Abstract
The chemotropic guidance cue netrin-1 promotes neurite outgrowth through its receptor Deleted in Colorectal Cancer (DCC) via activation of Rac1. The guanine nucleotide exchange factor (GEF) linking netrin-1/DCC to Rac1 activation has not yet been identified. Here, we show that the RhoGEF Trio mediates Rac1 activation in netrin-1 signaling. We found that Trio interacts with the netrin-1 receptor DCC in mouse embryonic brains and that netrin-1-induced Rac1 activation in brain is impaired in the absence of Trio. Trio−/− cortical neurons fail to extend neurites in response to netrin-1, while they are able to respond to glutamate. Accordingly, netrin-1-induced commissural axon outgrowth is reduced in Trio−/− spinal cord explants, and the guidance of commissural axons toward the floor plate is affected by the absence of Trio. The anterior commissure is absent in Trio-null embryos, and netrin-1/DCC-dependent axonal projections that form the internal capsule and the corpus callosum are defective in the mutants. Taken together, these findings establish Trio as a GEF that mediates netrin-1 signaling in axon outgrowth and guidance through its ability to activate Rac1.
During the development of the central nervous system, axons are guided to their targets in response to molecular cues that can be either membrane-bound factors or secreted molecules, acting over short or long distances. The neuronal growth cone is a specialized structure found at the tip of the axon that integrates attractive and repulsive signals elicited by these extracellular cues and responds to them by triggering signaling pathways that regulate growth cone motility (16, 18). Netrins are a family of secreted proteins that control axon outgrowth and guidance in multiple vertebrate and invertebrate species (3). Netrin-1 is a bifunctional molecule that attracts and repels different classes of axons. In vertebrates, netrin-1 was first shown to attract commissural axons of the developing spinal cord toward the ventral midline (21, 44). Since then, netrin-1 has been shown to promote outgrowth of a wide variety of axons, including growing cortical axons (30, 41). Two families of netrin-1 receptors in mammals have been identified: the Deleted in Colorectal Cancer (DCC) family, comprising DCC and neogenin, and the UNC-5 family of proteins (1, 20, 25). DCC mediates growth cone attraction induced by netrin-1 (1, 20, 25, 43) whereas the repulsive effect of netrin-1 is mediated by the UNC-5 family of netrin receptors, alone or in combination with DCC (17, 22, 35).
DCC is a transmembrane protein without any obvious catalytic activity in its intracellular domain, and for this reason, it was unclear until recently how the intracellular signaling machinery leading to axon outgrowth was initiated. This process has begun to be elucidated with the identification in cortical and commissural neurons of different DCC-binding proteins, including the protein tyrosine kinases focal adhesion kinase, Src, and Fyn; the Nck adaptor protein; and phosphatidylinositol transfer protein α (26, 27, 30, 40, 48). DCC acts as a tyrosine kinase-associated receptor. It is phosphorylated by Fyn on tyrosine 1418, and this phosphorylation event is required for netrin-1-induced axon outgrowth (26, 34). In addition, various signaling cascades are believed to be important for netrin-1-induced axon outgrowth and guidance including the mitogen-activated protein kinase and the phosphatidylinositol pathways (3). Numerous lines of evidence have established that guidance cues also influence the motility of the growth cone by remodeling the actin cytoskeleton through activation of the Rho family of GTPases (15). Small GTPases are molecular switches that oscillate between an inactive GDP-bound state and an active GTP-bound state, and they are activated by guanine nucleotide exchange factors (GEFs) that accelerate the GDP/GTP exchange (42). Cellular and genetic studies have shown that Rac, Cdc42, and RhoG promote neurite extension and growth cone motility in response to guidance cues, while RhoA mediates neurite retraction through growth cone collapse (10). We along with others have shown that the binding of netrin-1 to DCC activates the small GTPase Rac1 (28, 45) and that the adaptor protein Nck-1 is required for this activation (27). Rac1 activation is required for netrin-1-induced neurite outgrowth, but the GEF responsible for this activation has not yet been identified.
The multidomain protein Trio is the founding member of an intriguing family of GEFs that contains two GEF domains (GEFDs), with the first GEFD (GEFD1) activating Rac1 and RhoG and GEFD2 acting on RhoA (5, 6, 9). Genetic analysis of Trio orthologs in Caenorhabditis elegans (UNC-73) and in Drosophila melanogaster (D-Trio) have established Trio as a key component in the regulation of axon guidance and cell migration (2, 4, 29, 36, 46). Functional analysis indicates that the role of Trio in all organisms mainly depends on the catalytic activity of GEFD1. Moreover, D-Trio, the kinase Abl, the Abl substrate Ena, and the netrin receptor Frazzled have been shown to regulate axon guidance at the central nervous system midline in Drosophila (14). In mammals, we have shown that human Trio is a component of the nerve growth factor pathway leading to RhoG and Rac1 activation and neurite outgrowth in PC12 cells (11). Moreover, targeted disruption of Trio in mouse resulted in embryonic lethality between embryonic day 15.5 (E15.5) and birth, suggesting that Trio is required for late embryonic development, probably by playing essential roles in neural tissue and fetal skeletal muscle formation (37). However, the function of mammalian Trio in axon guidance remains unknown. In addition, the upstream signaling pathways leading to Trio activation in mammals are still unclear.
Here, we provide evidence that Trio is a key component of netrin-1 signaling in growth cone guidance. We show that Trio and DCC interact in embryonic brain lysates and that this association is probably mediated through the interaction with p21-activated kinase 1 (PAK1). Netrin-1-induced Rac1 activation is abolished in Trio−/− embryonic brains. Cortical neurons are defective in extending neurites in response to netrin-1, while they respond to glutamate stimulation. Likewise, netrin-1-induced axon outgrowth is also reduced in Trio−/− spinal cord explants. Finally, netrin-1- and DCC-dependent neuronal projections in the developing spinal cord and in the brain, such as the anterior commissure, the internal capsule, and the corpus callosum, are impaired in Trio-deficient mouse embryos.
MATERIALS AND METHODS
DNA constructs.
The green fluorescent protein (GFP) Trio constructs, GFP-RhoGA37, pRK5-DCC, pGEX4T2-DCC-C, pGEX2T-PAK1, and pRK5myc-PAK1 have been previously described (11, 24, 27, 28). The Nck-1 constructs have been generously provided by Louise Larose (McGill University, Montreal, Canada).
Genotyping of Trio-null mice.
Ablation of the Trio gene in mice has been previously described (37). Trio-heterozygous mice were kindly provided by Michel Streuli. To obtain Trio-null embryos, females of Trio+/− BALB/c mice were crossed with Trio+/− males. Midday on the day after coitus was considered E0.5. Genomic DNA from embryo tails was prepared for genotyping using a PCR method with specific oligonucleotides to detect the wild type (wt) or the Trio-null allele as previously described (37).
Cell culture and transfection.
Cortical neurons from E14.5 embryos were dissociated mechanically and plated on poly-l-lysine (25 μg/ml)-treated coverslips at a density of 250,000 cells/well in 24-well dishes. Neurons were cultured for the indicated times in neurobasal medium (Invitrogen) supplemented with 1% B27 (Invitrogen). Neurons were transfected with GFP or GFP-Trio constructs using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Cells were treated with the following reagents: recombinant netrin-1 (250 ng/ml; Sigma), glutamate (50 μM; Sigma), and blocking anti-DCC antibody (4 μg/ml; Calbiochem). N1E-115 neuroblastoma cells, COS-7 cells, and HEK-293 cells were cultured and transfected as previously described (28).
Immunoprecipitation. (i) Immunoprecipitation of proteins expressed in HEK-293 cells.
HEK-293 cells expressing GFP-Trio and DCC were lysed in buffer containing 20 mM HEPES, pH 7.5, 100 mM NaCl, 10% glycerol, 1% Triton X-100, 20 mM NaF, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1× complete protease inhibitor cocktail (Roche Diagnostics). One milligram of protein lysate was precleared with protein G-Sepharose beads at 4°C overnight. Then, the supernatants were incubated overnight at 4°C with 20 μl of protein G-Sepharose beads and 2.5 μg of anti-DCC antibodies (BD Biosciences) or normal mouse immunoglobulin Gs (IgGs). Beads were washed three times with ice-cold lysis buffer and boiled in sodium dodecyl sulfate (SDS) sample buffer, and the protein samples were resolved by SDS-polyacrylamide gel electrophoresis (PAGE).
(ii) Immunoprecipitation of endogenous Trio and DCC proteins from mouse embryonic brains.
Total brains of E18.5 mouse embryos were dissociated mechanically as described elsewhere (12, 31) before lysis in buffer containing 10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% NP-40, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. After centrifugation at 10,000 × g for 1 min, 1 mg of the cleared protein lysates was incubated for 3 h with 2 μg of normal rabbit IgGs or 2 μg of anti-Trio antibody (H120; Santa Cruz Inc.) and with 20 μl of protein G-Sepharose beads. The samples were washed three times with ice-cold lysis buffer and boiled in SDS sample buffer, and the protein samples were resolved by SDS-PAGE. The presence of Trio and DCC in the immunoprecipitates was revealed by Western blotting using the appropriate antibodies, anti-DCC (BD Biosciences Inc.) and polyclonal anti-Trio (39).
GST pull-down.
COS-7-transfected cells were lysed in buffer A (25 mM HEPES, pH 7.5, 1% NP-40, 10 mM MgCl2, 100 mM NaCl, 5% glycerol, 1 mM PMSF, 1× protease inhibitor cocktail). Lysates were then incubated for 2 h at 4°C with 30 μg of either glutathione S-transferase (GST), GST-PAK1, or GST-Nck-1 fusion proteins coupled to glutathione-Sepharose beads. Total cell lysates and GST pull-down-associated proteins were resolved by SDS-PAGE and transferred onto nitrocellulose. Membranes were immunoblotted with the following antibodies: polyclonal anti-Trio (39), anti-Nck (BD Transduction Laboratories Inc.), anti-DCC (BD Biosciences Inc.), anti-GFP (Molecular Probes Inc), and anti-PAK1 (Santa Cruz) antibodies.
Rac1 activation assay.
Total brains of E14.5 mouse embryos were dissociated mechanically, and one-half of the brain remained untreated whereas the other half was treated with netrin-1 (500 ng/ml) for the indicated times similarly to a method described elsewhere (12, 31). When indicated, samples were treated with blocking anti-DCC antibody (4 μg/ml; Calbiochem) for 10 min prior to netrin-1 stimulation. They were lysed in buffer A (see “GST pull-down”), and lysates were then subjected to centrifugation at 10,000 × g for 30 s at 4°C to remove insoluble materials. Active Rac1 was pulled down by incubating the supernatants for 1 h at 4°C with GST-PAK-PBD beads (Cytoskeleton Inc.). The beads were washed with 25 mM HEPES, pH 7.5, 1% NP-40, 30 mM MgCl2, 40 mM NaCl, and 1 mM dithiothreitol and resuspended in loading buffer. Protein samples from total cell lysates and from the GST pull-downs were resolved by SDS-PAGE and transferred onto nitrocellulose. Membranes were immunoblotted with anti-Rac1 antibody (BD Transduction Laboratories Inc).
Immunofluorescence.
Neurons were fixed and permeabilized as previously described (11). GFP-expressing cortical neurons were visualized using a Leica DMR microscope and a 40× PL APO lens. N1E-115 cells were fixed and permeabilized as already described (28). The cells were examined with an Axiovert 135 Carl Zeiss microscope using a 63× Plan-neofluor objective lens. Images were recorded with a digital camera (DVC Co.) and analyzed with Northern Eclipse software (Empix Imaging Inc.).
Neurite outgrowth analysis.
More than 100 cortical neurons were analyzed for each condition. For each neuron, the number of neurites was counted manually, and the length of the neurites was measured using MetaMorph and NeuronJ software programs (33) modified by Volker Backer, Montpellier RIO-Imaging (unpublished data). In N1E-115 cells, a neurite was defined as a process that measured at least the length of one cell body.
Explant assays.
Mouse dorsal spinal cord explants from wt, Trio+/−, or Trio−/− E11.5 embryos were dissected and cultured in three-dimensional collagen type I (BD Biosciences) gels as described previously (47). Recombinant chick netrin-1 protein was produced and purified as described previously (44). Netrin-1 (500 ng/ml) was added to the culture medium at the beginning of the culture period. Images were captured after 35 h with a digital camera on a Carl Zeiss Axiovert 135 microscope using a 10× phase-contrast objective lens. The total length of the axon bundles and number of axons growing out of the explants were quantified using Northern Eclipse software (Empix Imaging). The experiments were performed in a blinded fashion.
Spinal cord immunohistochemistry and brain histology.
Eosin staining and immunohistochemistry with antibodies to Nrp2 (1:100; R&D Systems) and to DCC (1:100; BD Biosciences) were performed on horizontal or coronal 70-μm-thick vibratome sections from E17 or E18.5 brains as previously described (12). Commissural axon projections were detected by immunohistochemistry using anti-DCC antibodies (1: 200; BD Biosciences) on 20-μm-thick cryostat transverse sections from E11.5 embryos as described previously (38). Quantification analysis of axon defasciculation in Trio−/− embryos was performed on sections positioned at the forelimb of wt and Trio−/− embryos.
RESULTS
A Trio mutant defective in Rac1 activation inhibits DCC-induced neurite outgrowth in N1E-115 neuroblastoma cells.
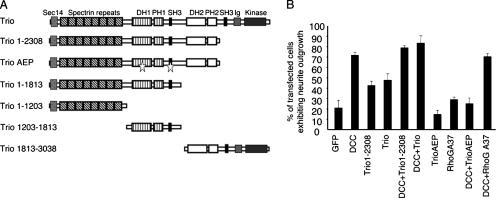
The chemotropic guidance cue netrin-1 attracts different types of neurons and activates the GTPase Rac1 through its receptor DCC. However, the GEF responsible for Rac1 activation remains unknown. We have previously demonstrated that netrin-1 but not DCC is constitutively expressed in N1E-115 neuroblastoma cells (28). The expression of DCC in these cells induces neurite outgrowth in a netrin-1- and Rac1-dependent manner. To determine whether Trio mediates netrin-1/DCC-induced neurite outgrowth, N1E-115 cells were cotransfected with DCC, and either Trio, Trio consisting of residues 1 to 2308 [Trio(1-2308)] and therefore lacking the kinase domain, or TrioAEP, a dominant negative form of Trio containing triple point mutations in the GEFD1 and the adjacent Src homology 3 (SH3) domain, which drastically reduces its in vitro exchange activity toward RhoG/Rac1 (11) (Fig. 1A). Trio and Trio(1-2308) were able to induce neurite outgrowth in N1E-115 cells, either alone or together with DCC. However, the expression of TrioAEP with DCC blocked the ability of DCC to induce neurite extension (Fig. 1B), suggesting that Trio mediates netrin-1/DCC-induced neurite outgrowth in N1E-115 cells. The GEFD1 domain of Trio has been previously shown to be active on both Rac1 and RhoG small GTPases (6). To determine whether RhoG is involved in netrin-1/DCC-induced neurite outgrowth, dominant negative RhoGA37 (RhoG with the point mutation A37) was expressed together with DCC in N1E-115 cells. RhoGA37 did not inhibit DCC-induced neurite outgrowth (Fig. 1B), in contrast to RacN17, which blocked the DCC effect on neurite extension (28). Therefore, these data strongly suggest that Trio plays a role in netrin-1/DCC-regulated neurite outgrowth via Rac1 and not RhoG.
FIG. 1.
Trio defective in Rac1 activation inhibits DCC-induced neurite outgrowth in N1E-115 neuroblastoma cells. (A) Schematic of Trio and Trio mutant proteins. TrioAEP is a dominant negative form of Trio and corresponds to Trio(1-2308) containing triple mutations (stars) in the GEFD1 and adjacent SH3 domain. DH, Dbl homology; PH, pleckstrin homology. (B) N1E-115 cells were transfected with the indicated plasmids, and cells exhibiting neurite outgrowth were counted 24 h after transfection. The values correspond to the average of at least three independent experiments. Error bars represent standard deviations.
Interaction of DCC and Trio.
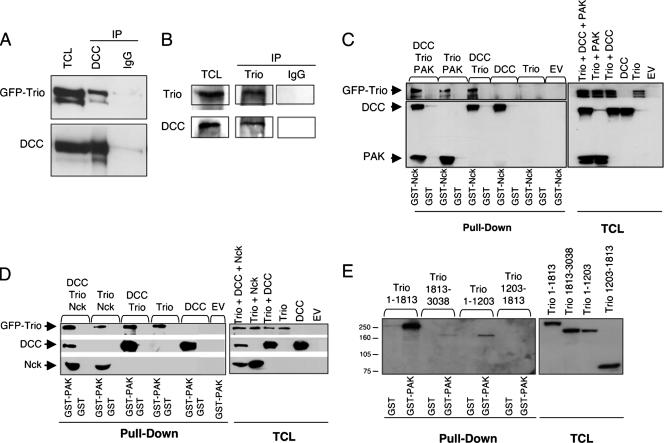
To determine whether Trio interacts with DCC, GFP-Trio was coexpressed with DCC in HEK-293 cells that do not express endogenous DCC (28, 45). We found that Trio was able to interact with DCC, with no band corresponding to Trio in immunoprecipitates using normal mouse IgGs (Fig. 2A). We next determined whether endogenous Trio and DCC proteins associate in mouse embryonic brains. DCC was detectable in Trio immunoprecipitates from embryonic brain lysates, which was not the case when normal rabbit IgGs were used for the immunoprecipitation (Fig. 2B). Stimulation of embryonic brains with netrin-1 did not significantly enhance Trio/DCC association (data not shown), suggesting that Trio binds constitutively to DCC.
FIG. 2.
Interaction of DCC and Trio. (A) Lysates of HEK-293 cells transfected with pEGFP-Trio and pRK5-DCC were submitted to immunoprecipitation using anti-DCC antibodies (DCC) or mouse IgG coupled to protein G-Sepharose beads. Immunoprecipitated proteins and 10% of the total cell lysates were submitted to SDS-PAGE, and GFP-Trio and DCC were detected by Western blotting using anti-DCC and anti-GFP antibodies. (B) Lysates of E18.5 mouse brains were submitted to immunoprecipitation using anti-Trio or normal rabbit IgGs coupled to protein G-Sepharose beads. Immunoprecipitated proteins and 10% of the total cell lysates were submitted to SDS-PAGE, and the presence of DCC and Trio was detected by Western blotting using the appropriate antibodies. (C and D) Lysates of COS-7 cells transfected with pRK5 (EV), pRK5-DCC, pEGFP-Trio, pRK5-HA-Nck-1, or pRK5myc-PAK1, alone or as indicated, were incubated with GST and with GST-Nck (C) or GST-PAK (D). GST pull-down proteins (pull-down) and 10% of the total cell lysates were submitted to SDS-PAGE, and proteins were detected by Western blotting analysis using anti-DCC, anti-GFP, anti-PAK1, and anti-Nck-1 antibodies. (E) Lysates of COS-7 cells transfected with pEGFP-Trio(1-1813), pEGFP-Trio(1813-3038), pEGFP-Trio(1-1203), or pEGFP-Trio(1203-1813) were incubated with GST or GST-PAK. GST pull-down proteins (pull-down) and 8% of the total cell lysates were submitted to SDS-PAGE, and proteins were detected by Western blotting analysis using anti-GFP antibodies. IP, immunoprecipitation; TCL, total cell lysates.
To characterize the interaction between Trio and DCC, we examined whether Trio associates with DCC via the adaptor protein Nck-1 and the serine/threonine protein kinase PAK1. Indeed, Rac, PAK, and DOCK, the Drosophila ortholog of Nck, interact genetically with D-Trio during axon guidance of Drosophila photoreceptors (36). Furthermore, PAK1 is known to interact with the second SH3 domain of Nck-1 (8) whereas the cytoplasmic domain of DCC interacts with the first and third SH3 domains of Nck-1 (27). We performed GST pull-down experiments using either recombinant GST-Nck-1 (Fig. 2C) or GST-PAK1 (Fig. 2D) and lysates from COS-7 cells expressing GFP-Trio, DCC, Nck-1, and PAK1, alone or together. Interestingly, we found that Trio alone interacted with PAK1 but not with Nck-1, whereas DCC was able to interact with both proteins (Fig. 2C and D). When PAK1 or DCC was coexpressed with Trio, Trio was detected in the GST-Nck-1 pull-down (Fig. 2C). Finally, when DCC, Trio, PAK1, and Nck-1 were expressed together, they were all detected in the GST-Nck-1 or GST-PAK1 pull-downs (Fig. 2C and D), suggesting that Trio may associate with DCC and Nck-1 indirectly via its interaction with PAK1.
To characterize further the interaction of Trio with PAK1, GST-PAK1 pull-down assays were performed with COS-7 cells expressing various deletion mutants of Trio (Fig. 1A). As shown in Fig. 2E, Trio(1-1813) and Trio(1-1203) were able to interact with GST-PAK1, whereas Trio(1203-1813) showed no interaction. Trio(1813-3038) was also detected in the GST-PAK1 pull-down although the interaction appeared much weaker than with Trio(1-1813). These results suggest that the N terminus extremity comprising the Sec-14 and the spectrin domains of Trio(1-1203) but not the GEFD1 or the first SH3 domains of Trio(1203-1813) mediates the interaction with PAK1. Additionally, a second region of Trio that contains the GEFD2, the second SH3, and the kinase domains of Trio(1813-3038) is capable of interacting with PAK1, although to a lesser extent. Thus, it seems that Trio, which is a large protein of 334 kDa with multiple signaling domains, has at least two separate regions that are able to mediate the interaction with PAK1.
Netrin-1-induced Rac1 activation is abolished in Trio−/− embryonic brains.
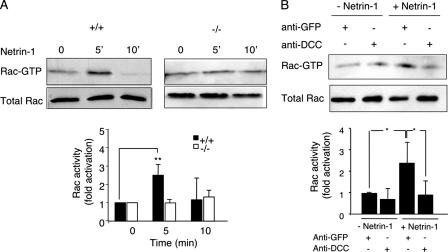
Netrin-1 binding to its receptor DCC has been shown in different cellular systems to induce rapid and robust Rac1 activation leading to neurite outgrowth (28, 45), but this has never been tested on endogenous Rac1 expressed in brain. We found that the addition of netrin-1 to wt embryonic mouse brains induced a rapid Rac1 activation with a peak at 5 min of stimulation (Fig. 3A). Netrin-1 activation of Rac1 occurred through DCC, as a blocking DCC antibody was able to suppress netrin-1-induced Rac1 activation in embryonic brains (Fig. 3B). We then tested whether the absence of Trio affects netrin-1-induced Rac1 activation by measuring the netrin-1 effect in embryonic brains of Trio−/− mice. As shown in Fig. 3A, netrin-1 failed to activate Rac1 in Trio-null embryos, consistent with Trio's being the GEF responsible for netrin-1-induced Rac1 activation through its receptor DCC.
FIG. 3.
Netrin-1-induced Rac1 activation is impaired in Trio null-embryonic brains. (A) GTP-loaded Rac1 was pulled down using GST-PAK-PBD from lysates of Trio+/+ or Trio−/− embryonic brains treated or not with netrin-1 for the indicated time. GTP-bound Rac1 was detected by Western blotting using anti-Rac1 antibodies (top). Total cell lysates probed for Rac1 indicated equal amounts of GTPase (bottom). Quantification of Rac1 activity corresponds to the average of at least three independent experiments (P < 0.01). (B) The experiment was as described in panel A, except that DCC blocking antibodies (4 μg/ml) were added before netrin-1 stimulation (5 min). Mouse anti-GFP antibodies were used as a negative control and had no effect on netrin-1-induced Rac1 activation. Quantification of Rac1 activity corresponds to the average of at least three independent experiments (P = 0.012, Student t test). For both panels, error bars represent standard deviations.
Trio is necessary for netrin-1-induced axon outgrowth in cortical neurons.
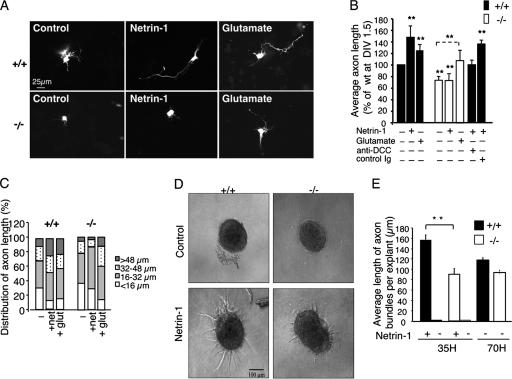
Netrin-1 has been described as an attractant for axons of cortical explants and dissociated cortical neurons, and DCC has been shown to be expressed in developing cortical neurons (30, 40, 41). To address whether Trio is required for netrin-1 to induce axon outgrowth of cortical neurons, we tested the effect of adding netrin-1 to wt or Trio-null dissociated cortical neurons. As shown in Fig. 4A, netrin-1 stimulated axon outgrowth of wt cortical neurons after 24 h in culture. This effect was mediated by the netrin-1 receptor DCC since a blocking DCC antibody completely abrogated the effect of netrin-1 (Fig. 4B). In contrast, netrin-1 failed to stimulate axon outgrowth of Trio-null cortical neurons (Fig. 4A and B). Consistently, analysis of the distribution of axon length in cortical neurons showed that netrin-1 significantly increased the percentage of long axons (>48 μm) in wt but not in mutant neurons (Fig. 4C). The slight increase in the percentage of intermediary axons (16 to 32 μm) observed for the mutant neurons treated with netrin-1 was not significant (Fig. 4C). The lack of a netrin-1 response of Trio-deficient neurons was not due to a defect in DCC expression as both wt and mutant cortical neurons expressed similar levels of DCC proteins (data not shown). We also observed that the average axon length of Trio-null neurons in the absence of netrin-1 was reduced by 20% compared to wt neurons (Fig. 4B). Nevertheless, Trio−/− neurons were able to extend neurites in response to glutamate (Fig. 4A and B), which has been shown to stimulate growth cone motility by different pathways including Ca2+-dependent activation of Rho GTPases (19, 23, 49). Even though the average axon length of Trio-null neurons was shorter than that of wt neurons (Fig. 4B), the ratio of glutamate-induced axon outgrowth versus the control was similar in both types of neurons (wt, 1.25; Trio−/−, 1.4), while this was not the case for the ratio of netrin-1-induced outgrowth versus the control for wt and mutant neurons (wt, 1.48; Trio−/−, 1). Analysis of the distribution of the axon length showed that glutamate stimulation of wt and mutant neurons induced the growth of long axons in both cases and significantly reduced the percentage of very short axons (Fig. 4C). These data show that Trio-null cortical neurons are able to induce axon extension in response to glutamate while they are specifically defective in netrin-1-induced axon outgrowth.
FIG. 4.
Trio is required for netrin-1-induced axon outgrowth. (A) Neurite outgrowth of Trio+/+ or Trio−/− cortical neurons expressing GFP at day in vitro 1.5 treated with control buffer, netrin-1, or glutamate for 24 h. Scale bar, 25 μm. (B) Quantification of average axon length of cortical neurons presented in panel A. Values are represented as a percentage of average axon length of wt cortical neurons at day in vitro (DIV) 1.5 incubated with control buffer. When indicated, neurons were incubated with control Igs or DCC blocking antibodies before netrin-1 addition. **, P <0.001 for the comparison to wt neurons expressing GFP, except for the dotted line that refers to GFP-transfected Trio-null neurons (n = 8 for +/+ and n = 10 for −/− embryos). (C) Distribution of axon length from panel B. (D) E11.5 dorsal spinal cord explants from Trio+/+ or Trio−/− embryos were incubated with control buffer or netrin-1 for 35 h. Scale bar, 100 μm. (E) Quantification of the average length of axon bundles per explant after a 35-h incubation with netrin-1 (n = 10 for Trio+/+, and n = 4 for Trio−/− embryos; **, P < 0.001) or after 70 h in the absence of netrin-1 (n = 3 for +/+ and −/− embryos).
Netrin-1-induced axon outgrowth is reduced in Trio−/− dorsal spinal cord explants.
To further demonstrate the involvement of Trio in netrin-1/DCC-induced axon outgrowth, we added netrin-1 to dorsal spinal cord explants dissected from E11.5 Trio−/− embryos. As shown in Fig. 4D, explants from wt dorsal spinal cords treated for 35 h with netrin-1 showed robust axon outgrowth compared to untreated controls. In contrast, when explants from Trio−/− dorsal spinal cords were cultured in the presence of netrin-1, commissural axon outgrowth showed a 56% reduction compared to wt explants (Fig. 4D and E). To determine whether the reduced response of Trio−/− spinal cord explants was due to a general defect in axon outgrowth, wt or Trio−/− explants were cultured for 70 h in the absence of netrin-1. As shown in Fig. 4E, both wt and Trio−/− explants were able to produce axon outgrowth in a netrin-1-independent manner. These findings demonstrate that Trio is required for netrin-1 to promote commissural axon outgrowth.
Trio-deficient mouse embryos show defects in spinal cord and brain development.
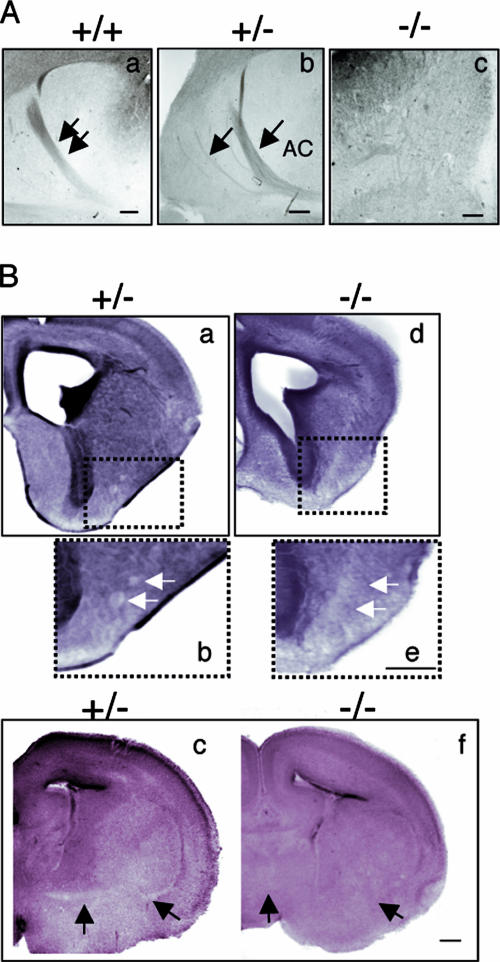
To determine the role of Trio in netrin-1 function in vivo, we next examined the axonal projections of the commissural neurons in the spinal cord of wt and Trio-null littermates by immunostaining with anti-DCC antibodies (Fig. 5A). In wt embryos, commissural axons are directed ventrally toward the floor plate of the developing spinal cord, which secretes the chemoattractant netrin-1 (Fig. 5A and B) (21). In Trio−/− embryos, commissural axons could reach the floor plate, but they appeared defasciculated in the ventral spinal cord (Fig. 5A to F), suggesting that Trio plays a role in the guidance of these axons. In addition to defects in the developing spinal cord, the netrin-1- and DCC-null mice also present defects in several projections of the brain, namely, the anterior commissure, the hippocampal commissure, the corpus callosum, and the thalamo-cortical reciprocal projections in the internal capsule (13, 43). Therefore, we examined the anterior commissure in sections of Trio-null brains (Fig. 6). Serial horizontal sections of the whole brain were analyzed, and while anterior and posterior branches forming the anterior commissure were present in the wt sections, they were totally absent in the homozygous mutant embryos in all sections tested (Fig. 6A, compare frames a and c). Interestingly, the heterozygous mutant embryos presented an intermediate phenotype, as the anterior branch of the commissure could form but was highly defasciculated, with several roots exiting the cortex at lateral positions (Fig. 6A, frame b). To confirm that the anterior commissure was absent in Trio−/− embryos, we analyzed different coronal sections of Trio+/− and Trio−/− embryonic brains. As shown in Fig. 6B, defasciculated fibers were present in the heterozygous embryos but were completely absent in the Trio-null embryos (Fig. 6B, compare frames a and b to d and e). Thus, similar to DCC and netrin-1, Trio is required for the formation of the anterior commissure.
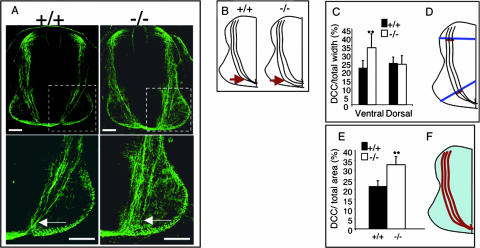
FIG. 5.
Commissural axon projections are defective in Trio-null embryos. (A) In the upper frames, trajectories of commissural axons are visualized using anti-DCC antibodies in sections of Trio+/+ or Trio−/− E11.5 embryos. The lower frames show enlargements of the corresponding images. Scale bar, 80 μm. (B) On the left is a schematic representing normal commissural axons that project from the dorsal spinal cord toward the ventral floor plate. In Trio−/− embryos (right), commissural axons are defasciculated when they reach the ventral floor plate (arrows in both A and B). (C and D) The thickness of axon bundles in the dorsal and ventral spinal cords was quantified by measuring the width of the DCC-stained axons (red) relative to the width of the spinal cord (blue) as depicted in panel D. (E and F) Axon defasciculation in Trio−/− embryos was quantified by measuring the DCC-stained area (red) relative to the total area of the spinal cord (blue) as depicted in panel F. P < 0.001, Student t test. Error bars represent standard deviations (n = 5 for +/+ and n = 7 for −/− embryos).
FIG. 6.
The anterior commissure is absent in Trio-null embryos. (A) Neuropilin 2 (Nrp2) immunostaining on horizontal serial brain sections from E17 +/+ (a), +/− (b), or −/− (c) embryos (n = 6 for +/+ and −/− embryos; n = 4 for +/− embryos). In the heterozygous embryos, the anterior branch of the commissure (AC) is defasciculated, which is illustrated by several roots exiting the cortex at lateral positions (arrows in frame b). In the Trio-null embryos, the commissure is absent (c). Scale bar, 80 μm. (B) Eosin staining on coronal brain sections from E17 +/− (a, b, and c) and −/− (d, e, and f) embryos. Defasciculated fibers are present in the heterozygous embryos (b, white arrows), whereas they are absent in the Trio-null embryos (e). In more posterior sections, anterior commissural fibers are detected in the heterozygous (c, black arrows) but not in Trio-null brains (f). Scale bar, 300 μm.
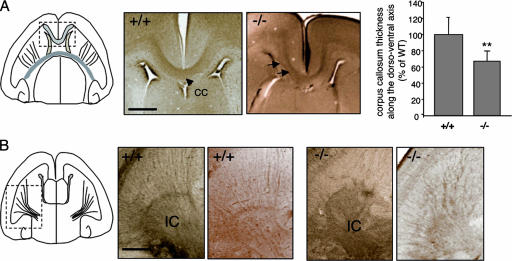
We next examined the corpus callosum in Trio-null brains. We observed subtle disorganizations of the Trio−/− corpus callosum in horizontal sections, with a few defasciculated fibers being visible (Fig. 7A). However, the reconstruction of the tract of the corpus callosum with horizontal sections revealed that Trio−/− corpus callosum thickness was decreased by 35% compared to wt corpus callosum in the dorso-ventral axis (Fig. 7A). Therefore, the corpus callosum is abnormal in Trio-deficient mice. Finally, we examined the organization of axon projections in the internal capsule in horizontal brain sections. Consistent with the defects observed in netrin-1 mutant mice (7), DCC staining revealed that the internal capsule was strongly disorganized in Trio−/− mice. The axonal projections formed a parallel array of fibers in the wt internal capsule but not in the Trio−/− mice, where they formed irregular and intermingled bundles (Fig. 7B). The defects observed in the Trio-null embryos were not due to a problem with the expression of netrin-1 since in both wt and Trio-null embryos netrin-1 had the same expression patterns (data not shown). Thus, these results indicate that Trio plays a significant role in netrin-1/DCC-dependent projections in the developing spinal cord and brain.
FIG. 7.
Defects in the corpus callosum and internal capsule in Trio-null embryos. (A) DCC immunostaining on horizontal brain sections from E18.5 Trio+/+ and Trio−/− embryos showing the corpus callosum (CC) region. In the Trio-null embryos, the corpus callosum appears slightly abnormal with some defasciculated fibers (arrows) in the horizontal sections. The right panel represents the quantification of corpus callosum thickness along the dorsoventral axis in Trio+/+ and Trio−/− embryos. Quantification has been obtained by counting the number of horizontal sections in which the corpus callosum is present and dividing by the total number of sections. The corpus callosum thickness of the Trio-null embryos is expressed relative to the thickness of the wt corpus callosum along the dorsoventral axis (n = 5 for Trio+/+ embryos; n = 8 for Trio−/− embryos; P < 0.05). Scale bar, 50 μm. (B) DCC immunostaining on horizontal brain sections from E18.5 Trio+/+ and Trio−/− embryos showing the internal capsule (IC) region. DCC-positive fibers are clearly disorganized in the internal capsule of Trio−/− embryos. Two different examples are shown. This defect was observed in 8 out of 9 Trio−/− embryos. Scale bar, 50 μm.
DISCUSSION
The findings presented here support a role for the RhoGEF Trio in axon outgrowth and guidance in mammals. We show that Trio and DCC interact in the embryonic brain, most likely independently of netrin-1. Coexpression of DCC and Trio with Nck-1 and PAK1 suggests that the Trio-DCC interaction probably occurs via the interaction of Trio with PAK1. Furthermore, the N terminus region of Trio comprising the Sec-14 and the spectrin domains mediates the interaction with PAK1. However, it still remains to be determined whether the interaction between Trio and PAK1 is direct. Altogether, these data are consistent with the results obtained in D. melanogaster, where D-Trio genetically interacts with DOCK, PAK, and Rac in controlling axon guidance of photoreceptors (36). Since Nck-1 binds to DCC through its first and third SH3 domains (27) and PAK1 binds to the second SH3 domain of Nck-1 (8), it is tempting to postulate that a cascade of molecular events implicating Nck-1-PAK interaction serves to bridge Trio to DCC. The mechanisms by which Trio becomes activated when netrin-1 binds to DCC remain unknown but may involve phosphorylation by focal adhesion kinase or the Src family kinase Fyn (32, 34).
We show here for the first time that netrin-1 treatment of embryonic brains stimulates Rac1 activity. This Rac1 activation is completely abolished in the absence of Trio, suggesting that Trio-related kalirin does not compensate for the lack of Trio in brain. Trio also activates nucleotide exchange on both RhoG and Rac1 through its first GEFD1 domain (6). Unfortunately, we could not determine whether netrin-1 is able to stimulate RhoG activity in mouse brains because of the lack of specific anti-RhoG antibodies. However, altering the specific RhoG pathway did not inhibit DCC-induced neurite outgrowth in N1E-115 cells, suggesting that it is unlikely that RhoG mediates DCC-induced Rac1 activation.
We have previously shown that human Trio plays a role in nerve growth factor-induced neurite outgrowth (11), but the role of mammalian Trio in axon outgrowth and guidance remained poorly characterized. We took advantage of the Trio-null mice to examine the axon outgrowth and guidance of Trio-deficient neurons. Our findings argue for a specific role of the GEF Trio in axon outgrowth induced by netrin-1. The cortical neurons of the Trio-null mice are defective in extending neurites in response to netrin-1 while they are able to extend neurites in response to glutamate, which has been proposed to act through different signaling pathways, including Ca2+-dependent activation of Rho GTPases (19, 23, 49). Likewise, commissural axon outgrowth from Trio−/− spinal cord explants is also reduced in response to netrin-1, while netrin-1-independent outgrowth is not affected. These data show that Trio-null neurons are not completely defective in neurite outgrowth but are specifically impaired in their axon response to netrin-1.
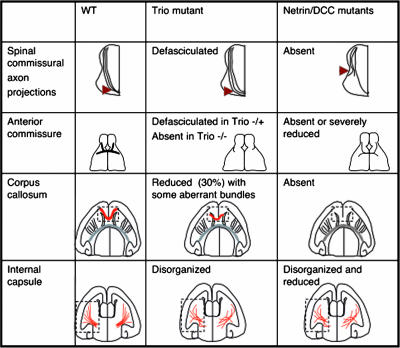
Netrin-1- and DCC-null mice present several defects in the developing spinal cord and brain commissures (13, 43). We have compared these defects with those observed in the Trio-null mice (Fig. 8). Interestingly, Trio−/− embryos show defects in the anterior commissure that are more severe than those observed in DCC- and netrin-1-deficient mice, suggesting that Trio plays a prominent role in brain morphogenesis. In addition, the observation that Trio-heterozygous mutant embryos present an intermediate phenotype with defasciculation of the axons of the anterior commissure suggests that Trio is involved in not only netrin-1-induced axon outgrowth but also guidance. The spinal commissural axons of the netrin-1- and DCC-mutant mice also show major deviations from normal trajectories only in the ventral part of the spinal cord. In the absence of Trio, the phenotype is milder, but the axon bundles are clearly defasciculated when they approach the floor plate, showing that Trio plays a role in the netrin-1-dependent pathfinding of commissural axons. Similarly, Trio contributes to the guidance of cortico-cortical projections along the corpus callosum although corpus callosum defects appear milder in Trio−/− embryos than in netrin-1 or DCC mutant mice. In the case of the corpus callosum, one could speculate that two populations of axons can be differentiated among the cortical axons projecting along the corpus callosum: one population in which the netrin-1 response is totally dependent on Trio and which would thus be defective in Trio−/− brains and another population that is not dependent on Trio and could thus project normally. Alternatively, Trio could be partially redundant with another protein in all these neurons. In addition to cortico-cortical projections, cortical axons also project to subcortical targets, including the thalamus and the spinal cord. These subcortical projections navigate in the internal capsule where reciprocal thalamic projections also extend in route toward the cortex. Netrin-1 has also been implicated in both cortico-thalamic and thalamo-cortical axon guidance (7, 41). Interestingly, we have detected a disorganization of the internal capsule in Trio−/− brains, which is also observed in netrin-1-deficient mice, supporting the hypothesis that Trio is implicated in both reciprocal pathways. In conclusion, the defects presented in the Trio−/− mice are observed in the same classes of axons that are affected in netrin-1- and DCC-deficient animals, even though the phenotypes are generally milder, except for the anterior commissure (Fig. 8).
FIG. 8.
Comparison of the phenotypes observed in the Trio-null embryos with netrin-1- and DCC-deficient embryos. Four different netrin-1- and DCC-dependent neuronal projections were examined in the Trio-null embryos, namely, the spinal commissural axon projections, the anterior commissure, the corpus callosum, and the internal capsule. The figure shows the comparison between the phenotypes observed in Trio-, netrin-1-, and DCC-deficient embryos (7, 13, 43).
Much evidence now suggests that several intracellular pathways, including mitogen-activated protein kinase, phosphatidylinositol signaling, tyrosine phosphorylation, and activation of Rac1, act in concert to mediate the response of axons to netrin-1 (3). Therefore, it is not surprising that the in vivo phenotypes of the Trio-null embryos do not reproduce exactly those seen in the netrin-1- and DCC-null mice because the Trio-null embryos are defective in Rac1 activation but not in the other netrin-1-activated signaling pathways. Future studies will help to define how these signaling pathways are interconnected in order to achieve a directed response of axons to netrin-1.
In conclusion, our study shows that Trio mediates netrin-1/DCC-induced Rac1 activation and that the role of mammalian Trio in axon guidance reflects the conserved signaling mechanisms involved in neural development throughout evolution.
Acknowledgments
We thank Jean-Michel Bellanger, Susanne Schmidt, Jean-Antoine Girault, Stéphane Richard, John J. Bergeron, and Eric Danek for helpful discussions and for critical reading of the manuscript and Tim Kennedy and Frédéric Charron for advice on spinal cord dissection. We also thank C. Jacquet and M. Plays for their technical assistance in maintaining our Trio−/− mice housed in the animal facility of the IGMM/IFR122 and Pierre Travo and all the members of the RIO-Imaging facility for their constant interest and support.
This work was supported by grants from the CNRS (A.D.), The Ligue Nationale Contre le Cancer équipe labelisée (A.D.), the CIHR (N.L.-V. and J.F.C.), FQRNT (J.F.C.), and the FRSQ-INSERM (A.D. and N. L.-V.). A.B.M. was supported by a predoctoral fellowship from the Ministère de la Recherche et de l'Enseignement Supérieur, followed by an Association pour la Recherche contre le Cancer fellowship. N.L.-V. is a recipient of a CIHR new investigator award. J.F.C. holds a Canada Research Chair Tier II.
Footnotes
Published ahead of print on 22 January 2008.
REFERENCES
- 1.Ackerman, S. L., L. P. Kozak, S. A. Przyborski, L. A. Rund, B. B. Boyer, and B. B. Knowles. 1997. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature 386838-842. [DOI] [PubMed] [Google Scholar]
- 2.Awasaki, T., M. Saito, M. Sone, E. Suzuki, R. Sakai, K. Ito, and C. Hama. 2000. The Drosophila trio plays an essential role in patterning of axons by regulating their directional extension. Neuron 26119-131. [DOI] [PubMed] [Google Scholar]
- 3.Barallobre, M. J., M. Pascual, J. A. Del Rio, and E. Soriano. 2005. The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res. Brain Res. Rev. 4922-47. [DOI] [PubMed] [Google Scholar]
- 4.Bateman, J., H. Shu, and D. Van Vactor. 2000. The guanine nucleotide exchange factor Trio mediates axonal development in the Drosophila embryo. Neuron 2693-106. [DOI] [PubMed] [Google Scholar]
- 5.Bellanger, J. M., J. B. Lazaro, S. Diriong, A. Fernandez, N. Lamb, and A. Debant. 1998. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene 16147-152. [DOI] [PubMed] [Google Scholar]
- 6.Blangy, A., E. Vignal, S. Schmidt, A. Debant, C. Gauthier-Rouviere, and P. Fort. 2000. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J. Cell Sci. 113729-739. [DOI] [PubMed] [Google Scholar]
- 7.Braisted, J. E., S. M. Catalano, R. Stimac, T. E. Kennedy, M. Tessier-Lavigne, C. J. Shatz, and D. D. O'Leary. 2000. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J. Neurosci 205792-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buday, L., L. Wunderlich, and P. Tamas. 2002. The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal. 14723-731. [DOI] [PubMed] [Google Scholar]
- 9.Debant, A., C. Serra-Pages, K. Seipel, S. O'Brien, M. Tang, S. H. Park, and M. Streuli. 1996. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate Rac- specific and Rho-specific guanine nucleotide exchange factor domains. Proc. Natl. Acad. Sci. USA 935466-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickson, B. J. 2001. Rho GTPases in growth cone guidance. Curr. Opin. Neurobiol. 11103-110. [DOI] [PubMed] [Google Scholar]
- 11.Estrach, S., S. Schmidt, S. Diriong, A. Penna, A. Blangy, P. Fort, and A. Debant. 2002. The human Rho-GEF Trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr. Biol. 12307-312. [DOI] [PubMed] [Google Scholar]
- 12.Falk, J., A. Bechara, R. Fiore, H. Nawabi, H. Zhou, C. Hoyo-Becerra, M. Bozon, G. Rougon, M. Grumet, A. W. Puschel, J. R. Sanes, and V. Castellani. 2005. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron 4863-75. [DOI] [PubMed] [Google Scholar]
- 13.Fazeli, A., S. L. Dickinson, M. L. Hermiston, R. V. Tighe, R. G. Steen, C. G. Small, E. T. Stoeckli, K. Keino-Masu, M. Masu, H. Rayburn, J. Simons, R. T. Bronson, J. I. Gordon, M. Tessier-Lavigne, and R. A. Weinberg. 1997. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 386796-804. [DOI] [PubMed] [Google Scholar]
- 14.Forsthoefel, D. J., E. C. Liebl, P. A. Kolodziej, and M. A. Seeger. 2005. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the netrin receptor Frazzled in Drosophila. Development 1321983-1994. [DOI] [PubMed] [Google Scholar]
- 15.Govek, E. E., S. E. Newey, and L. Van Aelst. 2005. The role of the Rho GTPases in neuronal development. Genes Dev. 191-49. [DOI] [PubMed] [Google Scholar]
- 16.Guan, K. L., and Y. Rao. 2003. Signalling mechanisms mediating neuronal responses to guidance cues. Nat. Rev. Neurosci. 4941-956. [DOI] [PubMed] [Google Scholar]
- 17.Hong, K., L. Hinck, M. Nishiyama, M. M. Poo, M. Tessier-Lavigne, and E. Stein. 1999. A ligand-gated association between cytoplasmic domains of UNC-5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97927-941. [DOI] [PubMed] [Google Scholar]
- 18.Huber, A. B., A. L. Kolodkin, D. D. Ginty, and J. F. Cloutier. 2003. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 26509-563. [DOI] [PubMed] [Google Scholar]
- 19.Jin, M., C. B. Guan, Y. A. Jiang, G. Chen, C. T. Zhao, K. Cui, Y. Q. Song, C. P. Wu, M. M. Poo, and X. B. Yuan. 2005. Ca2+-dependent regulation of rho GTPases triggers turning of nerve growth cones. J. Neurosci. 252338-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keino-Masu, K., M. Masu, L. Hinck, E. D. Leonardo, S. S. Chan, J. G. Culotti, and M. Tessier-Lavigne. 1996. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell 87175-185. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy, T. E., T. Serafini, J. R. de la Torre, and M. Tessier-Lavigne. 1994. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78425-435. [DOI] [PubMed] [Google Scholar]
- 22.Killeen, M., J. Tong, A. Krizus, R. Steven, I. Scott, T. Pawson, and J. Culotti. 2002. UNC-5 function requires phosphorylation of cytoplasmic tyrosine 482, but its UNC-40-independent functions also require a region between the ZU-5 and death domains. Dev. Biol. 251348-366. [DOI] [PubMed] [Google Scholar]
- 23.Kreibich, T. A., S. H. Chalasani, and J. A. Raper. 2004. The neurotransmitter glutamate reduces axonal responsiveness to multiple repellents through the activation of metabotropic glutamate receptor 1. J. Neurosci. 247085-7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamarche, N., N. Tapon, L. Stowers, P. D. Burbelo, P. Aspenstrom, T. Bridges, J. Chant, and A. Hall. 1996. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87519-529. [DOI] [PubMed] [Google Scholar]
- 25.Leonardo, E. D., L. Hinck, M. Masu, K. Keino-Masu, S. L. Ackerman, and M. Tessier-Lavigne. 1997. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 386833-838. [DOI] [PubMed] [Google Scholar]
- 26.Li, W., J. Lee, H. G. Vikis, S. H. Lee, G. Liu, J. Aurandt, T. L. Shen, E. R. Fearon, J. L. Guan, M. Han, Y. Rao, K. Hong, and K. L. Guan. 2004. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat. Neurosci. 71213-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, X., M. Meriane, I. Triki, M. Shekarabi, T. E. Kennedy, L. Larose, and N. Lamarche-Vane. 2002. The adaptor protein Nck-1 couples the netrin-1 receptor DCC (deleted in colorectal cancer) to the activation of the small GTPase Rac1 through an atypical mechanism. J. Biol. Chem. 27737788-37797. [DOI] [PubMed] [Google Scholar]
- 28.Li, X., E. Saint-Cyr-Proulx, K. Aktories, and N. Lamarche-Vane. 2002. Rac1 and Cdc42 but not RhoA or Rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (deleted in colorectal cancer) in N1E-115 neuroblastoma cells. J. Biol. Chem. 27715207-15214. [DOI] [PubMed] [Google Scholar]
- 29.Liebl, E. C., D. J. Forsthoefel, L. S. Franco, S. H. Sample, J. E. Hess, J. A. Cowger, M. P. Chandler, A. M. Shupert, and M. A. Seeger. 2000. Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF trio reveal trio's role in axon pathfinding. Neuron 26107-118. [DOI] [PubMed] [Google Scholar]
- 30.Liu, G., H. Beggs, C. Jurgensen, H. T. Park, H. Tang, J. Gorski, K. R. Jones, L. F. Reichardt, J. Wu, and Y. Rao. 2004. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat. Neurosci. 71222-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llambi, F., F. C. Lourenco, D. Gozuacik, C. Guix, L. Pays, G. Del Rio, A. Kimchi, and P. Mehlen. 2005. The dependence receptor UNC-5H2 mediates apoptosis through DAP-kinase. EMBO J. 241192-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medley, Q. G., E. G. Buchbinder, K. Tachibana, H. Ngo, C. Serra-Pages, and M. Streuli. 2003. Signaling between focal adhesion kinase and trio. J. Biol. Chem. 27813265-13270. [DOI] [PubMed] [Google Scholar]
- 33.Meijering, E., M. Jacob, J. C. Sarria, P. Steiner, H. Hirling, and M. Unser. 2004. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 58167-176. [DOI] [PubMed] [Google Scholar]
- 34.Meriane, M., J. Tcherkezian, C. A. Webber, E. I. Danek, I. Triki, S. McFarlane, E. Bloch-Gallego, and N. Lamarche-Vane. 2004. Phosphorylation of DCC by Fyn mediates Netrin-1 signaling in growth cone guidance. J. Cell Biol. 167687-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merz, D. C., H. Zheng, M. T. Killeen, A. Krizus, and J. G. Culotti. 2001. Multiple signaling mechanisms of the UNC-6/netrin receptors UNC-5 and UNC-40/DCC in vivo. Genetics 1581071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newsome, T. P., S. Schmidt, G. Dietzl, K. Keleman, B. Asling, A. Debant, and B. J. Dickson. 2000. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell 101283-294. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien, S. P., K. Seipel, Q. G. Medley, R. Bronson, R. Segal, and M. Streuli. 2000. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc. Natl. Acad. Sci. USA 9712074-12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okada, A., F. Charron, S. Morin, D. S. Shin, K. Wong, P. J. Fabre, M. Tessier-Lavigne, and S. K. McConnell. 2006. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature 444369-373. [DOI] [PubMed] [Google Scholar]
- 39.Portales-Casamar, E., A. Briancon-Marjollet, S. Fromont, R. Triboulet, and A. Debant. 2006. Identification of novel neuronal isoforms of the Rho-GEF Trio. Biol. Cell 98183-193. [DOI] [PubMed] [Google Scholar]
- 40.Ren, X. R., G. L. Ming, Y. Xie, Y. Hong, D. M. Sun, Z. Q. Zhao, Z. Feng, Q. Wang, S. Shim, Z. F. Chen, H. J. Song, L. Mei, and W. C. Xiong. 2004. Focal adhesion kinase in netrin-1 signaling. Nat. Neurosci. 71204-1212. [DOI] [PubMed] [Google Scholar]
- 41.Richards, L. J., S. E. Koester, R. Tuttle, and D. D. O'Leary. 1997. Directed growth of early cortical axons is influenced by a chemoattractant released from an intermediate target. J. Neurosci. 172445-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossman, K. L., C. J. Der, and J. Sondek. 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6167-180. [DOI] [PubMed] [Google Scholar]
- 43.Serafini, T., S. A. Colamarino, E. D. Leonardo, H. Wang, R. Beddington, W. C. Skarnes, and M. Tessier-Lavigne. 1996. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 871001-1014. [DOI] [PubMed] [Google Scholar]
- 44.Serafini, T., T. E. Kennedy, M. J. Galko, C. Mirzayan, T. M. Jessell, and M. Tessier-Lavigne. 1994. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78409-424. [DOI] [PubMed] [Google Scholar]
- 45.Shekarabi, M., and T. E. Kennedy. 2002. The netrin-1 receptor DCC promotes filopodia formation and cell spreading by activating Cdc42 and Rac1. Mol. Cell Neurosci. 191-17. [DOI] [PubMed] [Google Scholar]
- 46.Steven, R., T. J. Kubiseski, H. Zheng, S. Kulkarni, J. Mancillas, A. Ruiz Morales, C. W. Hogue, T. Pawson, and J. Culotti. 1998. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell 92785-795. [DOI] [PubMed] [Google Scholar]
- 47.Tessier-Lavigne, M., M. Placzek, A. G. Lumsden, J. Dodd, and T. M. Jessell. 1988. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature 336775-778. [DOI] [PubMed] [Google Scholar]
- 48.Xie, Y., Y. Q. Ding, Y. Hong, Z. Feng, S. Navarre, C. X. Xi, X. J. Zhu, C. L. Wang, S. L. Ackerman, D. Kozlowski, L. Mei, and W. C. Xiong. 2005. Phosphatidylinositol transfer protein-alpha in netrin-1-induced PLC signalling and neurite outgrowth. Nat. Cell Biol. 71124-1132. [DOI] [PubMed] [Google Scholar]
- 49.Zheng, J. Q., J. J. Wan, and M. M. Poo. 1996. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J. Neurosci. 161140-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]