomic DNA was prepared as described in reference 16. 2DGE was performed as described in references 2 and 5, with the following modifications. Genomic DNA was digested with HinfI and RsaI, extracted with phenol-chloroform, and precipitated with ethanol. Ten micrograms of HinfI/RsaI-digested genomic DNA was separated on a 0.5% agarose gel in 1× Tris-borate-EDTA at 1 V/cm for 18 h at room temperature. The gel was stained in 1× Tris-borate-EDTA containing 0.3 μg/ml ethidium bromide for 30 min, and then the lanes were cut and placed at 90° to the direction of electrophoresis, and 1.0% agarose containing 0.3 μg/ml ethidium bromide was poured around the first-dimension lane. The second dimension was run at 4 V/cm for 4 h at room temperature. The DNA was transferred to Hybond membrane by Southern blotting and hybridized with a (CCCTAA)4 probe. After hybridization, excess probe was washed from the membrane and the pattern of hybridization was visualized on X-ray film by autoradiography and PhosphorImager (Molecular Dynamics) scanning of the membrane. A circularized lambda DNA marker was generated by ligating HindIII-digested lambda DNA (NEB) at 5 ng/μl overnight at 16°C. The ligated lambda DNA was extracted with phenol-chloroform and precipitated with ethanol. Five hundred nanograms per lane was used for agarose gel electrophoresis. To generate the lambda DNA probe, HindIII-digested lambda DNA was digested with BstEII and extracted with phenol-chloroform. Two micrograms of the BstEII-digested lambda DNA was radiolabeled with T4 polynucleotide kinase and [γ-32P]ATP.
Immunofluorescence microscopy.
Telomerase-positive normal diploid fibroblasts (passage 27, AG06814) and telomerase-positive WS fibroblasts (passage 23, AG00780) were infected with a vector-, TRF2ΔB-, or TRF2ΔBΔM-expressing lentivirus and processed simultaneously. Transduced cells were trypsinized and plated on Permanox-coated chamber slides (∼80% confluence). Cells were grown in medium supplemented with puromycin (3 μg/ml). Cells were fixed at various time points (24 h, 48 h, and 72 h) by incubation in 3.5% (wt/vol) paraformaldehyde in 1× phosphate-buffered saline (PBS) for 20 min at room temperature and washed three times with 1× PBS. Following fixation, cells were permeabilized immediately or fixed cells were stored at 4°C in 1× PBS until use. Cells were permeabilized with 0.5% (vol/vol) Triton X-100 in 1× PBS for 7 min at room temperature and washed five times with 1× PBS. Blocking was performed by incubating cells in 15% (vol/vol) fetal bovine serum in 1× PBS for 2 h at room temperature. Cells were incubated in primary antibodies (53BP1, rabbit polyclonal antibody) and TRF1 (mouse monoclonal antibody) at the appropriate dilutions in 1× PBS at 4°C overnight and rinsed with 1× PBS five times. Cells were then incubated in secondary antibodies (donkey anti-rabbit antibody [Alexa Fluor 488] and donkey anti-mouse antibody [Alexa Fluor 594]; Molecular Probes, Eugene, OR) in 1× PBS for 2 h at room temperature. Cells were washed five times with 1× PBS and counterstained with 4′6′-diamidino-2-phenylindole (DAPI; final concentration of 1 μg/ml) for 1 min. Following the staining with DAPI, cells were washed three times with 1× PBS. Gel/Mount, an aqueous mounting medium with antifading agents (BioMedia Corp., Foster City, CA) was used for mounting. Controls with the secondary antibody alone gave no signal. Cells were viewed at ×100 magnification with a Nikon E600 fluorescence microscope. Images were processed with MetaMorph software (version 7.0r0). For each sample, an average of 150 cells were scored to calculate the percentage of cells with 53BP1 foci. While scoring, cells were categorized by the number of 53BP1 foci within the nucleus as one to three foci, four to seven foci, or eight or more foci. Percentages of foci in each category were plotted for each sample. The percentage of colocalization between 53BP1 and TRF1 was calculated by scoring at least 10 nuclei per sample. Results obtained were derived from three independent experiments.
Telomere length analysis.
Epitope-tagged TRF2, TRF2ΔB, and TRF2ΔBΔM were expressed in normal and WS fibroblasts by lentivirus transduction. The transduced cells were maintained in 5 μg/ml puromycin-containing medium for 3 days and then collected and analyzed 8 days after transduction. Genomic DNA was isolated by standard protocols, and equal amounts of DNA were digested with HinfI and RsaI. Two micrograms of DNA was separated on 0.8% agarose gels, transferred to Hybond membrane, and hybridized with a radiolabeled (TTAGGG)3 probe. Blots were exposed on films or PhosphorImager screens, and telomeric repeat signals were quantitated with ImageQuant software (Molecular Dynamics, Inc.). The telomeric signal was normalized to the loading control for each lane, and the normalized values for each sample were compared and expressed as telomere signal units relative to the respective vector-transduced sample. Median telomere restriction fragment length was estimated by comparing the median of the distribution of telomere values within each lane with the migration of a DNA standard.
Single-strand G-rich telomere length analysis.
Genomic DNA was digested with HinfI and RsaI and incubated with buffer and either exonuclease I (NEB) or S1 nuclease (Promega) for 12 h or 30 min, respectively. Each DNA sample was then extracted with phenol-chloroform, precipitated with ethanol, and resuspended in Tris-EDTA buffer. DNA (6 μg) was separated on 0.8% agarose gel. The gel was dried at 50°C for 3 h, washed with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and probed with a (CCCTAA)4 probe.
ChIPs.
Fibroblasts and WS cells transduced with control lentivirus or a lentivirus for the expression of Flag-TRF2 and Flag-TRF2ΔB were maintained in culture for 1 week and then harvested by trypsin treatment. Cells were washed with PBS and fixed in 1% formaldehyde in PBS for 60 min at room temperature. The ChIP assay was performed as described in reference 21, with minor modifications. Briefly, 300 μl of lysates was diluted with 1.2 ml of dilution buffer. Antibodies (the amount varied per antibody, from 2 to 5 μg) were added to the reaction mixture and incubated at 4°C overnight on a nutator. After extensive washes, bound chromatin was separated from the protein G beads with 200 μl of elution buffer (1% SDS, 0.1 M Na2CO3) twice. After addition of 16 μl of 5 M NaCl, cross-links were reversed by incubation of the reaction mixture at 65°C for 4 h. Samples were supplemented with 16 μl of 1 M Tris-HCl (pH 7.0), 8 μl of 0.5 M EDTA, and 20 μg DNase-free RNase A and incubated at 37°C for 30 min. Samples were then digested with 40 μg proteinase K at 37°C for 60 min and phenol extracted. After addition of 300 μl water, the DNA was precipitated with 0.8 ml propanol by overnight incubation at −20°C. The precipitated DNA was dissolved in 30 μl of water, denatured at 95°C for 5 min, and dot blotted onto Hybond membranes (80% was loaded for the detection of telomeric sequences, and 20% was loaded for the detection of Alu sequences). Membranes were treated with 1.5 M NaCl-0.5 N NaOH for 15 min and with 1 M NaCl-0.5 M Tris-HCl (pH 7.0) for 15 min. Hybridization was performed with radiolabeled (TTAGGG)5 or Alu probes. Membranes were washed four times in 2× SSC with 0.1% SDS, and signals were visualized by exposure to film and a PhosphorImager screen. The percentage of precipitated DNA was quantitated by ImageQuant software (Molecular Dynamics). Statistical significance was determined by a two-tailed Student t test.
Silencing vectors.
Scrambled and XRCC3-specific short hairpin RNA (shRNA) expression plasmids were purchased from Origene. Retroviruses were produced by transfecting packaging cells with shRNA expression plasmids. The medium was collected 2 days after transfection. The supernatants were passed through a 0.45-μm-pore-size filter and used to infect WS cells (telomerase positive). Cells were harvested, and DNA was analyzed by 2DGE at 10 and 12 days after retroviral transduction.
RESULTS
Overexpression of TRF2ΔB fails to induce senescence in WS cells.
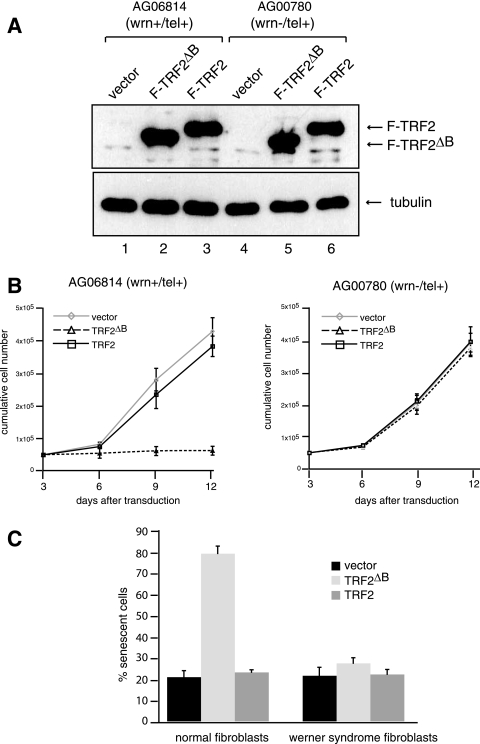
Previous studies have shown that expression of TRF2ΔB in both telomerase-positive and -negative mammalian cell lines limits cell proliferation and induces cellular senescence (33). To examine a possible role for WRN in this process, human normal and WS fibroblasts were telomerase immortalized and transduced with a lentivirus expressing Flag-TRF2 or Flag-TRF2ΔB or with a control lentivirus. The cultures were subjected to puromycin selection to eliminate uninfected cells, and expression of epitope-tagged wild-type and mutant TRF2 in the selected cells was demonstrated by immunoprecipitation and Western blot analysis with Flag antibody (Fig. 1A). The cells were subsequently cultured and monitored to analyze their growth properties. Normal fibroblasts expressing Flag-TRF2 did not show any detectable difference in growth rate or display morphological changes for 12 days after transduction compared to fibroblasts transduced with the control virus (Fig. 1B, left panel).
FIG. 1.
Overexpression of TRF2ΔB induces cell senescence of telomerase-positive normal but not WS fibroblasts. (A) Expression of Flag-TRF2 and Flag-TRF2ΔB in telomerase-positive (tel+) normal and WS fibroblasts. Telomerase-positive normal and WS fibroblasts were infected with a lentivirus for the expression of Flag-TRF2 or Flag-TRF2ΔB or a control lentivirus and cultured for 8 days. The expression of Flag-TRF2 and Flag-TRF2ΔB was analyzed by immunoblotting with an anti-Flag antibody. Antibodies against tubulin were used as a loading control. (B) Growth curves of normal and WS fibroblasts. Growth rates after 3 days of puromycin selection of telomerase-positive normal (left) and WS (right) fibroblasts infected with the indicated lentiviruses were measured by counting the cells every 3 days. Cells were seeded at a low density, and the medium was changed every 3 days. Values represent the mean ± the standard deviation of three experiments (n = 3). (C) Detection of SA-βgal activity. Normal and WS fibroblasts were cultured for 8 days after transduction, fixed, and stained. The SA-βgal-positive cells among 500 cells were counted. Values are the mean ± the standard deviation of three independent experiments (n = 3) carried out in duplicate.
In agreement with previous studies (34), normal fibroblasts expressing Flag-TRF2ΔB showed a dramatic growth defect. Notably, greater than 80% of Flag-TRF2ΔB-expressing fibroblasts showed a replicative senescence-like phenotype, exhibiting a flattened morphology, and stained positive for SA-βgal activity (Fig. 1C), while approximately 20% of the control vector- and Flag-TRF2-expressing fibroblasts were positive for SA-βgal. Importantly, when a similar analysis was carried out with WS fibroblasts, no significant difference in cell growth or the percentage of senescent cells was observed between cells transduced with the control virus or with a virus expressing either Flag-TRF2 or Flag-TRF2ΔB (Fig. 1B, right panel, and C). These results demonstrate that WS cells are refractory to the growth arrest and senescence induced by TRF2ΔB.
Lack of DNA damage response in WS cells expressing TRF2ΔB.
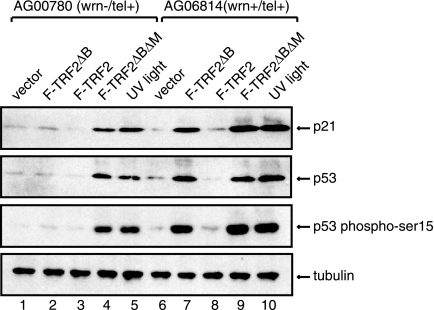
Overexpression of TRF2ΔB in normal human fibroblasts induces cell senescence through a DNA damage response pathway, which leads to the activation of the tumor suppressor protein p53 and TRF2ΔB formation of discrete foci of telomere-associated DNA damage factors, such as 53BP1, known as TIFs (32). To examine whether the failure to induce cellular senescence in WS fibroblasts overexpressing Flag-TRF2ΔB results from the lack of activation of the p53-dependent DNA damage response pathway, we examined the levels of p53 and p21 in cell lysates prepared from normal and WS cells transduced with a control lentivirus or a lentivirus which expresses Flag-TRF2 or Flag-TRF2ΔB. Western blot analysis shows that p53 and its downstream target, p21, are upregulated in normal fibroblasts expressing Flag-TRF2ΔB (Fig. 2). In contrast, WS cells expressing TRF2ΔB did not show a significant difference in the levels of p53 and p21 compared to normal fibroblasts.
FIG. 2.
Overexpression of TRF2ΔB induces a p53-dependent DNA damage response in normal but not WS fibroblasts. (A) The levels of p21, p53, and phosphorylation of p53 on serine 15 in WS (lanes 1 to 5) and normal (lanes 6 to 10) fibroblasts 8 days after transduction with the indicated lentiviruses or after UV irradiation (1 mJ/cm2) were assessed by Western blotting of cell lysates. Tubulin served as a loading control.
Significantly, the phosphorylation of p53 on serine 15, one of the characteristics of DNA damage-induced p53 activation, increased by more than 10-fold in normal fibroblasts expressing Flag-TRF2ΔB (Fig. 2, lane 7) but failed to show any increase in WS cells expressing Flag-TRF2ΔB (lane 2). However, the p53 response pathway was activated both in normal cells and in WS cells expressing TRF2ΔBΔM, a TRF2 mutant form missing both the basic amino-terminal region and the carboxyl-terminal DNA binding domain, which has been previously shown to induce p53 activation in response to telomere damage (33), and by UV irradiation (Fig. 2, lanes 4, 5, 9, and 10). These findings indicate that failure of TRF2ΔB to induce cellular senescence in telomerase-positive WS fibroblasts is not due to an intrinsic defect in the activation of the p53-dependent DNA damage response pathway.
To further compare and contrast the activation of the DNA damage pathway in response to TRF2ΔB between normal and WS fibroblasts, we monitored the formation of 53BP1 foci and their colocalization with TRF1 in cells transduced with the control vector- or TRF2ΔB-expressing lentivirus. As previously demonstrated by others (34), TRF2ΔB induced a significant number of 53BP1 foci in normal cells, and the majority of these foci colocalized with the telomeric factor TRF1 (Fig. 3; see Fig. S1 in the supplemental material), indicative of telomere dysfunction and induction of a DNA damage response. In contrast, WS fibroblasts did not show significant numbers of 53BP1 foci upon expression of TRF2ΔB, further demonstrating that, in contrast to normal fibroblasts, these cells are not responsive to TRF2ΔB. Importantly, the lack of 53BP1 foci was also not due to an intrinsic defect in the response to telomere-induced DNA damage, since expression of the dominant negative mutant form TRF2ΔBΔM in WS fibroblasts was sufficient to cause the accumulation of 53BP1 foci at telomeres (Fig. 3; see Fig. S1 in the supplemental material). Thus, taken together, these findings indicate that expression of TRF2ΔB does not elicit a DNA damage response in telomerase-positive WS fibroblasts and that this inability does not arise as a consequence of defective DNA damage-induced signaling.
FIG. 3.
Overexpression of TRF2ΔB induces TIFs in normal but not WS fibroblasts. 53BP1 foci and colocalization with the telomeric protein TRF1 in normal and WS fibroblasts 1 day after transduction of lentiviruses expressing the indicated proteins were detected with antibodies against 53BP1 (green) and TRF1 (red). Nuclei were visualized by DAPI staining. Arrows point at regions of 53BP1 and TRF1 colocalization. For the quantitation of 53BP1 foci and 53BP1 and TRF1 colocalization, see Fig. S1 in the supplemental material.
TRF2ΔB does not induce telomere shortening in WS cells.
To test whether lack of TIF formation was due to a failure to induce telomere shortening in WS fibroblasts upon overexpression of TRF2ΔB, we measured the average length of chromosome terminal restriction fragments in genomic DNA isolated from two telomerase-positive normal and WS fibroblasts 8 days after viral transduction by Southern blot analysis with a telomere-specific probe. As previously reported (34), normal cells transduced with a lentivirus expressing Flag-TRF2 did not show any significant change in mean telomere length compared to cells transduced with a control virus (Fig. 4, compare lanes 1 and 3), while expression of Flag-TRF2ΔB resulted in sudden telomere deletions (Fig. 4, lanes 2 and 9). Similar results were obtained with the colon carcinoma cell line HCT116 (see Fig. S2 in the supplemental material). In striking contrast to the results observed in normal cells, expression of Flag-TRF2ΔB did not affect mean telomere length in WS cells (Fig. 4, lanes 5, 6, 10, and 11). These findings demonstrate that TRF2ΔB-induced telomere shortening requires WRN.
FIG. 4.
TRF2ΔB induces telomere shortening in normal but not WS fibroblasts. Normal and WS fibroblasts expressing Flag-TRF2ΔB (lanes 2, 5, 9, and 11) and Flag-TRF2 (lanes 3 and 6), along with normal and WS fibroblasts transduced with control viruses (lanes 1, 4, 8, and 10), were harvested 8 days after lentivirus transduction. Equal amounts of genomic DNA digested with HinfI and RsaI were separated by electrophoresis on a 0.8% agarose gel and analyzed by Southern blotting with a radiolabeled (TTAGGG)3 probe. The molecular mass standards shown on right side were generated by digestion of lambda DNA with restriction endonuclease HindIII. Southern blot analyses were performed on three independent samples of normal and WS cells transduced with lentiviruses expressing the indicated proteins. The telomeric signal was normalized to the H1.1 gene probe for all lanes (see Fig. S2 in the supplemental material), and the normalized values ± standard deviations, expressed as the telomeric signal relative to the vector control for each cell line, from three independent experiments (n = 3) are shown below the blots.
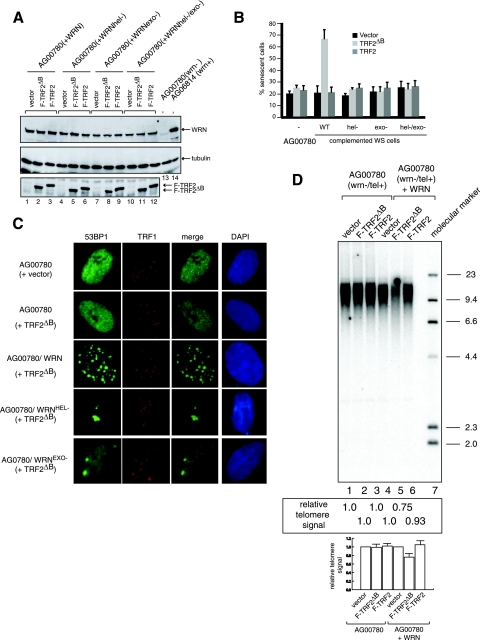
Expression of wild-type WRN but not the catalytically inactive forms of WRN reconstitutes TRF2ΔB-induced senescence, TIFs, and telomere shortening in WS cells.
As WS fibroblasts display genome instability, the failure to induce telomere deletion upon TRF2ΔB expression in these cells may be due to a secondary mutation. Thus, to substantiate the hypothesis that WRN is required for TRF2ΔB-induced telomere shortening and senescence, we genetically complemented WRN activities in telomerase-positive WS fibroblasts by expression of wild-type WRN. In parallel, to directly test the potential involvement of WRN helicase and exonuclease in this process, we expressed WRN protein variants bearing point mutations that inactivate helicase (K577M), exonuclease (E84A), or both activities (K577M/E84A) in telomerase-positive WS fibroblasts (18, 20). Western blot analysis with WRN antibody demonstrated that the wild-type and mutant forms of WRN were expressed at approximately the same level (Fig. 5A, top panel). The genetically complemented cell lines were then retransduced with a control lentivirus or a lentivirus expressing Flag-TRF2 or Flag-TRF2ΔB (Fig. 5A, bottom panel). Eight days after transduction, 70% of the wild-type WRN-complemented WS fibroblasts expressing Flag-TRF2ΔB stained positive for SA-βgal, demonstrating that TRF2ΔB-induced cellular senescence was reconstituted by the expression of a functional WRN protein (Fig. 5B). In contrast, overexpression of Flag-TRF2ΔB failed to increase the percentage of SA-βgal-positive cells in the lines expressing WRN variants lacking helicase, exonuclease, or both activities (Fig. 5B).
FIG. 5.
TRF2ΔB-induced cell senescence, TIFs, and telomere shortening are reconstituted in WS fibroblasts genetically complemented with wild-type WRN but not enzymatically deficient WRN variants. (A) Expression of wild-type and mutant forms of WRN in WS cells. WS cells were infected with lentiviruses for the expression wild-type, helicase-deficient, exonuclease-deficient, and helicase- and exonuclease-deficient forms of WRN and cultured for 2 weeks. The parental and genetically complemented cells lines were then transduced with a control virus or a virus for the expression of Flag-TRF2ΔB or Flag-TRF2. Analysis of protein expression was performed by preparation of nuclear extracts, followed by Western blotting with anti-WRN (top panel), antitubulin (middle panel), and anti-Flag (bottom panel) antibodies. (B) Detection of SA-βgal activity. Telomerase-positive WS fibroblasts transduced with the indicated lentiviruses were cultured for 8 days, fixed, and stained for SA-βgal. Five hundred cells of each line were analyzed in duplicate plates. Each bar represents the mean ± the standard deviation of three independent experiments (n = 3) carried out in duplicate. WT, wild type. (C) Detection of 53BP1 and TRF1 in WS fibroblasts consecutively transduced with lentiviruses expressing the indicated proteins with antibodies against 53BP1 (green) and TRF1 (red) 1 day after the second transduction. For the quantitation of 53BP1 foci and 53BP1 and TRF1 colocalization, see Fig. S1 in the supplemental material. (D) The parental and genetically complemented cell lines were transduced with control lentivirus (lanes 1 and 4) and lentiviruses for the expression of Flag-TRF2ΔB (lanes 2 and 5) or Flag-TRF2 (lanes 3 and 6). Cells were harvested 8 days after lentivirus transduction, and genomic DNA was isolated and digested with HinfI and RsaI. Equal amounts (2 μg) of digested genomic DNA were separated by electrophoresis on a 0.8% agarose gel, followed by Southern blot analysis with a radiolabeled (TTAGGG)3 probe. Southern blot analyses were performed on three independent samples of WS cells transduced with lentiviruses expressing the indicated proteins. The telomeric signal was normalized to the H1.1 gene probe for all lanes (see Fig. S2 in the supplemental material), and the normalized values ± standard deviations, expressed as the telomeric signal relative to the vector control for each cell line, from three independent experiments (n = 3) are shown below the blots.
To determine if cell senescence was triggered by telomere damage, the genetically complemented cell lines were analyzed by immunofluorescence with antibodies against 53BP1 and TRF1. Significantly, overexpression of Flag-TRF2ΔB in WS cells complemented with WRN resulted in the accumulation of a large number of 53BP1 foci (65% of the cells with more than eight foci per cell), ∼55% of which colocalized with TRF1 (Fig. 5C). Interestingly, although overexpression of Flag-TRF2ΔB in WS cells complemented with WRN lacking either exonuclease or helicase activity did not increase cell senescence, we observed a few very large 53BP1 foci in these cells (Fig. 5C). The potential role of these foci in telomere damage response is unclear, as their large size prevented unambiguous colocalization with TRF1. This suggests that a partially functional form of WRN can cooperate with TRF2ΔB to elicit a DNA damage response, although whether this signal is induced in response to loss of telomere integrity remains to be determined.
To test if TIF formation was coincident with telomere shortening, we isolated DNA from both the original and genetically complemented WS cell lines and analyzed telomere length by Southern blotting. The analysis revealed that overexpression of Flag-TRF2ΔB in WRN-complemented WS cells results in cells having shorter telomeres than the original WS cell line (Fig. 5D, lanes 2 and 5), while telomere length in WS cells expressing WRN variants lacking exonuclease, helicase, or both activities were not altered by overexpression of Flag-TRF2ΔB (see Fig. S3 in the supplemental material). Collectively, these results demonstrate that TRF2ΔB-induced telomere shortening, TIFs, and senescence require a fully functional WRN protein.
Telomeric circles are present at elevated levels in telomerase-positive WS fibroblasts in the absence of TRF2ΔB.
Telomere shortening induced by expression of TRF2ΔB in normal fibroblasts is accompanied by the formation of telomeric circles. Thus, formation of telomeric circles has been hypothesized to be the seminal event that leads to telomere shortening and cell senescence, which is triggered by TRF2ΔB. To test if the formation of telomeric circles is aberrant in WS cells which express TRF2ΔB, we studied the topology of telomeric DNA from telomerase-positive normal and WS fibroblasts transduced with a vector control- or TRF2ΔB-expressing lentivirus by 2DGE, which is an assay that allows the separation of DNA by size and shape and has been utilized to visualize extrachromosomal DNA circles (5, 6). Analysis of telomeric DNA isolated from normal fibroblasts expressing TRF2ΔB by 2DGE with a probe complementary to the G-rich telomeric strand showed the presence of a characteristic arc, whose migration is consistent with that of relaxed, double-stranded circles (Fig. 6A) (34). Telomeric circles are present at low levels in DNA isolated from normal fibroblasts but are a prominent feature of cells that utilize the alternative lengthening of telomere pathway to maintain telomere homeostasis (Fig. 6B) (34).
FIG. 6.
Telomeric circles are present in telomerase-positive WS fibroblasts in the absence of TRF2ΔB. DNA isolated from normal (A) and WS (C) fibroblasts transduced with lentiviruses expressing the indicated proteins was digested with HinfI and RsaI, separated by size and shape, blotted, and probed with a telomeric (CCCTAA) repeat probe. Arrows indicate arcs of telomeric DNA circles. Circularized λ × HindIII DNA fragments were used as molecular size markers (the 23- and 4.4-kb fragments have one cos end and do not circularize). Samples shown in panels A and C were run and processed in parallel under the same hybridization and washing conditions. (B) DNA isolated from ALT fibroblasts was separated by 2DGE and probed with a telomeric (CCCTAA)4 probe. The data shown are representative of at least three independent experiments. The approximate level of telomeric circles (expressed as a percentage of the total telomeric DNA) present in each sample was estimated (see Fig. S4 in the supplemental material) and is shown in the upper right corner of each panel. The samples shown in each panel were blotted, hybridized, washed, and analyzed simultaneously.
Importantly, we observed a prominent arc of double-stranded telomeric DNA circles in telomerase-positive WS cells, which both comigrated with circularized λ × HindIII DNA fragments and was resistant to exonuclease I and S1 nuclease treatment (see Fig. S5 in the supplemental material), thus demonstrating that this arc represents double-stranded telomeric DNA circles. Significantly, the levels of telomeric circles did not increase upon the expression of TRF2ΔB (Fig. 6C). As the current model proposes that telomeric-circle formation depends on recombination factors such as XRCC3, a member of the RecA/Rad51-related protein family, we determined the contribution of this protein to WS fibroblasts circle formation by using shRNA-based vectors. Downregulation of XRCC3 protein expression by more than 90% did not result in a significant reduction of telomeric circles in WS fibroblasts (see Fig. S6 in the supplemental material), suggesting that telomeric circles in WS cells are formed through an XRCC3-independent pathway.
To test whether reconstitution of WRN activities prevents accelerated telomeric-circle formation in WS fibroblasts, DNA from the genetically complemented cells was examined by 2DGE. Consistent with a protective function for WRN at telomeres, expression of catalytically active WRN resulted in a significant reduction of telomeric circles (Fig. 7A) while expression of a WRN variant lacking helicase activity did not appreciably affect the formation of telomeric circles (Fig. 7B). Expression of a WRN variant lacking exonuclease activity resulted in a small decrease in circle formation, possibly indicating a minor role for the exonuclease activity in this process. Furthermore, analysis of DNA isolated from WRN-complemented WS fibroblasts transduced with a lentivirus expressing Flag-TRF2ΔB by 2DGE demonstrated a restitution of the dramatic increase in telomeric-circle formation compared to WRN-complemented fibroblasts transduced with control lentivirus (Fig. 7C). These data demonstrate that WRN protects telomere integrity and inhibits telomere attrition by decreasing the rate of telomeric-circle formation.
FIG. 7.
Expression of wild-type WRN but not enzymatically deficient WRN variants in WS fibroblasts leads to a reduction in telomeric circles, which are reformed upon the overexpression of TRF2ΔB. (A) DNA isolated from WS fibroblasts transduced with a vector control lentivirus or a lentivirus expressing WRN was digested with HinfI and RsaI, separated by 2DGE, blotted, and probed with a telomeric (CCCTAA)4 probe. (B) DNA isolated from WS fibroblasts transduced with a lentivirus expressing a WRN variant lacking either exonuclease or helicase activity was digested with HinfI and RsaI, separated by 2DGE, blotted, and probed with a telomeric (CCCTAA)4 probe. Arrows show arcs of telomeric DNA circles. (C) DNA isolated from WRN-complemented WS fibroblasts was transduced with a control lentivirus or a lentivirus expressing TRF2ΔB, digested with HinfI and RsaI, separated by 2DGE, and probed with a telomeric (CCCTAA)4 probe. The samples shown in each panel were run and processed in parallel under the same hybridization and washing conditions. The approximate level of telomeric circles present in each sample (expressed as a percentage of the total telomeric DNA) was estimated (see Fig. S4 in the supplemental material) and is shown in the upper right corner of each panel. The samples shown in each panel were blotted, hybridized, washed, and analyzed simultaneously.
The amino-terminal basic domain of TRF2 is required for WRN binding.
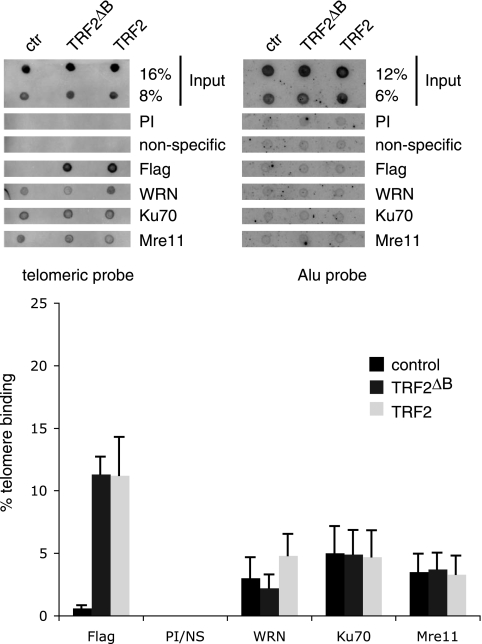
As absence of WRN results in circle formation, we next tested if the amino-terminal domain of TRF2 is required for WRN binding and assessed if this domain of TRF2 influences WRN recruitment to telomeres. Nuclear extracts were prepared from control fibroblasts and fibroblasts expressing Flag-TRF2 or Flag-TRF2ΔB and incubated with Flag antibodies immobilized on resin. Western blot analysis indicated that MRE11, RAD50, and NBS1, which are subunits of a protein complex that binds to TRF2 (37), coimmunoprecipitated with both Flag-TRF2 and Flag-TRF2ΔB (Fig. 8, lanes 5 and 6). In contrast, WRN was detected in the Flag immunoprecipitation reaction of extracts prepared from fibroblasts expressing Flag-TRF2 (Fig. 8, lane 6) but was absent from the immunoprecipitation of extracts prepared from fibroblasts expressing Flag-TRF2ΔB (Fig. 8, lane 5). These results demonstrate that the amino terminus of TRF2 is required for WRN binding and suggests that this domain of TRF2 may play an important role in establishing an appropriate network of protein-protein interactions important for the regulation of telomere length homeostasis.
FIG. 8.
The amino-terminal domain of TRF2 is required for binding to WRN. (A) Nuclear extracts were prepared from normal fibroblasts transduced with a lentivirus for the expression of Flag-TRF2 or Flag-TRF2ΔB and incubated with anti-Flag resin. After extensive washes, the immunoprecipitated proteins were eluted from the beads by treatment with high-salt buffer, separated by 8% SDS-polyacrylamide gel electrophoresis, and analyzed by Western blotting with antibodies against WRN, Nbs1, Mre11, and Rad50 (lanes 4 to 6). Lanes 1 to 3 represent 10% of the nuclear extracts used in the immunoprecipitations (IP) of extracts prepared from control cells (lane 1), cells expressing Flag-TRF2ΔB (lane 2), and cells expressing Flag-TRF2 (lane 3) fibroblasts. Arrows show the migration of relevant proteins.
Telomeric localization of WRN is influenced by TRF2.
As the amino-terminal domain of TRF2 is required for WRN binding, we predicted that WRN recruitment to telomeres would be reduced in normal fibroblasts expressing TRF2ΔB compared to TRF2. To test this idea, we performed ChIP assays on normal fibroblasts transduced with a control lentivirus or a lentivirus expressing Flag-TRF2 or Flag-TRF2ΔB. Seven days after transduction, cells were treated with formaldehyde and cross-linked DNA-protein complexes were immunoprecipitated with control antibodies (preimmune serum and anti-GST) and antibodies against a set of proteins found at telomeres, including WRN, MRE11, and Ku70. After reversal of the cross-linking, the presence of telomeric sequences in the immunoprecipitation products was assessed by dot blot analysis and hybridization with a telomeric probe. To control for the coprecipitation of nonspecific DNA, a probe for the Alu repeat sequence was used as a negative control. The results of this experiment demonstrate that the relative levels of MRE11 and Ku at telomeric sequences are not significantly affected by overexpression of Flag-TRF2 or Flag-TRF2ΔB in normal fibroblasts (Fig. 9). In contrast, a significant reduction in the amount of WRN present at telomeres was observed in cells expressing Flag-TRF2ΔB compared to Flag-TRF2-expressing cells (n = 4, P = 0.01 for a two-way comparison between control and Flag-TRF2ΔB- and Flag-TRF2-expressing cells). TRF2-overexpressing cells also show a significant increase in WRN bound to telomeres compared to control cells (n = 4, P = 0.02 for a two-way comparison between control and Flag-TRF2-expressing cells). The difference between control and Flag-TRF2ΔB-expressing cells demonstrated a trend toward reduced recruitment of WRN but did not reach statistical significance (n = 4, P = 0.1 for a two-way comparison between control and Flag-TRF2ΔB-expressing cells). None of the proteins tested were found to associate with the Alu repeat sequence (Fig. 9).
FIG. 9.
Telomeric association of WRN in fibroblasts is influenced by overexpression of Flag-TRF2 or Flag-TRF2ΔB. Extracts prepared from formaldehyde-treated normal and WS cells expressing Flag-TRF2, Flag-TRF2ΔB, and the vector control were subjected to ChIPs with the indicated antibodies. Coprecipitated DNA were released from the immune complex and analyzed by dot blot hybridization with a radiolabeled (TTAGGG)5 probe. The top panel shows representative dot blots of CHIP assays of cell lines transduced with control (ctr) and TRF2ΔB- and TRF2-specific lentiviruses. The indicated amounts of total input DNA and ChIP DNA were hybridized to telomere-specific and Alu-specific probes. The bottom panel shows results that were visualized by PhosphorImager and quantitated with ImageQuant software (Molecular Dynamics) and are expressed in relative binding units. PI and NS represent control immunoprecipitation reactions with preimmune serum (PI) and a nonspecific (NS) antibody against glutathione S-transferase. Data represent the mean and standard deviation from four independent ChIP assays (n = 4).
DISCUSSION
Telomere integrity is preserved through the association of telomeric repeat sequences with nuclear factors, which are essential to maintain chromosome stability and cell viability. Electron microscopy studies indicate that telomeres form a structure termed the t-loop, which caps the ends of chromosomes. Two telomere-specific factors, TRF1 and TRF2, bind to the TTAGGG repeats and serve as a docking site for the assembly of a multiprotein complex, termed shelterin, which has been proposed to play a key role in the formation and stabilization of the DNA end-capping structure (9). WRN has been shown to localize at telomeres and prevent telomere dysfunction (8, 25); however, the precise role of WRN at telomere ends has yet to be fully defined.
In this study, we demonstrate that WRN is required for TRF2ΔB-mediated telomere shortening and cell senescence. Specifically, we show that overexpression of TRF2ΔB in telomerase-positive WS fibroblasts fails to induce TIFs and cell senescence. The lack of a DNA damage response in WS cells expressing TRF2ΔB is unlikely to be the result of an intrinsic defect in the signaling pathway that is activated by telomere attrition, since WS cells are capable of forming TIFs in response to the overexpression of dominant negative TRF2ΔBΔM. Rather, our data show that a p53-mediated DNA damage response is absent from telomerase-positive WS cells because TRF2ΔB overexpression fails to induce telomere shortening.
Complementation studies demonstrate that both the exonuclease and helicase activities of WRN are required to reconstitute the full spectrum of phenotypes induced by TRF2ΔB in WS cells, including telomere shortening, formation of TIFs, and cell senescence. Interestingly, WS fibroblasts expressing TRF2ΔB, when reconstituted with a exonuclease or helicase mutant form of WRN, did not demonstrate telomere shortening or cell senescence but did display a few 53BP1 foci, whose colocalization with TRF1 was difficult to assess due to their large size. These results suggest that each enzymatic activity of WRN may partially cooperate with TRF2ΔB to induce a DNA damage response, although the full significance of this finding remains to be further investigated.
To understand why telomere shortening was not occurring in WS cells expressing TRF2ΔB, we tested if telomere circles, which are a key intermediate in TRF2ΔB-mediated telomere shortening, were present in these cells. Unexpectedly, we observed elevated levels of telomeric circles in telomerase-positive WS cells and that their level was not enhanced by expression of TRF2ΔB. The process of rapid telomere deletions by TRF2ΔB has been shown to be dependent on recombination factors such as XRCC3 (34). To begin addressing the mechanism of circle formation in WS cells, we examined whether HR factor XRCC3 contributes to this process. We used RNA interference to silence XRCC3 and found that a more than 90% reduction in protein levels did not appreciably influence the formation of telomeric circles in WS cells (see Fig. S6 in the supplemental material). Although we cannot exclude the possibility that residual activity or complementary activities contribute to circle formation in these cells, these data suggest that circles in WS cells are generated through an alternative, XRCC3-independent mechanism. Significantly, recent studies have demonstrated that inactivation of Ku, a heterodimeric factor that functionally interacts with WRN in both plants and humans (7, 17, 19), induces the formation of circles in Arabidopsis (36). This study also showed that circle formation in Ku-deficient plants is not suppressed by the inactivation of several genes involved in the HR pathway (36). These findings suggest a potential functional relationship between the telomeric circles in WRN- and Ku-deficient cells. The role of Ku in suppressing telomeric circles in human cells and the precise mechanism of circle formation in WS cells will be investigated in future studies.
We further observed that reconstitution with fully functional WRN was sufficient to inhibit telomeric-circle formation in WS cells. In contrast, expression of a helicase mutant form of WRN was unable to suppress circle formation, while expression of an exonuclease mutant protein resulted in a minor reduction of telomeric circles. These results demonstrate that WRN plays a key role in maintaining telomere topology and length and that both helicase and exonuclease activities are involved in telomere regulation, although the helicase activity seems to play a prominent role. It is possible that, in the absence of WRN exonuclease activity, unchecked telomerase activity may generate telomeres with long 3′ overhangs which could more efficiently lead to the formation of intermediates required for the formation of circles. Consistent with this idea, we have observed that telomerase-positive WS cells display a significant increase in G-strand overhang signal compared to telomerase-negative WS cells (data not shown). Moreover, lack of WRN helicase activity may facilitate the formation of secondary structures such as G quadruplexes within the t-loops, which may favor the stabilization of key intermediates that are processed to generate telomeric circles. Our results therefore emphasize the important regulatory role that WRN plays in telomere homeostasis and suggest that the absence of WRN exonuclease and helicase activities may serve to stabilize key intermediates in the enzymatic reaction that lead to telomeric-circle formation in vivo. We did not detect telomeric circles in telomerase-negative WS cells. While it is possible that telomerase activity is required for the formation of telomeric circles in WS cells, we cannot rule out the possibility that circles are difficult to detect or resolve by 2DGE since these cells have relatively short telomeres.
We next demonstrated that the amino terminus of TRF2 is required for WRN binding. These data, when taken together with our finding that WRN is required to inhibit telomere circle formation, predict that the ability of TRF2ΔB to elicit telomeric-circle formation and telomere shortening may reflect, at least in part, the reduced recruitment of WRN to telomeres when TRF2ΔB is expressed. Consistent with this idea, we demonstrate a significant reduction of WRN recruitment to telomeres in normal fibroblasts overexpressing TRF2ΔB compared to cells overexpressing TRF2. A trend toward reduced WRN recruitment to telomeres in normal cells was also observed compared to normal cells overexpressing TRF2ΔB. As even one or a few critically shortened telomeres are sufficient to trigger telomere dysfunction (14), it is possible that relatively small changes in global WRN recruitment to telomeres, as assessed by the ChIP assay, may lead to shortening of telomeres and cell senescence. It is therefore likely that the analysis of a very large number of samples would be required to increase the significance of this latter comparison. Taken together, our data are consistent with the hypothesis that one possible mechanism that may contribute to telomere circle formation and shortening induced by TRF2ΔB is the reduced recruitment of WRN to telomere ends. Clearly, reduced recruitment of WRN to telomeres by itself is not sufficient to elicit telomere shortening and the molecular mechanisms leading to shortening by TRF2ΔB remain to be further elucidated. It is interesting that telomerase-positive WS cells, in spite of increased telomeric-circle formation, have long telomeres. We therefore speculate that WRN may have a role in regulating TERT activity by an as-yet-unrecognized mechanism, and further studies are under way to better understand this possible functional relationship between WRN and telomerase.
An important concept that emerges from this study is that WRN has a key protective function at telomeres which influences telomere topology and inhibits accelerated attrition of telomeres by decreasing the rate of telomeric-circle formation. In these tasks, WRN may be aided by functional interactions with one or more members of the shelterin complex, including TRF2. A better understanding of how this set of protein-protein interactions influences telomere topology and homeostasis will provide important insights into the molecular mechanisms that maintain genome stability.
Supplementary Material
Acknowledgments
We thank members of the Comai and Reddy labs for valuable suggestions during the course of this study.
This investigation was supported by a National Institutes of Health grant (R01 AG023873) awarded to L.C. and was conducted in a facility constructed with support from Research Facilities Improvement Program grant no. C06 RR014514-01, C06 RR10600-01, and C06 CA62528 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Published ahead of print on 22 January 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bilaud, T., C. Brun, K. Ancelin, C. E. Koering, T. Laroche, and E. Gilson. 1997. Telomeric localization of TRF2, a novel human telobox protein. Nat. Genet. 17236-239. [DOI] [PubMed] [Google Scholar]
- 2.Brewer, B. J., and W. L. Fangman. 1987. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell 51463-471. [DOI] [PubMed] [Google Scholar]
- 3.Broccoli, D., A. Smogorzewska, L. Chong, and T. de Lange. 1997. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 17231-235. [DOI] [PubMed] [Google Scholar]
- 4.Chong, L., B. van Steensel, D. Broccoli, H. Erdjument-Bromage, J. Hanish, P. Tempst, and T. de Lange. 1995. A human telomeric protein. Science 2701663-1667. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, S., and S. Lavi. 1996. Induction of circles of heterogeneous sizes in carcinogen-treated cells: two-dimensional gel analysis of circular DNA molecules. Mol. Cell. Biol. 162002-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, S., A. Regev, and S. Lavi. 1997. Small polydispersed circular DNA (spcDNA) in human cells: association with genomic instability. Oncogene 14977-985. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, M. P., A. Machwe, D. K. Orren, R. M. Brosh, D. Ramsden, and V. A. Bohr. 2000. Ku complex interacts with and stimulates the Werner protein. Genes Dev. 14907-912. [PMC free article] [PubMed] [Google Scholar]
- 8.Crabbe, L., R. E. Verdun, C. I. Haggblom, and J. Karlseder. 2004. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science 3061951-1953. [DOI] [PubMed] [Google Scholar]
- 9.de Lange, T. 2005. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 192100-2110. [DOI] [PubMed] [Google Scholar]
- 10.Dimri, G. P., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, Monica Peacocke, and Judith Campisi. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 929363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein, C. J., G. M. Martin, A. L. Schultz, and A. G. Motulsky. 1966. Werner's syndrome: a review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine (Baltimore) 45177-221. [DOI] [PubMed] [Google Scholar]
- 12.Gebhart, E., R. Bauer, U. Raub, M. Schinzel, K. W. Ruprecht, and J. B. Jonas. 1988. Spontaneous and induced chromosomal instability in Werner syndrome. Hum. Genet. 80135-139. [DOI] [PubMed] [Google Scholar]
- 13.Griffith, J. D., L. Comeau, S. Rosenfield, R. M. Stansel, A. Bianchi, H. Moss, and T. de Lange. 1999. Mammalian telomeres end in a large duplex loop. Cell 97503-514. [DOI] [PubMed] [Google Scholar]
- 14.Hemann, M. T., M. A. Strong, L. Y. Hao, and C. W. Greider. 2001. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 10767-77. [DOI] [PubMed] [Google Scholar]
- 15.Karlseder, J., D. Broccoli, Y. Dai, S. Hardy, and T. de Lange. 1999. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 2831321-1325. [DOI] [PubMed] [Google Scholar]
- 16.Karlseder, J., A. Smogorzewska, and T. de Lange. 2002. Senescence induced by altered telomere state, not telomere loss. Science 2952446-2449. [DOI] [PubMed] [Google Scholar]
- 17.Li, B., and L. Comai. 2000. Functional interaction between Ku and the Werner syndrome protein in DNA end processing. J. Biol. Chem. 27528349-28352. [DOI] [PubMed] [Google Scholar]
- 18.Li, B., and L. Comai. 2001. Requirements for the nucleolytic processing of DNA ends by the Werner syndrome protein-Ku70/80 complex. J. Biol. Chem. 2769896-9902. [DOI] [PubMed] [Google Scholar]
- 19.Li, B., N. Conway, S. Navarro, L. Comai, and L. Comai. 2005. A conserved and species-specific functional interaction between the Werner syndrome-like exonuclease atWEX and the Ku heterodimer in Arabidopsis. Nucleic Acids Res. 336861-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, B., S. Navarro, N. Kasahara, and L. Comai. 2004. Identification and biochemical characterization of a Werner's syndrome protein complex with Ku70/80 and poly(ADP-ribose) polymerase-1. J. Biol. Chem. 27913659-13667. [DOI] [PubMed] [Google Scholar]
- 21.Loayza, D., and T. De Lange. 2003. POT1 as a terminal transducer of TRF1 telomere length control. Nature 4231013-1018. [DOI] [PubMed] [Google Scholar]
- 22.Machwe, A., L. Xiao, and D. K. Orren. 2004. TRF2 recruits the Werner syndrome (WRN) exonuclease for processing of telomeric DNA. Oncogene 23149-156. [DOI] [PubMed] [Google Scholar]
- 23.Nikitina, T., and C. L. Woodcock. 2004. Closed chromatin loops at the ends of chromosomes. J. Cell Biol. 166161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogburn, C. E., J. Oshima, M. Poot, R. Chen, K. E. Hunt, K. A. Gollahon, P. S. Rabinovitch, and G. M. Martin. 1997. An apoptosis-inducing genotoxin differentiates heterozygotic carriers for Werner helicase mutations from wild-type and homozygous mutants. Hum. Genet. 101121-125. [DOI] [PubMed] [Google Scholar]
- 25.Opresko, P. L., M. Otterlei, J. Graakjaer, P. Bruheim, L. Dawut, S. Kolvraa, A. May, M. M. Seidman, and V. A. Bohr. 2004. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol. Cell 14763-774. [DOI] [PubMed] [Google Scholar]
- 26.Opresko, P. L., C. von Kobbe, J. P. Laine, J. Harrigan, I. D. Hickson, and V. A. Bohr. 2002. Telomere-binding protein TRF2 binds to and stimulates the Werner and Bloom syndrome helicases. J. Biol. Chem. 27741110-41119. [DOI] [PubMed] [Google Scholar]
- 27.Ozgenc, A., and L. A. Loeb. 2005. Current advances in unraveling the function of the Werner syndrome protein. Mutat. Res. 577237-251. [DOI] [PubMed] [Google Scholar]
- 28.Pichierri, P., A. Franchitto, P. Mosesso, and F. Palitti. 2000. Werner's syndrome cell lines are hypersensitive to camptothecin-induced chromosomal damage. Mutat. Res. 45645-57. [DOI] [PubMed] [Google Scholar]
- 29.Poot, M., K. A. Gollahon, M. J. Emond, J. R. Silber, and P. S. Rabinovitch. 2002. Werner syndrome diploid fibroblasts are sensitive to 4-nitroquinoline-N-oxide and 8-methoxypsoralen: implications for the disease phenotype. FASEB J. 16757-758. [DOI] [PubMed] [Google Scholar]
- 30.Poot, M., K. A. Gollahon, and P. S. Rabinovitch. 1999. Werner syndrome lymphoblastoid cells are sensitive to camptothecin-induced apoptosis in S-phase. Hum. Genet. 10410-14. [DOI] [PubMed] [Google Scholar]
- 31.Poot, M., J. S. Yom, S. H. Whang, J. T. Kato, K. A. Gollahon, and P. S. Rabinovitch. 2001. Werner syndrome cells are sensitive to DNA cross-linking drugs. FASEB J. 151224-1226. [DOI] [PubMed] [Google Scholar]
- 32.Takai, H., A. Smogorzewska, and T. de Lange. 2003. DNA damage foci at dysfunctional telomeres. Curr. Biol. 131549-1556. [DOI] [PubMed] [Google Scholar]
- 33.van Steensel, B., A. Smogorzewska, and T. de Lange. 1998. TRF2 protects human telomeres from end-to-end fusions. Cell 92401-413. [DOI] [PubMed] [Google Scholar]
- 34.Wang, R. C., A. Smogorzewska, and T. de Lange. 2004. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119355-368. [DOI] [PubMed] [Google Scholar]
- 35.Yu, C. E., J. Oshima, Y. H. Fu, E. M. Wijsman, F. Hisama, R. Alisch, S. Matthews, J. Nakura, T. Miki, S. Ouais, G. M. Martin, J. Mulligan, and G. D. Schellenberg. 1996. Positional cloning of the Werner's syndrome gene. Science 272258-262. [DOI] [PubMed] [Google Scholar]
- 36.Zellinger, B., S. Akimcheva, J. Puizina, M. Schirato, and K. Riha. 2007. Ku suppresses formation of telomeric circles and alternative telomere lengthening in Arabidopsis. Mol. Cell 27163-169. [DOI] [PubMed] [Google Scholar]
- 37.Zhu, X.-D., B. Kuster, M. Mann, J. H. J. Petrini, and T. de Lange. 2000. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat. Genet. 25347-352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.