Abstract
During cellular stress, translation persists or increases for a number of stress-responsive proteins, including cellular inhibitor of apoptosis 2 (cIAP2). The cIAP2 transcript includes a very long (2.78-kb) 5′ untranslated region (UTR) with an unusually high number of upstream AUGs (uAUGs), i.e., 64, and a stable predicted secondary structure (ΔG ≅ −620 kcal/mol) that should completely block conventional scanning-dependent translation initiation. This region did not facilitate internal ribosome entry in vitro or when RNA reporter transcripts were transfected into cells. However, several structural features within the cIAP2 5′ UTR were observed to be nearly identical to those required for ribosome shunting in cauliflower mosaic virus RNA and are well conserved in cIAP2 orthologs. Selective mutation revealed that the cIAP2 mRNA mediates translation exclusively via ribosome shunting that bypasses 62 uAUGs. In addition, shunting efficiency was altered by stress and was greatly facilitated by a conserved RNA folding domain (1,470 to 1,877 nucleotides upstream) in a region not scanned by shunting ribosomes. This arrangement suggests that regulation of cIAP2 shunting may involve recruitment of RNA binding proteins to modulate the efficiency of translation initiation.
The antiapoptotic cellular inhibitor of apoptosis protein 2 (cIAP2) (also called BIRC3) is a direct inhibitor of active caspases and also possesses E3 ubiquitin ligase activity, which mediates the polyubiquitylation and degradation of the key cell death proteins Smac/Diablo and RIP (20, 32). Largely localized to the mitochondria, cIAP2 also directs the monoubiquitylation of caspases 3 and 7 as well as DEDD, which in the latter case relocalizes the proapoptotic DEDD from the nucleus to the cytoplasm (21, 27). cIAP2 is an oncogene, and its overexpression not only has been associated with neoplastic progression in a variety of cancers (7, 11, 22, 26) but also may be an early event in the progression of pancreatic cancer (12). Thus, its expression is tightly regulated both at the transcriptional and translational levels. Indeed, in certain instances, cIAP2 mRNA is present in the absence of cIAP2 protein (1, 30).
The majority of cellular mRNAs are translated through m7G cap-dependent recruitment of the 40S ribosomal subunit to the 5′ end of a mRNA, followed by linear scanning downstream to the first AUG codon in proper initiation context, where 60S subunit joining and translation begin (24). However, three major alternative mechanisms are used by ribosomes to facilitate or regulate access to AUGs found further from the 5′ cap. First, the presence of a few (one to four) short upstream open reading frames (uORFs) in the 5′ untranslated region (UTR) serve to downregulate translation of the mRNA but allow relative activation when eIF2-Met-tRNAi-GTP ternary complexes are limited due to increases in phosphorylated eIF2α. For ATF4 and GCN4 mRNAs, this is really a regulated reinitiation mechanism that allows more ribosomes to bypass uORFs to reach the true start codon, thereby activating its translation during amino acid starvation or endoplasmic reticulum (ER) stress (14, 25). Second, ribosomes may bypass the m7G cap entirely and be recruited to the mRNA by an internal ribosome entry site (IRES) present in the mRNA (23). A third alternative initiation mechanism, ribosome shunting, has been characterized primarily in viruses, including Sendai virus, cauliflower mosaic virus (CaMV), rice tungro bacilliform virus, and adenovirus (Ad) (6, 13, 35, 45). The mechanism involves the translocation of a ribosome from an upstream shunt donor region to a downstream shunt acceptor on an mRNA, often bypassing multiple uORFs and regions of stable secondary structure.
During cellular stress, the cap-dependent scanning mechanism for translation initiation is rapidly inhibited by multiple mechanisms (8). Under these circumstances, alternative initiation mechanisms facilitated by uORFs and IRES elements activate translation of a variety of proteins that are critical to the cellular stress response (23). Similarly, shunting within the 216-nucleotide (nt) 5′ UTR of human HSP70 mRNA stimulates the translation of this protein during heat shock, although the predominant mechanism for HSP70 translation in unstressed cells remains cap-dependent scanning (46).
Unconventional mechanisms of translational initiation have most often been observed in transcripts containing a long, highly structured 5′ UTR that inhibits ribosome scanning. The mRNA transcript coding for cIAP2 fits this description, as it contains a very large (2.83-kb) 5′ UTR that contains 64 uAUGs. It seemed highly unlikely to us that the predominant mechanism of translation initiation on this long leader was conventional ribosome scanning. Thus, we examined this 5′ UTR for its ability to mediate translation initiation via an alternative mechanism.
The 5′ UTR of cIAP2 displayed no IRES activity, but analysis of predicted secondary structures revealed a remarkable similarity to structural elements required for ribosome shunting in CaMV (18, 33, 34, 47). Selective mutagenesis of these regions confirmed that cIAP2 translation is indeed mediated by a ribosome shunt that is highly similar to the CaMV shunt in both structure and function. We propose a model in which the 43S ribosome binds the cIAP2 mRNA 5′ cap, scans only a short distance before translating a short uORF, and then is shunted across the base of a highly stable RNA stem. This allows the ribosome to bypass 62 of the 64 uAUGs present in the cIAP2 5′ UTR and places the shunted 40S ribosome just upstream from the cIAP2 start codon. This shunt mechanism tightly regulates cIAP2 translation, and we show that cIAP2 translation efficiency is rapidly altered by cell stress in 293T cells, being either activated or repressed by different stressors. To our knowledge, this is the first description of a mammalian mRNA that translates exclusively by shunting.
MATERIALS AND METHODS
Cell culture.
Cells were maintained in Dulbecco's modified Eagle medium (Invitrogen) and 10% fetal bovine serum (HyClone Labs, Logan, UT). Growth was at 37°C, 5.0% CO2, and 95% relative humidity, and cells were split 1:5 every 2 to 3 days at <85% confluence.
Construct assembly.
All constructs were derived from a pRL-HCV-FL backbone, as described previously (38). This construct contains a 35-bp poly(A) region with a 3′ AgeI site downstream of FLuc. The cIAP2 5′ UTR was amplified via reverse transcription-PCR (RT-PCR) from total RNA of HeLa S3 cells and inserted between the RLuc and FLuc cistrons to form pRL-cIAP2-FL. This sequence corresponds to the 5′ UTR of the cIAP2 transcript described in GenBank record NM_001165, except for one less thymidine in a poly(T) region spanning nt 142 to 150. The original cIAP2 start codon was retained and inserted in frame with FLuc, creating a FLuc ORF with six extra N-terminal codons. Monocistronic p-cIAP2-FL was constructed by inverse PCR to eliminate RLuc. Construction of php-cIAP2-FL included a 143-bp region that forms a stable RNA hairpin (ΔG = −60 kcal/mol) inserted at a unique NheI site located immediately upstream from the cIAP2 5′ UTR. Other clones were obtained using site-directed mutagenesis to alter specific bases or inverted PCR to delete specific regions. All clones were verified by both restriction digestion and sequencing.
In vitro transcription and translation.
In vivo stability of in vitro-transcribed mRNA was facilitated by a 35-nt poly(A) region present in all constructs that included a unique AgeI site at its 3′ end. Each construct was linearized by AgeI, and in vitro transcription was performed using T7 RNA polymerase (New England Biolabs) in a typical reaction mixture containing either an ARCA cap analog [3′-O-Me-7-methyl-G(5′)ppp(5′)guanosine] or an ApppG cap analog [adenosyl(5′)ppp(5′)guanosine) (New England Biolabs) at a ratio of 4:1 versus GTP. Translations were performed with fresh, nonnucleased HeLa translation extracts. Reporter luciferase activity was quantified with a luciferase assay kit (Promega).
Transient transfection of mRNA.
To avoid splicing artifacts in vivo, we transcribed m7G-capped and polyadenylated reporter mRNAs in vitro, transfected them into cells, and measured FLuc production after 7 h. We have shown that these reporter mRNAs are stable for at least 7 h following transfection (38). In monocistronic experiments, cotransfection of an RLuc reporter mRNA containing a short (40-nt) 5′ UTR (pRL) controlled for transfection efficiency and also provided an indicator of conventional scanning-mediated translation. Cells were plated at 1.25 × 105/well in 12-well plates and attached overnight. Transfections combined 500 ng/well mRNA with 3 μl DIMRE C reagent (Invitrogen) and 50 ng/well pT7-RLuc RNA and included 2% fetal bovine serum. Luciferase activity was measured as described above. Treatment with various stress-inducing agents was maintained during mRNA transfection.
RNA analysis.
RNA secondary structure predictions were computed using the Web-based Mfold algorithm (v. 3.1) at http://frontend.bioinfo.rpi.edu/applications/mfold/. Capped and polyadenylated RNAs were radiolabeled with [32P]ATP (∼2 μCi/100 μl) during in vitro runoff transcription with T7 RNA polymerase and were used to assess concentration, integrity, and stability of transcripts following transfection into cells. Stability of radiolabeled RNAs in transfected 293T cells was determined by harvesting cells at specific times posttransfection and extracting RNA immediately into TRIzol reagent (Invitrogen). RNA samples were analyzed by separation on glyoxal-dimethyl sulfoxide agarose gels and transferred to Hybond-N+ nylon membranes before exposure to X-ray film.
Northern analysis to assess endogenous expression of cIAP2 transcript was performed by isolating poly(A) mRNA on oligo(dT) cellulose and then running 2 μg RNA/well on a 1% NaHPO4-buffered gel denatured by the established glyoxal-dimethyl sulfoxide method. RNA was then transferred to Hybond N+ nylon (GE Healthcare) and exposed to film. Following transfer, cIAP2 mRNA was detected with an α-32P-labeled RNA probe complementary to the last 500 bases of the cIAP2 coding region. The probe was hybridized 2 h at 70°C in Rapid Hyb buffer (GE Healthcare), followed by autoradiography.
RT-PCR was used to assess endogenous cIAP2 transcripts from whole-cell RNA isolated using Trizol (Invitrogen) according to the manufacturer's protocol. Primers representing nt 1 to 22 (sense) and nt 2761 to 2783 (antisense) were incorporated into RT-PCR protocols with avian myeloblastosis virus reverse transcriptase (Promega) which was extended at 42°C for dual cycles. PCR amplification was performed with Accuprime Pfx polymerase (Invitrogen) for 25 cycles. RNase H scission of in vitro-transcribed cIAP2-FL RNA was performed by annealing an antisense primer to nt 1386 to 1412 of the cIAP2 5′ UTR for 30 min at 30°C and then incubating with RNase H (0.5 U) (Invitrogen) for 30 min at 30°C.
Immunoblotting for cIAP2.
Cells were lysed in radioimmunoprecipitation assay buffer, and 30 μg of total protein resolved via 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to polyvinylidene difluoride, blocked 1 h in Tris-buffered saline with Tween plus 3% milk, probed with 1:500 anti-cIAP2 polyclonal antibody (Ab) (clone H-85; Santa Cruz Biotechnologies) followed by a horseradish peroxidase-conjugated anti-rabbit immunoglobulin F(ab′)2 fragment (catalog no. 515-036-072; Jackson Immunoresearch Labs), and detected on film using a luminescent substrate. Normalization for protein loading was performed using a monoclonal Ab specific to α-tubulin (catalog no. T6199; Sigma).
Detecting homology between cIAP2 orthologs.
BLAST searches were performed using the ENSEMBL genome browser (Wellcome Trust/Sanger Institute). Where mRNA transcript data remain incomplete and the sequence of the full 5′ UTR is currently unknown (i.e., dog, opossum), the homologies shown were derived from genomic sequences immediately upstream from the coding region of each cIAP2 ortholog.
RESULTS
The cIAP2 gene produces a transcript of approximately 5.5 kb that is known to be expressed in nearly 20 different normal human tissues (3, 10, 30, 40). This transcript is also the sole species expressed in both large-cell lymphoma and mesothelioma tumors (15, 22). A second, smaller cIAP2 transcript of 2.7 kb (containing a 223-nt 5′ UTR) is reportedly expressed in normal testis tissue (10). However, this observation was not replicated by a different laboratory using testis-derived mRNA derived from the same commercial source (36).
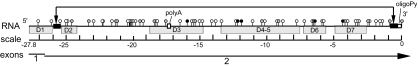
The 5.5-kb cIAP2 transcript contains an very large 5′ UTR of 2.78 kb, including a very high number of upstream AUGs (64 uAUGs) ahead of the true cIAP2 start codon (Fig. 1). Six of these AUGs are within perfect initiation context, while 13 other AUGs are in good context. We found that this 5′ UTR also is predicted by Mfold analysis to contain a thermodynamically stable stem structure that can loop out 90% of the 5′ UTR, including 62 of the 64 uAUGs (Fig. 1). We sought to determine how such a lengthy and complex leader could effectively mediate translation.
FIG. 1.
Linear diagram of cIAP2 5′ UTR features, showing location of 64 upstream AUG codons (circles). uAUGs contained within perfect or good Kozak consensus sequences are depicted as black or gray, respectively. Also depicted are an internal oligopyrimidine (oligoPy) tract and poly(A) sequence (white boxes), seven large stem-loop folding domains (D1 to D7, predicted by Mfold analysis), and two inverted repeat sequences (black boxes) that are predicted to form a highly stable RNA stem structure and bring the two ends of the 5′ UTR into close proximity. A scale with hatch marks at 10-nt intervals is shown below the RNA.
The cIAP2 5′ UTR mediates translation in a cap-dependent manner and not via an IRES.
We examined cIAP2 translation by placing the 5′ UTR of the cIAP2 transcript immediately upstream from a firefly luciferase (FLuc) reporter gene (p-cIAP2-FL) (Fig. 2A). A dicistronic construct was also created by placing a Renilla luciferase (RLuc) reporter gene upstream from the cIAP2 5′ UTR (pRL-cIAP2-FL). Reporter constructs were transcribed in vitro to produce m7G-capped and poly(A)-tailed transcripts, and equimolar amounts were translated in nonnucleased HeLa cell translation extracts for 30 min. Despite its immense length and complexity, the 5′ UTR of cIAP2 initiated translation at approximately 25% the rate of a short (40-nt) unstructured 5′ UTR in a monocistronic context (p-FL), both in vitro (Fig. 2B, left) and when transfected into 293T cells for 7 h (Fig. 2B, right). However, insertion of a stable RNA hairpin structure (ΔG = −60 kcal/mol) at the 5′ end of the cIAP2 5′ UTR to prevent cap-dependent ribosome scanning (php-cIAP2-FL) inhibited FLuc production by 98% (Fig. 2C, lane 2). Likewise, replacing the m7G cap with an ApppG cap analog that cannot bind eIF4E largely inhibited translation of the wild-type 5′ UTR in both the absence (lane 3) and presence (lane 4) of an upstream stable RNA hairpin. In agreement with the in vitro results, transfection of these same reporter transcripts into 293T cells likewise demonstrated a strong translation cap dependency (Fig. 2D).
FIG. 2.
The cIAP2 5′ UTR does not mediate translation by internal ribosome entry. (A) m7G-capped and polyadenylated mRNA reporter transcripts were utilized to assess the relative cap dependence of translation mediated by the cIAP2 5′ UTR. (B) Equimolar amounts of p-FL and p-cIAP2-FL reporter mRNAs were either translated in nonnucleased HeLa cell extracts for 30 min (in vitro) or transfected into 293T cells for 7 h (in vivo), followed by determination of Luc activity. (C and D) cIAP2 reporter mRNA transcripts that included either an unconventional ApppG cap or a stable hairpin structure added to the 5′ end were translated in HeLa cell extracts (C) or transfected into 293T cells for 7 h (D). (E) Dicistronic cIAP2 mRNA reporter transcripts were transfected into 293T cells that were either untreated or treated for 6 h with 1 μM thapsigargin (note different scale), followed by cell lysis and Luc activity measurement. The FL/RL ratio is displayed as a measure of IRES activity. The total RLuc activity for pRL-HCV-FL was 3.7 × 105 relative light units in untreated cells and 4.3 × 104 relative light units in thapsigargin-treated cells. All error bars represent standard deviations of the mean.
We also transfected dicistronic RNAs containing the cIAP2 5′ UTR into 293T cells to test for potential IRES activity (graphed as the ratio of IRES-mediated FL initiation versus cap-dependent RL initiation) (Fig. 2E). In comparison to a dicistronic reporter RNA containing the HCV IRES (or a negative control containing an inverted HCV IRES), potential IRES-mediated translation was not detected (Fig. 2E, left). Likewise, when 293T cells were treated with 1 μM thapsigargin for 6 h to induce ER stress (beginning 1 h posttransfection), no stress-induced cIAP2 IRES activity was detected (Fig. 2E, right).
Having ruled out the presence of an IRES, we sought an alternative explanation for how this complex 5′ UTR could be efficiently translated in a cap-dependent manner. When Zuker's Mfold algorithm (47) was used to determine thermodynamically stable RNA secondary structure, several structural features were detected within the cIAP2 5′ UTR that are remarkably similar to features required for ribosome shunting in the plant pararetroviruses (19). The best-characterized pararetroviral shunt is located in the 35S RNA of CaMV, which requires a short uORF in an average-to-good initiation context immediately preceding a highly stable stem structure (Fig. 3A). Not only were these same structural features found within the cIAP2 5′ UTR, a context nearly identical to the CaMV shunt, but they were also extremely well conserved in the transcripts for many putative cIAP2 orthologs (Fig. 3B). A search of the Ensembl database revealed a high degree of sequence homology in the putative cIAP2 5′ UTRs from species such as dog (Canis familiaris), opossum (Monodelphis domestica), and chicken (Gallus gallus), among others (Fig. 3B). Although the presence of an extended cIAP2 5′ UTR has yet to be confirmed in many species due to a lack of expressed sequence tag data, the widespread conservation of the short uORF and stable stem structure known to be required for shunting in CaMV strongly suggested that shunting may be a relatively ubiquitous mechanism for regulating cIAP2 expression in higher vertebrates.
FIG. 3.
Structural features within the cIAP2 5′ UTR are highly conserved and closely resemble those of the CaMV shunt. (A) Diagram of structural elements previously deemed critical for shunting within the CaMV 35S RNA, including a short uORF in good context immediately preceding a stable RNA stem. (B) Diagram of similar well-conserved structural elements found in the 5′ UTRs of cIAP2 orthologs derived from human (H. sapiens), dog (C. familiaris), opossum (M. domestica), and domestic chicken (G. gallus). Homology within putative cIAP2 orthologs was detected as described in Materials and Methods.
The cIAP2 5′ UTR mediates translation via ribosome shunting.
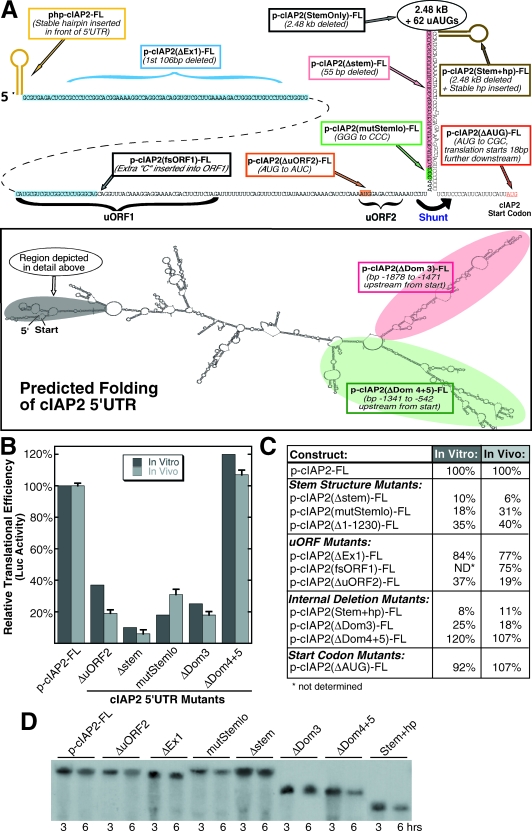
We tested the cIAP2 5′ UTR for its ability to support ribosome shunting by selectively mutating the structural features known to be essential for efficient shunting in CaMV (Fig. 4A). These cIAP2 5′ UTR mutant reporters were in vitro transcribed and then translated in vitro using nonnucleased HeLa cell extracts (Fig. 4B). We also assessed the activity of these cIAP2 5′ UTR mutants in vivo by transfecting equimolar amounts of m7G-capped and polyadenylated RNA directly into cells for 6 h, and then measuring Luc activity as an indicator of translational efficiency (Fig. 4B and C). The relative stability of cIAP2 reporter RNAs in cells was monitored by transfecting radiolabeled RNA, which was extracted at time points and analyzed on denaturing glyoxal gels (Fig. 4D). All cIAP2 RNAs used in this study displayed relatively equivalent decay rates in vivo.
FIG. 4.
Selective mutation reveals that the cIAP2 5′ UTR translates by ribosome shunting. (A) The boxed diagram shows the Mfold-predicted structure of the cIAP2 5′ UTR. The region shaded in gray is expanded in the close-up diagram at the top. Translational regulation within the cIAP2 5′ UTR was examined by making selective mutations to several key structural features. Colored or shaded regions indicate large domains or features removed or altered by mutagenesis and are identified by boxed legends. All mutant 5′ UTRs were positioned upstream from an FLuc reporter and transcribed in vitro (as described in Materials and Methods). Equimolar amounts of cIAP2 mutant reporter mRNAs were either translated in nonnucleased HeLa cell lysates for 30 min (in vitro) or transiently transfected into 293T cells for 7 h (in vivo). (B) The relative translational efficiency (i.e., Luc accumulation) of several key mutants is depicted. Error bars represent standard deviations of the mean. (C) Summary of the relative translational efficiency of all cIAP2 5′ UTR mutants. For each mutant, in vitro results are averages of duplicate reactions, while in vivo results are averages of three separate experiments. p-cIAP2(1-1230)-FL contains a deletion of the first 1,230 nt of the cIAP2 5′ UTR. (D) Autoradiograph of a nylon membrane showing stability of [α-32P]ATP-labeled capped and polyadenylated transcripts in transfected cells at the indicated times postinfection.
A region of double-stranded RNA that forms a stable stem is an essential structural element for all known ribosome shunts and seems to facilitate ribosome transfer from the shunt donor region to the shunt acceptor. We selectively deleted 55 nt (bp −2521 to −2576) from the cIAP2 5′ UTR in a region that comprises the front half of the conserved stable stem region depicted in Fig. 3B [Fig. 4A, p-cIAP2(Δstem)-FL]. While this deletion encompassed less than 2% of the total length of the 5′ UTR, translation was inhibited by over 90% (Fig. 4B and C). To confirm that this inhibition was due to a loss of stem stability, a more conservative mutation was made by changing three adjacent guanosines to cytidines (bp −2577 to −2580) within the lower portion of the stable stem [Fig. 4A, p-cIAP2(mutStemlo)-FL]. Even this seemingly minor change inhibited translation by approximately 75% (Fig. 4B and C), demonstrating that stable base-pairing within this region is essential for efficient cIAP2 translation.
Ribosome shunting in CaMV requires the translation of a short uORF located immediately upstream from the stable stem (Fig. 3A) (19). To examine the role of the analogous uORF located within the cIAP2 5′ UTR (nt −2605 to −2602), the start codon of uORF2 was mutated from AUG to AUC [Fig. 4A, p-cIAP2(ΔuORF2)-FL]. This one nucleotide change inhibited translational efficiency by 63% in vitro and 81% in vivo (Fig. 4B and C), demonstrating that similar to CaMV, a small uORF located immediately upstream from a stable stem plays a critical role in facilitating cIAP2 translation.
Intriguingly, deletion of the first 1,230 nt of the 5′ UTR [Fig. 4C, p-cIAP2(Δ1-1230)-FL] reduced the total length of the 5′ UTR by 44% yet inhibited translation by a more modest 60 to 65%. We had hypothesized that this deletion should inhibit nearly all translation by removing the entire shunt donor region, including uORF2 and the front half of the stable stem. Nevertheless, it seems possible that a less-efficient alternate shunting mechanism may have been utilized by this mutant, since 27 uAUGs would remain, as well as other stable stem structures that presumably inhibit scanning. Indeed, Hemmings-Miezczak and Hohn demonstrated that these two features are all that is required to permit a low-level of constitutive ribosome shunting (16). To further explore scanning versus shunting translation mechanisms, cIAP2-FL RNA was annealed with a DNA primer specific for nt 1386 to 1412 of the 5′ UTR, near putative RNA folding domains 3 and 4 (Fig. 4A), and then treated with RNase H to degrade the short (26-nt) RNA region complementary to the primer, producing a noncontiguous RNA reporter. Translation of capped undigested and digested cIAP2-FL RNAs in vitro showed RNase H treatment did not destroy translation, as would be expected if ribosome scanning were required on a contiguous RNA through the entire 2,783 nt; rather, translation levels were maintained at control levels (data not shown).
The uORF that facilitates cIAP2 translation is the second uORF in the 5′ UTR. Thus, the scanning 43S ribosome would presumably need to bypass the first uORF (located at nt −2702 to −2646) by leaky scanning in order to reach the shunt donor site. We investigated whether uORF1 affects cIAP2 translational efficiency by adding a single base to create a frameshift mutation that increased the length of uORF1 from 19 to 37 codons [Fig. 4A, p-cIAP2(fsORF1)-FL]. Translation initiated at uORF1 in this mutant would bypass both uORF2 and the putative shunt donor site, terminating 17 bases downstream from the end of uORF2. When tested, this frameshift mutation inhibited translation in vivo by only 25%, indicating that either a majority of scanning ribosomes bypassed uORF1 to initiate translation downstream at uORF2 or translation of an elongated uORF1 also allowed shunting at a point downstream from uORF2 (Fig. 4C). Given the poor initiation context of uORF1, and the fact that shorter uORFs appear more conducive to shunting (17), it seems likely that most scanning ribosomes bypass uORF1.
The cIAP2 5′ UTR is comprised of two exons: the first is 109 nt in length and contains the start of uORF1, while the second extends for 3,507 bases and includes the putative shunt donor and acceptor regions, as well as the first 834 nt of the cIAP2 coding region (Fig. 1). Despite its small size, genetic homology searching suggests that exon 1 may be conserved among mammalian orthologs of cIAP2 (data not shown). To assess the effect of this region on translational efficiency, we selectively deleted exon 1 [Fig. 4A, p-cIAP2(ΔEx1)-FL] from the cIAP2 5′ UTR. While an increase in translational efficiency was anticipated due to the simultaneous deletion of uORF1, translation was actually inhibited by ∼20% (Fig. 4C). Thus, despite harboring the inhibitory uORF1, exon 1 appears to slightly facilitate cIAP2 translation by an undefined mechanism.
The conserved stable stem and short uORF2 together comprise only a small portion of the cIAP2 5′ UTR. An additional ∼2.48 kb (and 62 uAUGs) are present that are looped out above the conserved stable stem and presumably are bypassed by the shunting ribosome. To assess the potential role of this sizable region in regulating cIAP2 translation, we first deleted it in its entirety and added an additional stable hairpin (ΔG = −60 kcal/mol) that blocks scanning ribosomes (Fig. 2C and D) atop the cIAP2 stable stem. This deletion construct RNA [Fig. 4A, p-cIAP2(Stem+hp)-FL] displayed dramatically inhibited translation (by ∼90%) (Fig. 4C). These results imply that the stable stem alone was not sufficient to enable efficient cIAP2 shunting, and that the additional 2.48 kb of sequence present above the stem serves a purpose other than simply preventing ribosome scanning.
We next looked for conserved regions present in the 5′ UTRs of orthologous cIAP2 transcripts. The highest homology fell within an area we defined as putative RNA folding domain 3 (Dom 3). In fact, the canine cIAP2 ortholog contained two highly homologous regions within Dom 3 of 208 nt (80.7% identity to human cIAP2) and 120 nt (73.3% identity to human cIAP2) (data not shown). Consequently, we selectively deleted Dom 3 (bp −1878 to −1471) [Fig. 4A, bottom, p-cIAP2(ΔDom 3)-FL] and observed a profound inhibition of FLuc translation of approximately 80% (Fig. 4B and C). In direct contrast, the combined deletion of two nonconserved putative RNA folding domains we termed “Domains 4+5”, (bp −1341 to −542) [Fig. 4A, bottom, p-cIAP2(ΔDom 4+5)-FL] did not affect constitutive translational efficiency (Fig. 4B and C).
Lastly, we examined whether the location of the cIAP2 start codon was critical for maintaining cIAP2 translational efficiency. We reasoned that if a shunted ribosome were to land upstream of the start codon and then encounter it via conventional scanning, moving the start codon further downstream would not affect translational efficiency. Yet, if the start codon placement were critical for shunting, moving the start codon might inhibit cIAP2 translation. Our reporter constructs contained the cIAP2 start codon fused in frame six codons upstream from the FLuc start codon. Thus, deletion of the cIAP2 start codon would cause a shunted ribosome to initiate translation 18 nt further downstream. The resulting mutant [Fig. 4A, pcIAP2(ΔAUG)-FL] showed no significant difference in translational efficiency versus the wild-type leader (Fig. 4C), suggesting that after landing, the shunted ribosome can scan to the next codon in good context to initiate translation.
Translation mediated by the cIAP2 5′ UTR is modulated by cellular stress.
Since the IRES-mediated translation of several antiapoptotic proteins is modulated by stress, we sought to determine whether the efficiency of cIAP2 shunting was also stress responsive. We avoided transfecting DNA reporter constructs, since during the course of our studies, we observed that drug-induced stress caused an unexpected splicing of our DNA reporters (data not shown). Instead, equimolar amounts of capped and polyadenylated cIAP2 reporter RNAs were transfected into 293T cells or MCF-7 cells. Then, 1 h posttransfection, cells were treated with various stress-inducing substances for 6 h before being harvested and assayed for relative Luc reporter activity. An RLuc reporter mRNA containing a short (40-nt) 5′ UTR (Fig. 5A, p-RL) was cotransfected to monitor conventional scanning-mediated translation initiation. In both cell lines, translation mediated by the cIAP2 5′ UTR (Fig. 5A, p-cIAP2-FL) increased in response to low-level sodium arsenite (NaArs) treatment. Six hours of treatment with levels of NaArs too low to induce stress granules (5 to 20 μM) increased cIAP2 5′ UTR-mediated FLuc production nearly threefold in 293T cells (Fig. 5B), while a longer (16-h) treatment with 1 to 8 μM NaArs stimulated cIAP2 5′ UTR-mediated translation nearly fivefold in MCF-7 cells (Fig. 5C). Although short-term treatment with NaArs often stimulated scanning-mediated cellular translation slightly, this stimulation never exceeded about 1.5-fold in either cell line (Fig. 5B and C). Etoposide treatment also stimulated cIAP2 5′ UTR-mediated translation, increasing FLuc accumulation in 293T cells nearly twofold after 6 h treatment with 25 to 100 μM (Fig. 5B and C). Once again, this increase occurred despite little change in conventional scanning-mediated cellular translation during treatment. MCF-7 cells proved more sensitive to etoposide treatment, and control FLuc translation declined with increased doses. Etoposide also stimulated cIAP 5′ UTR-mediated translation compared to the control RNA, despite an overall decrease in translation at high doses.
FIG. 5.
Translational efficiency of the cIAP2 5′ UTR is regulated by stress. (A) Cartoon of reporter constructs that were transcribed in vitro (as described in Materials and Methods). (B and C) Equimolar amounts of m7G-capped and polyadenylated reporter transcripts were transfected into 293T (B) or MCF-7 (C) cells, followed by measurement of FLuc activity after 7 h. Cotransfection of an RLuc-encoding transcript with a 40-nt unstructured 5′ UTR measured scanning-mediated translation initiation. For each graph, cIAP2 5′ UTR-dependent translation (FLuc, squares) is shown versus scanning-mediated translation (RLuc, circles) measured in the same cells. 293T cells were treated with drug 1 h posttransfection for 6 h, while caspase 3-deficient MCF-7 cells were treated at 16 h, followed by reporter mRNA transfection for 7 h in the absence of drug. (D) Autoradiograph of a nylon membrane showing stability of [α-32P]ATP-labeled capped and polyadenylated transcripts in transfected 293 cells at 6 h posttransfection. Thapsi, thapsigargin; Etopo, etoposide.
However, not all stress-inducing agents stimulated cIAP2 5′ UTR-mediated translation, as treatment with 40 to 80 μM hydrogen peroxide (H2O2) for 16 h caused a significant decrease in 5′ UTR-mediated translation in both MCF-7 and 293T cells versus scanning-mediated cellular translation (Fig. 5B and C). Likewise, thapsigargin treatment significantly decreased cIAP2 5′ UTR-mediated translation relative to scanning-dependent translation in both cell types (Fig. 5B and C). Analysis of transfected cIAP2 reporter RNA in 293T cells treated with stress agents revealed no significant differences in RNA stability (Fig. 5D).
Deletion of conserved internal domains inhibits constitutive, but not stress-modulated cIAP2 shunting.
To determine how stress altered shunting efficiency within the cIAP2 5′ UTR, we specifically deleted either the internal RNA folding domain Dom 3 or Dom 4+5. We reasoned that the highly conserved Dom 3 might attract RNA-binding proteins that could modulate the efficiency of shunting-mediated translation initiation during stress. As seen previously, etoposide treatment did not affect overall cellular translation rates by 6 h, as indicated by the cotransfected RLuc reporter. Meanwhile, etoposide induced translation mediated by the wild-type cIAP2 5′ UTR (p-cIAP2-FL) nearly 2.5-fold (Fig. 6). Notably, while deletion of conserved Dom 3 [p-cIAP2(ΔDom3)-FL] inhibited constitutive shunting by 80% (as previously observed), this did not prevent etoposide treatment from eliciting a similar induction in translation (Fig. 6). Etoposide treatment caused a similar absolute increase in ΔDom 3-mediated translation compared to the wild-type 5′ UTR; however, the increase in translation efficiency of the ΔDom 3 mutant was much higher due to its significantly lower baseline translation rate. In stark contrast, deletion of the nonconserved Dom 4+5 [p-cIAP2(ΔDom 4+5)-FL] had no significant effect on either constitutive shunting or etoposide-mediated inducibility (Fig. 6). Thus, Dom 3 appears to play a significant role in facilitating constitutive shunting, although neither Dom 3 nor Dom 4+5 appears to mediate the stimulation of shunting efficiency caused by etoposide treatment.
FIG. 6.
Deletion of conserved internal domains does not alter stress-modulated cIAP2 shunting. The translational efficiency of the cIAP2 5′ UTR deletion mutants p-cIAP2(ΔDom3)-FL and p-cIAP2(ΔDom 4+5)-FL was compared to that of the wild-type cIAP2 5′ UTR both before and after treatment with 200 μM etoposide (Etopo) in 293T cells. An RLuc reporter with a 40-nt 5′ UTR was cotransfected to monitor conventional, cap-dependent, scanning-mediated translation, and the ratio of cIAP2 5′ UTR-mediated shunting (FLuc) to scanning-mediated translation (RLuc) 4 to 7 h posttransfection (i.e., 3 to 6 h posttreatment) is shown. Error bars represent standard deviations of the mean.
Stress-induced changes in endogenous cIAP2 protein are consistent with cIAP2 translation being controlled by a stress-modulated ribosome shunt.
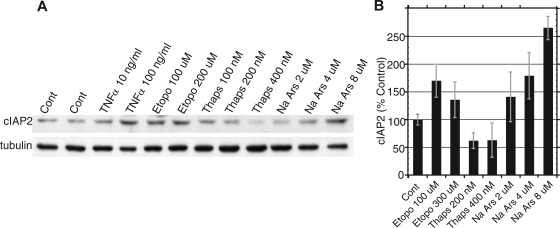
To confirm the physiological significance of our reporter data, we first attempted to assess the translation rate of endogenous cIAP2 in drug-treated cells via pulse-labeling, followed by immunoprecipitation with cIAP2 Ab. Unfortunately, none of the available cIAP2 antibodies we tested were able to immunoprecipitate endogenous cIAP2. Thus, we examined endogenous cIAP2 protein levels in response to the same drug treatments that altered translation efficiency in our cIAP2 reporter constructs. 293T cells were treated with increasing concentrations of etoposide, thapsigargin, or NaArs for 18 h, followed by cell lysis and immunoblotting for cIAP2 (Fig. 7). These treatments caused changes in cIAP2 levels that correlated well with the changes in translational efficiency we had previously observed in vivo following drug treatment (Fig. 2). Tumor necrosis factor alpha was used as a positive control to stimulate cIAP2 levels through known transcriptional activation (Fig. 7). Etoposide treatment likewise increased cIAP2 levels, and thapsigargin treatment caused a dose-dependent decrease in cIAP2 levels, while increasing NaArs concentrations correlated with an increase in cIAP2 levels (Fig. 7).
FIG. 7.
Stress-induced changes in endogenous cIAP2 protein levels correlate with changes in cIAP2 shunting efficiency. (A) Representative immunoblot of endogenous cIAP2 protein levels in 293T cells following 18 h of treatment with increasing concentrations of etoposide (Etopo), thapsigargin (Thaps), or NaArs. Tumor necrosis factor alpha (TNFα) was used as a positive control. (B) Protein band density was quantified by densitometry, and the cIAP2 levels (normalized to α-tubulin) for three experiments are graphed. Error bars represent standard deviations of the mean. Cont, control.
Endogenous cIAP2 mRNA expression is consistent with a stress-modulated ribosome shunt.
Previous work established that stress-induced splicing can remove secondary structure from a complex 5′ UTR to permit increased translation (5). Also, a smaller (2.7-kb) cIAP2 transcript (containing a 223-nt 5′ UTR) has been reported (GenBank NM_182962), although evidence for its expression in vivo is questionable. To ensure that stress-induced alternative splicing did not produce a shorter endogenous cIAP2 5′ UTR capable of mediating translation by conventional cap-dependent scanning, we performed Northern analysis of poly(A) RNA isolated from untreated 293T cells and RNA from cells that had been treated with 40 μM etoposide, 8 μM NaArs, or 1 μM thapsigargin for 7 h. We simultaneously wanted to determine whether the 5.5-kb transcript of cIAP2 (containing the 2.8-kb 5′ UTR) was the sole cIAP2 transcript in several cell lines. Thus, poly(A) RNA from Jurkat (T cells), MCF-7 (breast carcinoma), and HeLa (cervical carcinoma) were also examined (Fig. 8).
FIG. 8.
Stress does not alter either the transcription or splicing of endogenous cIAP2 transcript in 293T cells. (A) Polyadenylated RNA was isolated from several cell lines, including 293T, Jurkat, MCF-7, and HeLa. Cells were untreated or treated for 7 h with thapsigargin (1 μM) (Thapsi), etoposide (40 mM) (Etopo), or NaArs (8 μM). Northern analysis was performed using 2 μg mRNA/lane and a 500-bp RNA probe complementary to the C-terminal coding region of cIAP2 (top). cIAP2 mRNA expression levels were normalized to β-actin (bottom), and these values are listed under each lane. (B) RT-PCR analysis of total cellular RNA from 293T or HeLa cells treated with the same concentrations of stress agents as in panel A for 7 h. The probes used amplify the entire 5′ cIAP2 UTR. The first seven lanes used RNA isolated from cells, and the next two lanes used in vitro-transcribed cIAP2 RNA; however, reverse transcriptase was not included in the samples in the ninth lane. cIAP2 plasmid DNA was used as a positive control. M, DNA markers. RT-PCR of total cell RNA using GAPDH-specific probes for loading controls is shown at the bottom.
As expected, a 5.5- to 6.0-kb cIAP2 transcript was observed in all of the cell lines, although only at a trace level in Jurkat cells (Fig. 8). More importantly, in 293T cells, neither the size nor relative levels of this cIAP2 transcript were significantly altered (compared to actin mRNA loading controls) following any of the above-mentioned treatments. The smaller (2.7-kb) cIAP2 transcript was not observed in any of the cell lines utilized.
DISCUSSION
The expression of cIAP2 protein is highly regulated, undoubtedly reflecting its importance in controlling cell fate. cIAP2 levels are often induced by environmental stress (9, 28), while its stability is regulated both by Omi-mediated cleavage (39), as well as cIAP1-mediated ubiquitylation and degradation (4).
Adding an additional layer of control, we show here that the 2.78-kb 5′ UTR of the cIAP2 mRNA regulates cIAP2 translation, not by an IRES, but through a ribosome shunting mechanism that appears well-conserved in the cIAP2 orthologs of marsupials and other mammals, as well as birds (Fig. 3). To our knowledge, this represents the first example of a mammalian transcript, be it eukaryotic or viral, that apparently translates exclusively by shunting. Consequently, the cIAP2 shunt also demonstrates for the first time that cellular stress can alter shunting efficiency, rather than simply altering the percentage of translation initiation that occurs by shunting. For example, shunting within the 216-nt 5′ UTR of HSP70 facilitates its translation during heat shock because heat stress restricts conventional scanning-mediated translation initiation, thereby increasing the percentage of total HSP70 translation that is shunt mediated (46). However, the HSP70 transcript is relatively short, and it has not been proposed to shunt exclusively, even during stress.
Efficient shunting in the cIAP2 5′ UTR appears to require several structural features that are nearly identical to those required for shunting in the 35S mRNA of CaMV, including (i) a 5′ cap structure, (ii) a three-codon uORF at the shunt donor site, and (iii) a stable double-stranded RNA stem beginning 6 to 7 bp downstream from the uORF (37). We have found that these features are all likewise required for efficient translation mediated by the cIAP2 5′ UTR. Although it remains unclear exactly how these structural features facilitate cIAP2 shunting, recent studies examining shunting in the pararetroviruses CaMV and rice tungro bacilliform virus may provide clues. Shunting in CaMV is enhanced by the viral protein TAV, which facilitates translation reinitiation (37). TAV can replace eIF4B in the initiation complex, but only after translation of a short uORF. It then seems to facilitate CaMV shunting by helping to retain eIF3 and other initiation factors both during and after translation of the uORF that is required for CaMV shunting (37). Our data suggest that the efficiency of cIAP2 shunting during stress may also be controlled by one or more trans-acting regulatory factors.
Postulating that the extreme length of the cIAP2 5′ UTR may provide numerous sites for the binding of regulatory factors, we selectively deleted regions within the 2.5 kb of sequence that is looped out by the conserved stable stem of the cIAP2 shunt. Within this region, the highly conserved Dom 3 appeared to be important for cIAP2 shunting, as its selective deletion inhibited constitutive shunting by 80% (Fig. 4B and C). The role of this domain may involve recruitment of an RNA-binding protein or components of the translational apparatus, but this remains to be seen. That the deletion of the nonconserved Dom 4+5 had no significant effect on constitutive cIAP2 shunting reinforces the apparent importance of Dom 3 in this process. Surprisingly, however, neither of these deletions prevented the stress-induced changes in shunting efficiency we observed earlier, suggesting that the determinants controlling this reside elsewhere.
Translation initiation by shunting may be favored over conventional ribosome scanning in a cellular environment where stress induces eIF4E dephosphorylation, leading to postulated instability between the components comprising the initiation factor eIF4F (45, 46). However, this theory is inadequate to fully explain the translational advantage provided by many shunts. For example, both Ad and CaMV shunts are facilitated by trans-acting proteins derived from the viral genome that stimulate shunting severalfold (31, 44). The unusual length and complexity of the cIAP2 5′ UTR suggest that the regulation of its shunting should prove even more elaborate, potentially involving trans-acting factors that either facilitate or inhibit cIAP2 shunting during stress. Indeed, we observed an increase in cIAP2 shunting efficiency after etoposide treatment in the absence of significant global inhibition of translation, supporting the involvement of one or more trans-acting factors (Fig. 5B to D). It seems clear that for cIAP2, the advantage of shunting is how it enables the cell to quickly modulate cIAP2 protein expression during cell stress.
We treated cells with relatively low concentrations of stress-inducing agents and harvested them at relatively early times in order to observe the cellular antiapoptotic response, including potential changes in cIAP2 shunt-mediated translation. Surprisingly, cIAP2 shunting was rapidly inhibited by treatment with thapsigargin and H2O2, and it may be that cIAP2 shunting is preferentially inhibited by stresses that induce eIF2α phosphorylation. Thapsigargin selectively inhibits the sarcoplasmic-endoplasmic Ca2+ ATPase, which induces the unfolded-protein response due to increased cytoplasmic Ca2+ and lowered ER Ca2+. The unfolded-protein response leads to PERK-mediated phosphorylation of the α subunit of initiation factor eIF2, preventing the recycling of the ternary complex and inhibition of translation (43). Similar to thapsigargin, H2O2-induced stress also leads to rapid phosphorylation of eIF2α following an increase in cytosolic Ca2+ (41).
If cIAP2 shunting truly depends upon translation of uORF2, downstream translation initiation at the true cIAP2 start codon would require ribosomal reinitiation. In this instance, reinitiation would necessitate prompt reacquisition of ternary complex, since the shunted 43S ribosome presumably scans just 20 bases further before reaching the true cIAP2 start codon. Therefore, delays in ternary complex reacquisition caused by increased eIF2α phosphorylation might prevent initiation at the true cIAP2 start codon.
Etoposide treatment significantly stimulated cIAP2 shunting efficiency; however, etoposide inhibits translation not by increasing phosphorylation of eIF2α but rather by increasing the sequestration of the cap-binding protein eIF4E by 4E-BP1. Etoposide inhibits topoisomerase II, leading to DNA damage and p53 activation. Activated p53 causes rapid dephosphorylation of 4E-BP1, which then sequesters eIF4E and inhibits translation initiation (42). Even so, the strong enhancement of cIAP2 shunting in response to etoposide seems unlikely to be attributable solely to sequestration of eIF4E, as conventional scanning-mediated translation appeared to be uninhibited after 6 h of etoposide treatment (a relatively early time point). It seems more likely that etoposide treatment induces the expression and/or activity of a trans-acting stimulator of cIAP2 shunting efficiency, although this remains to be confirmed.
A similar scenario seems likely for NaArs-mediated stimulation of cIAP2 shunting. Although NaArs treatment increases oxidative stress, inhibits DNA repair, and can also induce eIF2α phosphorylation at higher concentrations (29), NaArs treatment at lower concentrations can actually stimulate cell metabolism (2). In agreement with this, we never observed a decrease in conventional scanning-mediated translation following low-level NaArs treatment in either 293T cells or MCF-7 cells (Fig. 5B and C). Certainly, the next step in examining how stress modulates cIAP2 shunting efficiency will be to search for proteins that specifically bind to the cIAP2 5′ UTR in either the presence or absence of cellular stress.
Acknowledgments
We thank Sarah Snider for excellent technical assistance.
This work was supported by NIH grants GM-59803 and AI-50237. K.W.S. was supported by Ruth L. Kirschstein NRSA Postdoctoral Training Grant AI-07471.
Footnotes
Published ahead of print on 14 January 2008.
REFERENCES
- 1.Bureau, F., G. Seumois, F. Jaspar, A. Vanderplasschen, B. Detry, P. P. Pastoret, R. Louis, and P. Lekeux. 2002. CD40 engagement enhances eosinophil survival through induction of cellular inhibitor of apoptosis protein 2 expression: Possible involvement in allergic inflammation. J. Allergy Clin. Immunol. 110443-449. [DOI] [PubMed] [Google Scholar]
- 2.Chen, H., J. Liu, C. Q. Zhao, B. A. Diwan, B. A. Merrick, and M. P. Waalkes. 2001. Association of c-myc overexpression and hyperproliferation with arsenite-induced malignant transformation. Toxicol. Appl. Pharmacol. 175260-268. [DOI] [PubMed] [Google Scholar]
- 3.Chu, Z. L., T. A. McKinsey, L. Liu, J. J. Gentry, M. H. Malim, and D. W. Ballard. 1997. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κB control. Proc. Natl. Acad. Sci. USA 9410057-10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conze, D. B., L. Albert, D. A. Ferrick, D. V. Goeddel, W. C. Yeh, T. Mak, and J. D. Ashwell. 2005. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol. Cell. Biol. 253348-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox, J. S., and P. Walter. 1996. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87391-404. [DOI] [PubMed] [Google Scholar]
- 6.Curran, J., and D. Kolakofsky. 1988. Scanning independent ribosomal initiation of the Sendai virus X protein. EMBO J. 72869-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai, Z., W. G. Zhu, C. D. Morrison, R. M. Brena, D. J. Smiraglia, A. Raval, Y. Z. Wu, L. J. Rush, P. Ross, J. R. Molina, G. A. Otterson, and C. Plass. 2003. A comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis cIAP1 and cIAP2 as candidate oncogenes. Hum. Mol. Genet. 12791-801. [DOI] [PubMed] [Google Scholar]
- 8.Dever, T. E. 2002. Gene-specific regulation by general translation factors. Cell 108545-556. [DOI] [PubMed] [Google Scholar]
- 9.Dong, Z., J. Z. Wang, F. Yu, and M. A. Venkatachalam. 2003. Apoptosis-resistance of hypoxic cells: multiple factors involved and a role for IAP-2. Am. J. Pathol. 163663-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duckett, C. S., V. E. Nava, R. W. Gedrich, R. J. Clem, J. L. Van Dongen, M. C. Gilfillan, H. Shiels, J. M. Hardwick, and C. B. Thompson. 1996. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 152685-2694. [PMC free article] [PubMed] [Google Scholar]
- 11.Durkop, H., B. Hirsch, C. Hahn, and H. Stein. 2006. cIAP2 is highly expressed in Hodgkin-Reed-Sternberg cells and inhibits apoptosis by interfering with constitutively active caspase-3. J. Mol. Med. 84132-141. [DOI] [PubMed] [Google Scholar]
- 12.Esposito, I., J. Kleeff, I. Abiatari, X. Shi, N. Giese, F. Bergmann, W. Roth, H. Friess, and P. Schirmacher. 2006. Overexpression of cellular inhibitor of apoptosis protein 2 is an early event in pancreatic cancer progression. J. Clin. Pathol. 60885-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Futterer, J., Z. Kiss-Laszlo, and T. Hohn. 1993. Nonlinear ribosome migration on cauliflower mosaic virus 35S RNA. Cell 73789-802. [DOI] [PubMed] [Google Scholar]
- 14.Gebauer, F., and M. W. Hentze. 2004. Molecular mechanisms of translational control. Nat. Rev. Mol. Cell. Biol. 5827-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon, G. J., K. Appasani, J. P. Parcells, N. K. Mukhopadhyay, M. T. Jaklitsch, W. G. Richards, D. J. Sugarbaker, and R. Bueno. 2002. Inhibitor of apoptosis protein-1 promotes tumor cell survival in mesothelioma. Carcinogenesis 231017-1024. [DOI] [PubMed] [Google Scholar]
- 16.Hemmings-Mieszczak, M., and T. Hohn. 1999. A stable hairpin preceded by a short open reading frame promotes nonlinear ribosome migration on a synthetic mRNA leader. RNA 51149-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemmings-Mieszczak, M., T. Hohn, and T. Preiss. 2000. Termination and peptide release at the upstream open reading frame are required for downstream translation on synthetic shunt-competent mRNA leaders. Mol. Cell. Biol. 206212-6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemmings-Mieszczak, M., G. Steger, and T. Hohn. 1998. Regulation of CaMV 35 S RNA translation is mediated by a stable hairpin in the leader. RNA 4101-111. [PMC free article] [PubMed] [Google Scholar]
- 19.Hohn, T., S. Corsten, D. Dominguez, J. Futterer, D. Kirk, M. Hemmings-Mieszczak, M. Pooggin, N. Scharer-Hernandez, and L. Ryabova. 2001. Shunting is a translation strategy used by plant pararetroviruses (Caulimoviridae). Micron 3251-57. [DOI] [PubMed] [Google Scholar]
- 20.Hu, S., and X. Yang. 2003. Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J. Biol. Chem. 27810055-10060. [DOI] [PubMed] [Google Scholar]
- 21.Huang, H., C. A. Joazeiro, E. Bonfoco, S. Kamada, J. D. Leverson, and T. Hunter. 2000. The inhibitor of apoptosis, cIAP2, functions as a ubiquitin-protein ligase and promotes in vitro monoubiquitination of caspases 3 and 7. J. Biol. Chem. 27526661-26664. [DOI] [PubMed] [Google Scholar]
- 22.Hubinger, G., C. Schneider, D. Stohr, H. Ruff, D. Kirchner, C. Schwanen, M. Schmid, L. Bergmann, and E. Muller. 2004. CD30-induced up-regulation of the inhibitor of apoptosis genes cIAP1 and cIAP2 in anaplastic large cell lymphoma cells. Exp. Hematol. 32382-389. [DOI] [PubMed] [Google Scholar]
- 23.Jackson, R. J. 2005. Alternative mechanisms of initiating translation of mammalian mRNAs. Biochem. Soc. Trans. 331231-1241. [DOI] [PubMed] [Google Scholar]
- 24.Kapp, L. D., and J. R. Lorsch. 2004. The molecular mechanics of eukaryotic translation. Annu. Rev. Biochem. 73657-704. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman, R. J. 2004. Regulation of mRNA translation by protein folding in the endoplasmic reticulum. Trends Biochem. Sci. 29152-158. [DOI] [PubMed] [Google Scholar]
- 26.Krajewska, M., H. Kim, C. Kim, H. Kang, K. Welsh, S. Matsuzawa, M. Tsukamoto, R. G. Thomas, N. Assa-Munt, Z. Piao, K. Suzuki, M. Perucho, S. Krajewski, and J. C. Reed. 2005. Analysis of apoptosis protein expression in early-stage colorectal cancer suggests opportunities for new prognostic biomarkers. Clin. Cancer Res. 115451-5461. [DOI] [PubMed] [Google Scholar]
- 27.Lee, J. C., G. X. Wang, O. Schickling, and M. E. Peter. 2005. Fusing DEDD with ubiquitin changes its intracellular localization and apoptotic potential. Apoptosis 101483-1495. [DOI] [PubMed] [Google Scholar]
- 28.Leong, W. F., H. C. Tan, E. E. Ooi, D. R. Koh, and V. T. Chow. 2005. Microarray and real-time RT-PCR analyses of differential human gene expression patterns induced by severe acute respiratory syndrome (SARS) coronavirus infection of Vero cells. Microbes Infect. 7248-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEwen, E., N. Kedersha, B. Song, D. Scheuner, N. Gilks, A. Han, J. J. Chen, P. Anderson, and R. J. Kaufman. 2005. Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J. Biol. Chem. 28016925-16933. [DOI] [PubMed] [Google Scholar]
- 30.O'Neill, A. J., B. T. Doyle, E. Molloy, C. Watson, D. Phelan, M. C. Greenan, J. M. Fitzpatrick, and R. W. Watson. 2004. Gene expression profile of inflammatory neutrophils: alterations in the inhibitors of apoptosis proteins during spontaneous and delayed apoptosis. Shock 21512-518. [DOI] [PubMed] [Google Scholar]
- 31.Park, H. S., A. Himmelbach, K. S. Browning, T. Hohn, and L. A. Ryabova. 2001. A plant viral “reinitiation” factor interacts with the host translational machinery. Cell 106723-733. [DOI] [PubMed] [Google Scholar]
- 32.Park, S. M., J. B. Yoon, and T. H. Lee. 2004. Receptor interacting protein is ubiquitinated by cellular inhibitor of apoptosis proteins (c-IAP1 and c-IAP2) in vitro. FEBS Lett. 566151-156. [DOI] [PubMed] [Google Scholar]
- 33.Pooggin, M. M., J. Futterer, K. G. Skryabin, and T. Hohn. 1999. A short open reading frame terminating in front of a stable hairpin is the conserved feature in pregenomic RNA leaders of plant pararetroviruses. J. Gen. Virol. 802217-2228. [DOI] [PubMed] [Google Scholar]
- 34.Pooggin, M. M., T. Hohn, and J. Futterer. 1998. Forced evolution reveals the importance of short open reading frame A and secondary structure in the cauliflower mosaic virus 35S RNA leader. J. Virol. 724157-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pooggin, M. M., L. A. Ryabova, X. He, J. Futterer, and T. Hohn. 2006. Mechanism of ribosome shunting in rice tungro bacilliform pararetrovirus. RNA 12841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothe, M., M. G. Pan, W. J. Henzel, T. M. Ayres, and D. V. Goeddel. 1995. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 831243-1252. [DOI] [PubMed] [Google Scholar]
- 37.Ryabova, L. A., M. M. Pooggin, and T. Hohn. 2005. Translation reinitiation and leaky scanning in plant viruses. Virus Res. 11952-62. [DOI] [PubMed] [Google Scholar]
- 38.Sherrill, K. W., M. P. Byrd, M. E. Van Eden, and R. E. Lloyd. 2004. BCL-2 translation is mediated via internal ribosome entry during cell stress. J. Biol. Chem. 27929066-29074. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasula, S. M., S. Gupta, P. Datta, Z. Zhang, R. Hegde, N. Cheong, T. Fernandes-Alnemri, and E. S. Alnemri. 2003. Inhibitor of apoptosis proteins are substrates for the mitochondrial serine protease Omi/HtrA2. J. Biol. Chem. 27831469-31472. [DOI] [PubMed] [Google Scholar]
- 40.Stehlik, C., R. de Martin, I. Kumabashiri, J. A. Schmid, B. R. Binder, and J. Lipp. 1998. Nuclear factor (NF)-κB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J. Exp. Med. 188211-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan, S., N. Somia, P. Maher, and D. Schubert. 2001. Regulation of antioxidant metabolism by translation initiation factor 2α. J. Cell Biol. 152997-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tilleray, V., C. Constantinou, and M. J. Clemens. 2006. Regulation of protein synthesis by inducible wild-type p53 in human lung carcinoma cells. FEBS Lett. 5801766-1770. [DOI] [PubMed] [Google Scholar]
- 43.Wu, J., and R. J. Kaufman. 2006. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 13374-384. [DOI] [PubMed] [Google Scholar]
- 44.Xi, Q., R. Cuesta, and R. J. Schneider. 2004. Tethering of eIF4G to adenoviral mRNAs by viral 100k protein drives ribosome shunting. Genes Dev. 181997-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yueh, A., and R. J. Schneider. 1996. Selective translation initiation by ribosome jumping in adenovirus-infected and heat-shocked cells. Genes Dev. 101557-1567. [DOI] [PubMed] [Google Scholar]
- 46.Yueh, A., and R. J. Schneider. 2000. Translation by ribosome shunting on adenovirus and hsp70 mRNAs facilitated by complementarity to 18S rRNA. Genes Dev. 14414-421. [PMC free article] [PubMed] [Google Scholar]
- 47.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 313406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]